Abstract
Rationale
Acute tryptophan depletion (ATD) transiently lowers central serotonin levels and can induce depressive mood states and cognitive defects. Previous studies have shown that ATD impairs object recognition in rats.
Objectives
As individual differences exist in central serotonin neurotransmission, the impact of ATD may vary accordingly. In this experiment, we investigated the hypothesis that male serotonin transporter knockout (SERT−/−), rats marked by a lower SERT function, are more vulnerable to the effects of ATD in an object recognition task than male wildtype (SERT+/+) and heterozygous (SERT+/−) rats.
Materials and methods
Twelve male SERT+/+, SERT+/−, and SERT−/− rats were treated with standard dose and low-dose ATD using a gelatine-based protein–carbohydrate mixture lacking tryptophan. In the control treatment, l-tryptophan was added to the mixture. Four hours after treatment, the rats were subjected to the object recognition task. In addition, the effects of ATD on plasma amino acid concentrations were measured, and concentrations of 5-HT and 5-HIAA were measured in the frontal cortex and hippocampus of these rats.
Results
Plasma TRP levels and central 5-HT and 5-HIAA levels were decreased in all genotypes after ATD, but effects were stronger in SERT−/− rats. The standard dose of ATD impaired object recognition in all genotypes. SERT−/− and SERT+/− rats were more vulnerable to low dose of ATD in the object recognition task compared to SERT+/+ rats.
Conclusions
These results indicate a greater sensitivity to ATD in SERT−/− and SERT+/− rats, which may be related to stronger central depletion effects in these rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Memory is a multifaceted cognitive function relating to the acquisition and storage of information for shorter or longer periods of time and the subsequent retrieval of this information. The role of the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) in learning and memory has been demonstrated in both humans and animals (Buhot 1997; Buhot et al. 2000; Meneses 1999; Riedel et al. 1999; Schmitt et al. 2000). Studies using the method of acute tryptophan depletion (ATD), which results in lower peripheral and central levels of tryptophan and 5-HT (Biggio et al. 1974; Fernstrom and Wurtman 1997; Gessa et al. 1974; Moja et al. 1989; Stancampiano et al. 1997), consistently report impaired memory in healthy volunteers (Park et al. 1994; Riedel et al. 1999; Sambeth et al. 2007; Schmitt et al. 2000) and rats (Jans et al. 2007a; Lieben et al. 2004b; Rutten et al. 2007). More specifically, ATD studies have shown impairments of long-term memory performance (Riedel et al. 2002) and reduced ability to actively recall, as well as recognize, words from a previously presented word list (Riedel et al. 1999). Whereas consolidation of new information into long-term memory appeared to be compromised by a reduction of central 5-HT activity; short-term memory functions were intact. Several studies have subsequently confirmed that 5-HT is specifically involved in long-term memory functioning (for review, see Sambeth et al. 2007; Schmitt et al. 2006). Interestingly, ATD did not affect long-term memory retrieval or recognition when the depletion was induced after learning and consolidation of a word list (Schmitt et al. 2000). Thus, these results suggest that consolidation of new information into long-term memory requires normal 5-HT functioning.
The serotonin transporter (SERT) has an important role in the re-uptake of 5-HT from the synapse, returning it to the presynaptic neuron where it can be degraded or retained for future release. In fact, the SERT has an essential role in serotonergic neurotransmission as it determines the magnitude and duration of the 5-HT signal in the synaptic cleft. We recently developed a SERT knockout (SERT−/−) rat using N-ethyl-N-nitrosurea-driven mutagenesis (Smits et al. 2004; Smits et al. 2006). This animal has a premature stopcodon (TGC>TGA) introduced at position 3924 in the third exon encoding the second extracellular loop of the SERT protein. Consistent with the absence of SERT in these rats, northern blot analysis revealed that the mutation resulted in nonsense-mediated decay of the mutant SERT transcript and showed reduced SERT mRNA transcript in the SERT heterozygous knockout (SERT+/−) rat (Homberg et al. 2007a). In addition, [3H]citalopram (SSRI) binding to brain slices of SERT−/− rats is completely absent, whereas in SERT+/− rats, citalopram binding was reduced by approximately 40%. Moreover, extracellular 5-HT levels in the hippocampus of SERT−/− are nine-fold elevated (Homberg et al. 2007a; Olivier et al. 2008), whereas in SERT+/− rats, extracellular 5-HT levels are similar to wildtype littermates (SERT+/+) (Olivier et al. unpublished data). However, intracellular 5-HT levels were reduced by approximately 75–50% in SERT−/− rats and by 45–55% in SERT+/− rats in several brain areas (Homberg et al. 2007a).
In humans, a polymorphism in the 5-HT transporter gene-linked promoter region (5-HTTLPR) results in individual differences in SERT expression and function (Heils et al. 1996; Lesch et al. 1996). Several studies have shown that 5-HTTLPR genotype can influence behavioral responses to ATD (Marsh et al. 2006; Neumeister et al. 2006; Roiser et al. 2006; Walderhaug et al. 2007). As wildtype (SERT+/+), heterozygous (SERT+/−), and knockout (SERT−/−) rats exhibit large differences in central 5-HT neurotransmission, the impact of ATD may vary accordingly in these rats. In this study, these different types of rat were subjected to two different doses of ATD—a standard dose and a low dose—and tested for memory in the object recognition task. Animals with lower SERT function were hypothesized to be more vulnerable to the effects of ATD on object recognition memory as they rely more heavily on 5-HT synthesis than animals in which the re-uptake mechanism is fully functional. The magnitude of the depletion was determined by measuring plasma amino acid concentrations of TRP and five other large neutral amino acids (LNAAs: valine, leucine, isoleucine, phenylalanine, tyrosine) that compete with TRP for transport across the blood–brain barrier. The ratio of TRP and these other LNAAs (TRP/ΣLNAA ratio) is thought to be a more sensitive index of brain tryptophan availability than plasma TRP (Fernstrom 1981; Wurtman et al. 1980) because this ratio determines the amount of tryptophan that can enter the brain. Moreover, 5-HT and 5-HIAA concentrations were measured in the frontal cortex and the hippocampus, the brain regions involved in cognition and memory (Dalley et al. 2004; Heidbreder and Groenewegen 2003; Squire and Zola-Morgan 1991; Wurtman et al. 1980).
