Abstract
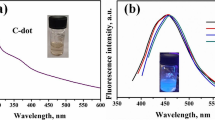
Here, a fast and eco-friendly one-pot hydrothermal technique is utilized for the synthesis of nitrogen/sulfur-co-doped fluorescent carbon quantum dots (NS-CQDs) from a simple precursor of citric acid (CA) and thiosemicarbazide (TSC). The obtained NS-CQDs exhibited strong blue emission under UV light, with fluorescence quantum yield (QY) of ~37.8%. The Commission internationale de l’eclairage (CIE) coordinates originated at (0.15, 0.07), which confirmed the blue fluorescence of the synthesized NS-CQDs. Interestingly, the prepared NS-CQDs were successfully used as a selective nanoprobe for the monitoring of environmentally hazardous explosive picric acid (PA) in different nitro- and non-nitro-aromatic derivatives of PA. The mechanism of the NS-CQDs was also explored, and was posited to occur via the fluorescence resonance electron transfer (FRET) process and non-fluorescent complex formation. Importantly, this system possesses excellent biocompatibility and low cytotoxicity in HeLa cervical cancer cells; hence, it can potentially be used for PA detection in analytical, environmental, and pathological applications. Furthermore, the practical applicability of the proposed sensing system to pond water demonstrated the feasibility of our system along with good recovery.

Graphical abstract








Similar content being viewed by others
References
Liu SG, Li N, Li NB, Xiao N, Luo HQ, Fan YZ, Ju YJ. Adenosine-derived doped carbon dots: From an insight into effect of N/P co-doping on emission to highly sensitive picric acid sensing. Anal Chim Acta 2018;1013:63–70.
Li J, Zhang L, Li P, Zhang Y, Dong C. One step hydrothermal synthesis of carbon nanodots to realize the fluorescence detection of picric acid in real samples. Sensor Actuat B: Chem. 2018;258:580–8.
Ye Q, Yan F, Shi D, Zheng T, Wang Y, Zhou X, Chen L. N, B-doped carbon dots as a sensitive fluorescence probe for Hg2+ ions and 2,4,6-trinitrophenol detection for bioimaging. J Photochem Photo B: Bio 2016;162:1–13.
Hu L, Sun Y, Zhou Y, Bai L, Zhang Y, Han M, et al. Nitrogen and sulfur co-doped chiral carbon quantum dots with independent photoluminescence and chirality. Inor Chem Front. 2017;4(6):946–53.
Jiang X, Qin D, Mo G, Feng J, Yu C, Mo W, et al. Ginkgo leaf-based synthesis of nitrogen-doped carbon quantum dots for highly sensitive detection of salazosulfapyridine in mouse plasma. J Pharm Biomed Anal. 2019;164:514–9.
Xu J, Sahu S, Cao L, Anilkumar P, Tackett KN, Qian H, et al. Carbon nanoparticles as chromophores for photon harvesting and photoconversion. ChemPhysChem. 2011;12(18):3604–8.
Li H, He X, Kang Z, Huang H, Liu Y, Liu J, et al. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chem Int Ed. 2010;49(26):4430–4.
Wang F, Pang S, Wang L, Li Q, Kreiter M, Liu CY. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem Mater. 2010;22(16):4528–30.
Zhou J, Booker C, Li R, Zhou X, Sham TK, Sun X, et al. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J Am Chem Soc. 2007;129(4):744–5.
Yang F, Zhao M, Zheng B, Xiao D, Wu L, Guo Y. Influence of pH on the fluorescence properties of graphene quantum dots using ozonation pre-oxide hydrothermal synthesis. J Mater Chem. 2012;22(48):25471–9.
Yang ZC, Wang M, Yong AM, Wong SY, Zhang XH, Tan H, et al. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem Commun. 2011;47(42):11615–7.
Mondal TK, Dinda D, Saha SK. Nitrogen, sulphur co-doped graphene quantum dot: An excellent sensor for nitroexplosives. Sensor Actuat B: Chem. 2018;257:586–93.
Wan Y, Wang M, Zhang K, Fu Q, Gao M, Wang L, et al. Facile and green synthesis of fluorescent carbon dots from the flowers of Abelmoschus manihot (Linn.) Medicus for sensitive detection of 2,4,6-trinitrophenol and cellular imaging. Microchem J. 2019;148:385–96.
