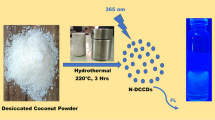
Abstract
Concentration-dependent photoluminescence carbon dots (CDs) have been successfully synthesized through the one-step hydrothermal treatment of o-phthalic acid and ethylenediamine. The CDs possessed higher fluorescence quantum yield, up to 39.22%, exhibiting distinguished optical property, water solubility, and stability. The CDs that emit strong blue-green fluorescence can visually identify and determine tetracycline (TC), oxytetracycline (OTC), and chlortetracycline (CTC). TC quenched the fluorescence of CDs at 500 nm owing to the inner filter effect; OTC behaved similarly, but the emission wavelength of CDs was red-shifted to 515 nm. Inversely, once CTC was introduced to CDs solution, the fluorescence increased and the emission peak was blue-shifted to 450 nm. Bandgap transition and electrostatic interaction were proposed to be the mechanisms for the detection of OTC and CTC by CDs. Wide linear relationships were established for TC, OTC, and CTC with the limits of detection to be 50 nM, 36 nM, and 373 nM, respectively. Furthermore, the nanoscale probe constructed by this system has been applied to detect tetracyclines (TCs) in complex samples with satisfying recoveries (93.2–114%) and was designed as a portable test strip sensor for visually on-site TCs of honey sample screening. Accordingly, the preparation process of the nano fluorescent probe is simple and environmentally friendly, and the probe has a specific recognition ability for tetracyclines. The synthesized CDs in this work provide a new orientation for fast, effective, and visual real-time detection of tetracycline in actual samples.
Graphical abstract








Similar content being viewed by others
References
Jiang X, Qin D, Mo G, Feng J, Zheng X, Deng B. Facile preparation of boron and nitrogen codoped green emission carbon quantum dots for detection of permanganate and captopril. Anal Chem. 2019;91(17):11455–60. https://doi.org/10.1021/acs.analchem.9b02938.
Granados JAO, Thangarasu P, Singh N, Vazquez-Ramos JM. Tetracycline and its quantum dots for recognition of Al(3+) and application in milk developing cells bio-imaging. Food Chem. 2019;278:523–32. https://doi.org/10.1016/j.foodchem.2018.11.086.
Meyer VK, Chatelle CV, Weber W, Niessner R, Seidel M. Flow-based regenerable chemiluminescence receptor assay for the detection of tetracyclines. Anal Bioanal Chem. 2020;412(14):3467–76. https://doi.org/10.1007/s00216-019-02368-y.
Granados-Chinchilla F, Rodriguez C. Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem. 2017;2017:1315497. https://doi.org/10.1155/2017/1315497.
Sachi S, Ferdous J, Sikder MH, Azizul Karim Hussani SM. Antibiotic residues in milk: past, present, and future. J Adv Vet Anim Res. 2019;6(3):315–32. https://doi.org/10.5455/javar.2019.f350.
Ramezani M, Mohammad Danesh N, Lavaee P, Abnous K, Mohammad Taghdisi S. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens Bioelectron. 2015;70:181–7. https://doi.org/10.1016/j.bios.2015.03.040.
Feng MX, Wang GN, Yang K, Liu HZ, Wang JP. Molecularly imprinted polymer-high performance liquid chromatography for the determination of tetracycline drugs in animal derived foods. Food Control. 2016;69:171–6. https://doi.org/10.1016/j.foodcont.2016.04.050.
Shariati S, Yamini Y, Esrafili A. Carrier mediated hollow fiber liquid phase microextraction combined with HPLC-UV for preconcentration and determination of some tetracycline antibiotics. J Chromatogr Sci. 2009;877(4):393–400. https://doi.org/10.1016/j.jchromb.2008.12.042.
Ahmed S, Ning J, Peng D, Chen T, Ahmad I, Ali A, et al. Current advances in immunoassays for the detection of antibiotics residues: a review. Food Agric Immunol. 2020;31(1):268–90. https://doi.org/10.1080/09540105.2019.1707171.
Wang Y, Sun Y, Dai H, Ni P, Jiang S, Lu W, et al. A colorimetric biosensor using Fe3O4 nanoparticles for highly sensitive and selective detection of tetracyclines. Sensors Actuators B Chem. 2016;236:621–6. https://doi.org/10.1016/j.snb.2016.06.029.
Ibarra IS, Rodriguez JA, Miranda JM, Vega M, Barrado E. Magnetic solid phase extraction based on phenyl silica adsorbent for the determination of tetracyclines in milk samples by capillary electrophoresis. J Chromatogr A. 2011;1218(16):2196–202. https://doi.org/10.1016/j.chroma.2011.02.046.
Xiong Y, Zhou H, Zhang Z, He D, He C. Molecularly imprinted on-line solid-phase extraction combined with flow-injection chemiluminescence for the determination of tetracycline. Analyst. 2006;131(7):829–34. https://doi.org/10.1039/b606779b.