Experimental procedures
Animals
The serotonin transporter knockout rat (Slc6a41Hubr) has been generated, bred, and reared in the Central Animal Laboratory of the Radboud University of Nijmegen. Experimental animals were derived from crossing SERT+/− rats that were outcrossed for four or five generations. Twelve male SERT+/+, SERT+/−, and SERT−/− littermates (age: 3 to 5 months old) were used in this experiment. After weaning at the age of 21 days, ear cuts were taken for genotyping. Genotyping was performed at the Hubrecht Institute (Utrecht, The Netherlands), and the procedure has been described elsewhere (Homberg et al. 2007a). During the experiment, all animals were individually housed in standard Macrolon® type 3 cages (42 × 26 × 20 cm) in temperature-controlled rooms (21 ± 1°C) with standard 12/12-h day/night cycle (lights on at 7.00 a.m.) and food (Sniff, long cut pellet, Bio Services, Uden, The Netherlands) and water available ad libitum.
Drugs and chemicals
The gelatin hydrolysate (Solugel P®) was obtained from PB Gelatins (Tessenderlo, Belgium). Glucodry 210 was obtained from Tate & Lyle (Koog aan de Zaan, The Netherlands). Kaliumchloride (KCl) and calciumchloride-dihydrate (CaCl2·2H2O) were purchased from Merck (Darmstadt, Germany). l-Tryptophan was obtained from Sigma (Zwijndrecht, The Netherlands).
Treatment
During a period of 2 weeks preceding the experiment, the rats were handled and habituated to oral injections with normal tap water (up to 10 ml/kg). The experiment consisted of blood sample collection (right after the handling period), object recognition task, and brain sample collection (1 week after object recognition task). On blood and brain sampling days, rats were treated with a protein–carbohydrate mixture containing l-TRP (TRP+ group, 0.30% TRP of the total protein) or lacking l-TRP (TRP− 100 g group). In all treatment conditions, rats received two oral injections of 10 ml/kg with a 90-min interval. Blood samples were taken at baseline (10 min before the first injection) and 4 h after the first injection. Brain samples were taken without treatment and 4 h after the first treatment. The composition of the nutritional mixture is shown in Table 1. In the object recognition task, rats were tested without treatment, with TRP+, with TRP− 100 g (standard-dose ATD), and with TRP− 40 g (low-dose ATD), respectively, with a 2-day interval. The TRP− 40 g condition is a mixture containing 40 g instead of the standard 100 g of Solugel P protein per 100 ml. Because TRP was absent and amino acids were given in a lower concentration, this resulted into milder tryptophan depletion. On each testing day, behavioral testing was conducted 4 h after the first oral administration. Treatment always took place between 8.30 and 12.00 h, and the object recognition task was performed between 12.30 and 17.00 h. The rats were fasted from 14 h prior to treatment until the testing period was completed. This was done to minimize the availability of TRP from food. At the end of each testing day, the animals had ad libitum access to food.
Biochemistry
Plasma amino acid levels
For the determination of plasma amino acid levels, blood samples were taken at baseline (T0; i.e., 10 min before the first oral administration) and repeated 4 h after the first administration (T4). Blood sampling was done via a tail-incision method (Fluttert et al. 2000). Promptly after collection of blood in a sodium heparin tube (Microvette® CB 300, Sarstedt, Germany), the samples were kept on ice. After centrifugation of the blood samples (at 4°C for 15 min at 3,000×g), plasma samples were stored at −70°C. Plasma amino acid concentrations were determined with a fully automated high-performance liquid chromatography (HPLC). The concentrations of the total plasma amino acids are expressed as micromoles per liter.
Brain 5-HT and 5-HIAA levels
Animals were decapitated, and tissue samples (frontal cortex and hippocampus) were dissected from the brain, weighed, and stored at −80°C until further use. The tissue samples were homogenized in 250 μl of an ice-cold solution containing 5 μM clorgyline, 5 μg/ml glutathione, and 0.6 μM Nω-methylserotonin (internal standard) using a potter tube. To 100 μl homogenate, 25 μl of 2 M HClO4 was added and mixed. Then, 20 μl of 2.5 M potassium acetate was added and again mixed. After 15 min in ice water, the homogenates were centrifuged for 15 min at 15,000×g (4°C). The supernatants were diluted ten times with water before HPLC analysis. The concentration of 5-HT and 5-HIAA in the tissue extracts were measured by HPLC with ECD. The HPLC system consisted of a pump model P100, an autosampler model AS300 (both from Thermo Separation Products, Waltham, MA, USA), an ERC-3113 degasser (Erma CR. Inc. Tokyo, Japan), an ESA Coulochem II detector with 5011 analytical cell set at potential +450 mV (ESA Inc. Bedford MA, USA), a BD 41 chart recorder (Kipp & zn, The Netherlands), and a column (150 mm × 4.6 mm i.d.) packed with Hypersil BDS C18, 5-μm particle size (Alltech Associates, USA). The mobile phase solution consisted of 50 mM citric acid, 50 mM phosphoric acid, 0.1 mM EDTA, 45 μl/l dibutylamine, and 77 mg/l 1-octanesulfonic acid sodium salt, 10% methanol; the pH of the buffer was adjusted to 3.4 with NaOH. Separation was performed at room temperature using a flow rate of 0.7 ml/min. The concentration of each compound was calculated by comparison with both the internal and the external standards. The limit of detection (signal/noise ratio 3:1) was 0.3 nM. Concentrations are expressed as nanomoles per gram. The 5-HIAA/5-HT turnover was calculated, which can be used as an index of 5-HT system activity.
