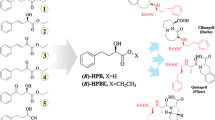
Abstract
Ethyl (S)-4-chloro-3-hydroxybutanoate ester ((S)-CHBE) is a precursor of enantiopure intermediates used for the production of chiral drugs, including the cholesterol-lowering 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitors (statins). The asymmetric reduction of ethyl 4-chloro-3-oxobutanoate ester (COBE) to (S)-CHBE by biocatalysis has several positive attributes, including low cost, mild reaction conditions, high yield, and a high level of enantioselectivity. During genome database mining of the yeast Pichia stipitis, our group found two novel carbonyl reductases (PsCRI and PsCRII) that have a promising future for the industrial production of (S)-CHBE with >99% enantiomeric excess. This review covers the main process of biosynthesis of (S)-CHBE: screening of microorganisms that catalyze the reduction of COBE to (S)-CHBE (I); gene cloning, expression, and characterization of carbonyl reductases for the production of (S)-CHBE in Escherichia coli (II); development of cofactor generation systems for regenerating cofactors (III); and biocatalysis of COBE to (S)-CHBE by recombinant E. coli (IV).




Similar content being viewed by others
References
Asako H, Shimizu M, Itoh N (2009) Biocatalytic production of (S)-4-bromo-3-hydroxybutyrate and structurally related chemicals and their applications. Appl Microbiol Biotechnol 84:397–405
Asako H, Shimizu M, Makino Y, Itoh N (2010) Biocatalytic reduction system for the production of chiral methyl (R)/(S)-4-bromo-3-hydroxybutyrate. Tetrahedron Asymmetr Lett 51:2664–2666
Barrios-González J, Miranda RU (2009) Biotechnological production and applications of statins. Appl Microbiol Biotechnol 85:869–883
Bühler B, Park JB, Blank LM, Schmid A (2008) NADH availability limits asymmetric biocatalytic epoxidation in a growing recombinant Escherichia coli strain. Appl Environ Microbiol 74:1436–1446
Cao H, Lan M, Ye Q, Zang G, Yan M, Wang Y, Zhang YY, Li XM, Xu L, Xiong J, OuYang PK, Ying HJ (2010) Purification and characterization of a novel NADH-dependent carbonyl reductase from Pichia stipitis involved in biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresour Technol. doi:10.1016/j.biortech.2010.08.072
Casar Z (2010) Historic overview and recent advances in the synthesis of super-statins. Curr Org Chem 14:816–845
Chakraborty AA, Phadke RP, Chaudhary FA, Shete PS, Rao BS, Jasani KD (2005) Optimization of redox reactions employing whole cell biocatalysis. World J Microbiol Biotechnol 21:221–227
Goldberg K, Schroer K, Lütz S, Liese A (2007) Biocatalytic ketone reduction—a powerful tool for the production of chiral alcohols—part I: processes with isolated enzymes. Appl Microbiol Biotechnol 76:237–248
Gouet P, Robert X, Courcelle E (2003) ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res 31:3320–3323
Gough S, Dostal L, Howe A, Deshpande M, Scher M, Rosazza JNP (2005) Production of pyruvate from lactate using recombinant Pichia pastoris cells as catalyst. Process Biochem 40:2597–2601
Hanniga G, Makridesa SC (1998) Strategies for optimizing heterologous protein expression in Escherichia coli. Trends Biothchnol 16:54–60
He JY, Sun ZH, Ruan WQ, Xu Y (2006) Biocatalytic synthesis of ethyl (S)-4-chloro-3-hydroxy-butanoate in an aqueous-organic solvent biphasic system using Aureobasidium pullulans CGMCC 1244. Process Biochem 41:244–249
Hummel W (1999) Large-scale applications of NAD(P)-dependent oxidoreductases: recent developments. Trends Biothchnol 17:487–492
Hummel W, Abokitse K, Drauz K, Rollmann C, Gröger H (2003) Towards a large-scale asymmetric reduction process with isolated enzymes: expression of an (S)-alcohol dehydrogenase in E. coli and studies on the synthetic potential of this biocatalyst. Adv Synth Catal 345:713–715
