Abstract
Key message
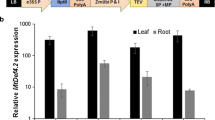
Coexpression of two antifungal genes ( NPR1 and defensin ) in transgenic peanut results in the development of resistance to two major fungal pathogens, Aspergillus flavus and Cercospora arachidicola.
Abstract
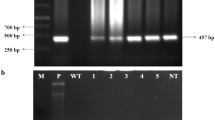
Fungal diseases have been one of the principal causes of crop losses with no exception to peanut (Arachis hypogeae L.), a major oilseed crop in Asia and Africa. To address this problem, breeding for fungal disease resistance has been successful to some extent against specific pathogens. However, combating more than one fungal pathogen via breeding is a major limitation in peanut. In the present study, we demonstrated the potential use of co-overexpression of two genes, NPR1 and defensin isolated from Brassica juncea and Trigonella foenum-graecum respectively; that offered resistance towards Aspergillus flavus in peanut. The transgenic plants not only resisted the mycelial growth but also did not accumulate aflatoxin in the seeds. Resistance was also demonstrated against another pathogen, Cercospora arachidicola at varied levels; the transgenic plants showed both reduction in the number of spots and delay in the onset of disease. PCR, Southern and Western blot analysis confirmed stable integration and expression of the transgenes in the transgenic plants. The combinatorial use of the two pathogen resistance genes presents a novel approach to mitigate two important fungal pathogens of peanut.







Similar content being viewed by others
References
Arthur E, Waltking DW, Chan D, Dunn EJ, Humphries Kandler H, Sizoo E (2006) Liquid chromatographic analysis of aflatoxin using post-column photochemical derivatization: collaborative study. J AOAC Int 89:678–692
Broekaert WF, Terras FR, Cammue BP, Osborn RW (1995) Plant defensins: novel antimicrobial peptides as components of the host defence system. Plant Physiol 108:1353–1358
Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254:1194–1197
Cao H, Li X, Dong X (1998) Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc Natl Acad Sci USA 95:6531–6536
Chern MS, Fitzgerald HA, Yadav RC, Canlas PE, Dong X, Ronald PC (2001) Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J 27:101–113
Dana MM, Pintor-Toro JA, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142:722–730
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Emani C, Garcia JM, Lopata-Finch E, Pozo MJ, Uribe P, Kim DJ, Sunilkumar G, Cook DR, Kenerley CM, Rathore KS (2003) Enhanced fungal resistance in transgenic cotton expressing an endochitinase gene from Trichoderma virens. Plant Biotechnol J 1:321–336
Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261:754–756
Ghag SB, Shekhawat UKS, Ganapathi TR (2012) Petunia floral defensins with unique prodomains as novel candidates for development of Fusarium Wilt resistance in transgenic banana plants. PLoS One 7(6):e39557
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. J Plant Mol Biol 25:989–994
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Jha S, Chattoo BB (2010) Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res 19:373–384
Kumar V, Parkhi V, Kenerley CM, Rathore KS (2009a) Defence related gene expression and enzyme activities in transgenic cotton plants expressing an endochitinase gene from Trichoderma virens in response to interaction with Rhizoctonia solani. Planta 230:277–291
Kumar AM, Sundaresha S, Rohini S (2009b) Resistance to Alternaria leaf spot disease in transgenic safflower (Carthamus tictorius L.) harboring a rice chitinase gene. Trans Plant J 3:113–118
Kumar V, Joshi SG, Bell AA, Rathore KS (2013) Enhanced resistance against Thielaviopsis basicola in transgenic cotton plants expressing Arabidopsis NPR1 gene. Transgenic Res 22:359–368
Lin WC, Lu CF, Wu JW, Cheng ML, Lin YM, Yang NS, Black L, Green SK, Wang JF, Cheng CP (2004) Transgenic tomato plants expressing the Arabidopsis AtNPR1 gene display enhanced resistance to a spectrum of fungal and bacterial diseases. Transgenic Res 13:567–581
Makandar R, Essig JS, Schapaugh MA, Trick HN, Shah J (2006) Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol Plant Microbe Interact 19:123–129
Malnoy M, Jin Q, Borejsza-Wysocka EE, He SY, Aldwinckle HS (2007) Overexpression of the Apple MpNPR1 gene confers increased disease resistance in Malus domestica. Mol Plant Microbe Interact 20:1568–1580
Meur G, Budatha M, Srinivasan T, Rajesh Kumar KR, Dutta Gupta A, Kirti PB (2008) Constitutive expression of Arabidopsis NPR1 confers enhanced resistance to the early instars of Spodoptera litura in transgenic tobacco. Physiol Plant 133:765–775