Wang T, Mei Q, Tao Z, Wu H, Zhao M, Wang S, et al. A smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens Bioelectron. 2020;148:111791. https://doi.org/10.1016/j.bios.2019.111791.
Li X, Wang H, Zhang Y, Cao Q, Chen Y. A GSH-responsive PET-based fluorescent probe for cancer cells imaging. Chin Chem Lett. 2020;5938:1–4. https://doi.org/10.1016/j.cclet.2020.10.047.
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, et al. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed. 2013;52(14):3953–7. https://doi.org/10.1002/anie.201300519.
Jana J, Lee HJ, Chung JS, Kim MH, Hur SH. Blue emitting nitrogen-doped carbon dots as a fluorescent probe for nitrite ion sensing and cell-imaging. Anal Chim Acta. 2019;1079:212–9. https://doi.org/10.1016/j.aca.2019.06.064.
Sun M, Qu S, Hao Z, Ji W, Jing P, Zhang H, et al. Towards efficient solid-state photoluminescence based on carbon-nanodots and starch composites. Nanoscale. 2014;6(21):13076–81. https://doi.org/10.1039/c4nr04034a.
Wang Y, Kalytchuk S, Wang L, Zhovtiuk O, Cepe K, Zboril R, et al. Carbon dot hybrids with oligomeric silsesquioxane: solid-state luminophores with high photoluminescence quantum yield and applicability in white light emitting devices. Chem Commun. 2015;51(14):2950–3. https://doi.org/10.1039/c4cc09589h.
Shen Z, Zhang C, Yu X, Li J, Wang Z, Zhang Z, et al. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J Mater Chem. 2018;6(36):9636–41. https://doi.org/10.1039/c8tc02982b.
Lu C, Su Q, Yang X. Ultra-long room-temperature phosphorescent carbon dots: pH sensing and dual-channel detection of tetracyclines. Nanoscale. 2019;11(34):16036–42. https://doi.org/10.1039/c9nr03989a.
Uriarte D, Domini C, Garrido M. New carbon dots based on glycerol and urea and its application in the determination of tetracycline in urine samples. Talanta. 2019;201:143–8. https://doi.org/10.1016/j.talanta.2019.04.001.
Fu Y, Huang L, Zhao S, Xing X, Lan M, Song X. A carbon dot-based fluorometric probe for oxytetracycline detection utilizing a Forster resonance energy transfer mechanism. Spectrochim Acta A Mol Biomol Spectrosc. 2021;246:118947. https://doi.org/10.1016/j.saa.2020.118947.
Xing X, Huang L, Zhao S, Xiao J, Lan M. S,N-doped carbon dots for tetracyclines sensing with a fluorometric spectral response. Microchem J. 2020;157:105065. https://doi.org/10.1016/j.microc.2020.105065.
Fu Y, Zhao S, Wu S, Huang L, Xu T, Xing X, et al. A carbon dots-based fluorescent probe for turn-on sensing of ampicillin. Dyes Pigments. 2020;172:107846. https://doi.org/10.1016/j.dyepig.2019.107846.
Miao H, Wang Y, Yang X. Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale. 2018;10(17):8139–45. https://doi.org/10.1039/c8nr02405g.
Zhao N, Wang Y, Hou S, Zhao L. Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Microchim Acta. 2020;187(6):351. https://doi.org/10.1007/s00604-020-04328-1.
Wang S, Yong W, Liu J, Zhang L, Chen Q, Dong Y. Development of an indirect competitive assay-based aptasensor for highly sensitive detection of tetracycline residue in honey. Biosens Bioelectron. 2014;57:192–8. https://doi.org/10.1016/j.bios.2014.02.032.
Meng L, Lan C, Liu Z, Xu N, Wu Y. A novel ratiometric fluorescence probe for highly sensitive and specific detection of chlorotetracycline among tetracycline antibiotics. Anal Chim Acta. 2019;1089:144–51. https://doi.org/10.1016/j.aca.2019.08.065.
Li W, Zhang X, Miao C, Li R, Ji Y. Fluorescent paper-based sensor based on carbon dots for detection of folic acid. Anal Bioanal Chem. 2020;412(12):2805–13. https://doi.org/10.1007/s00216-020-02507-w.
Jia P, Bu T, Sun X, Liu Y, Liu J, Wang Q, et al. A sensitive and selective approach for detection of tetracyclines using fluorescent molybdenum disulfide nanoplates. Food Chem. 2019;297:124969. https://doi.org/10.1016/j.foodchem.2019.124969.
Qu S, Wang X, Lu Q, Liu X, Wang L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew Chem Int Ed. 2012;51(49):12215–8. https://doi.org/10.1002/anie.201206791.
Ye C, Qin Y, Huang P, Chen A, Wu FY. Facile synthesis of carbon nanodots with surface state-modulated fluorescence for highly sensitive and real-time detection of water in organic solvents. Anal Chim Acta. 2018;1034:144–52. https://doi.org/10.1016/j.aca.2018.06.003.
Liu S, Tian J, Wang L, Zhang Y, Qin X, Luo Y, et al. Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv Mater. 2012;24(15):2037–41. https://doi.org/10.1002/adma.201200164.
