Abstract
Canola (Brassica napus), an agriculturally important oilseed crop, can be significantly affected by diseases such as sclerotinia stem rot, blackleg, and alternaria black spot resulting in significant loss of crop productivity and quality. Cysteine-rich antimicrobial peptides isolated from plants have emerged as a potential resource for protection of plants against phytopathogens. Here we report the significance of an antimicrobial peptide, PmAMP1, isolated from western white pine (Pinus monticola), in providing canola with resistance against multiple phytopathogenic fungi. The cDNA encoding PmAMP1 was successfully incorporated into the genome of B. napus, and it’s in planta expression conferred greater protection against Alternaria brassicae, Leptosphaeria maculans and Sclerotinia sclerotiorum. In vitro experiments with proteins extracted from transgenic canola expressing Pm-AMP1 demonstrated its inhibitory activity by reducing growth of fungal hyphae. In addition, the in vitro synthesized peptide also inhibited the growth of the fungi. These results demonstrate that generating transgenic crops expressing PmAMP1 may be an effective and versatile method to protect susceptible crops against multiple phytopathogens.









Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Broekaert WF, Cammue BPA, De Bolle MF, Thevissen K, De Samblanx GW, Osborn RW (1997) Antimicrobial peptides from plants. Crit Rev Plant Sci 16:297–323
Cook J (2006) Towards cropping systems that enhance productivity and sustainability. PNAS 103:18389–18394
De Caleya RF, Gonzalez-Pascual B, Garcia-Olmedo F, Carbonero P (1972) Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Microbiol 23:998–1000
De Gray G, Rajasekaran K, Smith F, Sanford J, Daniell H (2001) Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol 127:852–862
Dokken FL, Bouchard AJ, Bassendowski KA, Boyle T, Cowell LE, Gugel RK et al (2009) Survey of canola diseases in Saskatchewan, 2008. Can Plant Dis Surv 89:113–114
Ekramoddoullah AKM, Liu J–J, Zamani A (2006) Cloning and characterization of a putative antifungal peptide gene (Pm-AMP1) in Pinus monticola. Phytopathology 96:165–170
Falla T, Karunaratne N, Hancock R (1996) Mode of action of the antimicrobial peptide indolicidin. J Biol Chem 271:19293–19303
Ferket KK, Levery SB, Park C, Cammue BP, Thevissen K (2003) Isolation and characterization of Neurospora crassa mutants resistant to antifungal plant defensins. Fungal Genet Biol 40:176–185
Ferreira RB, Moneiro S, Freitas R, Santos CN, Chen Z, Batista LM, Duarte J, Borges A, Teixeira AR (2007) The role of plant defence proteins in fungal pathogenesis. Mol Plant Pathol 8:677–700
Gao A, Hakimi SM, Mittanck CA et al (2000) Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotechnol 18:1307–1310
Garcia-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P (1998) Plant defense peptides. Biopolymers 47:479–491
Hammami R, Ben Hamida J, Vergoten G, Fliss I (2009) PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acid Res 37:D963–D968
Hancock REW (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1:156–164
Kazan K, Rusu A, Marcus JP, Goulter KC, Manners JM (2002) Enhanced quantitative resistance to Leptospharia maculans conferred by expression of a novel antimicrobial peptide in canola (Brassica napus L.). Mol Breeding 10:63–70
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee SC, Hwang IS, Choi HW, Hwang BK (2008) Involvement of the pepper antimicrobial protein CaAMP1 gene in broad spectrum disease resistance. Plant Physiol 148:1004–1020
Li Q, Lawrence CB, Xing HY, Babbitt RA, Bass WT, Maiti IB, Everett NP (2001) Enhanced disease resistance conferred by expression of an antimicrobial magainin analog in transgenic tobacco. Planta 212:635–639
Manners JM (2009) Primitive defence: the MiAMP1 antimicrobial peptide family. Plant Mol Biol Rep 27:237–242
