Abstract
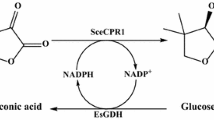
A cofactor regeneration system for enzymatic biosynthesis was constructed by coexpressing a carbonyl reductase from Pichia stipitis and a glucose dehydrogenase from Bacillus megaterium in Escherichia coli Rosetta (DE3) PlySs. Transformants containing the polycistronic plasmid pET-PII-SD2-AS1-B exhibited an activity of 13.5 U/mg protein with 4-chloro-3-oxobutanoate ethyl ester (COBE) as the substrate and an activity of 14.4 U/mg protein with glucose as the substrate; NAD(H) was the coenzyme in both cases. Asymmetric reduction of COBE to (S)-4-chloro-3-hydroxybutanoate ethyl ester [(S)-CHBE] with more than 99% enantiomeric excess was demonstrated by transformants. Furthermore, the paper made a comparison of crude enzyme catalysis and whole-cell catalysis in an aqueous monophasic system and a water/organic solvent biphasic system. In the water/n-butyl acetate system, the coexpression system produced 1,398 mM CHBE in the organic phase, which is the highest yield ever reported for CHBE production by NADH-dependent reductases from yeasts. In this case, the molar yield of CHBE was 90.7%, and the total turnover number, defined as moles (S)-CHBE formed per mole NAD+, was 13,980.





Similar content being viewed by others
References
Asako H, Shimizu M, Itoh N (2009) Biocatalytic production of (S)-4-bromo-3- hydroxybutyrate and structurally related chemicals and their applications. Appl Microbiol Biotechnol 84:397–405
Bare G, Jacques J, Hubert RR, Thonart PH (1991) Bioconversion of a L-carnitine precursor in a one- and two-phase system. Appl Biochem Biotechnol 28(29):445–456
Barrios-González J, Miranda RU (2010) Biotechnological production and application of statins. Appl Microbiol Biotechnol 85:869–883
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chakraborty AA, Phadke RP, Chaudhary FA, Shete PS, Rao BS, Jasani KD (2005) Optimization of redox reactions employing whole cell biocatalysis. World J Microbiol Biotechnol 21:221–227
Goldberg K, Schroer K, Lütz S, Liese A (2007) Biocatalytic ketone reduction-a powerful tool for the production of chiral alcohols-Part I: processes with isolated enzymes. Appl Microbiol Biotechnol 76:237–248
Gough S, Dostal L, Howe A, Deshpande M, Scher M, Rosazza JNP (2005) Production of pyruvate from lactate using recombinant Pichia pastoris cells as catalyst. Process Biochem 40:2597–2601
He JY, Sun ZH, Ruan WQ, Xu Y (2006) Biocatalytic synthesis of ethyl (S)-4-chloro-3-hydroxy-butanoate in an aqueous-organic solvent biphasic system using Aureobasidium pullulans CGMCC 1244. Process Biochem 41:244–249
Hummel W (1999) Large-scale applications of NAD(P)-dependent oxidoreductases: recent developments. Trends Biothchno l17:487–492
Ishige T, Honda K, Shimizu S (2005) Whole organism biocatalysis. Curr Opin Chem Biol 9:174–180
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Karanewsky DS, Badia MC, Ciosek CPJ, Robl JF, Sofia MJ, Simpkins LM, DeLange B, Harrity TW, Biller SA, Gorden EM (1990) Phosphorus-containing inhibitors of HMG-CoA reductase. 1.4-[(2-arylethyl)-hydroxyphosphinyl]-3-hydroxybutanoic acids: a new class of cell-selective inhibitors of cholesterol biosynthesis. J Med Chem 33:2925–2956
Kataoka M, Yamamoto K, Kawabata H, Wada M, Kita K, Yanase H, Shimizu S (1999) Stereoselective reduction of ethyl 4-chloro-3-oxobtanoate by Escherichia coli transformant cells coexpressing the aldehyde reductase gene and glucose dehydrogenase genes. Appl Microbiol Biotechnol 51:486–490
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Lee SH, Park OJ (2009) Uses and production of chiral 3-hydroxy-c-butyrolactones and structurally related chemicals. Appl Microbiol Biotechnol 84:817–828
