Abstract
Globally, substantial research into endophytic microbes is being conducted to increase agricultural and environmental sustainability. Endophytic microbes such as bacteria, actinomycetes, and fungi inhabit ubiquitously within the tissues of all plant species without causing any harm or disease. Endophytes form symbiotic relationships with diverse plant species and can regulate numerous host functions, including resistance to abiotic and biotic stresses, growth and development, and stimulating immune systems. Moreover, plant endophytes play a dominant role in nutrient cycling, biodegradation, and bioremediation, and are widely used in many industries. Endophytes have a stronger predisposition for enhancing mineral and metal solubility by cells through the secretion of organic acids with low molecular weight and metal-specific ligands (such as siderophores) that alter soil pH and boost binding activity. Finally, endophytes synthesize various bioactive compounds with high competence that are promising candidates for new drugs, antibiotics, and medicines. Bioprospecting of endophytic novel secondary metabolites has given momentum to sustainable agriculture for combating environmental stresses. Biotechnological interventions with the aid of endophytes played a pivotal role in crop improvement to mitigate biotic and abiotic stress conditions like drought, salinity, xenobiotic compounds, and heavy metals. Identification of putative genes from endophytes conferring resistance and tolerance to crop diseases, apart from those involved in the accumulation and degradation of contaminants, could open new avenues in agricultural research and development. Furthermore, a detailed molecular and biochemical understanding of endophyte entry and colonization strategy in the host would better help in manipulating crop productivity under changing climatic conditions. Therefore, the present review highlights current research trends based on the SCOPUS database, potential biotechnological interventions of endophytic microorganisms in combating environmental stresses influencing crop productivity, future opportunities of endophytes in improving plant stress tolerance, and their contribution to sustainable remediation of hazardous environmental contaminants.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
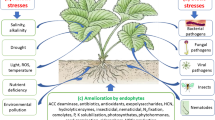
Plants interact with diverse microbial species thriving in the rhizosphere and phyllosphere, thereby resulting in altered vital biological activities together with defense strategies against various abiotic and biotic stresses [43, 78, 101, 178]. Rhizosphere and phyllosphere plant growth–promoting bacteria (PGPB) and mycorrhizal fungi in the rhizosphere are capable to induce growth of the plants directly by increasing macronutrient and mineral uptake and concentrations of essential hormones and/or indirectly through minimizing the negative impacts of a myriad of pathogens [18, 35, 79, 147, 161, 162, 171, 186, 240, 263] (Fig. 1).
Overview of the plant–microbe interactions at phyllospheric and rhizospheric zone: endophytic microbes and rhizospheric microbes are capable to induce growth of the plants directly by increasing macronutrient and mineral uptake or indirectly through plant protection against pathogens. Naturally synthesized bioactive compounds with antimicrobial activities can be exploited in various sectors, especially in the agricultural and medicinal sectors
The microbial species surviving on plant surfaces are epiphytes, whereas endophytes are those that inhabit the plant tissues [149, 203, 253]. In 1866, De Barry introduced the term “endophyte” for those organisms, including bacteria, fungi, or their associations multiplying intracellularly or intercellularly into host plants at least once in a lifetime without producing any marked signs of disease. Recent studies have illustrated that the growth and development of host plants depend to a greater extent on such symbiotic microbial species [55]. For example, in the most widely studied endosymbiotic association of rhizobium and legume, the bacterial counterpart is reported to regulate and meet the host plant nitrogen requirement [200, 201].
Endophytes facilitate the successful establishment of symbiotic association via the synthesis and secretion of plant growth–promoting compounds responsible for host adaptation under given environmental conditions. Several fungal, bacterial, and actinomycetes species are described to participate in the synthesis and secretion of biologically active compounds and secondary metabolites [7, 14, 46, 56, 64, 144, 189, 198, 230, 273].
Biomolecules belonging to classes of alkaloids, phenols, peptides, etc. synthesized by bacterial endosymbionts show a promising future in agriculture and medicine [163, 215]. For example, microbially synthesized bio-insecticide azadirachtin was found to be an effective inhibitor toward the desert locust (Schistocerca gregaria) [33]. Since its first discovery, azadirachtin has been found to be effective against more than 200 insect species and has become an active component of many commercial pesticides, including TreeAzin, AzaMax, BioNEEM, AzaGuard, and AzaSol [38, 59, 62, 80, 85, 94, 156, 196]. Many experimental investigations have reported the differential impact of factors such as specific host tissue, climatic conditions, and soil characteristics on bioactive compounds synthesized by endophytic microbial species [205]. The clue about the important role of endophytic microorganisms in the governance of the composition of metabolic products of host plants has attracted plant biologists to decipher the complexities of endophytic associations to improve crop plants.
Based on life strategies, endophytic bacteria were classified as facultative, obligate, opportunistic, and passenger endophytes [84] (Fig. 2). Currently, different biotic factors (e.g., insects and phytopathogens) and abiotic stress (e.g., extreme temperatures, salinity, drought, flood, low/excess nutrients, and organic/inorganic contamination) resulting from climate change have emerged as important limiting factors for agricultural and horticultural crop productivity worldwide [274]. Biotic stress has been estimated to reduce annual production of about 30% of crops [66]. In particular, combined effects of multiple abiotic stress factors such as drought and heat in a particular stage of growth of the plant are more detrimental than individual stress factors. Apart from abiotic stress factors, plants are constantly challenged with biological stresses through pathogenic bacteria, viruses, fungi, insects, and pests, causing considerable losses in food productivity worldwide [76, 152, 202, 234]. Various approaches, such as the selection of tolerant varieties, molecular breeding, and genetic engineering are being used to improve crop varieties against different stressors. However, the majority of these methods are time consuming, costly, and not well accepted in some areas [12]. Therefore, to neutralize the negative consequences of various factors connected with abiotic and biotic stress, host plants have developed many biological mechanisms that can function simultaneously. In this context, the mutualistic association arising from interconnections between the host and the microbe is considered an effective and sustainable means of improving plant development and growth [54, 132, 173, 195].
Categorization of endophytic bacteria based on their lifestyle. Opportunistic endophytes: they are bacteria which occasionally enter plants for their own needs. Passenger endophytes: they are bacteria which enter the plant by chance. Obligate endophytes: they are bacteria which are strictly bound to life inside a plant. Facultative endophytes: they are bacteria which can live inside plants and in other habitats also
Unlike other plant growth–promoting microorganisms, endophytes have a direct relation with plants. They possess rapid adaptability under given conditions of biotic and abiotic stress, thereby improving host plant growth and survivability [9, 25, 61, 101, 149]. Furthermore, endophytic microbes can be an integral part of the rhizospheric region with the potential to synthesize and secrete metabolic products and enzymes [27, 188]. They facilitate in neutralizing harmful impacts of plant pathogens. They may also allow the host plant to multiply even in polluted soil by degradation of contaminants in a manner similar to those harbored by plant growth–promoting rhizobacteria (PGPR) [31, 37]. The application of high-throughput current “omics”-based technology such as gene sequencing, metabolomics, and microarray could comprehend the complex associations existing between plants and their endophytes and can be a promising tool for sustainable environmental development [40, 105]. Their high colonization efficacy and stability against abiotic stress make them a potential candidate for environmental management [12, 47, 116, 128].
The novelty of the present review is the current understanding pertaining to the colonization strategy of endophytes into host plants and their promising role in the alleviation of multiple abiotic and biotic environmental constraints limiting crop productivity. Noteworthy, the review has included comprehensive bibliometric information using the “SCOPUS” research database to illustrate the current research trend in the area of endophyte and possible implications in environmental stress management. In addition, the extensive information dealing with the possible roles of endophytes in eco-friendly removal of contaminants of hazardous nature including heavy metals, and diverse organic pollutants along with the future opportunities of endophytic microbes in crop improvement under changing climatic conditions, not considered in previously published reviews, are extensively taken into account.
Study Design
This review was designed after a literature search and analysis using the following criteria to provide a critical, effective, and comprehensive analysis of the literature on endophytic microbes. A search was carried out with the SCOPUS database considering titles, abstracts, and keywords fields of all available literature. The search contained only two keywords: “endophytic” (or “endophyte”) and “stress.” It showed the publication of 2949 papers starting from 1960. To highlight the more recent results, the review was specifically addressed to the publications of the last 10 years (from 2012 until December 3, 2022), resulting in a total of 2532 publications.
To obtain a suitable and systematic synthesis of all bibliographic information, including the article title, abstract, authors, and keywords, a cluster analysis was performed using VOSviewer software (“VOSviewer version 1.6.16,” 2020).
Figure 3 reports the cluster analysis provided from the co-occurrence network of keywords of the papers extracted from the SCOPUS platform. The results can be grouped into five clusters. The first cluster (241 items), highlighted by green balls, is devoted to stress factors and adaptation. Keywords are related to abiotic stress (e.g., salinity and drought) and biotic (pathogen).
The keywords co-occurrence network, obtained from the articles extracted by SCOPUS, selecting two keywords: “endophytic” (or "endophyte") and “stress,” in the titles, abstracts, and keywords fields of all available literature. The review was specifically addressed to the publications of the last 10 years (from 2012 until December 3, 2022). The map highlights the most frequently used bibliographic terms to understand the most active research fields that are grouped into 5 clusters. Data analysis was performed by “VOSviewer version 1.6.16,” 2020
Cluster 2 (224 items) keywords highlight the reactivity of endophytes, the endophytic production of metabolites, and the antibacterial activity of the obtained bioactive compounds (blue balls highlight this cluster). The third cluster is represented by yellow balls (192 items), and mainly concerns colonization mechanisms, with several keywords devoted to culture, and bacterial and fungi growth.
Cluster 4 (175 items), represented by violet balls, is devoted to remediation, with keywords related to contamination and detoxification. The keywords contaminants refer to heavy metals and organic pollutants. Finally, cluster 5 (168 items), represented by red balls, is mainly devoted to genome and genetic expressions.
Based on the study design, the review was conceived in the following sections:
Impact of Stress Conditions on the Plant
The green revolution remarkably improved food availability in developing and developed countries. However, the indiscriminate use of chemical fertilizers reduced the biodiversity of soil microorganisms and frequently resulted in the loss of beneficial microbes necessary for soil health [126, 138]. Meanwhile, the predicted expansion of the human population beyond 10 billion in the next half a century requires doubling food production [232]. Therefore, ensuring stable global food production and supply is among the main challenges of the twenty-first century.
The biotic and abiotic stresses have a negative impact on agricultural productivity. Biotic stress includes pathogens that cause plant diseases (e.g., fungi, bacteria, viruses, and nematodes) and insects that feed on plant parts and compete with plants to get nutrients [178]. Phytopathogens can cause various plant diseases such as leaf spot, necrosis, wilt, head rot, fruit rot, root rot, and black foot [122, 146, 165]. In addition, insect feeding can cause bore formation on leaves, stems, flowers, and bark. Some insects are also potential vectors of microbial pathogens, so the disease becomes epiphytotic to healthy plant populations.