Husken LE, Dalm MCF, Tramper J, Wery J, de Bont JAM, Beeftink R (2001) Integrated bioproduction and extraction of 3-methylcatechol. J Biotechnol 88:11–19
Inoue K, Makino Y, Itoh N (2005) Production of (R)-chiral alcohols by a hydrogen-transfer bioreduction with NADH-dependent Leifsonia alcohol dehydrogenase (LSADH). Tetrahedron Asymmetr 16:2539–2549
Ishige T, Honda K, Shimizu S (2005) Whole organism biocatalysis. Curr Opin Chem Biol 9:174–180
Itoh N, Asako H, Banno K, Makino Y, Shinohara M, Dairi T, Wakita R, Shimizu M (2004) Purification and characterization of NADPH-dependent aldo-keto reductase specific for β-keto esters from Penicillium citrinum, and production of methyl (S)-4-bromo-3-hydroxybutyrate. Appl Microbiol Biotechnol 66:53–62
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilizations—aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Karanewsky DS, Badia MC, Ciosek CP Jr, Robl JF, Sofia MJ, Simpkins LM, Delange B, Harrity TW, Biller SA, Gordon EM (1990) Phosphorus-containing inhibitors of HMG-CoA reductase. 1. 4-[(2-arylethyl)hydroxyphosphinyl]-3-hydroxy-butanoic acids: a new class of cell-selective inhibitors of cholesterol biosynthesis. J Med Chem 33:2952–2956
Kataoka M, Doi Y, Sim TS, Shimizu S, Yamada H (1992a) A novel NADPH-dependent carbonyl reductase of Candida macedoniensis: purification and characterization. Arch Biothem Biophys 294:469–474
Kataoka M, Shimizu S, Yamada H (1992b) Distribution and immunological characterization of microbial aldehyde reductases. Arch Microb 157:279–283
Kataoka M, Rohani LPS, Yamamoto K, Wada M, Kawabata H, Kita K, Yanase H, Shimizu S (1997) Enzymatic production of ethyl (R)-4-chloro-3-hydroxybutanoate: asymmetric reduction of ethyl 4-chloro-3-oxobutanoate by an Escherichia coli transformant expressing the aldehyde reductase gene from yeast. Appl Microbiol Biotechnol 48:699–703
Kataoka M, Kita K, Wada M, Yasohara Y, Hasegawa J, Shimizu S (2003) Novel bioreduction system for the production of chiral alcohols. Appl Microbiol Biotechnol 62:437–445
Kataoka M, Hoshino-Hasegawa A, Thiwthong R, Nanami H, Ishige T, Shimizu S (2006) Gene cloning of an NADPH-dependent menadione reductase from Candida macedoniensis, and its application to chiral alcohol production. Enzyme Microb Technol 38:944–951
Kita K, Kataoka M, Shimizu S (1999a) Diversity of 4-chloroacetoacetate ethyl ester—reducing enzymes in yeasts and their application to chiral alcohol synthesis. J Biosci Bioeng 6:591–598
Kita K, Nakase K, Yanase H, Kataoka M, Shimizu S (1999b) Purification and characterization of new aldehyde reductases from Sporobolomyces salmonicolor AKU4429. J Mol Catal B Enzym 6:305–313
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Lee SH, Park OJ (2009) Uses and production of chiral 3-hydroxy-γ-butyrolactones and structurally related chemicals. Appl Microbiol Biotechnol 84:817–828
Liljeblad A, Kallinen A, Kanerva LT (2009) Biocatalysis in the preparation of the statin side chain. Curr Org Synth 6:362–379
Liu WF, Wang P (2007) Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnol Adv 25:369–384
Makrides S (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetr 20:513–557
Matsuyama A, Yamamoto H, Kobayashi Y (2002) Practical application of recombinant whole-cell biocatalysts for the manufacturing of pharmaceutical intermediates such as chiral alcohols. Org Process Res Dev 6:558–561
Michael M (2005) Chemoenzymatic synthesis of building blocks for statin side chains. Angew Chem Int Ed 44:362–365
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetr 14:2659–2681