Behavior
Object recognition task
The object recognition task was performed as described elsewhere (Ennaceur and Delacour 1988; Prickaerts et al. 2002). The apparatus consisted of a square arena (100 × 100 × 40 cm), with an open top, dark walls, and a dark floor. Testing was carried out in dimmed white light. We used four different sets of objects that could not be displaced by the rat. Each object was available in triplicate. The different objects were (1) a bowl with handle made of green china (maximum diameter of 15 cm and a height of 9 cm); (2) a cubic box (12 × 12 × 7 cm) made of polyvinyl, with a pink topping; (3) a china trapezium cylinder (maximum diameter of 12 cm and minimum diameter of 10.5 cm) with a dish on top (diameter, 12 cm); and (4) a brown tinned cylinder (diameter, 9.5 cm and height, 15 cm).
One day preceding testing, the animals were adapted to the procedure, i.e., they were allowed to explore the apparatus (without any objects) for 3 min. In the following days, the rats were tested twice. A testing session is comprised of two 3-min trials, with a 1-h interval between trials. Two objects were placed in a symmetrical position about 10 cm away from the black wall. A rat was always placed in the apparatus facing one corner, which was the same for all rats. During the first trial, the apparatus contained two identical objects. After the first exploration period, the rat was put back in its home cage. One hour later, the rat was put back in the apparatus for the second trial but now with dissimilar objects: a familiar one and a new one. The duration of exploring each object in both trials was recorded manually on a personal computer. Exploration was defined as directing the nose to the object at a distance of no more than 2 cm and/or touching the object with the nose. Sitting on the object was not considered as exploratory behavior. In order to avoid the presence of olfactory trails, the objects were thoroughly cleaned between trials with a 70% ethanol solution. Moreover, each object was available in triplicate so that none of the two objects from the first trial had to be used as the familiar object in the second trial. In addition, all combinations and locations of objects were used in a balanced manner to reduce potential biases due to preferences for particular locations or objects.
The basic measures in the object recognition task were the times spent by rats exploring an object during trial 1 and trial 2. The discrimination index d2 [(exploration new object during trial 2 − exploration familiar object during trial 2)/total exploration time during trial 2] was calculated for each treatment condition (see Rutten et al. 2007). The d2 is a relative index of discrimination between new and familiar objects because it corrects for total exploration time in trial 2 (see Şık et al. 2003). Rats that explored less than 5 s in any of the trials or explored only one of the objects were removed from analysis to avoid possible erroneous conclusions (see Şık et al. 2003).
Statistical analysis
For all variables, treatment effects were analyzed using parametric statistics (ANOVA). Plasma amino acid concentrations were analyzed with repeated measures ANOVA, with factors genotype, treatment, and time. Brain 5-HT, 5-HIAA, and 5-HIAA/5-HT turnovers were analyzed with two-way ANOVA, with factors treatment and genotype. Where appropriate, post hoc testing with Bonferroni correction was used. In the object recognition task, effects of treatment and genotype on exploration times in each trial was analyzed using a two-way ANOVA. Where appropriate, post hoc testing with Bonferroni correction was used. We compared the d2 values of untreated testing and treatment conditions with d2 values of a virtual control group (see Şık et al. 2003). The virtual control group had a d2 of zero, meaning that there was no object recognition. The number of animals and SEM were similar to those of our treatment groups. Comparison with this virtual control group is used to evaluate more reliably whether discrimination performance differs from zero in a certain treatment condition. The d2 values were compared with ANOVA, and a one-sided Dunnett post hoc test was used to test whether d2 in a treatment condition was higher than in the virtual control group, which would indicate that the rats are able to discriminate the objects.
Results
Plasma amino acid concentrations
To determine the effects of the treatment conditions, plasma amino acid concentrations were measured, and the TRP/ΣLNAA ratio was calculated for each measurement, treatment, and genotype (Fig. 1). Plasma TRP levels and the plasma TRP/ΣLNAA ratio decreased over the 4 h [Time: TRP: F(1, 29) = 45.20, p < 0.001; ratio: F(1, 29) = 31.43, p < 0.001]. Plasma TRP levels and the TRP/ΣLNAA ratio were lower in the TRP− 100 g group compared to the TRP+ group [Treatment: TRP: F(1, 29) = 77.46, p < 0.001; ratio: F(1, 29) = 84.91, p < 0.001]. There was no Time × Genotype × Treatment interaction effect and no Genotype × Treatment interaction effect on TRP or on the ratio (F’s < 2.15, ns). A Time × Treatment interaction effect was found on TRP [F(1, 29) = 206.17, p < 0.001] and on the TRP/ΣLNAA ratio [F(1, 29) = 181.17, p < 0.001]. Further analysis showed that in the TRP+ condition, there was a significant increase of TRP and the TRP/ΣLNAA ratio over the 4 h [TRP: F(1, 13) = 26.26, p < 0.001; ratio: F(1, 13) = 23.01, p < 0.001], whereas in the TRP− 100 g condition, these were significantly decreased [TRP: (1,14) = 223.26, p < 0.001; ratio: F(1, 14) = 222.13, p < 0.001]. There was no Time × Genotype effect and no effect of genotype on TRP levels and on the TRP/ΣLNAA ratio. Thus, TRP− 100 g ATD resulted in strong depletion of plasma TRP and the TRP/ΣLNAA ratio, whereas TRP+ treatment caused an increase in TRP and the TRP/ΣLNAA ratio. These effects were similar in all genotypes.
