Abstract
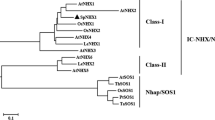
We previously cloned a vacuolar Na+/H+ antiporter gene (OsNHX1) from rice (Oryza sativa). Here we identified four additional NHX-type antiporter genes in rice (OsNHX2 through OsNHX5) and performed molecular and functional analyses of those genes. The exon–intron structure of the OsNHX genes and the phylogenetic tree of the OsNHX proteins suggest that the OsNHX proteins are categorized into two subgroups (OsNHX1 through OsNHX4 and OsNHX5). OsNHX1, OsNHX2, OsNHX3, and OsNHX5 can suppress the Na+, Li+, and hygromycin sensitivity of yeast nhx1 mutants and their sensitivity to a high K+ concentration. The expression of OsNHX1, OsNHX2, OsNHX3, and OsNHX5 is regulated differently in rice tissues and is increased by salt stress, hyperosmotic stress, and ABA. When we studied the expression of β-glucuronidase (GUS) driven by either the OsNHX1 or the OsNHX5 promoter, we observed activity in the stele, the emerging part of lateral roots, the vascular bundle, the water pore, and the basal part of seedling shoots with both promoters. In addition, each promoter had a unique expression pattern. OsNHX1 promoter–GUS activity only was localized to the guard cells and trichome, whereas OsNHX5 promoter–GUS activity only was localized to the root tip and pollen grains. Our results suggest that the members of this gene family play important roles in the compartmentalization into vacuoles of the Na+ and K+ that accumulate in the cytoplasm and that the differential regulation of antiporter gene expression in different rice tissues may be an important factor determining salt tolerance in rice.








Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ABRE:
-
ABA-responsive element
- bZIP:
-
Basic-domain leucine zipper
- EST:
-
Expressed sequence tag
- GUS:
-
β-Glucuronidase
- kb:
-
Kilobase
- 4-MU:
-
4-Methylumbelliferone
- 4-MUG:
-
4-Methylumbelliferyl-β-d-glucuronide
- PAC:
-
P1-derived artificial chromosome
- RACE:
-
Rapid amplification of cDNA ends
- RGRP:
-
Rice Genome Research Program
References
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis thaliana. Science 285:1256–1258
Axelsen KB, Palmgren MG (2001) Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol 126:696–706
Bowers K, Levi BP, Patel FI, Stevens TH (2000) The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast. Mol Biol Cell 11:4277–4294
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254
Counillon L, Franchi A, Pouysségur J (1993) A point mutation of the Na+/H+ exchanger gene (NHE1) and amplification of the mutated allele confer amiloride resistance upon chronic acidosis. Proc Natl Acad Sci USA 90:4508–4512
Fukuda A, Nakamura A, Tanaka Y (1999) Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim Biophys Acta 1446:149–155
Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y (2004a) Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol 45:146–159
Fukuda A, Chiba K, Maeda M, Nakamura A, Maeshima M, Tanaka Y (2004b) Effect of salt and osmotic stresses on the expression of genes for the vacuolar H+-pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter from barley. J Exp Bot 55:585–594
Fukuoka H, Ogawa T, Mitsuhara I, Iwai T, Isuzugawa K, Nishizawa Y, Gotoh Y, Nishizawa Y, Tagiri A, Ugaki M, Ohshima M, Yano H, Murai N, Niwa Y, Hibi T, Ohashi Y (2000) Agrobacterium-mediated transformation of monocot and dicot plants using the NCR promoter derived from soybean chlorotic mottle virus. Plant Cell Rep 19:815–820
Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana transporters, AtNHX1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96:1480–1485
Gietz RD, Schiestl RH (1995) Transforming yeast with DNA. Methods Mol Cell Biol 5:255–269
Goff SA, Ricke D, Lan T-H, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchison D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong J, Miguel T, Paszkowski U, Zhang S, Colbert M, Sun W, Chen L, Cooper B, Park S, Wood TC, Mao L, Quail P, Wing R, Dean R, Yu Y, Zharkikh A, Shen R, Sahasrabudhe S, Thomas A, Cannings R, Gutin A, Pruss D, Reid J, Tavtigian S, Mitchell J, Eldredge G, Scholl T, Miller RM, Bhatnagar S, Adey N, Rubano T, Tusneem N, Robinson R, Feldhaus J, Macalma T, Oliphant A, Briggs S (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296:92–100
Hamada A, Hibino T, Nakamura T, Takabe T (2001) Na+/H+ antiporter from Synechocystis species PCC 6803, homologous to SOS1, contains an aspartic residue and long C-terminal tail important for the carrier activity. Plant Physiol 125:437–446
Hobo T, Asada M, Kowyama Y, Hattori T (1999) ACGT-containing abscisic acid response element (ABRE) and coupling element 3 (CE3) are functionally equivalent. Plant J 19:679–689