Liu S, Shi F, Chen L, Su X. Bovine serum albumin coated CuInS2 quantum dots as a near-infrared fluorescence probe for 2, 4, 6-trinitrophenol detection. Talanta. 2013;116:870–5.
Deng X, Huang X, Wu D. Förster resonance-energy-transfer detection of 2,4,6-trinitrophenol using copper nanoclusters. Anal and Bioanal Chem. 2015;407(16):4607–13.
Chen BB, Liu ZX, Zou HY, Huang CZ. Highly selective detection of 2, 4, 6-trinitrophenol by using newly developed terbium-doped blue carbon dots. Analyst. 2016;141(9):2676–81.
Wang B, Mu Y, Zhang C, Li J. Blue photoluminescent carbon nanodots prepared from zeolite as efficient sensors for picric acid detection. Sensor Actuat B: Chem. 2017;253:911–7.
Khan ZM, Saifi S, Aslam Z, Khan SA, Zulfequar M. A facile one step hydrothermal synthesis of carbon quantum dots for label-free fluorescence sensing approach to detect picric acid in aqueous solution. J Photochem and Photobio A: Chem. 2020;388:112201.
Rong M, Lin L, Song X, Zhao T, Zhong Y, Yan J, Wang Y, Chen X. A label-free fluorescence sensing approach for selective and sensitive detection of 2,4,6-trinitrophenol (TNP) in aqueous solution using graphitic carbon nitride nanosheets. Anal chem. 2015 20;87(2):1288–96.
Wang M, Fu Q, Zhang K, Wan Y, Wang L, Gao M, et al. A magnetic and carbon dot based molecularly imprinted composite for fluorometric detection of 2,4,6-trinitrophenol. Micro Acta. 2019;186(2):86.
Tian M, Wang Y, Zhang Y. Synthesis of fluorescent nitrogen-doped carbon quantum dots for selective detection of picric acid in water samples. J Nano Nanotec. 2018;18(12):8111–7.
Wang Y, Chang X, Jing N, Zhang Y. Hydrothermal synthesis of carbon quantum dots as fluorescent probes for the sensitive and rapid detection of picric acid. Anal Methods. 2018;10(23):2775–84.
Barron L, Gilchrist E. Ion chromatography-mass spectrometry: a review of recent technologies and applications in forensic and environmental explosives analysis. Anal Chim Acta. 2014;806:27–54.
Srinivasan P, Gunasekaran M, Kanagasekaran T, Gopalakrishnan R, Ramasamy P. 2,4,6-Trinitrophenol (TNP): An organic material for nonlinear optical (NLO) applications. J Cryst Growth. 2006;289(2):639–46.
Ko H, Chang S, Tsukruk VV. Porous substrates for label-free molecular level detection of nonresonant organic molecules. ACS Nano. 2009;3(1):181–8.
Ho MY, D’Souza N, Migliorato P. Electrochemical aptamer-based sandwich assays for the detection of explosives. Anal chem. 2012 15;84(10):4245–7.
Babaee S, Beiraghi A. Micellar extraction and high performance liquid chromatography-ultra violet determination of some explosives in water samples. Anal Chim Acta. 2010;662(1):9–13.
Siddique AB, Pramanick AK, Chatterjee S, Ray M. Amorphous carbon dots and their remarkable ability to detect 2,4,6-trinitrophenol. Sci Reports. 2018;8(1):1–10.
Peng Y, Zhang A-J, Dong M, Wang Y-W. A colorimetric and fluorescent chemosensor for the detection of an explosive—2,4,6-trinitrophenol (TNP). Chem Commun. 2011;47(15):4505–7.
Hussain M, Nafady A, Sherazi ST, Shah MR, Alsalme A, Kalhoro MS, et al. Cefuroxime derived copper nanoparticles and their application as a colorimetric sensor for trace level detection of picric acid. RSC Adv. 2016;6(86):82882–9.
Zhang X, Hu J, Wang B, Li Z, Xu S, Ma X. A chiral zinc(II) metal-organic framework as high selective luminescent sensor for detecting trace nitro explosives picric acid and Fe3+ ion. J Solid St Chem. 2019;269:459–64.
Lu X, Zhang G, Li D, Tian X, Ma W, Li S, et al. Thiophene aromatic amine derivatives with two-photon activities as probes for the detection of picric acid and pH. Dye Pig. 2019;170:107641.