Marcus JP, Goulter KC, Green JL, Harrison SJ, Manners JM (1997) Purification and characterization of an antimicrobial peptide from Macadamia integrifolia. Eur J Biochem 244:743–749
Marshall E, Costa LM, Marsoce JG (2011) Cysteine-Rich Peptides (CRPs) mediate diverse aspects of cell–cell communication in plant reproduction and development. J Exp Bot 62:1677–1686
McManus AM, Nielsen KJ, Marcus JP et al (1999) MiAMP1, a novel protein from Macadamia integrifolia adopts a Greek key beta barrel fold unique amongst plant antimicrobial proteins. J Mol Biol 293:629–638
Meena PD, Awasthi RP, Chattaopadhyay C, Kolte SJ, Kumar A (2010) Alternaria blight: a chronic disease in rapersepeed–mustard. J Oilseed Brassica 1:1–11
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8:238–242
Montesinos E (2007) Antimicrobial peptides and plant disease control. FEMS Microbiol Lett 270:1–11
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Oh IS, Park AR, Bae MS, Kwon SJ, Kim YS, Lee JE, Kang NY, Lee S, Cheong H, Park OK (2005) Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell 17:2832–2847
Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S (2000) Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat Biotechnol 18:1162–1166
Pillay V, Polya GM, Spangenberg GC (2011) Otimization of an in vitro antifungal protein assay for the screening of potential antifungal proteins against Leptosphaeria maculans. J Microbiol Methods 84:121–127
Pino LE, Lombardi-Crestana S, Azevedo MS et al (2010) The Rg1 allele as a valuable tool for genetic transformation of the tomato ‘Micro-Tom’ model system. Plant Methods 6:23. doi:10.1186/1746-4811-6-23
Pope SJ, Varney PL, Sweet JB (1989) Susceptibility of cultivars of oilseed rape to Sclerotinia sclerotiorum and the effect of infection on yield. Asp Appl Biol 23:451–456
Powers JS, Hancock REW (2003) The relationship between peptide structure and antibacterial activity. Peptides 24:1681–1691
Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ et al (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol 139:1902–1913
Schardl CL, Byrd AD, Benzion G, Altschuler MA, Hilderbrand DF, Hunt AG (1987) Design and construction of a versatile system for the expression of foreign genes in plants. Gene 61:1–11
Selitrennikoff CP (2001) Antifungal proteins. Appl Environ Microbiol 67:2883–2894
Sharma N, Rahman MH, Strelkov S, Thiagarajah M, Bansal VK, Kav NNV (2007) Proteome-level changes in two Brassica napus lines exhibiting differential responses to the fungal pathogen Alternaria brassicae. Plant Sci 172:95–110
Spelbrink RG, Dilmac N, Allen A, Smith TJ, Shah DM, Hockerman GH (2004) Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol 135:2055–2067
Srivastava S, Rahman MH, Shah S, Kav NN (2006) Constitutive expression of the pea ABA-responsive 17 (ABR17) cDNA confers multiple stress tolerance in Arabidopsis thaliana. Plant Biotechnol J 4:529–549
Takai K, Sawasaki T, Endo Y (2010) Practical cell-free protein synthesis system using purified wheat embryos. Nat Protocol 5:227–238
Terras FRG, Goderis IJ, Van Leuven F, Vanderleyden J, Cammue BPA, Broekaert WF (1992a) In vitro antifungal activity of a radish (Raphanus sativus L.) seed protein homologous to nonspecific lipid transfer proteins. Plant Physiol 100:1055–1058
Terras FRG, Schoofs HME, De Bolle MFC, Van Leuven F, Rees SB, Vanderleyden J, Cammue BPA, Broekaert WF (1992b) Analysis of two novel classes of antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem 267:15301–15309
Terras FRG, Eggermont K, Kovaleva V et al (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Thevissen K, Kristensen H–H, Thomma BPHJ, Cammue BPA, François IEJA (2007) Therapeutic potential of antifungal plant and insect defensins. Drug Discovery Today 12:966–971
Wang Y, Nowak G, Culley D, Hadwiger LA, Fristensky B (1999) Constitutive expression of pea defense gene DRR206 confers resistance to blackleg (Leptosphaeria maculans) disease in transgenic canola (Brassica napus). Mol Plant-Microbe Interact 12:410–418