The main abiotic stress includes salinity, drought, nutrient-deficient, temperature (low/high), flood, heavy metal contamination stress, and ultraviolet radiation, which massively limit the overall yield and growth of crop plants [86, 231, 238, 246, 264, 271]. Drought stress alters the diffusion of nutrients, and the relationship between plants and water, and hampers normal functions, altering the plant morphological and physiological features. For example, drought stress can decrease chlorophyll content and cause an excess of reactive oxygen species (ROS) that can damage nucleic acids, proteins, and lipids [3, 41, 241, 255, 256]. Furthermore, salinity stress reduces the growth of plants and productivity through specific ion toxicity and osmotic effects that lead to nutritional imbalance, changes in morphology and biochemistry, and a decrease in photosynthesis [102, 137, 214]. In addition, the acidic condition can produce a nutrient deficiency in plants, leading to an acute loss of the physiological growth and development sequence. Heavy metals have a similar effect on plants,they are released into the soil, water, and atmosphere as a result of various anthropogenic activities such as industrialization, mining, and agricultural activities such as the use of fungicides, pesticides, and fertilizers, including organic ones. The concentration of heavy metals in the environment depends on different activities, then it can become toxic when it exceeds acceptable limits [199]. Finally, high- and low-temperature stress diminishes enzyme functioning, cell division, and excessive denaturation of membranous proteins that leads to cell death when the condition persists in the case of long-term conditions [28, 169]. Therefore, researchers need to develop sustainable microbe-based strategies to cope with difficult stress situations for food security and crop productivity. In this regard, endophytic microorganisms are the alternative that can contribute to plant health, nutrient supply, soil productivity, and protection against biotic and abiotic stress [49, 174, 176, 183].
Colonization of Endophytes in the Host Plant
Plant endophyte colonization cannot be considered an abrupt phenomenon, but a series of complex and organized events determined by chemotactic responses. The intracellular colony development mechanism adopted by bacteria and fungi is almost the same, but their strategies and modes differ considerably. For example, bacterial endophytes colonize intercellularly the host plant system vasculature, whereas fungal endophytes colonize inter- and intracellularly within the entire root system [103, 114, 129, 149, 179, 259]. The entry and colonization of endophytes involve different mechanisms comprising of (1) host availability and identification through a receptor and specific plant protein interaction, and (2) interaction with the phyllosphere followed by entry into the cellular environment (Fig. 4). Successful colonization by microbial endophytes is influenced by various factors such as the host plant genotype, the type of plant tissue, the microbial taxon and species, as well as abiotic and biotic stresses [135, 136]. Plant root exudates serve as chemical signals to attract bacterial endophytes. Bacteria use flagella to move toward the root surface and eventually leading to interaction with the plant system through pili and fibers [34, 113, 153]. During the moving process from the rhizosphere environment to the endosphere region, microbial endophytes can rapidly adapt to the contrasting environment (e.g., redox status, oxygen availability, nutrient composition, and the osmotic balance of the host cell system). Furthermore, microbial endophytes invading the endosphere region must cope with the host’s antioxidant defense machinery to internalize and colonize successfully [32, 113, 170]. In conclusion, the successful endophyte invasion and colonization within the host plant are largely determined by the timely identification of signaling substances, quorum responses, the potential to invade host defense machinery, and, most strikingly, the efficiency of tuning up with the entirely different complex host cellular system [119, 153].
Entry and colonization of endophytic microorganisms in host plants. The successful colonization of the host plant by endophytes is a crucial component of advantageous plant–microbe interactions. Entry and colonization of endophytes into the host plant include several events that occur within the host plant, including endophytic population entrance, motility, transmission, and multiplication
Role of Endophytes in the Management of Abiotic Stress
Endophytes and Their Role in Mitigation of Drought and Temperature Stress
Plants in natural environments are bound to expose to different abiotic stresses. Drought is one of the main limiting factors for the growth and productivity of crops around the world [58, 67, 177, 231]. Under water-limiting conditions, crop growth and productivity in the early stages are arrested due to low energy supply, low water uptake, and hindered functions of enzymes [52, 60, 121]. Furthermore, all considerable characters of plant–water relations, such as leaf relative water content (RWC), phenology, osmotic potential, water potential, pressure potential, photosynthesis, respiration, nutrition uptake, and rate of transpiration, are significantly impacted by drought, leading to decreased crop productivity (Fig. 5) [69, 83, 229]. Considerable research has been conducted for the development of resistance in various model and crop plant species using conventional and molecular techniques that are tedious and expensive. Therefore, researchers are seeking a sustainable approach and numerous studies recognize that plant-associated microbes have tremendous potential to develop resistance against drought.
An overview of plant response to abiotic stress (left): prolonged abiotic stress (drought, salinity, and heavy metals) causes regeneration of ROS, desiccation, cellular dehydration, hormonal imbalance etc. that limit plant growth and productivity. Endophytic mediated abiotic stress tolerance mechanism (right): under abiotic conditions, endophytes trigger the production of osmolytes (proline, glycine betaine, etc.), secretion of phytohormones (IAA, cytokinins, GAs), and induce gene expressions for plant defense
The literature so far revealed that endophytes induce tolerance to drought by certain molecular and biochemical changes in plants [70, 208, 265, 269]. In field tests, the Bukholderia phytofirmans PsJN bacteria endophyte was inoculated in wheat plants that maintained metabolic balance due to higher antioxidant activity compared to control under drought conditions (Table 1) [164]. Furthermore, inoculation of the Piriformospora indica fungal endophyte also demonstrates drought resistance by upregulating antioxidant enzymes, drought-regulated genes, and CAS mRNA levels in drought-challenged leaves [223]. The pot experiment conducted on rice inoculated with Trichoderma harzianum TH-56 showed better drought tolerance by modulating SOD, proline, lipid peroxidation, and growth attributes, and the level of DHN/AQU transcript, under drought stress [181].
The accumulation of total soluble sugars, glucose, fructose, and starch content during endophyte infection plays an important role in increasing the resistance and improving plant tolerance to drought stress. Bacillus subtilis B26 has been found to reduce the negative effects of drought stress, which was linked to an increased level of starch content and total soluble sugars in inoculated stressed Brachypodium distachyon [71] and in Phleum pratense grasses [70]. The inoculation of the Bacillus subtilis B26 endophytic bacterium with Phleum pratense was found to have a significant effect on metabolism of plants. For instance, higher levels of fructans and sucrose, and key amino acids such as glutamic acid, glutamine, and asparagine were found in the roots and shoots of plants colonized compared to non-colonized ones. Furthermore, inoculation of plants with endophytes resulted in an increased level of a non-protein amino acid, i.e., gamma-aminobutyric acid (GABA), in shoots and roots [70, 92]. A Trichoderma hamatum DIS 219b fungal endophyte delayed the onset of drought response in Theobroma cacao by changing gene expression, possibly corresponding to changes in net photosynthesis, stomatal conductance, and green fluorescence emissions [21]. A recent study indicated that Ampelomyces sp. colonized tomato plants and improved the promotion of plant growth under drought conditions, representing a sustainable form of biofertilizer that could improve agronomic production [160]. The recent finding revealed that P. indica confers drought tolerance by the regulation of promoter genes, resulting in morphophysiological changes in tomatoes [19]. In summary, the endophyte-mediated drought resistance mechanism is based on phytohormone production, antioxidant-mediated ROS scavenging activity, induction of microbial genes, and accumulation of compatible solutes (Fig. 5).
In turn, to alleviate heat/temperature stress (HS), some studies have identified the potential role of plant hormones and other secondary metabolites produced by fungi endophytes such as Paecilomyces formosus LWL1 in the Dongjin japonica rice cultivar. This fungus protected rice plants against HS compared to the control, as shown by lower endogenous stress signaling compounds, such as jasmonic acid (34.57%) and abscisic acid (25.71%), and the overall protein content increased (18.76–33.22%) [245]. The Rhizopus oryzae endophytic fungus inoculated in soybean (Glycine max L.) and sunflower (Helianthus annuus L.) also has the potential to alleviate thermal stress. Namely, both crops also showed low levels of abscisic acid (ABA), while high levels of catalase (CAT), ascorbic acid oxidase (AAO), phenolics, proline, sugars, flavonoids, lipids, and proteins were also observed. It was also found that the endophytic fungus stimulates chlorophyll content, length of shoots and roots, and dry and fresh biomass compared to uninoculated plants [97]. Aspergillus japonicus EuR-26 endophytic fungus isolated from the Euphorbia indica L. wild plant (Euphorbiaceae) also mediated the growth of host plants under normal and heat-stress conditions. Namely, A. japonicus–associated sunflower and soybean seedlings improved the growth of plant biomass and other plant traits and food quality (flavonoids, phenolic, proteins, soluble sugars, and lipids) under the stress of high temperature (40 °C) compared to plants without endophyte [96]. These types of phenomena are also observed in wild plants, e.g., in the desert plant Cullen plicatum (Delile) C.H.Stirt. (Fabaceae) which, if it is a co-inhabitant with another endophytic fungus, Thermomyces lanuginosus, copes much better with heat stress in its natural environment [11].
Endophytic Microorganisms and Their Role in Alleviating Salinity Stress
Salinity is one of the most important environmental problems affecting plant productivity in dry and semi-dry climates [6, 216, 102, 133, 260]. The high salt content of the soil has been described as the result of natural and human activities leading to soil sodium salt accumulation. Furthermore, soil high salt concentration is frequently correlated with the reduction in seedling formation and imbalance in cellular homeostasis culminating in diminished photosynthetic activities [13, 204, 222, 267].
Endophytic microorganisms develop strategies against salinity, similar to drought-resistant mechanisms. Endophytes stimulate the synthesis of antioxidant enzymes to balance various free radicals and maintain the normal functioning of the cell under salinity stress (Table 1). For example, inoculation of poplar tree with Curvularia sp. stimulates plant production of ascorbate peroxidase (APX) and superoxide dismutase (SOD) [180]. Furthermore, exposure of endophytic microbes to high salinity may stimulate the synthesis of the ACC deaminase. For instance, Barnawal et al. [23] observed an increase in the growth rate of salt-sensitive spider plants (Chlorophytum sp., Asparagaceae) with the presence of the bacterium Brachybacterium paraconglomeratum that produce ACC deaminase and diminishing the negative impact of gaseous hormone ethylene. Similar studies on the involvement of ACC deaminase for improved rice plant growth and stress mitigation were recently described [98, 193, 254].
In addition, osmolyte production was also recorded in maintaining the sodium–potassium ratio to overcome the osmotic effect of salinity (Table 1).
The pot experiment demonstrated that colonization with P. pseudoalcaligenes improved Arabidopsis sp. growth under salt stress conditions by likely modulating the expression levels of K+ and Na+ ion channels and genes involved in Na+/K+ homeostasiss [4]. Colonization of P. indica in salinity-sensitive Brassica rapa (= B. campestris subsp. chinensis) confers salinity tolerance by significantly higher production of antioxidant enzymes such as catalase (CAT), peroxidase (POD), and SOD and increased the plant hormone level such as gibberellic acid (GA) and salicylic acid (SA) [107]. Further study by Ravi et al. (2022) suggested that fungal root endophyte (Fusarium haematococcum) can resist salt stress and produces extracellular enzymes such as amylase, cellulase, and protease under in vitro conditions in addition to antioxidant production [192].
Recently, Eida et al. [50] have illustrated the role of endophytes isolated from desert plants in mitigating plant stress caused in the soil by the high salt content. The model plant Arabidopsis thaliana exposed to different salt levels exhibited tolerance to salinity after inoculation of isolated endophytes. Recent findings of Zhang et al. [266] concluded that apart from higher antioxidative enzymes of proline content, upregulation of key genes involved in IAA synthase and ethylene signaling were observed in B. cereus KP120 inoculated with A. thaliana under salt-stressed condition. In addition, a number of recent research have shown that isolated endophytes are very effective in enhancing physiological performance, plant growth, root and shoot biomass, symbiotic performance, energy production, osmoregulation, Na+ sequestration, and ion homeostasis under salt-stressed conditions [30, 48, 110, 111, 123, 125, 157, 160, 219].
