Abstract
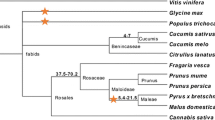
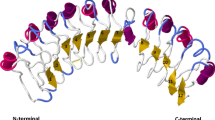
Degenerate oligonucleotide primers, designed based on conserved regions of several serine-threonine kinases (STK) previously cloned in tomato and Arabidopsis, were used to isolate STK candidates in wild and cultivated strawberries. Seven distinct classes of STKs were identified from three related wild species, i.e., Fragaria vesca, Fragaria chiloensis, and Potentilla tucumanensis, and seven different Fragaria × ananassa cultivars. Alignment of the deduced amino acid sequences and the Pto R protein from tomato revealed the presence of characteristic subdomains and conservation of the plant STK consensus and other residues that are crucial for Pto function. Based on identity scores and clustering in phylogenetic trees, five groups were recognized as Pto-like kinases. Strawberry Pto-like clones presented sequences that were clearly identified as the activation segments contained in the Pto, and some of them showed residues previously identified as being required for binding to AvrPto. Some of the non-Pto-like kinases presented a high degree of identity and grouped together with B-lectin receptor kinases that are also involved in disease resistance. Statistical studies carried out to evaluate departure from the neutral theory and nonsynonymous/synonymous substitutions suggest that the evolution of STK-encoding sequences in strawberries is subjected mainly to a purifying selection process. These results represent the first report of Pto-like STKs in strawberry.



Similar content being viewed by others
References
Aarts MG, te Lintel Hekkert B, Holub BT, Beynon JL, Stiekema WJ, Pereira A (1998) Identification of R-gene homologous DNA fragments genetically linked to disease resistance loci in Arabidopsis thaliana. Mol Plant-Microbe Interact 11:251–258
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bernal A, Pollack J, Pan Q, Rose L, Kozik A (2005) Functional analysis of the plant resistance gene Pto using DNA shuffling. J Biol Chem 280:23073–23083
Bogdanove AJ (2002) Pto update: recent progress on an ancient plant defence response signalling pathway. Mol Plant Pathol 3:283–288
Chang JH, Tai YS, Bernal AJ, Lavelle DT, Staskawicz BJ (2002) Functional analyses of the Pto resistance gene family in tomato and the identification of a minor resistance determinant in a susceptible haplotype. Mol Plant-Microbe Interact 15:281–291
Chen X, Shang J, Chen D, Lei C, Zou Y, Zhai W Liu G, Xu J, Ling Z, Ma B, Wang Y, Zhao X, Li S, Zhu L (2006) A B lectin receptor kinase gene conferring rice blast resistance. Plant J 46:794–804
Cui Y, Bi Y, Brugière N, Arnoldo M, Rothstein S (2000) The S locus glycoprotein and the S receptor kinase are sufficient for self-pollen rejection in Brassica. Proc Natl Acad Sci USA 97:3713–3717
Dangl JL, Jones JD (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
Dayhoff MO, Schwartz RM, Orcutt BC (1978) A model of evolutionary change in proteins. Matrices for detecting distant relationships. In: Dayhoff MO (ed) Atlas of protein sequence and structure. Vol. 5. National Biomedical Research Foundation, Washington, DC, pp 345–358
Denoyes-Rothan B, Guérin G, Lerceteau-Köller E, Risser G (2005) Inheritance of resistance to Colletotrichum acutatum in Fragaria × ananassa. Phytopathology 95:405–412
DeVries JS, Andriotis VME, Wu AJ, Rathjen JP (2006) Tomato Pto encodes a functional N-myristoylation motif that is required for signal transduction in Nicotiana benthamiana. Plant J 45:31–45
Ellis J, Dodds P, Pryor T (2000) Structure, function and evolution of plant disease resistance genes. Curr Opin Plant Biol 3:278–284
Flor HH (1956) The complementary genic systems in flax and flax rust. Adv Genet 8:29–54
Fluhr R (2001) Sentinels of disease. Plant resistance genes. Plant Physiol 127:1367–1374
Frederick RD, Thilmony RL, Sessa G, Martin GB (1998) Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol Cell 2:241–245
Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133:693–709
Galletta GJ, Smith BJ, Gupton CL (1993) Strawberry parent clones US70, US159, US292, and US438 resistant to anthracnose crown rot. Hortscience 28:1055–1056