Brain 5-HT and 5-HIAA concentrations
To determine the central effects of the treatment, concentrations of 5-HT and 5-HIAA were determined in the frontal cortex and in the hippocampus, and for both structures, the turnover (5-HIAA/5-HT) was calculated (Table 2).
5-HT
In the frontal cortex, a treatment effect on 5-HT was found [F(2, 66) = 41.46, p < 0.001]. Post hoc testing revealed that 5-HT levels were lower in the TRP− 100 g condition than in the untreated condition and the TRP+ condition (Fig. 2a). Furthermore, 5-HT concentrations in the TRP+ condition were higher than in the untreated rats. Moreover, a genotype effect on 5-HT in the frontal cortex [F(2, 66) = 73.76, p < 0.001] was found. Post hoc analysis showed that 5-HT levels in SERT−/− rats were lower than in SERT+/− and SERT+/+ rats. No Treatment × Genotype interaction effect on 5-HT was found in the frontal cortex. In the hippocampus, similar effects were found to the prefrontal cortex. In this structure also, a treatment effect on 5-HT [F(2, 66) = 17.85, p < 0.001] was found, with lower 5-HT levels in the TRP− 100 g condition than in the untreated condition and in the TRP+ condition (Fig. 2a). Similar to the frontal cortex, 5-HT concentrations in the hippocampus were higher in the TRP+ condition than in the untreated rats. Also, a genotype effect on 5-HT was found in the hippocampus [F(2, 66) = 67.25, p < 0.001], with lower 5-HT levels in SERT−/− rats compared with SERT+/− and SERT+/+ rats. No Treatment × Genotype interaction effects were found on 5-HT in the hippocampus.
In order to test our specific hypotheses, we also analyzed the effects of treatment on behavior in each experimental group separately. In the frontal cortex, 5-HT levels in the TRP− 100 g group were significantly lower than in the TRP+ group in all genotypes [Treatment: SERT+/+: F(2, 20) = 9.80, p < 0.001; SERT+/−: F(2, 21) = 12.75, p < 0.001; SERT−/−: F(2, 21) = 27.88, p < 0.001]. In SERT+/+ and SERT+/− rats, 5-HT levels were higher in the TRP+ group than in the untreated rats, which was not the case in SERT−/− rats. In SERT−/− rats, 5-HT was lower in the TRP− 100 g condition than in untreated rats, which was not the case in SERT+/+ and SERT+/− rats. In the hippocampus, there was a treatment effect on 5-HT in SERT+/− [F(2, 21) = 9.29, p < 0.001] and in SERT−/− rats [F(2, 21) = 20.88, p < 0.001] but not in SERT+/+ rats. In SERT+/− rats, 5-HT levels in the TRP+ condition were higher than in untreated and TRP− 100 g treated rats. In SERT−/− rats, 5-HT levels in the TRP− 100 g condition were lower than in the TRP+ condition and in untreated rats.
5-HIAA
A treatment effect on 5-HIAA was found in the frontal cortex [F(2, 66) = 23.98, p < 0.001; see Fig. 2b]. Post hoc testing showed that 5-HIAA concentrations in the TRP− 100 g condition were lower than in the untreated rats and in the TRP+ condition. In the frontal cortex, a genotype effect was found on 5-HIAA in the frontal cortex [F(2, 66) = 34.03, p < 0.001]. Post hoc analysis showed that 5-HIAA levels in SERT−/− were lower than in SERT+/− and SERT+/+ rats. No Treatment × Genotype interaction effects on 5-HIAA were found in the frontal cortex. Similar to the frontal cortex, a treatment effect on 5-HIAA was also found in the hippocampus [F(2, 66) = 34.35, p < 0.001]. As shown in Fig. 2b, 5-HIAA concentrations in the TRP− 100 g condition were lower than in the untreated rats and the TRP+ condition. In the hippocampus, 5-HIAA levels were higher in untreated rats than in TRP+ treated rats. Moreover, a genotype effect on 5-HIAA was found in the hippocampus [F(2, 66) = 35.30, p < 0.001], showing lower 5-HIAA levels in SERT−/− rats compared with SERT+/− and SERT+/+ rats. No Treatment × Genotype interaction effects on 5-HIAA levels were found in the hippocampus.
In all genotypes, there was a treatment effect on 5-HIAA in the frontal cortex [SERT+/+: F(2, 20) = 18.69, p < 0.001; SERT+/−: F(2, 21) = 4.29, p < 0.05; SERT−/−: F(2, 21) = 10.66, p < 0.001]. Post hoc testing revealed that in SERT+/+ rats, 5-HIAA was lower in the TRP− 100 g group compared to the TRP+ group (p < 0.01) and the untreated group (p < 0.001). In SERT+/− and SERT−/− rats, 5-HIAA levels were lower in TRP− 100 g compared to untreated rats (SERT+/−: p < 0.05; SERT−/−: p < 0.001). For the hippocampus also, there was a treatment effect in all genotypes in the hippocampus as well [SERT+/+: F(2, 20) = 28.19, p < 0.001; SERT+/−: F(2, 21) = 7.16, p < 0.001; SERT−/−: F(2, 21) = 11.91, p < 0.001]. Post hoc testing showed lower 5-HIAA levels in SERT+/+ and SERT+/− rats in the TRP− 100 g group compared with the TRP+ (SERT+/+: p < 0.001; SERT+/−: p < 0.05) and untreated group (SERT+/+: p < 0.001; SERT+/−: p < 0.01). In SERT−/− rats, 5-HIAA was higher in the untreated rats compared with TRP+ (p < 0.05) and TRP− 100 g (p < 0.001) treated rats.