Role of Endophytes in the Management of Biotic Stress
Plants are often exposed to harmful molecules produced by microorganisms. These molecules alter plant metabolism, causing diseases and significant crop loss [53, 76, 217]. Beneficial interactions between plants and microbes play an important role in plant protection against phytopathogens. Plant-beneficial microorganisms release elicitors that alter biochemical and physiological plant properties in changing environments [5, 34, 99]. Plants have physical and chemical barriers able to react to pathogens: they activate signal transduction in response to pathogen attacks directed to induce defenses. Important mechanisms of tolerance to biotic and abiotic stress are ROS production, antioxidative defense, and oxidative burst [72, 87, 151, 218, 261]. Like rhizosphere microbes, endophytes trigger direct and indirect mechanisms of disease resistance (Fig. 6). Direct mechanisms include the production of antimicrobial compounds and the lytic enzymes of the cell wall of fungi are capable of inhibiting plant pathogen growth and act as biological controls (Table 2). For example, a study suggested that chitinase produced by endophytic Streptomyces sp. can control plant pathogenic fungi [187].
An overview of plant response to biotic stress (left): pathogen infection causes photosystem damage, ROS regeneration, and impaired cell division that lead to reduced plant growth and development. Endophytic mediated biotic stress tolerance mechanism (right): endophytes trigger defense mechanisms directly by the production of antimicrobial compounds and indirectly through the production of lytic enzymes, activation of systemic defense responses involving jasmonic acid (JA), oligogalacturonoids (OGAs), and salicylic acid (SA) signaling pathways
Many fungal and bacterial endophytes produce antimicrobial compounds with strong antifungal and antibacterial activities that could be antagonistic to plant pathogens [7, 51, 108, 141, 142, 145, 155, 228]. For example, endophytes Pseudomonas sp. isolated from Artemisia sp. roots (Asteraceae) known to produce the antibiotic DAPG (2,4-diacetylphloroglucinol) can also induce the defense of plants against pathogens such as Verticillium dahliae, Colletotrichum gloeosporioides, Fusarium oxysporum, and Phytophthora capsici (Table 2) [39]. In addition, the DAPG-producing bacterium Paracoccus halophilus G062 can aggressively colonize stems and leaves, and further suppress pathogen establishment [10]. Populus trichocarpa and Salix sitchensis (both Salicaceae) are dominant endophytes taxonomically affiliated with Burkholderia, Rahnella, Pseudomonas, and Curtobacterium genera. These genera are well known for producing antifungal compounds (e.g., occidiofungin and hydrogen cyanide) with proven biocontrol activities against soil-borne plant pathogens, including Fusarium culmorum, Rhizoctonia solani AG-8, Pythium ultimum, and Gaeumannomyces graminis var. tritici [103].
Like bacterial endophytes, it has been reported that fungal endophytes produce antimicrobial compounds. For instance, Soliman et al. [220] reported that the Paraconiothyrium endophyte strain SSM001 inhibits the growth of Heterobasidion annosum, Phaeolus schweinitzii, and Perenniporia subacida wood-decaying fungal species. Furthermore, the 3,11,12-trihydroxycadalene (sesquiterpenes derivatives) produced from the endophytic fungus Phomopis cassiae isolated from Senna spectabilis (DC.) H.S.Irwin & Barneby (= Cassia spectabilis DC., Fabaceae) has been reported as a strong antifungal agent against Cladosporium cladosporioides and C. sphaerospermum [213]. Similarly, Flueggea suffruticosa (= Securinega suffruticosa, Phyllanthaceae) and Cucurbita pepo (Cucurbitaceae) were colonized by fungal endophytic isolates that inhibited the growth of respective pathogens of plants [45, 235].
A variety of microbial phyla, including Pseudomonas sp., Bacillus sp., and Trichoderma sp., have been shown to lead to systemic resistance in plants against pathogen attacks [117, 150, 168, 175, 184]. Microorganisms activate defense reaction mechanisms that involve the induction of systemic acquired resistance (SAR) and systemic resistance (ISR) pathways. SAR is activated by pathogen infection, which is connected with the activation of salicylic acid signaling and the accumulation of pathogenesis-related proteins (PR). For example, activation of β-1,3-glucanase (PR 2) was increased in oilseed rape infected with Plasmodiophora brassicae after colonization with Heteroconium chaetospira, a dark septate endophyte [120]. Similarly, the endophyte Fusarium solani, recovered from tomato, triggered ISR across the Septoria lycopersici tomato foliar pathogen and activated the expressions of PR7 and PR5 in roots [106]. Experimental studies on resistance induction mediated by the endophyte Serendipita indica revealed that Blumeria graminis f. sp. hordei inoculation resulted in induction of gene expressions (notably Hsp70, PR1, PR2, and PR5, and barley chemically induced 7 (BCI-7)) in barley foliage, which is supposed to be involved in various functions including defense reactions and protein synthesis and apoptosis [158].
Role of Endophytic Microorganisms in Phytoremediation
Phytoremediation of Heavy Metals
Currently, the management of environmental pollutants based on living agents has achieved considerable progress worldwide. Pollutant removal by photosynthetic organisms (e.g., phytoremediation) has emerged as an attractive and light-driven decontamination technique and also an emerging green sustainable technology [44, 63, 65, 89, 118, 185, 221, 226, 233, 236]. However, the low multiplication rate along with the low amount of cell mass, phytotoxic impacts, and release of pollutants of gaseous nature are the main drawbacks associated with phytoremediation technology, making the process inefficient at field scale [26, 73, 242, 252]. The solution to these limitations lies in the development of microbe-assisted phytoremediation. Previous studies have illustrated the use of rhizosphere-dwelling microbes to improve pollutant removal [77, 81, 190, 250, 275]. Furthermore, it was suggested that endophytes could facilitate phytoremediation more efficiently [44, 112, 154].
The negative impacts of heavy metals on plants can be described as reduced crop productivity resulting from changes in growth rate, nutrient accumulation capacity, and leaf area. In addition, heavy metal pollutions can cause considerable changes in community structure of diverse microbial populations and function associated with host plants [29, 42, 224]. Numerous studies have discussed the impact of various heavy metals on the diversity of endophytes, biological processes, and biomass production [57, 68, 123, 125, 127, 172].
However, current studies dealing with the interactions between hyperaccumulator plants and endophytes have attracted attention worldwide because of inherent pollutant removal ability and possibilities for large-scale applications [91, 109, 123, 125, 130, 131, 197, 225, 243]. Furthermore, hyperaccumulators sequester a significantly high content of hazardous heavy metals and therefore create the internal environmental conditions suitable for the development of metal resistance in endophytes exposed to high heavy metal concentrations [172].
In terms of endophytic application, various metal-resistant endophytic bacteria were isolated from leaves, stem, and roots of plant hyperaccumulators, including Thlaspi caerulescens, Th. goesingense, Alyssum bertolonii (all Brassicaceae), and Nicotiana tabacum (Solanaceae) (Table 3). The association of these endophytes with hyperaccumulators suggests the widespread habitat choice of these microbes. For example, Thlaspi goesingense stems under field conditions harbor different bacteria including α-proteobacteria, γ-proteobacteria, Acidobacterium sp., Bacillus sp., Blastococcus sp., Curtobacterium sp., Desulfitobacterium metallireductans, Flavobacterium sp., Holophaga sp., M. mesophilicum, M. extorquens, Plantibacter flavus, Propionibacterium acnes, Rhodococcus sp., and Sphingomonas sp. These isolates were shown to be resistant to nickel (Ni) concentrations between 5 and 12 mM [95]. The same results were obtained in the field site experiment that the total Ni uptake by Alyssum serpyllifolium (Brassicaceae) was significantly enhanced by heavy metal–resistant endophytic bacterial strains Microbacterium sp., Pseudomonas sp., and Staphylococcus sp. [24]. In the line of the same experiment, Ma et al. [134] found that inoculation with the plant growth–promoting Pseudomonas sp. A3R3 endophytic bacterium significantly increased Ni uptake by 10% in A. serpyllifolium. In a later experiment, Achromobacter piechaudii was documented to sequester more than 60% of zinc (Zn), lead (Pb), and cadmium (Cd) from the corresponding hyperaccumulators, namely, Sedum plumbizincicola (Crassulaceae), Alnus firma (Betulaceae), and Solanum nigrum (Solanaceae), respectively [135, 136]. Similarly, another study reported arsenic (As)-tolerant Bacillus sp. endophytes isolated from the leaves, stem, and root of Pteris vittata and P. multifida (Pteridaceae) [270] and concluded that bacteria with less biomass had greater tolerance to As. Surprisingly, fungal endophytes Fusarium sp. CBRF44, Alternaria sp. CBSF68, and Penicillium sp. CBRF65 isolated from the hyperaccumulators Brassica napus (Brassicaceae) showed significant tolerance to Cd and Pb [211]. This finding supported the result of Zhu et al. [272] where dark septate endophytes Phialophora mustea inoculated tomato roots established remarkable tolerance to Cd and Zn and promoted the tomato seedlings’ growth under all metal stresses tested.
In addition, evidence of phytoremediation of Pb by plants grown in soils contaminated by heavy metals has also been confirmed. [209, 210] reported that Brassica napus inoculated with Pseudomonas fluorescens G10 improved the total uptake of Pb from 76 to 131% of the shoot, while it was 59 to 80% (p < 0.05) for Microbacterium sp. G16, respectively. Mastretta et al. [148] supported the same finding and reported that Sanguibacter sp. Cd-resistant endophyte inoculated Nicotiana tabacum (Solanaceae) increased Cd concentration in shoot tissues. Yamaji et al. [257] revealed that Clethra barbinervis (Clethraceae) could tolerate high metal concentrations (Zn, 21–2600 μg/g; Cu, 2–1123 μg/g; Pb, 32–1506 μg/g) due to the support of root fungal endophytes, including Rhizodermea veluwensis, Phialocephala fortinii, and Rhizoscyphus sp. through K uptake promotion, growth enhancement, and decrease of heavy metal concentrations. Further studies revealed that the metal resistance mechanisms in endophytes surviving within hyperaccumulators can be attributed to activities such as metal extracellular precipitation, intracellular storage and sequestration [20, 212], conversion of hazardous metal into less or non-hazardous forms [270], and surface binding/detachment of metal [82, 130, 131].
In addition, some endophytes were isolated from different parts of non-hyperaccumulators, such as Salix caprea (Salicaceae) and Oryza sativa (Poaceae). The reported metal-resistant endophytes belonged to numerous taxa, including Burkholderia sp., Methylobacterium oryzae, Frigoribacterium sp., Microbacterium sp., and Sphingomonas sp. (Table 3). Kuffner et al. [115] revealed that inoculation of Salix caprea with Microbacterium sp., Frigoribacterium sp., Sphingomonas sp., and Methylobacterium sp. increase leaves Cd and Zn accumulation. Sharma et al. [207] concluded that seed endophytes FXZ2 inoculation in Dysphania ambrosioides induced increased Zn/Cd tolerance by changing Zn/Cd speciation in rhizospheric soils, as well as exogenous production of phytohormones to promote growth, lowering oxidative damage while enhancing antioxidant properties. Enhanced metal bioaccumulation in the inoculated plant was attributed to siderophores, indole acetic acid, and ACC deaminase secretion.
In general, the basic mechanism of metal adsorption involves two distinct steps: (1) passive binding/loading of metals onto the wall of dead/inactive cell without integrating energy [239] and (2) active removal (bioaccumulation), involving the movement of metals through the plasma membrane driven by energy input and followed by intracellular storage [143].
Phytoremediation of Water and Soil Contaminated with Organic Pollutants
Industrialization and intensive agriculture are the main sources of hazardous contaminants that have deteriorated the quality of the natural ecosystem [15]. Even a small quantity of contaminants can reduce plant growth performance coupled with significant changes in soil microbe physiological processes, thus affecting critical soil biological processes [1, 139].