Zhang C, Zhang S, Yan Y, Xia F, Huang A, Xian Y. Highly fluorescent polyimide covalent organic nanosheets as sensing probes for the detection of 2,4,6-trinitrophenol. ACS Appl Mater and Inter. 2017;9(15):13415–21.
Pal A, Sk MP, Chattopadhyay A. Conducting carbon dot-polypyrrole nanocomposite for sensitive detection of picric acid. ACS Appl Mater and Inter. 2016;8(9):5758–62.
Pramanik S, Bhalla V, Kumar M. Mercury assisted fluorescent supramolecular assembly of hexaphenylbenzene derivative for femtogram detection of picric acid. Anal Chim Acta. 2013;793:99–106.
Wang M, Zhang H, Guo L, Cao D. Fluorescent polymer nanotubes as bifunctional materials for selective sensing and fast removal of picric acid. Sensor Actuat B: Chem. 2018;274:102–9.
Zhang JR, Yue YY, Luo HQ, Li NB. Supersensitive and selective detection of picric acid explosive by fluorescent Ag nanoclusters. Analyst. 2016;141(3):1091–7.
Liu B, Tong C, Feng L, Wang C, He Y, Lü C. Water-soluble polymer functionalized CdTe/ZnS quantum dots: a facile ratiometric fluorescent probe for sensitive and selective detection of nitroaromatic explosives. Chem Eur J. 2014;20(8):2132–7.
Hou X-G, Wu Y, Cao H-T, Sun H-Z, Li H-B, Shan G-G, et al. A cationic iridium(iii) complex with aggregation-induced emission (AIE) properties for highly selective detection of explosives. Chem Commun. 2014;50(45):6031–4.
Zhao Z, Zhang J, Wang Y, Chen L, Zhang Y. Hydrothermal synthesis of fluorescent nitrogen-doped carbon quantum dots from ascorbic acid and valine for selective determination of picric acid in water samples. Int J Env Anal Chem. 2016;96(14):1402–13.
Han C, Wang R, Wang K, Xu H, Sui M, Li J, et al. Highly fluorescent carbon dots as selective and sensitive “on-off-on” probes for iron(III) ion and apoferritin detection and imaging in living cells. Biosens Bioelectron. 2016;83:229–36.
Bano D, Kumar V, Singh VK, Hasan SH. Green synthesis of fluorescent carbon quantum dots for the detection of mercury(ii) and glutathione. New J Chem. 2018;42(8):5814–21.
Zhao Y, Zou S, Huo D, Hou C, Yang M, Li J, et al. Simple and sensitive fluorescence sensor for methotrexate detection based on the inner filter effect of N, S co-doped carbon quantum dots. Anal Chim Acta. 2019;1047:179–87.
Liao S, Zhao X, Zhu F, Chen M, Wu Z, Yang H, Chen X. Novel S, N-doped carbon quantum dot-based" off-on" fluorescent sensor for silver ion and cysteine. Talanta. 2018;180:300–308.
Song Z, Quan F, Xu Y, Liu M, Cui L, Liu J. Multifunctional N, S co-doped carbon quantum dots with pH-and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon. 2016;104:169–178.
Zhu C, Zhai J, Dong S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem Commun. 2012;48(75):9367–9.
Chandra S, Singh VK, Yadav PK, Bano D, Kumar V, Pandey VK, et al. Mustard seeds derived fluorescent carbon quantum dots and their peroxidase-like activity for colorimetric detection of H2O2 and ascorbic acid in a real sample. Anal Chim Acta. 2019;1054:145–56.
Xue M, Zhang L, Zou M, Lan C, Zhan Z, Zhao S. Nitrogen and sulfur co-doped carbon dots: a facile and green fluorescence probe for free chlorine. Sensor Actuat B: Chem. 2015;219:50–6.
Sun Y, Shen C, Wang J, Lu Y. Facile synthesis of biocompatible N, S-doped carbon dots for cell imaging and ion detecting. RSC Adv. 2015;5(21):16368–75.
Bano D, Kumar V, Singh VK, Chandra S, Singh DK, Yadav PK, et al. A facile and simple strategy for the synthesis of label free carbon quantum dots from the latex of Euphorbia milii and its peroxidase-mimic activity for the naked eye detection of glutathione in a human blood serum. ACS Sustain Chem Eng. 2019;7(2):1923–32.