West JS, Kharbanda P, Barbetti MJ, Fitt BDL (2001) Epidemiology and management of Leptosphaeria maculans (phoma stem canker) in Australia, Canada and Europe. Plant Pathol 50:10–27
Wise AA, Liu Z, Binns AN (2006) Three methods for the introduction of foreign DNA into Agrobacterium. Methods Mol Biol 343:43–53
Yajima W, Rahman MH, Das D, Suresh MR, Kav NN (2008) Detection of Sclerotinia sclerotiorum using a monomeric and dimeric single-chain fragment variable (scFv) antibody. J Agric Food Chem 56:9455–9463
Yajima W, Verma SS, Shah S, Rahman MH, Liang Y, Kav NNV (2010) Expression of anti-sclerotinia scFV in transgenic Brassica napus enhances tolerance against stem rot. New Biotechnol 27:816–821
Yeung EC, Saxena PK (2005) Histological techniques. In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants. Springer, Dordrecht, pp 517–537
Yevtushenko DP, Misra S (2007) Comparison of pathogen-induced expression and efficacy of two amphibian antimicrobial peptides, MsrA2 and temporin A, for engineering wide-spectrum disease resistance in tobacco. Plant Biotechnol J 5:720–734
Zamany A, Liu J–J, Emramoddoullah A, Sniezko R (2011) Antifungal activity of a Pinus monticola antimicrobial peptide 1 (Pm-AMP1) and its accumulation in western white pine infected with Cronartium ribicola. Can J Microbiol 57:667–679
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhou M, Hu Q, Li Z, Li D, Chen CF, Luo H (2011) Expression of a novel antimcirobial peptide penaeidin4-1 in creeping bentgrass (Agrostis stolonifera L.) enhances plant fungal disease resistance. PLoS One 6:e24677. doi:10.1371/journal.pone.0024677
Acknowledgments
This research was supported by funding from Agriculture Funding Consortium, the Alberta Canola Producers Commission (ACPC), Alberta Crop Industry Development Fund (ACIDF) and the Canola Council of Canada which is gratefully acknowledged. We are also thankful to MBSU and the microscopy unit, as well as Department of Agricultural, Food and Nutritional Science of University of Alberta for providing research facilities. We would also like to thank A. Kamran for help with the statistical analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2012_9895_MOESM1_ESM.tif
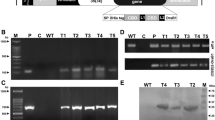
T-DNA of plant transformation vector pPKYLX-71. PmAMP1 cDNA driven by constitutive promoter CAMV35S and pea rbcS30 polyA terminator and neomycin phosphotransferase (nptII) coding gene between nos promoter and nos terminator are shown within the T-DNA; LB, left T-DNA border; RB, right T-DNA border (TIFF 462 kb)
11103_2012_9895_MOESM2_ESM.tif
Immunohistochemical analysis of Alternaria brassicae (a), Leptosphaeria maculans (b) and Sclerotinia sclerotiorum (c) showing the immunolocalization of PmAMP1. The insets show the transverse section of fungal mycelia. One representative control (A. brassicae without primary antibody) is shown (d) out of all six negative controls of 3 species with either primary or secondary antibody. The arrows indicate the cell walls of the fungal hyphae, which are not detected by the antibody (TIFF 8,714 kb)
11103_2012_9895_MOESM3_ESM.tif
Predicted three dimensional structure of PmAMP1 employing the tool available on the ExPASY-Pyhre2 server package. The putative structure shows the organization of the protein into β-strands which are furthered ordered into motifs similar to previously reported structure of the MiAMP1 (TIFF 2,475 kb)
Rights and permissions
About this article
Cite this article
Verma, S.S., Yajima, W.R., Rahman, M.H. et al. A cysteine-rich antimicrobial peptide from Pinus monticola (PmAMP1) confers resistance to multiple fungal pathogens in canola (Brassica napus). Plant Mol Biol 79, 61–74 (2012). https://doi.org/10.1007/s11103-012-9895-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9895-0