Hammond-Kosack KE, Jones JDG (1997) Plant disease resistance genes. Annu Rev Plant Physiol Plant Mol Biol 48:575–607
Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9:576–596
Hanks SK, Quinn AM (1990) Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol 200:38–62
Hardie DG (1999) Plant protein serine/threonine kinases: classification and functions. Annu Rev Plant Physiol Mol Biol 50:97–131
Haymes KM, Henken B, Davis TM, Van de Weg WE (1997) Identification of RAPD markers linked to a Phytophthora fragariae resistance gene (Rpf1) in the cultivated strawberry. Theoret Appl Genet 94:1097–1101
Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouze P, Brunak S (1996) Splice site prediction in Arabidopsis thaliana DNA by combining local and global sequence information. Nucleic Acids Res 24:3439–3452
Hofmann K, Bucher P, Falquet L, Bairoch A (1999) The PROSITE database, its status in 1999. Nucleic Acids Res 27: 215–219
Joyeux A, Fortin MG, Mayerhofer R, Good AG (1999) Genetic mapping of plant disease resistance gene homologues using a minimal Brassica napus L. population. Genome 42:735–743
Kanazin V, Marek LF, Shoemaker RC (1996) Resistance gene analogs are conserved and clustered in soybean. Proc Natl Acad Sci USA 93:11746–11750
Kim YJ, Lin NC, Martin GB (2002) Two distinct Pseudomonas effector proteins interact with the Pto kinase and activate plant immunity. Cell 109:589–598
Korber B (2000) HIV signature and sequence variation analysis. In: Rodrigo AG, Learn GH (eds) Computational analysis of HIV molecular sequences. Kluwer Academic, Dordrecht, Netherlands, pp 55–72
Leister D, Ballvora A, Salamini F, Gebhardt C (1996) A PCR-based approach for isolating pathogen resistance genes from potato with potential for wide application in plants. Nat Genet 14:421–442
Lerceteau-Kohler E, Roudeillac P, Markocic M, Guerin G, Praud K, Denoyes-Rothan B (2002) The use of molecular markers for durable resistance breeding in the cultivated strawberry (Fragaria x ananassa). Acta Hort 567:615–618
Loh YT, Martin GB (1995) The Pto bacterial resistance gene and the Fen insecticide sensitivity gene encode functional protein kinases with serine/threonine specificity. Plant Physiol 108:1735–1739
Martin GB (1999) Functional Analysis of plant disease resistance genes and their downstream effectors. Curr Opin Plant Biol 2:273–279
Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD (1993) Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262:1432–1435
Martin GB, Frary A, Wu T, Brommonschenkel S, Chunwongse J, Earle ED, Tanksley SD (1994) A member of the tomato Pto gene family confers sensitivity to fenthion resulting in rapid cell death. Plant Cell 6:1543–1552
Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the function of plant disease resistance proteins. Annu Rev Plant Biol 54:23–61
Martínez-Zamora MG, Castagnaro AP, Diaz-Ricci JC (2004) Isolation and diversity analysis of resistance gene analogues (RGAs) from cultivated and wild strawberries. Mol Genet Genomics 272:480–487
McHale L, Tan X, Koehl P, Michelmore RW (2006) Plant NBS-LRR proteins: adaptable guards. Genome Biol 7:212
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8:1113–1130
Morrisette PM (1989) The evolution of policy responses to stratospheric ozone depletion. Nat Resources J 29:793–820
Nei M, Gojobori T (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3:418–426
Nishiguchi M, Yoshida K, Sumizono T, Tazaki K (2002) A receptor-like protein kinase with a lectin-like domain from lombardy poplar: gene expression in response to wounding and characterization of phosphorylation activity. Mol Genet Genomics 267:506–514
Pan Q, Wendel J, Fluhr R (2000) Divergent evolution of plant NBS-LRR resistance gene homologues in dicot and cereal genomes. J Mol Evol 50:203–213
Parker JE, Coleman MJ, Szabo V, Frost LN, Schmidt R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JDG (1997) The arabidopsis downy mildew resistance gene RPP5 shares similarity to the Toll and interleukin-1 receptors with N and L6. Plant Cell 9:879–894
Pedley KF, Martin GB (2003) Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu Rev Phytopathol 41:215–243
Peraza-Echeverría S, James-Kay A, Canto-Canché B, Castillo-Castro E (2007) Structural and phylogenetic analysis of Pto-type disease resistance gene candidates in banana. Mol Genet Genomics 278:443–453