Phytoremediation can be used to detoxify or stabilize organic and inorganic pollutants. It is considered to be the most promising technology because it is the least disturbed at the site, cheap, and eco-friendly in nature compared to conventional remediation technologies [166, 247, 258]. Despite public acceptance, the application in the field of phytoremediation faces several obstacles, such as low biomass and slow growth, volatile contaminant evapotranspiration, and plant toxicity. Further research experiments revealed that microbe-assisted phytoremediation enhances the efficiency of phytoremediation due to its plant growth–promoting activity (e.g., siderophore production) [247]. Compared to rhizosphere microbes, endophytic microbes have been considered a better candidate for the remediation process due to their internal inhabitation that offers the opportunity to adaptation inside host cells [16, 262]. In addition, once plant growth–promoting endophytes (PGPEs) are formed in plant tissues, they are less susceptible to soil conditions’ changes but depend more on plant tissues and physiological status, such as plant health, plant growth stage, and the nutritional state [74, 191, 194, 200, 201].
Generally, the endophyte-associated phytoremediation process involves three distinct steps: (1) development, plant growth, and biomass production; (2) availability of pollutants to the host system; (3) rapid increase in endophyte population responsible for contaminant degradation. So far, many endophytic microorganisms are isolated from contaminated and non-contaminated soils capable of degrading herbicides and polyaromatic hydrocarbons pollutants (Table 4). Moore et al. (159) found that bacterial genera belonging to Arthrobacter, Pseudomonas, Bacillus, and Enterobacter recovered from different organs of poplar inhabiting near the automobile industries could remove a volatile organic compound BTEX, a component of petroleum product [159]. The mineralization of the herbicide 2,4-D was also documented by Pseudomonas putida VM1450 [75]. The results confirmed that 2,4-D was not detected in the soil of inoculated plants exposed to 7 or 13 mg of 2,4-D. Pseudomonas ITRI53 inoculated Lolium multiflorum var. taurus greatly degrades 68% of diesel-contaminated soil compared to control treatments [17]. Other bacterial endophytes such as Achromobacter xylosoxidans F3B and Pantoea sp. noted similar degradation capacity of diesel/petroleum products ITSI10 and inoculated in Arabidopsis thaliana and Italian ryegrass under controlled conditions, respectively [8, 90, 262]. Endophytic bacteria have also been studied to remove other aromatic compounds such as naphthalene and toluene. The inoculated pea plant with P. putida VM1441 (pNAH7) degraded 40% more naphthalene than the non-inoculated plant [74]. The toluene volatilization experiment suggested less toluene released from the leaves of the inoculated poplar plant with B. cepacia FX2 [244, 249]. Moreover, pyrene degradation increased by 43–65% in the live Enterobacter sp. 12J1 inoculated planted soils compared to dead bacterium inoculated planted soils [209, 210]. Furthermore, microbial species that catalyze the degradation of volatile organic contaminants, including trichloroethylene (TCE) degrading microbes, are described from Quercus robur (Fagaceae), Fraxinus excelsior (Oleaceae), and poplar growing in sites enriched with TCE [104, 248, 251]. The results of all these studies indicated that endophytic inoculation such as B. cepacia VM1468, P. putida W619-TCE, and Enterobacter PDN3, respectively, highly resist the release of TCE vapor in the environment, indicating the increased degradation efficiency.
In addition to soil remediation, plant endophyte associations have also been deployed to manage ground and surface water contaminated with organic contaminants (Table 5). An experimental investigation described a more than 50–70% reduction in toluene volatilization through inoculated yellow lupine with engineered B. cepacia VM 1330 compared to control plants grown in a hydroponic culture system [22]. Taghavi et al. [227] revealed that B. cepacia VM1468 inoculated poplar plants released five times less toluene in the air through the leaves. Furthermore, this study also concluded that horizontal gene transfer in natural endophytes could improve the phytoremediation of environmental contaminants. In addition, genetic modification of endophytes carrying foreign genes with degradation capacity has been proven to improve the phytoremediation of contaminants of aromatic and organic substances. An engineered P. putida W619TCE endophytic bacterium inoculated to poplar cuttings alleviated growth promotion and reduced TCE toxicity when grown in water that was contaminated with TCE [251].
Comprehensive research on endophytes proposed that the use of bacteria (preferably endophytes) that promote both plant growth and pollutant-degrading activities is superior to the use of bacteria that only promote plant growth or have pollutant-degrading activities. Therefore, an attempt is made to isolate and characterize endophytic bacteria that have plant growth–promoting and pollutant-degrading activities when growing on a contaminated site.
Conclusion and Future Perspectives
The application of microbial endophytes in agriculture, as well as environmental sustainability, is a growing research field. During the past two and a half decades, many studies have revealed rising interest in endophytic microbes. Endophytic microbes are known to improve host plant performance under abiotic and biotic stress conditions by altering the plants’ response. Recent advances in biotechnology and bioinformatic tools such as CRISPR (Clustered Regularly Interspaced Palindromic Repeats)–Cas system, RNA interference (RNAi), metabolomics, and next-generation sequencing systems have made the possibility of studying endophytes at the molecular level [167]. The present concept of isolation, purification, and characterization of endophytes and the research connecting biology to chemistry is now being developed. This opens new interdisciplinary dimensions and actively allows bachelor and master research students to participate in this domain of research. Research must focus on microbial endophytes to come up with new ideas to improve crop productivity on a pilot scale. Endophytes play an important role in producing a wide variety of naturally occurring secondary metabolites (such as tyrosol, saadamycin, and munumbicins) showing the industrial application in pharmaceutics and thus human health. In this regard, researchers from all over the world are continuously exploring hidden endophytic microbes for novel potent bioactive compounds that can be used as potential therapeutics. Figure 7 shows the importance of the biological activities of endophytic metabolites. Endophytes are reported to be a warehouse of new metabolites that can be widely used as antimicrobial, anticancer, immunosuppressant, antiarthritic, and anti-insect drugs. Although several bioactive compounds produced by endophytes, such as camptothecin, vinblastine, hypericin, and podophyllotoxin, have already been commercialized, novel bioactive compounds seem promising in the case of most pathogenic microorganisms in overcoming the problem of antibiotic resistance.
Biological activities of importance to humans present in endophytes’ metabolites. Endophytes have been reported to have the ability to produce novel metabolites which can serve as anticancer agents, glucosidase inhibitors (antidiabetic), and immunosuppressive agents; some of these endophytes also show antioxidant, antituberculosis, anti-inflammatory, and antimalarial activity, and serve as inhibitors of viruses
Taken together, new bioactive compounds emitted by endophytes, particularly endophytic actinomycetes, could make a significant contribution to the current and future challenges of agriculture, the environment, and medicine. To isolate and characterize new endophytes with specific features that could be useful for crop production, comprehensive bioprospecting research of endophytic microbes from various ecological niches (e.g., harsh habitats, the marine environment, etc.) is required. We anticipate a shift in practice in the future, with a greater emphasis on optimizing the interaction between plants and soil microorganisms and endophytes. However, molecular mechanisms that explain the interaction between plants and endophytes have yet to be discovered. They will open a new door to the isolation and characterization of new molecules for humans and provide a new way to improve crops and environmental sustainability.
Data Availability Statement
This is a review article. So, all the data are taken/extracted from the cited references or are furnished in the manuscript at the relevant place. The data that support the present study are available in the cited references.
References
Abatenh E, Gizaw B, Tsegaye Z, Wassie M (2017) The role of microorganisms in bioremediation – a review. Open j Environ Biol 2:38–46
Abd Allah EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi FON, ... Egamberdieva D (2018) Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Interact 13(1):37–44
Abdelaal K, AlKahtani M, Attia K, Hafez Y, Király L, Künstler A (2021) The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 10(6):520
Abdelaziz ME, Kim D, Ali S, Fedoroff NV, Al-Babli S (2017) The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci 263:107–115
Abdul Malik NA, Kumar IS, Nadarajah K (2020) Elicitor and receptor molecules: orchestrators of plant defense and immunity. Int J Mol Sci 21(3):963
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7(1):18
Adeleke BS, Babalola OO (2021) Pharmacological potential of fungal endophytes associated with medicinal plants: a review. J Fungi 7(2):147
Afzal M, Yousaf S, Reichenauer TG, Sessitsch A (2012) The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int J Phytoremediat 14:35–47
Aghai MM, Khan Z, Joseph MR, Stoda AM, Sher AW, Ettl GJ, Doty SL (2019) The effect of microbial endophyte consortia on Pseudotsuga menziesii and Thuja plicata survival, growth, and physiology across edaphic gradients. Front Microbiol 10:1353
Akhdiya A, Wahyudi AT, Munif A, Darusman LK (2014) Characterization of an endophytic bacterium g062 isolate with beneficial traits. Hayati J Biosci 21:187–196
Ali AH, Radwan U, El-Zayat S, El-Sayed MA (2019) The role of the endophytic fungus, Thermomyces lanuginosus, on mitigation of heat stress to its host desert plant Cullen plicata. Biologia Futura 70:1–7
Ali B, Hafeez A, Javed MA, Afridi MS, Abbasi HA, Qayyum A, Batool T, Ullah A, Marc RA, Al Jaouni SK, Alkhalifah DHM (2022) Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: a comprehensive review. S Afr J Bot 151:33–46
Almeida DM, Oliveira MM, Saibo NJ (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biol 40:326–345
Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N (2019) A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites 9(11):258
Anand U, Reddy B, Singh VK, Singh AK, Kesari KK, Tripathi P, Kumar P, Tripathi V, Simal-Gandara J (2021) Potential environmental and human health risks caused by antibiotic-resistant bacteria (ARB), antibiotic resistance genes (ARGs) and emerging contaminants (ECs) from municipal solid waste (MSW) landfill. Antibiotics 10(4):374
Andreolli M, Lampis S, Poli M, Gullner G, Biro B, Vallini G (2013) Endophytic Burkholderia fungorum DBT1 can improve phytoremediation efficiency of polycyclic aromatic hydrocarbons. Chemosphere 92:688–694