Oppermann U, Filling C, Jörnvall H (2001) Forms and functions of human SDR enzymes. Chem Biol Interact 130–132:699–705
Panke S, Wubbolts M (2005) Advances in biocatalytic synthesis of pharmaceutical intermediates. Curr Opin Chem Biol 9:188–194
Panke S, Held M, Wubbolts MG, Witholt B, Schmid A (2002) Pilot-scale production of (S)-styrene oxide from styrene by recombinant Escherichia coli synthesizing styrene monooxygenase. Biotechnol Bioeng 80:33–41
Patel RN (2001) Biocatalytic synthesis of intermediates for the synthesis of chiral drug substances. Curr Opin Biotechnol 12:587–604
Patel RN (2004) Biocatalytic synthesis of chiral pharmaceutical intermediates. Food Technol Biotechnol 42:305–325
Patel RN (2008) Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord Chem Rev 252:659–701
Patel JM (2009) Biocatalytic synthesis of atorvastatin intermediates. J Mol Catal B Enzym 61:123–128
Patel RN, McNamee CG, Benerjee A, Howell JM, Robison RS, Szarka LJ (1992) Stereoselective reduction of β-keto esters by Geotrichum candidum. Enzyme Microb Technol 14:731–738
Persson B, Kallberg Y, Oppermann U, Jörnvall H (2003) Coenzyme-based functional assignments of short-chain dehydrogenases/reductases (SDRs). Chem Biol Interact 143–144:271–278
Persson B, Kallberg Y, Bray JB, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL, Kedishvi N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U (2009) The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact 178:94–98
Pollard DJ, Woodley MJ (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Ran NQ, Zhao LS, Chen ZM, Tao JH (2008) Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale. Green Chem 10:361–372
Saratani Y, Uheda E, Yamamoto H, Nishimura A, Yoshizako F (2001) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by fungi. Biosci Biotechnol Biochem 65:1676–1679
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990a) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent–water diphasic system. Appl Environ Microbiol 56:2374–2377
Shimizu S, Kataoka M, Morishita A, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990b) Microbial asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to optical active ethyl 4-chloro-3-hydroxybutanoate. Biotechnol Lett 12:593–596
Shimizu S, Kataoka M, Kita K (1998a) Chiral alcohol synthesis with yeast carbonyl reductases. J Mol Catal B Enzym 5:321–325
Shimizu S, Kataoka M, Kita K (1998b) Chiral alcohol synthesis with microbial carbonyl reductases in a water–organic solvent two-phase system. Ann NY Acad Sci 864:87–95
Stewart JD (2001) Dehydrogenases and transaminases in asymmetric synthesis. Curr Opin Chem Biol 5:120–129
Tao JH, Xu JH (2009) Biocatalysis in development of green pharmaceutical processes. Curr Opin Chem Biol 13:43–50
Tao JH, Zhao LS, Ran NQ (2007) Recent advances in developing chemo enzymatic processes for active pharmaceutical ingredients. Org Process Res Dev 11:259–267
Van der Donk WA, Zhao HM (2003) Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol 14:421–426
Wackett LP (2004) Novel biocatalysis by database mining. Curr Opin Biotechnol 15:280–284
Wada M, Kataoka M, Kawabata H, Yasohara Y, Kizaki N, Hasegawa J, Shimizu S (1998) Purification and characterization of NADPH-dependent carbonyl reductase, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate, from Candida magnoliae. Biosci Biotech Biochem 62:280–285
Wada M, Kawabata H, Kataoka M, Yasohara Y, Kizaki N, Hasegawa J, Shimizu S (1999) Purification and characterization of an aldehyde reductase from Candida magnoliae. J Mol Catal B Enzym 6:333–339
Wohlgemuth R (2010) Asymmetric biocatalysis with microbial enzymes and cells. Curr Opin Microbiol 13:283–292
