Abstract
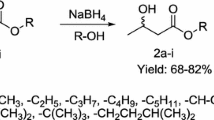
Asymmetric reduction of ethyl-4-chloro-3-oxobutanoate to (S)-ethyl-4-chloro-3-hydroxybutanoate in aqueous medium by resting cells of Candida parapsilosis ATCC 7330 was optimized. The influence of culture parameters (inoculum size, inoculum age and biocatalyst harvest time) and reaction parameters (co-substrate, resting cell, pH and substrate concentrations) on the asymmetric reduction were studied. It was found that these parameters significantly influenced the rate of the asymmetric reduction. Under the optimum conditions, the final concentration of (S)-ethyl-4-chloro-3-hydroxybutanoate, enantiomeric excess and the isolated yield of (S)-ethyl-4-chloro-3-hydroxybutanoate were 1.38 M (230 g/l), >99 and 95%, respectively. The space time yield was 115 mmol/lh, which is significantly higher than other whole cell biocatalysts reported so far.






Similar content being viewed by others
References
Jiang B, Liu JF, Zao SY (2003) Enantioselective synthesis for the antipodes of slagenins B and C. J Org Chem 68:2376–2384
Kita K, Kataoka M, Shimizu S (1999) Diversity of 4-chloroacetoacetate ethyl esters reducing enzymes in yeast and their application to chiral alcohol synthesis. J Biosci Bioengg 88:591–598
Imamoto T, Nishimura M, Koide A, Yoshida K (2007) t-BU-QuinoxP ligand: applications in asymmetric Pd-catalysed allylic substitution and Ru catalysed hydrogenation. J Org Chem 72:7413–7416
Kitamura M, Ohkuma T, Takaya H, Noyori R (1988) A practical asymmetric synthesis of carnitine. Tetrahedron Lett 29:1555–1556
Pai CC, Li YM, Zhou ZY, Chan ASC (2002) Synthesis of new chiral diphosphine ligand (Bisbenzodioxan phos) and its application in asymmetric catalytic hydrogenation. Tetrahedron Lett 43:2789–2792
Wan Y, Sun Y, Luo Y, Li D, Zhang Z (2005) Synthesis of a bulky and electron rich derivative of SEG phos and its application in Ru catalyzed enatioselective hydrogenation of beta keto esters. J Org Chem 70:1070–1072
Amidjojo M, Weuster-Botz D (2005) Asymmetric synthesis of chiral synthon ethyl-4-chloro-3-hydroxybutanoate using Lactobacillus kefir. Tetrahedron Asymmetr 16:899–901
Kizaki N, Yasohara Y, Hasegawa M, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure S-ethyl-4-chloro-3-oxobutanoate by E. coli transformed cells expressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Yang ZH, Yao SJ, Lin DQ (2004) Improving the stereoselectivity of asymmetric reduction of 3-oxo esters with pretreatments on Bakers’ yeast. Ind Eng Chem Res 43:4871–4875
Yashohara Y, Kizaki J, Hasegawa S, Takahashi M, Wada M, Kataoka S (1999) Synthesis of optically active ethyl-4-chloro-3-hydroxybutanoate by microbial reduction. Appl Microbiol Biotechnol 51:847–851
Heldge E, Rupert P, Cumther W, Weuster-Botz D (2004) Stereoselective reduction of ethyl-4-chloro acetoacetate with recombinant Pichia pastoris. Tetrahedron Asymmetr 15:3591–3593
He JY, Sum ZH, Ruan WQ, Xu Yan (2006) Biocatalytic synthesis of ethyl-(S)-4chloro-3-hydroxy-butanoate in an aqueous-organic solvent biphasic system using Aureobasidium pullulans CGMCC 1244. Process Biochem 41:244–249
Padhi SK, Titu D, Pandian NG, Chadha A (2006) Deracemisation of b-hydroxy esters using immobilised whole cells of Candida parapsilosis ATCC 7330: substrate specificity and mechanistic investigation. Tetrahedron Asymmetr 62:5133–5140
Titu D, Chadha A (2008) Preparation of optically pure alkyl 3-(hetero-2-yl)-3-hydroxypropanoates by Candida parapsilosis ATCC 7330 mediated deracemisation. J Mol Catal B Enzyme 52:168–172
Yoshihiko Y, Noriyuki K, Hasegawa K, Wada M, Kataoka M, Shimizu S (2001) Stereoselective reduction of alkyl 3-oxobutanoate by carbonyl reductase from Candida mangnoliae. Tetrahedron Asymmetr 12:1713–1718
Ifoeng CJ, Jurgen H, Adrie JJ, Jaap A, Andreas L, Joseph J (2002) Reduction of ethyl 3-oxobutanoate using non-growing baker’s yeast in a continuously operated reactor with cell retention. Enzyme Microb Technol 31:665–672
Zhang B, Pionnier S (2003) Cofactor recycling mechanism in asymmetric biocatalytic reduction of carbonyl compounds mediated by yeast: which is the efficient electron donor? Chemistry 9:3604–3610
Pankaj S, Gandhan SP, Banerjee UC (2006) Optimization of physicochemical parameters for the enhancement of carbonyl reductase production by Candida viswanathii. Bioprocess Biosyst Eng 29:149–156
Sreenath HK, Chapman TW, Jeffries TN (1986) Ethanol production from D-xylose in batch fermentation with Candida shehatar: process variable. Appl Microbiol Biotechnol 24:294–299
Shafiq S, Ali S, Ehsan A, Haq I (2003) Optimization of assay condition and inoculum size with kinetic analysis for biosynthesis of invertase by Sacchromyces cerevisiae. J Biol Sci 3:191–196
Ashwini LK, Pankaj S, Banerjee UC (2005) Biocatalytic synthesis of S-(-)-1-(1’-napthyl)-ethanol by a novel isolate of Candida viswanathii. J Mol Catal B Enzyme 35:1–6
Pankaj S, Banerjee UC (2005) Biotransformations for the production of the chiral drug (S)-duloxetine catalyzed by a novel isolate of Candida tropicalis. Appl Microbiol Biotechnol 67:771–777
Houng JY, Liau JS (2003) Applying slow release biocatalysis to the asymmetric reduction of ethyl-4-chloro-acetoacetate. Biotechnol Lett 25:17–21
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent-water diphaseic system. Appl Environ Microbiol 56:2374–2377
Acknowledgments
We thank the Department of Biotechnology, Government of India, for funding and the Sophisticated Analytical Instrumentation Facility (SAIF), IITM, for the NMR spectra.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kaliaperumal, T., Kumar, S., Gummadi, S.N. et al. Asymmetric synthesis of (S)-ethyl-4-chloro-3-hydroxybutanoate using Candida parapsilosis ATCC 7330. J Ind Microbiol Biotechnol 37, 159–165 (2010). https://doi.org/10.1007/s10295-009-0657-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0657-1