5-HIAA/5-HT
As seen in Fig. 2c, there was a Treatment × Genotype interaction effect on the 5-HIAA/5-HT turnover in the frontal cortex [F(4, 62) = 9.628, p < 0.001] caused by the high 5-HIAA/5-HT turnover rate of SERT−/− rats. A treatment effect was found on the turnover in the frontal cortex [F(2, 62) = 11.60, p < 0.001]. Post hoc testing revealed that the turnover was lower in the TRP+ condition than in the untreated rats and in the TRP− 100 g condition. Moreover, a genotype effect on 5-HIAA in the frontal cortex [F(2, 62) = 13.64, p < 0.001] was found. The turnover was higher in SERT−/− rats than in SERT+/− and SERT+/+ rats. Similarly to the frontal cortex, a Treatment × Genotype interaction effect on the 5-HIAA/5-HT turnover was also found in the hippocampus [F(4, 62) = 12.60, p < 0.001]. This effect seems to be the result of the high 5-HIAA/5-HT turnover rate in SERT−/− rats. Moreover, a treatment effect on the turnover was found in the hippocampus [F(2, 62) = 9.02, p < 0.001], again, with a lower turnover in the TRP+ condition compared with the untreated rats and the TRP− 100 g condition. Similar to the frontal cortex, a genotype effect on 5-HIAA/5-HT was found in the hippocampus [F(2, 62) = 28.62, p < 0.001], with a higher turnover in SERT−/− rats compared with SERT+/− and SERT+/+ rats.
In all genotypes, there was a treatment effect on 5-HIAA/5-HT turnover in the frontal cortex [SERT+/+: F(2, 20) = 4.64, p < 0.05; SERT+/−: F(2, 21) = 3.78, p < 0.05; SERT−/−: F(2, 21) = 16.37, p < 0.001]. Post hoc testing showed that in SERT+/+ rats, the 5-HIAA/5-HT was lower in the TRP− 100g group compared to the untreated group (p < 0.05). In SERT+/− rats, 5-HIAA/5-HT was lower in TRP+ rats than in untreated rats (p < 0.05). In SERT−/− rats, 5-HIAA/5-HT was higher in TRP− 100 g compared to untreated rats (p < 0.001) and TRP+ rats (p < 0.001). In all genotypes, there was a treatment effect in the hippocampus [SERT+/+: F(2, 20) = 7.31, p < 0.01; SERT+/−: F(2, 21) = 4.10, p < 0.05; SERT−/−: F(2, 21) = 15.12, p < 0.001]. Post hoc testing showed that in SERT+/+ rats, 5-HIAA/5-HT was lower in the TRP− 100 g group compared to the untreated group (p < 0.01). In SERT−/− rats, 5-HIAA/5-HT was higher in the TRP− 100 g treated rats compared to untreated rats (p < 0.01) and TRP+ treated rats (p < 0.001). In SERT+/− rats, no differences in 5-HIAA/5-HT were found.
Object recognition task
In order to test whether animals with lower SERT function are more vulnerable to the effects of ATD, we tested SERT+/+, SERT+/− and SERT−/− rats on object recognition memory. Exploration times of untreated rats and rats treated with TRP+, TRP− 100 g, and TRP− 40 g treated were compared (data not shown). There were no Genotype × Treatment interaction effects on exploration time in trial 1 or in trial 2. There was no effect of Genotype or Treatment on exploration time in trial 1. In trial 2, there was an effect of Genotype [F(2, 136) = 3.27, p < 0.05] and Treatment [F(3, 136) = 3.98, p < 0.01] on exploration time. Post hoc testing showed that in trial 2, exploration time was lower in the TRP− 100 g and TRP+ condition compared to the TRP− 40 g condition (p < 0.05). As mentioned before, d2 values of the untreated rats and the rats treated with TRP+, TRP− 100 g, and TRP− 40 g of the present study were compared with d2 values of a virtual control group with no object recognition (see Şık et al. 2003). The effects of Genotype and Treatment on discrimination index d2 in the object recognition task are shown in Fig. 3.
There was no Genotype × Treatment interaction effect [F(8, 138) = 1.28, ns] and no effect of Genotype on discrimination index d2 [F(2, 138) = 2.31, ns]. A treatment effect was found on the discrimination index d2 [F(4, 146) = 21.65, p < 0.001]; post hoc analysis showed that the untreated d2 and the d2 in the TRP+ and TRP− 40 g condition differed from the virtual control group with no object recognition. Only in the TRP− conditions that rats were unable to discriminate between the new and familiar object after a 1-hour interval. To evaluate whether SERT−/− rats were more vulnerable to ATD treatment than SERT+/− or SERT+/+ rats, treatment effects were analyzed within each genotype group. There was a treatment effect on d2 in all genotypes [SERT+/+ F(4, 50) = 17.61, p < 0.001; SERT+/− F(4, 43) = 6.00, p < 0.01; SERT−/− F(4, 45) = 4.92, p < 0.01]. In the SERT+/+, untreated rats and rats treated with TRP+ and TRP− 40 g were different from the virtual controls but TRP− 100 g was not. In the SERT+/−, only untreated rats and TRP+ treated rats were different from the virtual controls but rats treated with TRP− 100 g and TRP− 40 g were not. In the SERT−/− rats, only the untreated rats were different from the virtual controls whereas rats treated with TRP+, TRP− 100 g, and TRP− 40 g were not.