Woodley JM (2008) New opportunities for biocatalysis: making pharmaceutical processes greener. Trends Biotechnol 26:321–327
Yamamoto H, Kimoto N, Matsuyama A, Kobayashi Y (2002) Purification and properties of a carbonyl reductase useful for production of ethyl (S)-4-chloro-3-hydroxybutanoate from Kluyveromyces lactis. Biosci Biotechnol Biochem 66:1775–1778
Yamamoto H, Mitsuhashi K, Kimoto N, Matsuyama A, Easki N, Kobayashi Y (2004) A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 68:638–649
Yasohara Y, Kizaki N, Hasegawa J, Takahashi S, Wada M, Kataoka M, Shimizu S (1999) Synthesis of optically active ethyl 4-chloro-3-hydroxybutanoate by microbial reduction. Appl Microbiolo Biotechnol 51:847–851
Yasohara Y, Kizaki N, Hasegawa J, Wada M, Kataoka M, Shimizu S (2000) Molecular cloning and overexpression of the gene encoding an NADPH-dependent carbonyl reductase from Candida magnoliae, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate. Biosci Biotechnol Biochem 64(7):1430–1436
Ye Q, Yan M, Xu L, Cao H, Li ZJ, Chen Y, Li SY, Ying HJ (2009a) A novel carbonyl reductase from Pichia stipitis for the production of (S)-4-chloro-3-hydroxybutanoate ethyl. Biotechnol Lett 31:537–542
Ye Q, Yan M, Yao Z, Xu L, Cao H, Li ZJ, Chen Y, Li SY, Bai JX, Xiong J, Ying HJ, Ouyang PK (2009b) A new member of the short-chain dehydrogenases/reductases superfamily: purification, characterization and substrate specificity of a recombinant carbonyl reductase from Pichia stipitis. Bioresour Technol 100:6022–6027
Ye Q, Li XM, Yan M, Cao H, Xu L, Zhang YY, Chen Y, Xiong J, Pk O, Ying HJ (2010a) High-level production of heterologous proteins using untreated cane molasses and corn steep liquor in Escherichia coli medium. Appl Microbiol Biotechnol 87:517–525
Ye Q, Cao H, Yan M, Cao F, Zhang YY, Li XM, Xu L, Chen Y, Xiong J, Ouyang PK, Ying HJ (2010b) Construction and co-expression of a polycistronic plasmid encoding carbonyl reductase and glucose dehydrogenase for production of ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresour Technol 101:6761–6767
Ye Q, Cao H, Lan M, Yan M, Wang Y, He QT, Li J, Xu L, Chen Y, Xiong J, Ouyang PK, Ying HJ (2010c) Biosynthesis of (S)-4-chloro-3-hydroxybutanoate ethyl using Escherichia coli co-expressing a novel NADH-dependent carbonyl reductase and a glucose dehydrogenase. Bioresour Technol 101:8911–8914
Ye Q, Cao H, Zang G, Lan M, Yan M, Wang Y, Zhang YY, Li XM, Xu L, Xiong J, Ouyang PK, Ying HJ (2010d) Biocatalytic synthesis of (S)-4-chloro-3-hydroxybutanoate ethyl ester using a recombinant whole-cell catalyst. Appl Microbiol Biotechnol. doi:10.1007/s00253-010-2836-4
Zhang R, Zhu G, Zhang W, Cao S, Ou X, Li X, Bartlam M, Xu Y, Zhang XC, Rao Z (2008) Crystal structure of a carbonyl reductase from Candida parapsilosis with anti-prelog stereospecificity. Protein Sci 17:1412–1423
Zhao HM, van der Donk WA (2003) Regeneration of cofactors for use in biocatalysis. Curr Opin Biotechnol 14:583–589
Acknowledgements
This work was supported by the Major Basic Research Program of China (grant no. 2009CB724700) and the National Key Technology RD Program (grant no. 2008BAI63B07). Hanjie Ying was supported by the China National Funds for Distinguished Young Scientists (grant no. 21025625). Qi YE was supported by the Innovation Fund for Doctoral Dissertation of Nanjing University of Technology (BSCX200911). We also thank Prof. Ming Yan, Jian Xiong, and Lin Xu for revising the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Q., Ouyang, P. & Ying, H. A review—biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives. Appl Microbiol Biotechnol 89, 513–522 (2011). https://doi.org/10.1007/s00253-010-2942-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2942-3