Yang H, He L, Pan S, Liu H, Hu X. Nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of tannic acid. Spect Acta - Part A: Mol Bio Spect. 2019;210:111–9.
Bano D, Kumar V, Singh VK, Hasan SH. Green synthesis of fluorescent carbon quantum dots for the detection of mercury(II) and glutathione. New J Chem. 2018;42:5814–21.
Hu Y, Zhang L, Li X, Liu R, Lin L, Zhao S. Green preparation of S and N co-doped carbon dots from water chestnut and onion as well as their use as an off-on fluorescent probe for the quantification and imaging of coenzyme A. ACS Sustain Chem Eng. 2017;5(6):4992–5000.
Song Y, Zhu S, Xiang S, Zhao X, Zhang J, Zhang H, et al. Investigation into the fluorescence quenching behaviors and applications of carbon dots. Nanoscale. 2014;6(9):4676–82.
Dinda D, Gupta A, Shaw BK, Sadhu S, Saha SK. Highly selective detection of trinitrophenol by luminescent functionalized reduced graphene oxide through FRET mechanism. ACS Appl Mater Inter. 2014;6(13):10722–8.
Shi Z-Q, Guo Z-J, Zheng H-G. Two luminescent Zn(ii) metal–organic frameworks for exceptionally selective detection of picric acid explosives. Chem Commun. 2015;51(39):8300–3.
Liu Y, Gao M, Lam JWY, Hu R, Tang BZ. Copper-catalyzed polycoupling of diynes, primary amines, and aldehydes: A new one-pot multicomponent polymerization tool to functional polymers. Macromolecules. 2014;47(15):4908–19.
Sk MP, Chattopadhyay A. Induction coil heater prepared highly fluorescent carbon dots as invisible ink and explosive sensor. RSC Adv. 2014;4(60):31994–9.
Cheng F, An X, Zheng C, Cao S. Green synthesis of fluorescent hydrophobic carbon quantum dots and their use for 2,4,6-trinitrophenol detection. RSC Adv. 2015;5(113):93360–3.
Niu Q, Gao K, Lin Z, Wu W. Amine-capped carbon dots as a nanosensor for sensitive and selective detection of picric acid in aqueous solution via electrostatic interaction. Anal Methods. 2013;5(21):6228–33.
Lin L, Rong M, Lu S, Song X, Zhong Y, Yan J, et al. A facile synthesis of highly luminescent nitrogen-doped graphene quantum dots for the detection of 2,4,6-trinitrophenol in aqueous solution. Nanoscale. 2015;7(5):1872–8.
Peng D, Zhang L, Li FF, Cui WR, Liang RP, Qiu JD. Facile and green approach to the synthesis of boron nitride quantum dots for 2,4,6-trinitrophenol sensing. ACS Appl Mater Inter. 2018;10(8):7315–23.
Sun X, He J, Meng Y, Zhang L, Zhang S, Ma X, et al. Microwave-assisted ultrafast and facile synthesis of fluorescent carbon nanoparticles from a single precursor: preparation, characterization and their application for the highly selective detection of explosive picric acid. J Mater Chem A. 2016;4(11):4161–71.
Bano D, Kumar V, Chandra S, Singh VK, Mohan S, Singh DK, et al. Synthesis of highly fluorescent nitrogen-rich carbon quantum dots and their application for the turn-off detection of cobalt (II). Opt Mater. 2019 Jun 1;92:311–8.
Hebert RM, Jackovitz AM. Wildlife toxicity assessment for picric acid (2,4,6-trinitrophenol). Wildlife Toxicity Assessments of Chemicals of Military Concern. Elsevier Inc.; 2015;271–277 p.
Acknowledgments
This work was financially supported by the Indian Institute of Technology IIT (BHU), Varanasi, and MHRD New Delhi, India. The authors SC, DB, PP, VKS, and PKY acknowledge the Department of Chemistry and Central Instrument Facility (CIF), IIT (BHU), Varanasi, for providing an instrumentation facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 455 kb)
Rights and permissions
About this article
Cite this article
Chandra, S., Bano, D., Pradhan, P. et al. Nitrogen/sulfur-co-doped carbon quantum dots: a biocompatible material for the selective detection of picric acid in aqueous solution and living cells. Anal Bioanal Chem 412, 3753–3763 (2020). https://doi.org/10.1007/s00216-020-02629-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02629-1