Olli S, Kirti PB (2006) Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenum-graecum L. J Biochem Mol Biol 39:278–283
Osborn RW, De Samblanx GW, Thevissen K, Goderis I, Torrekens S, Van Leuven F, Attenborough S, Rees SB, Broekaert WF (1995) Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett 368:257–262
Parkhi V, Kumar V, Campbell LAM, Bell AA, Rathore KS (2010a) Expression of Arabidopsis NPR1 in transgenic cotton confers resistance to non-defoliating isolates of Verticillium dahliae but not the defoliating isolates. J Phytopathol 158:822–825
Parkhi V, Kumar V, Campbell LM, Bell AA, Shah J, Rathore KS (2010b) Resistance against various fungal pathogens and reniform nematode in transgenic cotton plants expressing Arabidopsis NPR1. Trans Res 19:959–975
Pieterse CM, Van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7(4):456–464
Punja ZK (2006) Recent developments toward achieving fungal disease resistance in transgenic plants. Can J Plant Pathol 28:S298–S308
Ramineni R, Sadumpati V, Khareedu VR, Vudem DR (2014) Transgenic pearl millet male fertility restorer line (ICMP451) and hybrid (ICMH451) expressing Brassica juncea nonexpressor of Pathogenesis Related Genes 1 (BjNPR1) exhibit resistance to downy mildew disease. PLoS One 9:e90839
Rivero M, Furman N, Mencacci N, Picca P, Toum L, Lentz E, Bravo-Almonacid F, Mentaberry A (2012) Stacking of antimicrobial genes in potato transgenic plants confers increased resistance to bacterial and fungal pathogens. J Biotechnol 157:334–343
Rohini VK, Rao KS (2001) Transformation of peanut (Arachi hypogaea L.) with tobacco chitinase gene: variable responses of transformants to leaf spot disease. Plant Sci 16:889–898
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Sadumpati V, Kalamburb M, Vudema DR, Kirti PB, Kharredu VR (2013) Transgenic indica rice lines, expressing Brassica juncea nonexpressor of pathogenesis-related genes 1 (BjNPR1), exhibit enhanced resistance to major pathogens. J Biotechnol 166:114–121
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: laboratory manual, 2nd edn. Coldspring Harbor Press, New York
Sundaresha S, Kumar MA, Rohini S, Math SA, Keshamma E, Chandrashekar SC, Udayakumar M (2010) Enhanced protection against two major fungal pathogens of peanut, Cercospora arachidicola and Aspergillus flavus in transgenic peanut over-expressing a tobacco β 1–3 glucanase. Eur J Plant Pathol 126:497–508
Thevissen K, Warnecke DC, Francois IEJA, Leipelt M, Heinz E, Ott C, Zähringer U, Thomma BPHJ, Ferket KKA, Cammue BPA (2004) Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem 279:3900–3905
Thirulogachandar V, Prachi P, Vaishnavi CS, Reddy MK (2011) An affinity-based genome walking method to find transgene integration loci in transgenic genome. Anal Biochem 416:196–201
Thomma BPHJ, Cammue BPA, Thevissen K (2002) Plant defensins. Planta 216:193–202
Vasavirama K, Kirti PB (2013) Constitutive expression of a fusion gene comprising Trigonella foenum-graecum defensin (Tfgd2) and Raphanus sativus antifungal protein (RsAFP2) confers enhanced disease and insect resistance in transgenic tobacco. Plant Cell Tissue Organ Cult 115:309–319
Verma SS, Yajima WR, Rahman MH, Shah S, Liu JJ, Ekramoddoullah AK, Kav NN (2012) A cysteine-rich antimicrobial peptide from Pinus monticola (PmAMP1) confers resistance to multiple fungal pathogens in canola (Brassica napus). Plant Mol Biol 79:61–74
Vijayan S, Kirti PB (2012) Mungbean plants expressing BjNPR1 exhibit enhanced resistance against the seedling rot pathogen, Rhizoctonia solani. Transgenic Res 21:193–200
Wally O, Jayaraj J, Punja ZK (2009) Broad-spectrum disease resistance to necrotrophic and biotrophic pathogens in transgenic carrots (Daucus carota L.) expressing an Arabidopsis NPR1 gene. Planta 231:131–141
Zhang X, Francis MI, Dawson WO, Graham JH, Orbović V, Triplett EW, Mou Z (2010) Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur J Plant Pathol 128:91–100
Acknowledgments
The authors wish to acknowledge financial support from NABARD (National Bank for Agriculture and Rural Development), India and Biotech Hub, Department of Biotechnology, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Prasad.
S. Sundaresha, S. Rohini and V. K. Appanna have made equal contributions to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sundaresha, S., Rohini, S., Appanna, V.K. et al. Co-overexpression of Brassica juncea NPR1 (BjNPR1) and Trigonella foenum-graecum defensin (Tfgd) in transgenic peanut provides comprehensive but varied protection against Aspergillus flavus and Cercospora arachidicola . Plant Cell Rep 35, 1189–1203 (2016). https://doi.org/10.1007/s00299-016-1945-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1945-7