Hood EE, Helmer GL, Fraley RT, Chilton MD (1986) The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region pTiBo542 outside of T-DNA. J Bacteriol 168:1291–1301
Kumagai H, Kouchi H (2003) Gene silencing by expression of hairpin RNA in Lotus japonicus roots and root nodules. Mol Plant Microbe Interact 16:663–668
Lam E, Chua N-H (1991) Tetramer of a 21-base pair synthetic element confers seed expression and transcriptional enhancement in response to water stress and abscisic acid. J Biol Chem 266:17131–17135
Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, Ueno K, Mochizuki A, Tanimoto H, Tsugawa H, Otsuki Y, Ohashi Y (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37:49–59
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H (2005) Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280:1561–1572
Nass R, Rao R (1998) Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. J Biol Chem 273:21054–21060
Nass R, Cunningham KW, Rao R (1997) Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. J Biol Chem 272:26145–26152
Orlowski J, Grinstein S (1997) Na+/H+ exchangers of mammalian cells. J Biol Chem 272:22373–22376
Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57:1181–1199
Prior C, Potier S, Souciet J-L, Sychrova H (1996) Characterization of the NHA1 gene encoding a Na+/H+-antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett 387:89–93
Rob M, Roelfsema G, Hedrich R (2005) In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol 167:665–691
Rodriguez-Rosales MP, Jiang X, Gálvez FJ, Aranda MN, Cubero B, Venema K (2008) Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol 179:366–377
Sato Y, Sakaguchi M (2005) Topogenic properties of transmembrane segments of Arabidopsis thaliana NHX1 reveal a common topology model of the Na+/H+ exchanger family. J Biochem 138:425–431
Shi H, Zhu J-K (2002) Regulation of expression of the vacuolar Na+/H+ antiporter gene AtNHX1 by salt stress and abscisic acid. Plant Mol Biol 50:543–550
Shi H, Ishitani M, Kim C, Zhu J-K (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901
Shi H, Quintero FJ, Pardo JM, Zhu J-K (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115:327–334
Song C-P, Guo Y, Qiu Q, Lambert G, Galbraith DW, Jagendorf A, Zhu J-K (2004) A probable Na+(K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci USA 101:10211–10216
Sze H, Padmanaban S, Cellier F, Honys D, Cheng N-H, Bock KW, Conéjéro G, Li X, Twell D, Ward JM, Hirschi KD (2004) Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol 136:2532–2547
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson J, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Venema K, Belver A, Marín-Manzano MC, Rodríguez-Rosales MP, Donaire JP (2003) A novel intracellular K+/H+ antiporter related to Na+/H+ antiporters is important for K+ ion homeostasis in plants. J Biol Chem 278:22453–22459
Véry A-A, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54:575–603
Yamaguchi T, Apse MP, Shi H, Blumwald E (2003) Topological analysis of a plant vacuolar Na+/H+ antiporter reveals a luminal C terminus that regulates antiporter cation selectivity. Proc Natl Acad Sci USA 100:12510–12515
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30:529–539
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98:12832–12836
Zhu J-K (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Acknowledgments
We thank Dr. Rajini Rao (Johns Hopkins University, USA) for kindly providing the yeast strains, K601 and R100. We also thank Drs. I. Mitsuhara and Y. Ohashi for kindly providing the vectors, pTN2 and pE2113–GUS. The cDNA clones including OsNHX2 and OsNHX5 and PAC clones including OsNHX1 and OsNHX5 were gifts from the Rice Genome Research Program. We thank Drs. Y. Nagamura and M. Yano for technical advice. We also thank C. Tsuiki, K. Toyoshima, T. Kataoka, and S. Li for technical assistance. This work was supported by Grants-in-Aid from the Ministry of Agriculture, Forestry and Fisheries of Japan (Development of Innovative Transgenic Plants no.2113 and Rice Genome Project MP-2126).
Author information
Authors and Affiliations
Corresponding author
Additional information
The nucleotide sequences reported in this paper have been submitted to the DDBJ/EMBL/GenBank with accession numbers AB531435 (OsNHX2), AB531433 (OsNHX3), and AB531434 (OsNHX5).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukuda, A., Nakamura, A., Hara, N. et al. Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta 233, 175–188 (2011). https://doi.org/10.1007/s00425-010-1289-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1289-4