Discussion
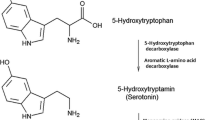
In the present study, the effects of standard-dose (TRP− 100 g) and low-dose (TRP− 40 g) ATD were examined in SERT+/+, SERT+/−, and SERT−/− rats in the object recognition task. The biochemical data showed plasma TRP depletion of 65% in SERT+/+, 61% in SERT+/−, and 55% in SERT−/− rats 4 h after standard-dose TRP−. This decrease in plasma TRP levels is in agreement with other ATD studies (Jans et al. 2007a; Lieben et al. 2004b). SERT−/− rats showed stronger depletion of 5-HT in the frontal cortex (63%) than SERT+/+ and SERT+/− (both 19%) rats. Similar results were found in the hippocampus, where SERT−/− rats also showed a stronger depletion (70%) compared to SERT+/− rats (18%) and SERT+/+ rats (13%). Rats treated with standard-dose TRP− showed lower 5-HT levels in both brain structures compared to TRP+ treated rats and untreated rats. These effects were most pronounced in SERT−/− rats, as only these rats showed significantly lower 5-HT in the standard-dose TRP− condition than in the untreated condition. In both the frontal cortex and hippocampus, standard-dose ATD decreased 5-HIAA to a similar extent in all genotypes. In SERT+/+ and SERT+/− rats, 5-HIAA/5-HT turnover was a bit lower in rats treated with TRP+ and standard-dose TRP− compared with the untreated condition, but in SERT−/− rats, this turnover ratio was much higher in the standard-dose TRP− group than in the TRP+ group and the untreated group. In previous studies, SERT−/− animals exhibited an increased 5-HT turnover at basal levels in the cortex and caudate putamen, but not in the amygdala medial prefrontal cortex, and orbitofrontal cortex (Homberg et al. 2007a, b). In line with this, untreated SERT−/− animals in this study did not have increased 5-HIAA/5-HT turnover in the frontal cortex. Although the central effects of ATD were stronger in SERT−/− rats, plasma TRP and TRP/ΣLNAA levels decreased to a similar extent in all genotypes. Thus, different effects seen in SERT+/+, SERT+/−, and SERT−/− rats after ATD were found only in the brain and not in the periphery. Previous ATD studies reported a similar dissociation between peripheral and central effects (Jans et al 2007a; Jans et al. unpublished data). Although the reason for this difference is not known, it is interesting to note that 5-HT synthesis in brain and peripheral tissues functions differently. For example, tryptophan hydroxylase (TPH), the rate-limiting enzyme to form 5-hydroxytryptophan (5-HTP) from tryptophan, is controlled by a different isoform in the brain than in the periphery (for review, see Walther and Bader 2003). This difference in 5-HT synthesis might play a role in the different effects seen after ATD in brain and periphery.
The standard ATD dose (TRP− 100 g) is known to impair object recognition in Wistar rats (Jans et al. 2007a; Lieben et al. 2004b) and was thus expected to impair object recognition in all genotypes. The re-uptake mechanism in SERT+/+ rats is fully functional, whereas this mechanism is partly functional in SERT+/− rats and not functional in SERT−/− rats. Therefore, SERT+/− and SERT−/− rats rely more heavily on 5-HT synthesis than SERT+/+ rats. Consequently, it was hypothesized that differences in object recognition between the genotypes would occur only after the low-dose ATD treatment. It was found that all genotypes showed impaired object recognition after standard-dose ATD. The relatively mild depletion of the TRP− 40 g treatment impaired object recognition in SERT+/− and especially in SERT−/− rats but not in SERT+/+ rats, suggesting that SERT−/− and SERT+/− rats are more sensitive to the memory-impairing effects of low-dose ATD. In SERT+/+ rats, the d2 in the TRP− 40 g condition was lower than in the untreated condition but still different from the virtual control group with impaired object recognition. The stronger effects of the TRP− 40 g treatment in SERT−/− rats may be related to the stronger effects of TRP− treatment on central 5-HT levels. The same dose of TRP− 100 g had a stronger effect on central 5-HT levels in SERT−/− rats compared to SERT+/− and SERT+/+ rats. Notably, SERT−/− rats showed impaired object recognition after TRP+ treatment as well, although their untreated d2 indicated normal object recognition when untreated. This may be related to stress associated with the ATD procedure, such as the repeated oral injections. Chronic stress is known to impair object memory (Beck and Luine 1999), and there is some evidence to suggest that diminished SERT function is associated with increased stress responsivity in humans (for review, see Canli and Lesch 2007) and mice (Wellman et al. 2007). In this way, the injection stress may have resulted in mildly impaired object recognition memory in TRP+ treated SERT−/− rats.
Several 5-HT receptors are known to be involved in memory (for review, see Buhot 1997; Perez-Garcia and Meneses 2008). ATD can modify 5-HT receptors, for example, ATD in rats reduced 5-HT1A binding in the dorsal raphe but not in the cortex and hippocampus (Cahir et al. 2007). This decrease in autoreceptor binding may represent a compensatory intrinsic homeostatic response, attempting to counteract ATD-induced decreases in central 5-HT (Cahir et al. 2007). It is interesting to note that in SERT−/− rats, the 5-HT1A receptors are less sensitive due to the nine-fold increased extracellular 5-HT levels (Olivier et al. unpublished data). The stronger ATD effects on memory in SERT−/− rats might be a result of adaptations in 5-HT receptors.
ATD has been reported to impair cognitive performance in humans (Park et al. 1994; Riedel et al. 1999, 2002; Sambeth et al. 2007; Schmitt et al. 2000), and impaired object recognition has been found in Wistar rats (Lieben et al. 2004a, b; Jans et al. 2007a). The effects of low-dose ATD on memory in groups that differ in serotonergic functioning have not been studied extensively. Booij et al. (2005) found that ATD had a dose-dependent effect on selective attention (Stroop color–word interference) in remitted depressed patients, but no other cognitive effects of low-dose ATD were observed. The magnitude of the reduction of plasma tryptophan concentrations following ATD depends on the amount and composition of the amino acid mixture (Young et al. 1989). In human studies, it has been suggested that a threshold exists that needs to be exceeded before any behavioral effects occurs since studies in which the plasma tryptophan reduction was lower than 70% generally do not find any symptomatic effects (Van Der Does 2001). A similar threshold may also exist in the rat, although the level of the threshold is likely to be lower as rat studies in general show lower plasma TRP depletion than human studies. In the present study, SERT−/−, and to a smaller extent SERT+/− rats, showed impairment in recognition memory after low-dose ATD. Thus, it may be possible that in SERT−/−, and to a smaller extent in SERT+/− rats, the threshold is lower than in SERT+/+ rats, and less depletion of TRP is required to cause impaired memory.