Zhu C, Yang S, Wang G, Mo R, He P, Sun J, et al. A new mild, clean and highly efficient method for the preparation of graphene quantum dots without by-products. J Mater Chem B. 2015;3(34):6871–6. https://doi.org/10.1039/c5tb01093d.
Zheng M, Xie Z, Qu D, Li D, Du P, Jing X, et al. On-off-on fluorescent carbon dot nanosensor for recognition of chromium(VI) and ascorbic acid based on the inner filter effect. ACS Appl Mater Interfaces. 2013;5(24):13242–7. https://doi.org/10.1021/am4042355.
Zhu A, Qu Q, Shao X, Kong B, Tian Y. Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew Chem Int Ed. 2012;51(29):7185–9. https://doi.org/10.1002/anie.201109089.
Moniruzzaman M, Kim J. N-doped carbon dots with tunable emission for multifaceted application: solvatochromism, moisture sensing, pH sensing, and solid state multicolor lighting. Sensors Actuators B Chem. 2019;295:12–21. https://doi.org/10.1016/j.snb.2019.05.035.
Ba XX, Zhang L, Yin YL, Jiang FL, Jiang P, Liu Y. Luminescent carbon dots with concentration-dependent emission in solution and yellow emission in solid state. J Colloid Interface Sci. 2020;565:77–85. https://doi.org/10.1016/j.jcis.2020.01.007.
Gao MX, Liu CF, Wu ZL, Zeng QL, Yang XX, Wu WB, et al. A surfactant-assisted redox hydrothermal route to prepare highly photoluminescent carbon quantum dots with aggregation-induced emission enhancement properties. Chem Commun. 2013;49(73):8015–7. https://doi.org/10.1039/c3cc44624g.
Bao L, Zhang ZL, Tian ZQ, Zhang L, Liu C, Lin Y, et al. Electrochemical tuning of luminescent carbon nanodots: from preparation to luminescence mechanism. Adv Mater. 2011;23(48):5801–6. https://doi.org/10.1002/adma.201102866.
Chico J, van Holthoon F, Zuidema T. Ion suppression study for tetracyclines in feed. Chromatogr Res Int. 2012;2012:1–9. https://doi.org/10.1155/2012/135854.
Esmaelpourfarkhani M, Abnous K, Taghdisi SM, Chamsaz M. A fluorometric assay for oxytetracycline based on the use of its europium(III) complex and aptamer-modified silver nanoparticles. Microchim Acta. 2019;186(5):290. https://doi.org/10.1007/s00604-019-3389-6.
Qiao L, Qian S, Wang Y, Yan S, Lin H. Carbon-dots-based lab-on-a-nanoparticle approach for the detection and differentiation of antibiotics. Chemistry. 2018;24(18):4703–9. https://doi.org/10.1002/chem.201706056.
Lu W, Jiao Y, Gao Y, Qiao J, Mozneb M, Shuang S, et al. Bright yellow fluorescent carbon dots as a multifunctional sensing platform for the label-free detection of fluoroquinolones and histidine. ACS Appl Mater Interfaces. 2018;10(49):42915–24. https://doi.org/10.1021/acsami.8b16710.
Chandra S, Bano D, Pradhan P, Singh VK, Yadav PK, Sinha D, et al. Nitrogen/sulfur-co-doped carbon quantum dots: a biocompatible material for the selective detection of picric acid in aqueous solution and living cells. Anal Bioanal Chem. 2020;412(15):3753–63. https://doi.org/10.1007/s00216-020-02629-1.
Zhou C, He X, Ya D, Zhong J, Deng B. One step hydrothermal synthesis of nitrogen-doped graphitic quantum dots as a fluorescent sensing strategy for highly sensitive detection of metacycline in mice plasma. Sensors Actuators B Chem. 2017;249:256–64. https://doi.org/10.1016/j.snb.2017.04.092.
Yan Y, Liu JH, Li RS, Li YF, Huang CZ, Zhen SJ. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal Chim Acta. 2019;1063:144–51. https://doi.org/10.1016/j.aca.2019.02.047.
Li Y, Du Q, Zhang X, Huang Y. Ratiometric detection of tetracycline based on gold nanocluster enhanced Eu(3+) fluorescence. Talanta. 2020;206:120202. https://doi.org/10.1016/j.talanta.2019.120202.
Funding
We greatly acknowledge the financial support supplied from the National Natural Science Foundation of China (No. 21765014 and 21864018) and the Science and Technology Innovation Platform Project of Jiangxi Province (No. 20192BCD40001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Jiangxi Medical College, and their guidelines were followed in the whole study. The serum samples related to our research were collected from the patient. The urine samples involved in our research were from a healthy individual. Informed consent was obtained from all human participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 16509 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Liu, B., Huang, P. et al. Concentration-dependent photoluminescence carbon dots for visual recognition and detection of three tetracyclines. Anal Bioanal Chem 413, 2565–2575 (2021). https://doi.org/10.1007/s00216-021-03221-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03221-x