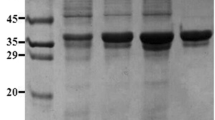
Abstract
(S)-4-chloro-3-hydroxybutanoate ((S)-CHBE) is an important chiral intermediate to synthesize the side chain of cholesterol-lowering drug atorvastatin. To biosynthesize the (S)-CHBE, a recombinant Escherichia coli harboring the carbonyl reductase and glucose dehydrogenase was successfully constructed. The recombinant E. coli was cultured in a 500-L fermentor; after induction and expression, the enzyme activity and cell biomass were increased to 23,661.65 U/L and 13.90 g DCW/L which was 3.24 and 2.60-folds compared with those in the 50 L fermentor. The biocatalytic process for the synthesis of (S)-CHBE in an aqueous-organic solvent system was constructed and optimized with a substrate fed-batch strategy. The ethyl 4-chloro-3-oxobutanoate concentration reached to 1.7 M, and the (S)-CHBE with yield of 97.2 % and enantiomeric excess (e.e.) of 99 % was obtained after 4-h reaction in a 50-L reactor. In this study, the space-time yield and space-time yield per gram of biomass (dry cell weight, DCW) were 413.17 mM/h and 27.55 mM/h/g DCW for (S)-CHBE production, respectively, which were the highest values as compared to previous reports. Finally, (S)-CHBE was extracted from the reaction mixture with 82 % of yield and 95 % of purity. This study paved the foundation for the upscale production of (S)-CHBE by biocatalysis method.









Similar content being viewed by others
References
An M, Cai P, Yan M, Hao N, Wang S, Liu H, Li Y, Xu L (2012) A novel reductase from Candida albicans for the production of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 76:1210–1212
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312
Applegate GA, Cheloha RW, Nelson DL, Berkowitz DB (2011) A new dehydrogenase from Clostridium acetobutylicum for asymmetric synthesis: dynamic reductive kinetic resolution entry into the Taxotere side chain. Chem Commun 47:2420–2422
Cho CW, Cho YHG, Chun JP, Roh KR, Shin JH, Yu HS (2002) Process for producing optically pure δ-hydroxy-β-ketoester derivatives. WO 2002096915 A1
Chung CT, Niemela SL, Miller RH (1989) One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci 86:2172–2175
Clark JH (1999) Green chemistry: challenges and opportunities. Green Chem 1:1–8
Engelking H, Pfaller R, Wich G, Weuster-Botz D (2004) Stereoselective reduction of ethyl 4-chloro acetoacetate with recombinant Pichia pastoris. Tetrahedron Asymmetry 15:3591–3593
Hoff BH, Anthonsen T (1999) Lipase-catalyzed resolution of esters of 4-chloro-3-hydroxybutanoic acid: effects of the alkoxy group and solvent on the enantiomeric ratio. Tetrahedron Asymmetry 10:1401–1412
Kaliaperumal T, Kumar S, Gummadi SN, Chadha A (2010) Asymmetric synthesis of (S)-ethyl-4-chloro-3-hydroxybutanoate using Candida parapsilosis ATCC 7330. J Ind Microbiol Biotechnol 37:159–165
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Madec J, Pfister X, Phansavath P, Ratovelomanana-Vidal V, Genet JP (2001) Asymmetric hydrogenation reactions using a practical in situ generation of chiral ruthenium-diphosphine catalysts from anhydrous RuCl3. Tetrahedron 57:2563–2568
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetry 20:513–557
Morikawa S, Nakai T, Yasohara Y, Nanba H, Kizaki N, Hasegawa J (2005) Highly active mutants of carbonyl reductase S1 with inverted coenzyme specificity and production of optically active alcohols. Biosci Biotechnol Biochem 69:544–552
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetry 14(18):2659–2681
Nie Y, Xiao R, Xu Y, Montelione GT (2011) Novel anti-Prelog stereospecific carbonyl reductases from Candida parapsilosis for asymmetric reduction of prochiral ketones. Org Biomol Chem 9:4070–4078
Qiu L, Qi J, Pai CC, Chan S, Zhou Z, Choi MC, Chan AS (2002) Synthesis of novel diastereomeric diphosphine ligands and their applications in asymmetric hydrogenation reactions. Org Lett 4:4599–4602
Roth BD (2002) The discovery and development of atorvastatin, a potent novel hypolipidemic agent. Prog Med Chem 40:1–22
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press
Shimoda K, Kubota N, Hamada H, Kobayashi T, Hamada H, Shafi SM, Nakajima N (2009) Production of (2R, 3S)-2-benzamidomethyl-3-hydroxybutanoates by immobilized plant cells of parthenocissus tricuspidata. Biochem Insights 2:1–3
Soni P, Banerjee UC (2005) Biotransformations for the production of the chiral drug (S)-duloxetine catalyzed by a novel isolate of Candida tropicalis. Appl Microbiol Biotechnol 67:771–777
Sundby E, De Zotti M, Anthonsen T (2003) The enantioselectivity of reduction of ethyl 4-halo-3-oxobutanoate catalyzed by Geotrichum candidum depends on the cofactor. J Mol Catal B Enzym 21:63–66
Wang LJ, Li CX, Ni Y, Zhang J, Liu X, Xu JH (2011) Highly efficient synthesis of chiral alcohols with a novel NADH-dependent reductase from Streptomyces coelicolor. Bioresour Technol 102:7023–7028
Yamamoto H, Mitsuhashi K, Kimoto N, Matsuyama A, Esaki N, Kobayashi Y (2004) A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 68:638–649
Yasohara Y, Kizaki N, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Stereoselective reduction of alkyl 3-oxobutanoate by carbonyl reductase from Candida magnoliae. Tetrahedron Asymmetry 12:1713–1718
Ye Q, Yan M, Yao Z, Xu L, Cao H, Li ZJ, Chen Y, Li SY, Bai JX, Xiong J, Ying HJ, Ouyang PK (2009) A new member of the short-chain dehydrogenases/reductases superfamily: Purification, characterization and substrate specificity of a recombinant carbonyl reductase from Pichia stipitis. Bioresour Technol 100:6022–6027
Ye Q, Cao H, Zang GL, Mi L, Yan M, Wang Y, Zhang YY, Li XM, Li JA, Xu L, Xiong JA, Ouyang PK, Ying HJ (2010) Biocatalytic synthesis of (S)-4-chloro-3-hydroxybutanoate ethyl ester using a recombinant whole-cell catalyst. Appl Microbiol Biotechnol 88:1277–1285
Ye Q, Ouyang PK, Ying H (2011) A review-biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives. Appl Microbiol Biotechnol 89:513–522
You ZY, Liu ZQ, Zheng YG (2014) Characterization of a newly synthesized carbonyl reductase and construction of a biocatalytic process for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with high space-time yield. Appl Microbiol Biotechnol 98:1671–1680
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (No. 2011CB710800), Natural Science Foundation of Zhejiang Province (No. R3110155) and Qianjiang Talent Project of Zhejiang Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 131 kb)
Rights and permissions
About this article
Cite this article
Liu, ZQ., Ye, JJ., Shen, ZY. et al. Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl Microbiol Biotechnol 99, 2119–2129 (2015). https://doi.org/10.1007/s00253-014-6245-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6245-y