Liu W, Wang P (2007) Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnol Adv 25:369–384
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetr 20:513–557
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetr 14:2659–2681
Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B, Jörnvall H (2003) Short-chaindehydrogenases/reductases (SDR): the 2002 update. Chem Biol Interact 143–144:247–253
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent-water diphasic system. Appl Environ Microbiol 56:2374–2377
Stewart JD (2001) Dehydrogenases and transaminases in asymmetric synthesis. Curr Opin Chem Biol 5:120–129
Van der Donk WA, Zhao HM (2003) Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol 14:421–426
Wada M, Kataoka M, Kawabata H, Yasohara Y, Kizaki N, Hasegawa J, Shimizu S (1998) Purification and charaterization of NADPH-dependent carbonyl reductase, involved in stereoselective reduction of ethyl 4-Chloro-3-oxobutanoate, from Candida magnoliae. Biosci Biotechnol Biochem 62:280–285
Yamamoto H, Mitsuhashi K, Kimoto N, Matsuyama A, Esaki N, Kobayashi Y (2004) A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 68:638–649
Yasohara Y, Kizaki N, Hasegawa J, kataoka M, Shimizu S (2000) Molecular cloning and overexpression of the gene encoding an NADPH-dependent carbonyl reductase from Candida magnoliae, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate. Biosci Biotechnol Biochem 64:1430–1436
Ye Q, Yan M, Xu L, Cao H, Li ZJ, Chen Y, Li SY, Ying HJ (2009a) A novel carbonyl reductase from Pichia stipitis for the production of (S)-4-chloro-3-hydroxybutanoate ethyl. Biotechnol Lett 31:537–542
Ye Q, Yan M, Yao Z, Xu L, Cao H, Li ZJ, Chen Y, Li SY, Bai JX, Xiong J, Ying HJ, Ouyang PK (2009b) A new member of the short-chain dehydrogenases/reductases superfamily: purification, characterization and substrate specificity of a recombinant carbonyl reductase from Pichia stipitis. Bioresour Technol 100:6022–6027
Ye Q, Li XM, Yan M, Cao H, Xu L, Zhang YY, Chen Y, Xiong J, Pk O, Ying HJ (2010a) High-level production of heterologous proteins using untreated cane molasses and corn steep liquor in Escherichia coli medium. Appl Microbiol Biotechnol 87:517–525
Ye Q, Cao H, Yan M, Cao F, Zhang YY, Li XM, Xu L, Chen Y, Xiong J, OuYang PK, Ying HJ (2010b) Construction and co-expression of a polycistronic plasmid encoding carbonyl reductase and glucose dehydrogenase for production of ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresour Technol 101:6761–6767
Ye Q, Cao H, Lan M, Yan M, Wang Y, He QT, Li J, XU L, Chen Y, Xiong J, OuYang PK, Ying HJ (2010c) Biosynthesis of (S)-4-chloro-3-hydroxybutanoate ethyl using Escherichia coli co-expressing a novel NADH-dependent carbonyl reductase and a glucose dehydrogenase. Bioresour Technol 101:8911–8914
Acknowledgements
This work was supported by the Major Basic Research Program of China (Grant No. 2009CB724700), National Key Technology RD Program (Grant No. 2008BAI63B07). Qi YE was supported by the Innovation Fund for Doctoral Dissertation of Nanjing University of Technology (BSCX200911) and the Program (KJ103736).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qi Ye, Hou Cao, and Lan Mi have equally contributed to this study.
Rights and permissions
About this article
Cite this article
Ye, Q., Cao, H., Zang, G. et al. Biocatalytic synthesis of (S)-4-chloro-3-hydroxybutanoate ethyl ester using a recombinant whole-cell catalyst. Appl Microbiol Biotechnol 88, 1277–1285 (2010). https://doi.org/10.1007/s00253-010-2836-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2836-4