Qin Y, Texeira da Silva J, Zhang L, Zhang Sh (2008) Transgenic strawberry: state of the art for improved traits. Biotechnol Adv 26:219–232
Rathjen JP, Chang JH, Staskawicz BJ, Michelmore RW (1999) Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of avrPto. EMBO J 18:3232–3240
Richter T, Ronald P (2002) The evolution of disease resistance genes. Plant Mol Biol 42:195–204
Rose LE, Michelmore RW, Langley CH (2007) Natural variation in the Pto pathogen resistance gene within species of wild tomato (Lycopersicon): II. Population genetics of Pto. Genetics 175:1307–1319
Rozas J, Sánchez-DelBarrio JC, Messegyer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Rudrabhatla P, Reddy MM, Rajasekharan R (2006) Genome-wide analysis and experimentation of plant serine/threonine/tyrosine-specific protein kinases. Plant Mol Biol 60:293–319
Sawyer S (1989) Statistical tests for detecting gene conversion. Mol Biol Evol 6:526–538
Schmidt HA, Strimmer K, Vingron M, von Haeseler A (2002) TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502–504
Scofield SR, Tobias CM, Rathjen JP, Chang JH, Lavelle DT, Michelmore RW, Staskawicz BJ (1996) Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274:2063–2065
Sessa G, D’Ascenzo M, Martin GB (2000a) The major site of the Pti1 kinase phosphorylated by the Pto kinase is located in the activation domain and is required for Pto–Pti1 physical interaction. Eur J Biochem 267:171–178
Sessa G, D’Ascenzo M, Martin GB (2000b) Thr38 and Ser198 are Pto autophosphorylation sites required for the AvrPto–Pto-mediated hypersensitive response. EMBO J 19:2257–2269
Shen KA, Meyers BC, Islam-Faridi N, Stelly DM, Michelmore RW (1998) Resistance gene candidates identified using PCR with degenerate oligonucleotide primers map to resistance gene clusters in lettuce. Mol Plant-Microbe Interact 11:815–823
Smith BJ, Black LL (1990) Morphological, cultural and pathogenic variation among Colletotrichum species isolated from strawberry. Plant Dis 74:69–76
Speulman E, Bouchez D, Holub EB, Beynon JL (1998) Disease resistance gene homologs correlate with disease resistance loci of Arabidopsis thaliana. Plant J 14:467–474
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–596
Tang X, Xie M, Kim YJ, Zhou J, Klessig DF, Martin GB (1999) Overexpression of Pto activates defense responses and confers broad resistance. Plant Cell 11:15–30
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vallad G, Rivkin M, Vallejos C, McClean P (2001) Cloning and homology modelling of a Pto-like protein kinase family of common bean (Phaseolus vulgaris L.). Theor Appl Genet 103:1046–1058
Vellicce GR, Díaz Ricci JC, Hernandez L, Castagnaro AP (2006) Enhanced resistance to Botrytis cinerea mediated by the transgenic expression of the chitinase gene ch5B in strawberry. Trans Res 15:57–68
Vleeshouwers VGAA, Martens A, van Dooijeweert W, Colon LT, Govers F, Kamoun S (2001) Ancient diversification of the Pto kinase family preceded speciation in Solanum. Mol Plant Microbe Interact 14:996–1005
Yu YG, Buss GR, Maroof MA (1996) Isolation of a superfamily of candidate disease-resistance genes in soybean based on a conserved nucleotide-binding site. Proc Natl Acad Sci USA 93:11751–11756
Wu A, Andriotis V, Durrant M, Rathjen JP (2004) A patch of surface-exposed residues mediates negative regulation of immune signaling by tomato Pto kinase. Plant Cell 16:2809–2821
Zhou J, Tang X, Martin GB (1997) The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO J 16:3207–3218
Acknowledgments
This paper was partially supported by Grants PICTO 04-759 BID 1728/OC-AR and CIUNT 26/D346. The work was carried out in compliance with the current laws regulating genetic experimentation in Argentina. G.M.Z. is a fellow of CONICET, and A.P.C. and J.C.D.R. are members of CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez Zamora, M.G., Castagnaro, A.P. & Díaz Ricci, J.C. Genetic Diversity of Pto-Like Serine/Threonine Kinase Disease Resistance Genes in Cultivated and Wild Strawberries. J Mol Evol 67, 211–221 (2008). https://doi.org/10.1007/s00239-008-9134-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-008-9134-0