Andria V, Reichenauer TG, Sessitsch A (2009) Expression of alkane monooxygenase (alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass (Lolium multiflorum L.) grown in diesel contaminated soil. Environ Pollut 157:3347–3350
Aragón W, Reina-Pinto JJ, Serrano M (2017) The intimate talk between plants and microorganisms at the leaf surface. J Exp Bot 68(19):5339–5350
Azizi M, Fard EM, Ghabooli M (2021) Piriformospora indica affect drought tolerance by regulation of genes expression and some morphophysiological parameters in tomato (Solanum lycopersicum L.). Sci Hortic 287:110260
Babu AG, Shea PJ, Sudhakar D, Jung IB, Oh BT (2015) Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J Environ Manage 151:160–166
Bae H, Sicher RC, Kim M, Kim SH, Strem MD, Melnick RL, Bailey BA (2009) The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J Exp Bot 60:3279–3295
Barac T, Taghavi S, Borremans B, Provoost A, Oeyen L, Colpaert JV, van der Lelie D (2004) Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat Biotechnol 22:583–588
Barnawal D, Bharati N, Tripathi A, Pandey SS, Chanotiya CS, Kalra A (2016) ACC-Deaminase-Producing Endophyte Brachybacterium paraconglomeratum Strain SMR20 Ameliorates Chlorophytum Salinity Stress via Altering Phytohormone Generation. J Plant Growth Reg 35:553–564. https://doi.org/10.1007/s00344-015-9560-3
Barzanti R, Ozino F, Bazzicalupo M, Gabbrielli R, Galardi F, Gonnelli C, Mengoni A (2007) Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii. Microb Eco 53:306–316
Bastías DA, Gianoli E, Gundel PE (2021) Fungal endophytes can eliminate the plant growth–defence trade-off. New Phytol 230(6):2105–2113
Beans C (2017) Core concept: phytoremediation advances in the lab but lags in the field. Proc Natl Acad Sci 114(29):7475–7477
Bhadra F, Gupta A, Vasundhara M, Reddy MS (2022) Endophytic fungi: a potential source of industrial enzyme producers. 3 Biotech 12(4):1–17
Bita CE, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273
Borymski S, Cycoń M, Beckmann M, Mur LA, Piotrowska-Seget Z (2018) Plant species and heavy metals affect biodiversity of microbial communities associated with metal-tolerant plants in metalliferous soils. Front Microbiol 9:1425
Bouzouina M, Kouadria R, Lotmani B (2021) Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J Appl Microbiol 130(3):913–925
Brader G, Compant S, Mitter B, Trognitz F, Sessitsch A (2014) Metabolic potential of endophytic bacteria. Curr Opin Biotechnol 27:30–37
Brotman Y, Landau U, Cuadros-Inostroza Á, Takayuki T, Fernie AR, Chet I, Viterbo A, Willmitzer L (2013) Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog 9(3):e1003221
Butterworth JH, Morgan ED (1968) Isolation of a substance that suppresses feeding in locusts. Chem Commun (London) 1:23–24
Chagas FO, de Cassia Pessotti R, Caraballo-Rodriguez AM, Pupo MT (2018) Chemical signaling involved in plant–microbe interactions. Chem Soc Rev 47(5):1652–1704
Chaudhry V, Runge P, Sengupta P, Doehlemann G, Parker JE, Kemen E (2021) Shaping the leaf microbiota: plant–microbe–microbe interactions. J Exp Bot 72(1):36–56
Chen C, Xin K, Liu H, Cheng J, Shen X, Wang Y, Zhang L (2017) Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci Rep 7:41564
Cherif-Silini H, Silini A, Chenari Bouket A, Alenezi FN, Luptakova L, Bouremani N, Nowakowska JA, Oszako T, Belbahri L (2021) Tailoring next generation plant growth promoting microorganisms as versatile tools beyond soil desalinization: a road map towards field application. Sustainability 13(8):4422
Christina A, Christapher V, Bhore SJ (2013) Endophytic bacteria as a source of novel antibiotics: an overview. Pharmacogn Rev 7(13):11
Chung BS, Aslam Z, Kim SW, Kim GG, Kang HS, Ahn JW, Chung YR (2008) Bacterial endophyte, Pseudomonas brassicacearum YC5480, isolated from the root of Artemisia sp. producing antifungal and phytotoxic compounds. The Plant Pathol J 24:461–468
Crandall SG, Gold KM, Jiménez-Gasco MDM, Filgueiras CC, Willett DS (2020) A multi-omics approach to solving problems in plant disease ecology. PLoS ONE 15(9):e0237975
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biol Med 122:4–20
DalCorso G, Fasani E, Manara A, Visioli G, Furini A (2019) Heavy metal pollutions: state of the art and innovation in phytoremediation. Int J Mol Sci 20(14):3412
Dastogeer KM, Tumpa FH, Sultana A, Akter MA, Chakraborty A (2020) Plant microbiome–an account of the factors that shape community composition and diversity. Curr Plant Biol 23:100161
Deng Z, Cao L (2017) Fungal endophytes and their interactions with plants in phytoremediation: a review. Chemosphere 168:1100–1106
Du W, Yao Z, Li J, Sun C, Xia J, Wang B, ... Ren L (2020) Diversity and antimicrobial activity of endophytic fungi isolated from Securinega suffruticosa in the Yellow River Delta. PloS One 15(3):e0229589
Duan X, Xu F, Qin D, Gao T, Shen W, Zuo S, Yu B, Xu J, Peng Y, Dong J (2019) Diversity and bioactivities of fungal endophytes from Distylium chinense, a rare waterlogging tolerant plant endemic to the Three Gorges Reservoir. BMC Microbiol 19(1):1–14
Dwibedi V, Rath SK, Joshi M, Kaur R, Kaur G, Singh D, Kaur G, Kaur S (2022) Microbial endophytes: application towards sustainable agriculture and food security. Appl Microbiol Biotechnol 106:5359–5389
Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd Allah EF (2017) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol 8:1887
Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan SED (2021) Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10(5):935
Eida AA, Alzubaidy HS, de Zélicourt A, Synek L, Alsharif W, Lafi FF, Hirt H, Saad MM (2019) Phylogenetically diverse endophytic bacteria from desert plants induce transcriptional changes of tissue-specific ion transporters and salinity stress in Arabidopsis thaliana. Plant Sci 280:228–240
Ek-Ramos MJ, Gomez-Flores R, Orozco-Flores AA, Rodríguez-Padilla C, González-Ochoa G, Tamez-Guerra P (2019) Bioactive products from plant-endophytic Gram-positive bacteria. Front Microbiol 10:463
Elliott J, Deryng D, Müller C, Frieler K, Konzmann M, Gerten D, Glotter M, Flörke M, Wada Y, Best N, Eisner S (2014) Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc Natl Acad Sci 111(9):3239–3244
Enebe MC, Babalola OO (2019) The impact of microbes in the orchestration of plants’ resistance to biotic stress: a disease management approach. Appl Microbiol Biotechnol 103(1):9–25
Etesami H, Beattie GA (2017) Plant-microbe interactions in adaptation of agricultural crops to abiotic stress conditions. In: Probiotics and Plant Health; Springer: Singapore. 163–200
Fadiji AE, Babalola OO (2020) Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J Biol Sci 27(12):3622
Fadiji AE, Babalola OO (2020) Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol 8:467
Fagorzi C, Checcucci A, DiCenzo GC, Debiec-Andrzejewska K, Dziewit L, Pini F, Mengoni A (2018) Harnessing rhizobia to improve heavy-metal phytoremediation by legumes. Genes 9(11):542
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Falade AO, Adewole KE, Ekundayo TC (2021) Therapeutic potentials of endophytes for healthcare sustainability. Egyptian J Basic Appl Sci 8(1):117–135
Falkenmark M (2013) Growing water scarcity in agriculture: future challenge to global water security. Philos Trans R Soc A 371(2002):20120410
Fan D, Subramanian S, Smith DL (2020) Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci Rep 10(1):1–18
Farder-Gomes CF, Saravanan M, Martinez LC, Plata-Rueda A, Zanuncio JC, Serrao JE (2022) Azadirachtin-based biopesticide affects the respiration and digestion in Anticarsia gemmatalis caterpillars. Toxin Rev 41(2):466–475
Feng NX, Yu J, Zhao HM, Cheng YT, Mo CH, Cai QY, Li YW, Li H, Wong MH (2017) Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci Total Environ 583:352–368
Figueiredo G, Gomes M, Covas C, Mendo S, Caetano T (2022) The unexplored wealth of microbial secondary metabolites: the Sphingobacteriaceae case study. Microb Ecol 83(2):470–481
Firdous J, Lathif NA, Mona R, Muhamad N (2019) Endophytic bacteria and their potential application in agriculture: a review. Indian J Agric Res 53(1):1–7
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoof LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Francini A, Sebastiani L (2019) Abiotic stress effects on performance of horticultural crops. Horticulturae 5(4):67
Franco-Franklin V, Moreno-Riascos S, Ghneim-Herrera T (2021) Are endophytic bacteria an option for increasing heavy metal tolerance of plants? A meta-analysis of the effect size. Front Environ Sci 8:294
Franco-Navarro JD, Díaz-Rueda P, Rivero-Núñez CM, Brumós J, Rubio-Casal AE, de Cires A, Colmenero-Flores JM, Rosales MA (2021) Chloride nutrition improves drought resistance by enhancing water deficit avoidance and tolerance mechanisms. J Exp Bot 72(14):5246–5261
Gagné-Bourque F, Bertrand A, Claessens A, Aliferis KA, Jabaji S (2016) Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front Plant Sci 7:584
Gagné-Bourque F, Mayer BF, Charron JB, Vali H, Bertrand A, Jabaji S (2015) Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS One 23(10):e0130456. https://doi.org/10.1371/journal.pone.0130456
Gechev T, Petrov V (2020) Reactive oxygen species and abiotic stress in plants. Int J Mol Sci 21(20):7433. https://doi.org/10.3390/ijms21207433
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2015) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176:20–30
Germaine KJ, Keogh E, Ryan D, Dowling DN (2009) Bacterial endophyte-mediated naphthalene phytoprotection and phytoremediation. FEMS Microbiol Lett 296:226–234
Germaine KJ, Liu X, Cabellos GG, Hogan JP, Ryan D, Dowling DN (2006) Bacterial endophyte-enhanced phytoremediation of the organochlorine herbicide 2,4-dichlorophenoxyacetic acid. FEMS Microbiol Ecol 57:302–310
Gimenez E, Salinas M, Manzano-Agugliaro F (2018) Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 10(2):391
Gkorezis P, Daghio M, Franzetti A, Van Hamme JD, Sillen W, Vangronsveld J (2016) The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: an environmental perspective. Front Microbiol 7:1836
Glick BR, Gamalero E (2021) Recent developments in the study of plant microbiomes. Microorganisms 9(7):1533
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica
Gómez OC, Luiz JHH (2018) Endophytic fungi isolated from medicinal plants: future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Appl Microbiol Biotechnol 102(21):9105–9119
Gunarathne V, Mayakaduwa S, Ashiq A, Weerakoon SR, Biswas JK, Vithanage M (2019) Transgenic plants: benefits, applications, and potential risks in phytoremediation. In: Transgenic plant technology for remediation of toxic metals and metalloids (pp. 89–102). Academic Press
Guo HJ, Luo SL, Chen L, Xiao X, Xi Q, Wei W, Zheng G, Liu C, Wan Y, Chen J, He Y (2010) Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Biores Technol 101:8599–8605
Hacke UG, Jacobsen AL, Pratt RB, Maurel C, Lachenbruch B, Zwiazek J (2012) New research on plant–water relations examines the molecular, structural, and physiological mechanisms of plant responses to their environment. New Phytol 196(2):345–348
Hardoim PR, van Overbeek LS, Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Harman G, Khadka R, Doni F, Uphoff N (2021) Benefits to plant health and productivity from enhancing plant microbial symbionts. Front Plant Sci 11:610065. https://doi.org/10.3389/fpls.2020.610065
Hartman K, Tringe SG (2019) Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem J 476(19):2705–2724