The results of this study indicate higher serotonergic vulnerability in SERT−/− and to a lesser extent SERT+/− rats than in SERT+/+ rats as low-dose ATD only affected memory in subjects that already have a disturbed 5-HT system. Serotonergic vulnerability means that minor changes in serotonergic functioning do not cause symptoms but make the system more vulnerable so that additional challenges of the serotonergic system may result in the occurrence of psychiatric symptoms (Jans et al. 2007b). Thus, serotonergic vulnerability can be demonstrated by challenging the 5-HT system, as is done with ATD; only vulnerable subjects will react to such manipulations with changes in behavior. Challenging the 5-HT system in vulnerable subjects can have stronger effects on the memory function of these subjects. ATD in SERT−/− rats may therefore be a good tool to investigate serotonergic vulnerability.
Some possible limitations of this study have to be mentioned. First, plasma amino acid concentrations and brain 5-HT and 5-HIAA levels after the low-dose TRP− 40 g treatment could not be determined in this study due to the limited number of rats that were available for this study. Previous standard-dose ATD studies have shown a robust reduction in plasma TRP (about 70%) and central tissue 5-HT (about 40–45%) concentrations in male Wistar rats (Lieben et al. 2004a). Lowering the concentration of Solugel protein in the TRP− mixture had dose-dependent effects on the plasma TRP/ΣLNAA ratio in previous research, and there was a positive correlation between the plasma TRP/ΣLNAA ratio and performance in the object recognition task (Lieben et al. unpublished data). We can therefore assume that the low-dose TRP− 40 g treatment resulted in milder TRP and 5-HT depletion. Further research may elucidate the exact level of peripheral and central depletion that is required to impair object recognition memory in SERT+/+, SERT+/−, and SERT−/− rats. Secondly, the TRP+ treatment resulted in increased levels of plasma TRP and brain 5-HT, suggesting an active control. Essentially, this means that we compared TRP depletion and mild TRP suppletion in all rats. This may have affected behavior in the TRP+ condition. Although these treatment effects in the TRP+ condition occurred to a similar degree in all genotypes, it cannot be excluded that the mild TRP suppletion had different effects in SERT+/+, SERT+/−, and SERT−/− rats. A third concern could be that the same group of rats were repeatedly subjected to ATD treatment (for the measurement of plasma levels, for the ORT, and eventually for the measurement of brain levels), although the treatment condition varied between tests. Previous ATD studies have shown that repeated treatment did not alter the effect of the treatment on the plasma TRP/ΣLNAA ratio and that plasma levels returned to baseline levels on the treatment day (Jans et al. unpublished data). We therefore assume that the treatment has had comparable biochemical effects throughout the study.
The aim of the present study was to investigate whether male SERT+/+, SERT+/−, and SERT−/− serotonin transporter rats differed in their response to ATD with respect to object memory. Without treatment, all rats showed normal object recognition memory. After a standard 100-g dose of ATD, object recognition memory was impaired in all genotypes. However, in the low-dose ATD condition, SERT+/− and SERT−/− rats showed increased responsiveness to the treatment than SERT+/+ rats by showing impaired object recognition after this relatively mild ATD treatment. Therefore, ATD in SERT−/− rats might be a valuable animal model to investigate serotonergic vulnerability. It can be concluded that there is a SERT gene–dosage effect with respect to the behavioral response to TRP depletion in the object recognition task. Because ATD decreased 5-HT levels in brain structures that play a crucial role in memory, such as the hippocampus and the frontal cortex, the outcome of the present study underlines the relevance of the serotonergic system in memory.