Hasanuzzaman M, Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Hasegawa S, Meguro A, Nishimura T, Kunoh H (2004) Drought tolerance of tissue-cultured seedlings of mountain laurel (Kalmia latifolia L.) induced by an endophytic actinomycete I. Enhancement of osmotic pressure in leaf cells. Actinomycetologica 18:43–47
He W, Megharaj M, Wu CY, Subashchandrabose SR, Dai CC (2020) Endophyte-assisted phytoremediation: mechanisms and current application strategies for soil mixed pollutants. Crit Rev Biotechnol 40(1):31–45
Ho YN, Mathew DC, Hsiao SC, Shih CH, Chien MF, Chiang HM, Huang CC (2012) Selection and application of endophytic bacterium Achromobacter xylosoxidans strain F3B for improving phytoremediation of phenolic pollutants. J Haz Mat 219:43–49
Hou D, Lin Z, Wang R, Ge J, Wei S, Xie R, Wang H, Wang K, Hu Y, Yang X, Lu L (2018) Cadmium exposure-sedum alfredii planting interactions shape the bacterial community in the hyperaccumulator plant rhizosphere. Appl Environ Microbiol 84(12):e02797-e2817
Hua MDS, Kumar RS, Shyur LF, Cheng YB, Tian Z, Oelmüller R, Yeh KW (2017) Metabolomic compounds identified in Piriformospora indica-colonized Chinese cabbage roots delineate symbiotic functions of the interaction. Sci Rep 7(1):1–14
Huang Z, Cai X, Shao C, She Z, Xia X, Chen Y, Jianxiang Y, Zhou S, Lin Y (2008) Chemistry and weak antimicrobial activities of phomopsins produced by mangrove endophytic fungus Phomopsis sp. ZSU-H76. Phytochem 69:1604–1608
Hug JJ, Bader CD, Remškar M, Cirnski K, Müller R (2018) Concepts and methods to access novel antibiotics from actinomycetes. Antibiotics 7(2):44
Idris R, Trifonova R, Puschenreiter M, Wenzel WW, Sessitsch A (2004) Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Environ Microbiol 70:2667–2677
Ismail, Hamayun M, Hussain A, Iqbal A, Khan SA, Lee I-J (2018) Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res Int 2018:7696831
Ismail I, Hussain A, Mehmood A, Qadir M, Husna, Iqbal A, Hamayun M, Khan N (2020) Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pak J Bot 52(5):1857–1865
Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A (2018) Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci Rep 8:1950
Jamiołkowska A (2020) Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 10(2):173
Jogawat A, Vadassery J, Verma N, Oelmüller R, Dua M, Nevo E, Johri AK (2016) PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Sci Rep 6(1):36765
Jones P, Garcia BJ, Furches A, Tuskan GA, Jacobson D (2019) Plant host-associated mechanisms for microbial selection. Front Plant Sci 10:862
Kamran M, Parveen A, Ahmar S, Malik Z, Hussain S, Chattha MS, Saleem MH, Adil M, Heidari P, Chen JT (2020) An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int J Mol Sci 21(1):148
Kandel SL, Joubert PM, Doty SL (2017) Bacterial endophyte colonization and distribution within plants. Microorganisms 5:77
Kang JW, Khan Z, Doty SL (2012) Biodegradation of trichloroethylene by an endophyte of hybrid poplar. Appl Environ Microbiol 12:3504–3507
Kaul S, Sharma T, Dhar MK (2016) “Omics” tools for better understanding the plant–endophyte interactions. Front Plant Sci 7:955
Kavroulakis N, Ntougias S, Zervakis GI, Ehaliotis C, Haralampidis K, Papadopoulou KK (2007) Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J Exp Bot 58:3853–3864
Khalid M, Hassani D, Liao J, Xiong X, Bilal M, Huang D (2018) An endosymbiont Piriformospora indica reduces adverse effects of salinity by regulating cation transporter genes, phytohormones, and antioxidants in Brassica campestris ssp. chinensis. Environ Exp Bot 153:89–99
Khalil AMA, Abdelaziz AM, Khaleil MM, Hashem AH (2021) Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett Appl Microbiol 72(3):263–274
Khan AR, Waqas M, Ullah I, Khan AL, Khan MA, Lee IJ, Shin JH (2017) Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ Exp Bot 135:126–135
Khan MA, Asaf S, Khan AL, Adhikari A, Jan R, Ali S, Imran M, Kim KM, Lee IJ (2020) Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol 22(5):850–862
Khan MA, Asaf S, Khan AL, Ullah I, Ali S, Kang SM, Lee IJ (2019) Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann Microbiol 69(8):797–808
Kong Z, Glick BR (2017) The role of plant growth-promoting bacteria in metal phytoremediation. Adv Microb Physiol 71:97–132
Kost T, Stopnisek N, Agnoli K, Eberl L, Weisskopf L (2014) Oxalotrophy, a widespread trait of plant-associated Burkholderia species, is involved in successful root colonization of lupin and maize by Burkholderia phytofirmans. Front Microbiol 4:1–9
Kowal J, Arrigoni E, Serra J, Bidartondo M (2020) Prevalence and phenology of fine root endophyte colonization across populations of Lycopodiella inundata. Mycorrhiza 30(5):577–587
Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, Gorfer M, Strauss J, Rivelli RA, Sessitsch A (2010) Culturable bacteria from Zn-and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108:1471–1484
Kumar S, Thakur N, Singh AK, Gudade BA, Ghimire D, Das S (2022) Microbes-assisted phytoremediation of contaminated environment: global status, progress, challenges, and future prospects. Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water, pp.555–570
Kupferschmied P, Maurhofer M, Keel C (2013) Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Front Plant Sci 4:287
Kurade MB, Ha YH, Xiong JQ, Govindwar SP, Jang M, Jeon BH (2021) Phytoremediation as a green biotechnology tool for emerging environmental pollution: a step forward towards sustainable rehabilitation of the environment. Chem Eng J 415:129040. https://doi.org/10.1016/j.cej.2021.129040
Kusari P, Kusari S, Spiteller M, Kayser O (2015) Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl Microbiol Biotechnol 99(13):5383–5390
Lahlali R, McGregor L, Song T, Gossen BD, Narisawa K, Peng G (2014) Heteroconium chaetospira induces resistance to clubroot via upregulation of host genes involved in jasmonic acid, ethylene, and auxin biosynthesis. PLoS ONE 9:e94144
Lamaoui M, Jemo M, Datla R, Bekkaoui F (2018) Heat and drought stresses in crops and approaches for their mitigation. Front Chem 6:26
Lamichhane JR, Venturi V (2015) Synergisms between plant disease complexes: a growing trend. Front Plant Sci 6:385
Li X, Geng X, Xie R, Fu L, Jiang J, Gao L, Sun J (2016) The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol Biofuels 9(1):1–12
Li X, He X, Hou L, Ren Y, Wang S, Su F (2018) Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci Rep 8:7896
Li X, Li W, Chu L, White JF Jr, Xiong Z, Li H (2016) Diversity and heavy metal tolerance of endophytic fungi from Dysphania ambrosioides, a hyperaccumulator from Pb–Zn contaminated soils. J Plant Interactions 11(1):186–192
Lin W, Lin M, Zhou H, Wu H, Li Z, Lin W (2019) The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 14(5):e0217018
Liu C, Lin H, Li B, Dong Y, Yin T (2020) Responses of microbial communities and metabolic activities in the rhizosphere during phytoremediation of Cd-contaminated soil. Ecotoxicol Environ Saf 202:110958
Liu C, Lin H, Li B, Dong Y, Qiu Y (2022) Screening endophyte with capability to improve phytoremediation efficiency from hyperaccumulators: a novel and efficient microfluidic method. Chemosphere 286:131723
Liu H, Carvalhais LC, Crawford M, Singh E, Dennis PG, Pieterse CM, Schenk PM (2017) Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol 8:2552
Luo SL, Chen L, Chen JL, Xiao X, Xu TY, Wan Y, Rao C, Liu CB, Liu YT, Lai C, Zeng GM (2011) Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 85(7):1130–1138
Luo SL, Wan Y, Xiao X, Guo H, Chen L, Xi Q, Zeng G, Liu C, Chen J (2011) Isolation and characterization of endophytic bacterium LRE07 from cadmium hyperaccumulator Solanum nigrum L. and its potential for remediation. Appl Microbiol Biotechnol 89:1637–1644
Lyu D, Msimbira LA, Nazari M, Antar M, Pagé A, Shah A, Monjezi N, Zajonc J, Tanney CA, Backer R, Smith DL (2021) The coevolution of plants and microbes underpins sustainable agriculture. Microorganisms 9(5):1036
Ma Y, Dias MC, Freitas H (2020) Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci 11:1750
Ma Y, Rajkumar M, Luo Y, Freitas H (2011) Inoculation of endophytic bacteria on host and non-host plants—effects on plant growth and Ni uptake. J Haz Mat 195:230–237
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage 174:14–25
Ma Y, Zhang C, Oliveira RS, Freitas H, Luo Y (2016) Bioaugmentation with endophytic bacterium E6S homologous to Achromobacter piechaudii enhances metal rhizoaccumulation in host Sedum plumbizincicola. Front Plant Sci 7:75
Machado RMA, Serralheiro RP (2017) Soil salinity: effect on vegetable crop growth management practices to prevent and mitigate soil salinization. Horticulture 3:30
Mącik M, Gryta A, Frąc M (2020) Biofertilizers in agriculture: an overview on concepts, strategies and effects on soil microorganisms. Adv Agron 162:31–87
MacKinnon G, Duncan HJ (2013) Phytotoxicity of branched cyclohexanes found in the volatile fraction of diesel fuel on germination of selected grass species. Chemosphere 90:952–957
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228
Mai PY, Levasseur M, Buisson D, Touboul D, Eparvier V (2020) Identification of antimicrobial compounds from Sandwithia guyanensis-associated endophyte using molecular network approach. Plants 9(1):47
Malhadas C, Malheiro R, Pereira JA, de Pinho PG, Baptista P (2017) Antimicrobial activity of endophytic fungi from olive tree leaves. World J Microbiol Biotechnol 33(3):46
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–262
Manganyi MC, Ateba CN (2020) Untapped potentials of endophytic fungi: a review of novel bioactive compounds with biological applications. Microorganisms 8(12):1934
Mao Z, Zhang W, Wu C, Feng H, Peng Y, Shahid H, Cui Z, Ding P, Shan T (2021) Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol 21(1):1–12
Martins PM, Merfa MV, Takita MA, De Souza AA (2018) Persistence in phytopathogenic bacteria: do we know enough? Front Microbiol 9:1099
Massoni J, Bortfeld-Miller M, Jardillier L, Salazar G, Sunagawa S, Vorholt JA (2020) Consistent host and organ occupancy of phyllosphere bacteria in a community of wild herbaceous plant species. ISME J 14(1):245–258
Mastretta C, Taghavi S, Van Der Lelie D, Mengoni A, Galardi F, Gonnelli C, Barac T, Boulet J, Weyens N, Vangronsveld J (2009) Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int J Phytoremediat 11:251–267
Mengistu AA (2020) Endophytes: colonization, behaviour, and their role in defense mechanism. Int J Microbiol 2020. https://doi.org/10.1155/2020/6927219
Miljaković D, Marinković J, Balešević-Tubić S (2020) The significance of Bacillus spp in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8(7):1037
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467
Misiou O, Koutsoumanis K (2022) Climate change and its implications for food safety and spoilage. Trends Food Sci Technol 126:142–152
Mitter B, Petric A, Shin MW, Chain PS, Hauberg-Lotte L, Reinhold-Hurek B, Nowak J, Sessitsch A (2013) Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front Plant Sci 4:120
Mitter EK, Kataoka R, de Freitas JR, Germida JJ (2019) Potential use of endophytic root bacteria and host plants to degrade hydrocarbons. Int J Phytorem 21(9):928–938
Mohamad OA, Li L, Ma JB, Hatab S, Xu L, Guo JW, Rasulov BA, Liu YH, Hedlund BP, Li WJ (2018) Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front Microbiol 9:924
Molina G, Pimentel MR, Bertucci TCP, Pastore GM (2012) Application of fungal endophytes in biotechnological processes. Chem Eng Trans 27:289–294
Molina-Montenegro MA, Acuña-Rodríguez IS, Torres-Díaz C, Gundel PE, Dreyer I (2020) Antarctic root endophytes improve physiological performance and yield in crops under salt stress by enhanced energy production and Na+ sequestration. Sci Rep 10(1):1–10