References
Beck KD, Luine VN (1999) Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res 830:56–71
Biggio G, Fadda F, Fanni P, Tagliamonte A, Gessa GL (1974) Rapid depletion of serum tryptophan, brain tryptophan, serotonin and 5-hydroxyindoleacetic acid by a tryptophan-free diet. Life Sci 14:1321–1329
Booij L, Van Der Does AJ, Haffmans PM, Riedel WJ, Fekkes D, Blom MJ (2005) The effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patients. J Psychopharmacol 19:267–275
Buhot MC (1997) Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol 7:243–254
Buhot MC, Martin S, Segu L (2000) Role of serotonin in memory impairment. Ann Med 32:210–221
Cahir M, Ardis T, Reynolds GP, Cooper SJ (2007) Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology (Berl) 190:497–506
Canli T, Lesch KP (2007) Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10:1103–1109
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31:47–59
Fernstrom JD (1981) Dietary precursors and brain neurotransmitter formation. Annu Rev Med 32:413–425
Fernstrom JD, Wurtman RJ (1997) Brain serotonin content: physiological regulation by plasma neutral amino acids. 1971. Obes Res 5:377–380
Fluttert M, Dalm S, Oitzl MS (2000) A refined method for sequential blood sampling by tail incision in rats. Lab Anim 34:372–378
Gessa GL, Biggio G, Fadda F, Corsini GU, Tagliamonte A (1974) Effect of the oral administration of tryptophan-free amino acid mixtures on serum tryptophan, brain tryptophan and serotonin metabolism. J Neurochem 22:869–870
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579
Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP (1996) Allelic variation of human serotonin transporter gene expression. J Neurochem 66:2621–2624
Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, Nieuwenhuizen OF, Cools AR, Ronken E, Cremers T, Schoffelmeer AN, Ellenbroek BA, Cuppen E (2007a) Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience 146:1662–1676
Homberg JR, Pattij T, Janssen MC, Ronken E, de Boer SF, Schoffelmeer AN, Cuppen E (2007b) Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur J Neurosci 26:2066–2073
Jans LA, Lieben CK, Blokland A (2007a) Influence of sex and estrous cycle on the effects of acute tryptophan depletion induced by a gelatin-based mixture in adult Wistar rats. Neuroscience 147:304–317
Jans LA, Riedel WJ, Markus CR, Blokland A (2007b) Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry 12:522–543
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
Lieben CK, Blokland A, Westerink B, Deutz NE (2004a) Acute tryptophan and serotonin depletion using an optimized tryptophan-free protein–carbohydrate mixture in the adult rat. Neurochem Int 44:9–16
Lieben CK, van OK, Deutz NE, Blokland A (2004b) Acute tryptophan depletion induced by a gelatin-based mixture impairs object memory but not affective behavior and spatial learning in the rat. Behav Brain Res 151:53–64
Marsh AA, Finger EC, Buzas B, Soliman N, Richell RA, Vythilingham M, Pine DS, Goldman D, Blair RJ (2006) Impaired recognition of fear facial expressions in 5-HTTLPR S-polymorphism carriers following tryptophan depletion. Psychopharmacology (Berl) 189:387–394
Meneses A (1999) 5-HT system and cognition. Neurosci Biobehav Rev 23:1111–1125
Moja EA, Cipolla P, Castoldi D, Tofanetti O (1989) Dose–response decrease in plasma tryptophan and in brain tryptophan and serotonin after tryptophan-free amino acid mixtures in rats. Life Sci 44:971–976
Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS (2006) Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry 63:978–986
Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA (2008) A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience 152:573–584
Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ (1994) Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 33:575–588
Perez-Garcia G, Meneses A (2008) Memory formation, amnesia, improved memory and reversed amnesia: 5-HT role. Behav Brain Res (in press)
Prickaerts J, van Staveren WC, Şık A, Markerink-van IM, Niewohner U, van der Staay FJ, Blokland A, de VJ (2002) Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 113:351–361
Riedel WJ, Klaassen T, Deutz NE, van SA, van Praag HM (1999) Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology (Berl) 141:362–369
Riedel WJ, Klaassen T, Schmitt JA (2002) Tryptophan, mood, and cognitive function. Brain Behav Immun 16:581–589
Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW, Sahakian BJ (2006) Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology 31:2264–2272
Rutten K, Lieben C, Smits L, Blokland A (2007) The PDE4 inhibitor rolipram reverses object memory impairment induced by acute tryptophan depletion in the rat. Psychopharmacology (Berl) 192:275–282
Sambeth A, Blokland A, Harmer CJ, Kilkens TO, Nathan PJ, Porter RJ, Schmitt JA, Scholtissen B, Sobczak S, Young AH, Riedel WJ (2007) Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: a pooled analysis of nine studies. Neurosci Biobehav Rev 31:516–529
Schmitt JA, Jorissen BL, Sobczak S, van Boxtel MP, Hogervorst E, Deutz NE, Riedel WJ (2000) Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J Psychopharmacol 14:21–29
Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ (2006) Serotonin and human cognitive performance. Curr Pharm Des 12:2473–2486
Şık A, van NP, Prickaerts J, Blokland A (2003) Performance of different mouse strains in an object recognition task. Behav Brain Res 147:49–54
Smits BM, Mudde J, Plasterk RH, Cuppen E (2004) Target-selected mutagenesis of the rat. Genomics 83:332–334
Smits BM, Mudde JB, van de Belt J, Verheul M, Olivier J, Homberg J, Guryev V, Cools AR, Ellenbroek BA, Plasterk RH, Cuppen E (2006) Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet Genomics 16:159–169
Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380–1386
Stancampiano R, Melis F, Sarais L, Cocco S, Cugusi C, Fadda F (1997) Acute administration of a tryptophan-free amino acid mixture decreases 5-HT release in rat hippocampus in vivo. Am J Physiol Regul Integr Comp Physiol 272:R991–R994
Van Der Does AJ (2001) The mood-lowering effect of tryptophan depletion: possible explanation for discrepant findings. Arch Gen Psychiatry 58:200–202
Walderhaug E, Magnusson A, Neumeister A, Lappalainen J, Lunde H, Refsum H, Landro NI (2007) Interactive effects of sex and 5-HTTLPR on mood and impulsivity during tryptophan depletion in healthy people. Biol Psychiatry 62:593–599
Walter DJ, Bader M (2003) A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66:1673–1680
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A (2007) Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27:684–691
Wurtman RJ, Hefti F, Melamed E (1980) Precursor control of neurotransmitter synthesis. Pharmacol Rev 32:315–335
Young SN, Ervin FR, Pihl RO, Finn P (1989) Biochemical aspects of tryptophan depletion in primates. Psychopharmacology (Berl) 98:508–511
Acknowledgements
We thank Wim Riedel, Edwin Cuppen, and Judith Homberg for cooperation and for their critical reading of the manuscript and Mark Verheul for genotyping of the rats. All experiments were carried out in accordance with institutional, national, and international guidelines for animal care and the Dutch law concerning welfare.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. D. A. Olivier and L. A. W. Jans contributed equally to this work.
This work was supported by the Dutch Ministry of Economic Affairs through the Innovation Oriented Research Program on Genomics (IGE1017).
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Olivier, J.D.A., Jans, L.A.W., Korte-Bouws, G.A.H. et al. Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology 200, 243–254 (2008). https://doi.org/10.1007/s00213-008-1201-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1201-0