Molitor A, Zajic D, Voll LM, Kühnemann JP, Samans B, Kogel KH, Waller F (2011) Barley leaf transcriptome and metabolite analysis reveals new aspects of compatibility and Piriformospora indica-mediated systemic induced resistance to powdery mildew. Mol Plant Microbe Interact 24:1427–1439
Moore FP, Barac T, Borremans B, Oeyen L, Vangronsveld J, van der Lelie D, Campbell CD, Moore ERB (2006) Endophytic bacterial diversity in poplar trees growing on a BTEX-contaminated site: the characterization of isolates with potential to enhance phytoremediation. Syst Appl Microbiol 29:539–556
Morsy M, Cleckler B, Armuelles-Millican H (2020) Fungal endophytes promote tomato growth and enhance drought and salt tolerance. Plants 9(7):877
Mowafy MA, Agha SM, Haroun AS, Abbas AM, Elbalkini M (2022) Insights in nodule-inhabiting plant growth promoting bacteria and their ability to stimulate Vicia faba growth. Egypt J Basic and Appl Sci 9:51–64. https://doi.org/10.1080/2314808X.2021.2019418
Müller T, Ruppel S (2014) Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol Ecol 87(1):2–17
Narayanan Z, Glick BR (2022) Secondary metabolites produced by plant growth-promoting bacterial endophytes. Microorganisms 10(10):2008
Naveed M, Hussain MB, Zahir ZA, Mitter B, Sessitsch A (2014) Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul 73:121–131
Nazarov PA, Baleev DN, Ivanova MI, Sokolova LM, Karakozova MV (2020) Infectious plant diseases: etiology, current status, problems and prospects in plant protection. Acta Naturae 12(3):46
Nedjimi B (2021) Phytoremediation: a sustainable environmental technology for heavy metals decontamination. SN Appl Sci 3(3):1–19
Nidhi S, Anand U, Oleksak P, Tripathi P, Lal JA, Thomas G, Kuca K, Tripathi V (2021) Novel CRISPR–Cas systems: an updated review of the current achievements, applications, and future research perspectives. Int J Mol Sci 22(7):3327
Nie P, Li X, Wang S, Guo J, Zhao H, Niu D (2017) Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET-and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis. Front Plant Sci 8:238
Nievola CC, Carvalho CP, Carvalho V, Rodrigues E (2017) Rapid responses of plants to temperature changes. Temperature 4(4):371–405
Noman M, Ahmed T, Ijaz U, Shahid M, Li D, Manzoor I, Song F (2021) Plant–microbiome crosstalk: dawning from composition and assembly of microbial community to improvement of disease resilience in plants. Int J Mol Sci 22(13):6852
Nwachukwu BC, Babalola OO (2021) Perspectives for sustainable agriculture from the microbiome in plant rhizosphere. Plant Biotechnol Reports 15:259–278
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14:1504
Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO (2019) Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol Biotechnol 103(3):1155–1166
Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J (2020) Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ 743:140682. https://doi.org/10.1016/j.scitotenv.2020.140682
Olowe OM, Akanmu AO, Asemoloye MD (2020) Exploration of microbial stimulants for induction of systemic resistance in plant disease management. Ann Appl Biol 177(3):282–293
Omomowo OI, Babalola OO (2019) Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 7(11):481
Osmolovskaya N, Shumilina J, Kim A, Didio A, Grishina T, Bilova T, Keltsieva OA, Zhukov V, Tikhonovich I, Tarakhovskaya E, Frolov A (2018) Methodology of drought stress research: experimental setup and physiological characterization. Int J Mol Sci 19(12):4089
Oukala N, Aissat K, Pastor V (2021) Bacterial endophytes: the hidden actor in plant immune responses against biotic stress. Plants 10(5):1012
Palmieri D, Vitale S, Lima G, Di Pietro A, Turrà D (2020) A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat Commun 11(1):1–11
Pan X, Qin Y, Yuan Z (2018) Potential of a halophyte-associated endophytic fungus for sustaining Chinese white poplar growth under salinity. Symbiosis 76:109–116
Pandey V, Ansari MW, Tula S, Yadav S, Sahoo RK, Shukla N, Bains G, Badal S, Chandra S, Gaur AK, Kumar A, Shukla A, Kumar J, Tuteja N (2016) Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 243:1251–1264
Passari AK, Mishra VK, Leo VV, Gupta VK, Singh BP (2016) Phytohormone production endowed with antagonistic potential and plant growth promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol Res 193:57–73
Patle PN, Navnage NP, Ramteke PR (2018) Endophytes in plant system: roles in growth promotion, mechanism and their potentiality in achieving agriculture sustainability. Int J Chem Std 6:270–274
Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM (2014) Induced systemic resistance by beneficial microbes. Ann Rev Phytopathol 52:347–375
Płociniczak T, Pacwa-Płociniczak M, Kwaśniewski M, Chwiałkowska K, Piotrowska-Seget Z (2020) Response of rhizospheric and endophytic bacterial communities of white mustard (Sinapis alba) to bioaugmentation of soil with the Pseudomonas sp. H15 strain. Ecotoxicol Environ Safety 194:110434
Pohjanen J, Koskimäki JJ, Sutela S, Ardanov P, Suorsa M, Niemi K, Sarjala T, Häggman H, Pirttilä AM (2014) Interaction with ectomycorrhizal fungi and endophytic Methylobacterium affects nutrient uptake and growth of pine seedlings in vitro. Tree Physiol 34(9):993–1005
Quecine MC, Araujo WL, Marcon J, Gai CS, Azevedo JL, Kleiner AAP (2008) Chitinolytic activity of endophytic Streptomyces and potential for biocontrol. Lett Appl Microbiol 47:486–491
Raghav D, Jyoti A, Siddiqui AJ, Saxena J (2022) Plant-associated endophytic fungi as potential bio-factories for extracellular enzymes: progress, challenges and strain improvement with precision approaches. J Appl Microbiol. https://doi.org/10.1111/jam.15574
Raimi A, Adeleke R (2021) Bioprospecting of endophytic microorganisms for bioactive compounds of therapeutic importance. Arch Microbiol 203(5):1917–1942
Rajkumar M, Ae N, Prasad MNV et al (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Rashid S, Charles TC, Glick BR (2012) Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol 61:217–224
Ravi RK, Valli PPS, Muthukumar T (2022) Physiological characterization of root endophytic Fusarium haematococcum for hydrolytic enzyme production, nutrient solubilization and salinity tolerance. Biocatalysis and Agricultural Biotechnology 43:102392
Reyad AMM, Radwan TEE, Hemida KA, Abo Al-Qassem NA, Ali RM (2017) Salt tolerant endophytic bacteria from Carthamus tinctorius and their role in plant salt tolerance improvement. Inter J Curr Sci Res 3:1467–1488
Rigobelo EC, Baron NC (2021) Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology 13(1):39–55
Rodriguez PA, Rothballer M, Chowdhury SP, Nussbaumer T, Gutjahr C, Falter-Braun P (2019) Systems biology of plant-microbiome interactions. Mol Plant 12(6):804–821
Rojas V, Rivas L, Cárdenas C, Guzmán F (2020) Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides. Molecules 25(24):5804
Salazar-Ramírez G, Flores-Vallejo RDC, Rivera-Leyva JC, Tovar-Sánchez E, Sánchez-Reyes A, Mena-Portales J, Sánchez-Carbente MDR, Gaitán-Rodríguez MF, Batista-García RA, Villarreal ML, Mussali-Galante P (2020) Characterization of fungal endophytes isolated from the metal hyperaccumulator plant Vachellia farnesiana growing in mine Tailings. Microorganisms 8(2):226
Salazar B, Ortiz A, Keswani C, Minkina T, Mandzhieva S, Pratap Singh S, Rekadwad B, Borriss R, Jain A, Singh HB, Sansinenea E (2022) Bacillus spp. as bio-factories for antifungal secondary metabolites: innovation beyond whole organism formulations. Microb Ecol. https://doi.org/10.1007/s00248-022-02044-2
Sandeep G, Vijayalatha KR, Anitha T (2019) Heavy metals and its impact in vegetable crops. Int J Chem Stud 7(1):1612–1621
Santoyo G, Hagelsieb GM, Orozco-Mosqued, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99
Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3(3):430–439
Schlechter RO, Miebach M, Remus-Emsermann MN (2019) Driving factors of epiphytic bacterial communities: a review. J Adv Res 19:57–65
Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, Gómez C, Mattson N, Nasim W, Garcia-Sanchez F (2020) Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 10(7):938
Sharma H, Rai AK, Dahiya D, Chettri R, Nigam PS (2021) Exploring endophytes for in vitro synthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol 7(2):175
Sharma VK, Li X, Wu G, Bai W, Parmar S, White JF, Li H (2019) Endophytic community of Pb-Zn hyperaccumulator Arabis alpina and its role in host plants metal tolerance. Plant Soil 439:397–411
Sharma VK, Parmar S, Tang W, Hu H, White JF Jr, Li H (2022) Effects of fungal seed endophyte FXZ2 on Dysphania ambrosioides Zn/Cd tolerance and accumulation. Front Microbiol 13:995830. https://doi.org/10.3389/fmicb.2022.995830
Sheibani-Tezerji R, Rattei T, Sessitsch A, Trognitz F, Mitter B (2015) Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. MBio 6(5):e00621-e715
Sheng X, Chen X, He L (2008) Characteristics of an endophytic pyrene-degrading bacterium of Enterobacter sp. 12J1 from Allium macrostemon Bunge. Int Biodeter Biodegr 62:88–95
Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156:1164–1170
Shi Y, Xie H, Cao L, Zhang R, Xu Z, Wang Z, Deng Z (2017) Effects of Cd- and Pb-resistant endophytic fungi on growth and phytoextraction of Brassica napus in metal-contaminated soils. Environ Sci Pollut Res 24:417–426
Shin M, Shim J, You Y, Myung H, Bang KS, Cho M, Kannan SK, Oh BT (2012) Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J Haz Mat 199–200:314–320
Silva GH, Teles HL, Zanardi LM, Young MCM, Eberlin MN, Hadad R, Pfenning LH, Costa-Neto CM, Castro-Gamboa I, da Silva Bolzani V, Araujo AR (2006) Cadinane sesquiterpenoids of Phomopsis cassiae, an endophytic fungus associated with Cassia spectabilis (Leguminosae). Phytochem 67:1964–1969
Singh D, Roy BK (2016) Salt stress affects mitotic activity and modulates antioxidant systems in onion roots. Braz J Bot 39:67–76
Singh N, Mishra SK, Kumar PR, Kumar N, Kumar D (2022) Role of endophyte metabolites in plant protection and other metabolic activities. In: Bacterial endophytes for sustainable agriculture and environmental management (pp. 213–233). Springer, Singapore
Singh VK, Singh AK, Singh PP, Kumar A (2018) Interaction of plant growth promoting bacteria with tomato under abiotic stress: a review. Agric Ecosyst Environ 267:129–140
Singh VK, Singh AK, Kumar A (2017) Disease management of tomato through PGPB: current trends and future perspective. 3Biotech 7:255–264
Soares C, Carvalho ME, Azevedo RA, Fidalgo F (2019) Plants facing oxidative challenges—a little help from the antioxidant networks. Environ Exp Bot 161:4–25
Soldan R, Mapelli F, Crotti E, Schnell S, Daffonchio D, Marasco R, Fusi M, Borin S, Cardinale M (2019) Bacterial endophytes of mangrove propagules elicit early establishment of the natural host and promote growth of cereal crops under salt stress. Microbiol Res 223:33–43
Soliman SSM, Greenwood JS, Bombarely A, Mueller LA, Tsao R, Mosser DD, Raizada MN (2015) An endophyte constructs fungicide-containing extracellular barriers for its host plant. Curr Biol 25:2570–2576
Song YQ, Shahir S, Abd Manan F (2021) Bacterial inoculant-assisted phytoremediation of heavy metal-contaminated soil: inoculant development and the inoculation effects. Biologia 76:2675–2685
Sugai T, Yannan W, Watanabe T, Satoh F, Qu L, Koike T (2019) Salt stress reduced the seedling growth of two larch species under elevated ozone. Front Forests Global Change 2:53
Sun JL, Johnson JM, Daguang C, Sherameti I, Oelmüllera R, Lou B (2010) Piriformospora indica confers drought tolerance in chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol 167:1009–1017
Sun X, Song B, Xu R, Zhang M, Gao P, Lin H, Sun W (2021) Root-associated (rhizosphere and endosphere) microbiomes of the Miscanthus sinensis and their response to the heavy metal contamination. J Environ Sci 104:387–398
Sura-de Jong M, Reynolds RJ, Richterova K, Musilova L, Staicu LC, Chocholata I, Cappa JJ, Taghavi S, van der Lelie D, Frantik T, Dolinova I (2015) Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Front Plant Sci 6:113
Syranidou E, Christofilopoulos S, Gkavrou G, Thijs S, Weyens N, Vangronsveld J, Kalogerakis N (2016) Exploitation of endophytic bacteria to enhance the phytoremediation potential of the wetland helophyte Juncus acutus. Front Microbiol 7:1016
Taghavi S, Barac T, Greenberg B, Borremans B, Vangronsveld J, van der Lelie D (2005) Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl Environ Microbiol 71:8500–8505
Tan XM, Zhou YQ, Zhou XL, Xia XH, Wei Y, He LL, Tang HZ, Yu LY (2018) Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci Rep 8(1):1–9
Tankari M, Wang C, Zhang X, Li L, Soothar RK, Ma H, Xing H, Yan C, Zhang Y, Liu F, Wang Y (2019) Leaf gas exchange, plant water relations and water use efficiency of Vigna unguiculata L. Walp. inoculated with rhizobia under different soil water regimes. Water 11(3):498
Techaoei S, Jirayuthcharoenkul C, Jarmkom K, Dumrongphuttidecha T, Khobjai W (2020) Chemical evaluation and antibacterial activity of novel bioactive compounds from endophytic fungi in Nelumbo nucifera. Saudi J Biol Sci 27(11):2883–2889
Teklić T, Parađiković N, Špoljarević M, Zeljković S, Lončarić Z, Lisjak M (2021) Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann Appl Biol 178(2):169–191
Tian J, Bryksa BC, Yada RY (2016) Feeding the world into the future–food and nutrition security: the role of food science and technology. Front Life Sci 9:55–166
Tiodar ED, Văcar CL, Podar D (2021) Phytoremediation and microorganisms-assisted phytoremediation of mercury-contaminated soils: challenges and perspectives. Int J Environ Res Public Health 18(5):2435
Trebicki P (2020) Climate change and plant virus epidemiology. Virus Res 286:198059
Tymon LS, Morgan P, Gundersen B, Inglis DA (2020) Potential of endophytic fungi collected from Cucurbita pepo roots grown under three different agricultural mulches as antagonistic endophytes to Verticillium dahliae in western Washington. Microbiol Res 240:126535
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Vargas L, Santa Brígida AB, Mota Filho JP, de Carvalho TG, Rojas CA, Vaneechoutte D, Bel MV, Farrinelli L, Ferreira PCG, Vandepoele K, Hemerly AS (2014) Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: a transcriptomic view of hormone pathways. PLoS ONE 9:e114744
Vaughan MM, Block A, Christensen SA, Allen LH, Schmelz EA (2018) The effects of climate change associated abiotic stresses on maize phytochemical defenses. Phytochem Rev 17(1):37–49
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Vishnupradeep R, Bruno LB, Taj Z, Karthik C, Challabathula D, Kumar A, Freitas H, Rajkumar M (2022) Plant growth promoting bacteria improve growth and phytostabilization potential of Zea mays under chromium and drought stress by altering photosynthetic and antioxidant responses. Environ Technol Innov 25:102154
Vurukonda SSKP, Vardharajula S, Shrivastava M, Ali SKZ (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24
Waigi MG, Sun K, Gao Y (2017) Sphingomonads in microbe-assisted phytoremediation: tackling soil pollution. Trends Biotechnol 35(9):883–899
Wan Y, Luo S, Chen J, Xiao X, Chen L, Zeng G, Liu C, He Y (2012) Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere 89(6):743–750
Wang Y, Li H, Zhao W, Xiaoli H, Jun C, Xiaolu G, Ming X (2010) Induction of toluene degradation and growth promotion in corn and wheat by horizontal gene transfer within endophytic bacteria. Soil Biol Biochem 2:1051–1057
Waqas M, Khan AL, Shahzad R, Ullah I, Khan AR, Lee I-J (2015) Mutualistic fungal endophytes produce phytohormones and organic acids that promote japonica rice plant growth under prolonged heat stress. J Zhejiang Univ-Sci B 16:1011–1018
Waqas MA, Khan I, Akhter MJ, Noor MA, Ashraf U (2017) Exogenous application of plant growth regulators (PGRs) induces chilling tolerance in short-duration hybrid maize. Environ Sci Pollut Res Int 24(12):11459
Wei J, Liu X, Wang Q, Wang C, Chen X, Li H (2014) Effect of rhizodeposition on pyrene bioaccessibility and microbial structure in pyrene and pyrene–lead polluted soil. Chemosphere 97:92–97
Weyens N, Croes S, Dupae J, Newman L, van der Lelie D, Carleer R, Vangronsveld J (2010) Endophytic bacteria improve phytoremediation of Ni and TCE co-contamination. Environ Pollut 158:2422–2427
Weyens N, Schellingen K, Beckers B, Janssen J, van der Ceulemans R, Lelie D, Taghavi S, Carleer R, Vangronsveld J (2013) Potential of willow and its genetically engineered associated bacteria to remediate mixed Cd and toluene contamination. J Soils Sediments 13:176–188
Weyens N, Thijs S, Popek R, Witters N, Przybysz A, Espenshade J, Gawronska H, Vangronsveld J, Gawronski SW (2015) The role of plant–microbe interactions and their exploitation for phytoremediation of air pollutants. Int J Mol Sci 16(10):25576–25604
Weyens N, Truyens S, Dupae J, Newman L, Taghavi S, van der Lelie D, Carleer R, Vangronsveld J (2010) Potential of the TCE-degrading endophyte Pseudomonas putida W619-TCE to improve plant growth and reduce TCE phytotoxicity and evapotranspiration in poplar cuttings. Environ Pollut 158:2915–2919
Weyens N, van der Lelie D, Taghavi S, Vangronsveld J (2009) Phytoremediation: plant-endophyte partnerships take the challenge. Curr Opin Biotechnol 20:248–254
White JF, Kingsley KL, Zhang Q, Verma R, Obi N, Dvinskikh S, Elmore MT, Verma SK, Gond SK, Kowalski KP (2019) Endophytic microbes and their potential applications in crop management. Pest Manag Sci 75(10):2558–2565
Win KT, Tanaka F, Okazaki K, Ohwaki Y (2018) The ACC deaminase expressing endophyte Pseudomonas spp. enhances NaCl stress tolerance by reducing stress-related ethylene production, resulting in improved growth, photosynthetic performance, and ionic balance in tomato plants. Plant Physiol Biochem 127:599–607
Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res Int 2019:9732325. https://doi.org/10.1155/2019/9732325
Yalcinkaya T, Uzilday B, Ozgur R, Turkan I, Mano JI (2019) Lipid peroxidation-derived reactive carbonyl species (RCS): their interaction with ROS and cellular redox during environmental stresses. Environ Exp Bot 165:139–149
Yamaji K, Watanabe Y, Masuya H, Shigeto A, Yui H, Haruma T (2016) Root fungal endophytes enhance heavy metal stress tolerance of clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PloS One 11(12):e0169089
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359
Yan L, Zhu J, Zhao X, Shi J, Jiang C, Shao D (2019) Beneficial effects of endophytic fungi colonization on plants. Appl Microbiol Biotechnol 103(8):3327–3340
Yang Y, Guo Y (2018) Unraveling salt stress signaling in plants. J Integr Plant Biol 60(9):796–804
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Yousaf S, Afzal M, Reichenauer TG, Brady CL, Sessitsch A (2011) Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ Pollut 159:2675–2683
Yu Z, Duan X, Luo L, Dai S, Ding Z, Xia G (2020) How plant hormones mediate salt stress responses. Trends Plant Sci 25(11):1117–1130
Zafar SA, Noor MA, Waqas MA, Wang X, Shaheen T, Raza M, Rahman MU (2018) Temperature extremes in cotton production and mitigation strategies. Past, present and future trends in cotton breeding 65–91
Zhang W, Xie Z, Zhang X, Lang D, Zhang X (2019) Growth-promoting bacteria alleviates drought stress of G. uralensis through improving photosynthesis characteristics and water status. J Plant Interactions 14(1):580–589
Zhang Y, Tian Z, Xi Y, Wang X, Chen S, He M, Chen Y, Guo Y (2022) Improvement of salt tolerance of Arabidopsis thaliana seedlings inoculated with endophytic Bacillus cereus KP120. J Plant Interactions 17(1):884–893
Zhao C, Zhang H, Song C, Zhu JK, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. The Innovation 1(1):100017
Zhou JY, Zhao XY, Dai CC (2014) Antagonistic mechanisms of endophytic Pseudomonas fluorescens against Athelia rolfsii. J Appl Microbiol 117(4):1144–1158
Zhou XR, Dai L, Xu GF, Wang HS (2021) A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci Rep 11(1):1–11
Zhu LJ, Guan DX, Luo J, Rathinasabapathi B, Ma LQ (2014) Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida. Chemosphere 113:9–16
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Zhu L, Li T, Wang C, Zhang X, Xu L, Xu R, Zhao Z (2018) The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ Sci Pollut Res 25:35232–35241
Zimowska B, Bielecka M, Abramczyk B, Nicoletti R (2020) Bioactive products from endophytic fungi of sages (Salvia spp.). Agriculture 10(11):543
Zörb C, Geilfus CM, Dietz KJ (2019) Salinity and crop yield. Plant biology 21:31–38
Zuzolo D, Guarino C, Tartaglia M, Sciarrillo R (2021) Plant-soil-microbiota combination for the removal of total petroleum hydrocarbons (TPH): an in-field experiment. Front Microbiol 11:3611
Acknowledgements
All authors are highly grateful to the authority of the respective departments and institutions for their support in carrying out this research. The graphical abstract and Figs. 2 and 7 were created with Biorender.com. The authors acknowledge Serena Ducoli for her support in the preparation of figures.
Funding
Open access funding provided by Università degli Studi di Brescia, Italy, within the CRUI-CARE Agreement. YM has received funding from the Portuguese Foundation for Science and Technology (FCT) for the project UIDB/04004/2020. The FCT supported the research contract of YM (SFRH/BPD/76028/2011). MK has received funding from The Daniel E. Koshland Fund.
Author information
Authors and Affiliations
Contributions
All authors of this manuscript have substantially contributed to the concept, literature mining, writing, and methodology of the review; provided critical feedback; and critically revised the manuscript. All authors contributed to the writing or revision of the final manuscript. UA: contributed to the study idea, planned and designed the review structure, literature survey, writing—original draft preparation, data validation, revised the tables and figures, arranged references, final draft. TP: literature survey, writing—review and editing, table and figure preparation, suggestions, response, responded to referees comments. NY, VKS, VT, KKC, AKS, KS, AK: literature survey, first draft preparation, writing—review and editing, table and figure preparation, suggestions, response, validation. EB: drafted study design section, writing—review and editing, figure preparation, data validation, response, supervision. YM: revised the manuscript, suggestions, response. MK: revised the manuscript, suggestions, response. AKS: conceptualization, revised the review structure, writing—review and editing, table and figure preparation, suggestions, formal interpretation, supervision, resources, final draft. All authors made a substantial contribution to the manuscript writing and revision, and approved it for publication.
Corresponding authors
Ethics declarations
Ethics Approval
This review article does not contain any studies with human participants or animals performed by any of the listed authors.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anand, U., Pal, T., Yadav, N. et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb Ecol 86, 1455–1486 (2023). https://doi.org/10.1007/s00248-023-02190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02190-1