Abstract
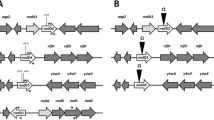
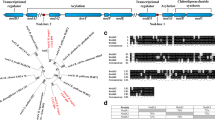
Rhizobium tropici CIAT 899 is a strain known by its ability to nodulate a broad range of legume species, to synthesize a variety of Nod factors, its tolerance of abiotic stresses, and its high capacity to fix atmospheric N2, especially in symbiosis with common bean (Phaseolus vulgaris L.). Genes putatively related to the synthesis of indole acetic acid (IAA) have been found in the symbiotic plasmid of CIAT 899, in the vicinity of the regulatory nodulation gene nodD5, and, in this study, we obtained mutants for two of these genes, y4wF and tidC (R. tropiciindole-3-pyruvic acid decarboxylase), and investigated their expression in the absence and presence of tryptophan (TRP) and apigenin (API). In general, mutations of both genes increased exopolysaccharide (EPS) synthesis and did not affect swimming or surface motility; mutations also delayed nodule formation, but increased competitiveness. We found that the indole-3-acetamide (IAM) pathway was active in CIAT 899 and not affected by the mutations, and—noteworthy—that API was required to activate the tryptamine (TAM) and the indol-3-pyruvic acid (IPyA) pathways in all strains, particularly in the mutants. High up-regulation of y4wF and tidC genes was observed in both the wild-type and the mutant strains in the presence of API. The results obtained revealed an intriguing relationship between IAA metabolism and nod-gene-inducing activity in R. tropici CIAT 899. We discuss the IAA pathways, and, based on our results, we attribute functions to the y4wF and tidC genes of R. tropici.





Similar content being viewed by others
Data Availability
The strains are freely available for distribution for research from our culture collection, on upon filling the forms required by the legislation. All results were informed in the manuscript and as supplementary material. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- API:
-
Apigenin
- EPS:
-
Exopolysaccharides
- IAA:
-
Indole acetic acid
- IAM:
-
Indole-3-acetamide
- IAN:
-
Indole-3-acetonitrile
- IPyA:
-
Indol-3-pyruvic acid
- LCOs:
-
Lipochitooligosaccharides
- TAM:
-
Tryptamine
- TRP:
-
Tryptophan
- YM:
-
Yeast-extract mannitol medium
References
Acosta-Jurado S, Navarro-Gómez P, Murdoch PS, Crespo-Rivas JC, Jie S, Cuesta-Berrio L, Cuesta-Berrio L, Ruiz-Sainz JE, Rodríguez-Carvajal M, Vinardell JM (2016) Exopolysaccharide production by Sinorhizobium fredii HH103 is repressed by genistein in a NodD1-dependent manner. PLoS One 11(8):e0160499
Akashi H, Gojobori T (2002) Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc Natl Acad Sci USA 99(6):3695–3700
Barradas CA, Hungria M (1989) Seleção de estirpes de Rhizobium para o feijoeiro. I—Precocidade para nodulação e fixação do nitrogênio. Turrialba 39:236–242
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Camerini S, Senatore B, Lonardo E, Imperlini E, Bianco C, Moschetti G, Rotino GL, Campion B, Defez R (2008) Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch Microbiol 190:67–77
Carreno-Lopez R, Campos-Reales N, Elmerich C, Baca BE (2000) Physiological evidence for differently regulated tryptophan dependent pathways for indole acetic acid synthesis in Azospirillum brasilense. Mol Gen Genet 264:521–530
Cerboneschi M, Decorosi F, Biancalani C, Ortenzi MV, Macconi S, Giovannetti L, Viti C, Campanella B, Onor M, Bramanti E, Tegli S (2016) Indole-3-acetic acid in plant–pathogen interactions: a key molecule for in planta bacterial virulence and fitness. Res Microbiol 167(9–10):774–787
Chan PK, Gresshoff PM (2009) Roles of plant hormones in legume nodulation. In: Doelle HW, DaSilva EJ (eds) Encyclopedia of life support systems (EOLSS): biotechnology. EOLSS Publishers, Oxford
Checcucci A, Azzarello E, Bazzicalupo M, De Carlo A, Emiliani G, Mancuso S, Spini G, Viti C, Mengoni A (2017) Role and regulation of ACC deaminase gene in Sinorhizobium meliloti: is it a symbiotic, rhizospheric or endophytic gene? Front Genet 8:6
Conforte VP, Echeverria M, Sánchez C, Ugalde RA, Menéndez AB, Lepek VC (2010) Engineered ACC deaminase-expressing free-living cells of Mesorhizobium loti show increased nodulation efficiency and competitiveness on Lotus spp. J Gen Appl Microbiol 56:331–338
Crozier A, Arruda P, Jasmim JM, Monteiro AM, Sandberg G (1988) Analysis of indole acetic acid and related indoles in culture medium from Azospirillum lipoferum and Azospirillum brasilense. Appl Environ Microbiol 54:2833–2837
del Cerro P, Rolla-Santos AAP, Gomes DF, Marks BB, Espuny MR, Rodríguez-Carvajal M, Soria-Díaz ME, Nakatani AS, Hungria M, Ollero FJ, Megías M (2015a) Opening the “black box” of nodD, nodD4 and nodD5 genes of Rhizobium tropici strain CIAT 899. BMC Genom 16:864
del Cerro P, Rolla-Santos AAP, Gomes DF, Marks BB, Pérez-Montaño F, Rodríguez-Carvajal M, Nakatani AS, Gil-Serrano A, Megías M, Ollero FJ, Hungria M (2015b) Regulatory nodD1 and nodD2 genes of Rhizobium tropici strain CIAT 899 and their roles in the early stages of molecular signaling and host-legume nodulation. BMC Genom 16:251
del Cerro P, Rolla-Santos AAP, Valderrama-Fernández R, Gil-Serrano A, Bellogín RA, Gomes DF, Pérez-Montaño F, Megías M, Hungria M, Ollero FJ (2016) NrcR, a new transcriptional regulator of Rhizobium tropici CIAT 899 involved in the legume root-nodule symbiosis. PLoS One 11:e0154029
Ding Y, Oldroyd GE (2009) Positioning the nodule, the hormone dictum. Plant Signal Behav 4:89–93
Donoso R, Leiva-Novoa P, Zúñiga A, Timmermann T, Recabarren-Gajardo G, González B (2016) Biochemical and genetic basis of indole acetic acid (auxin phytohormone) degradation by the plant growth promoting rhizobacterium Paraburkholderia phytofirmans PsJN. Appl Environ Microbiol 83:pii:e01991–16
Dusha I, Kondorosi A (1993) Genes at different regulatory levels are required for the ammonia control of nodulation in Rhizobium meliloti. Mol Gen Genet 240:435–444
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Gomes DF, Batista JSS, Rolla AAP, Silva LP, Bloch C, Galli-Terasawa LV, Hungria M (2014) Proteomic analysis of free-living Bradyrhizobium diazoefficiens: highlighting potential determinants of a successful symbiosis. BMC Genom 15:643
Gomes DF, Ormeño-Orrillo E, Hungria M (2015) Biodiversity, symbiotic efficiency, and genomics of Rhizobium tropici and related species. In: de Bruijn FJ (ed) Biological Nitrogen Fixation, v.2. Wiley, Hoboken, pp 747–756
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CL, Krishnamurthy L (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5:355–377
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195
Haag AF, Arnold MF, Myka KK, Kerscher B, Dall’Angelo S, Zanda M, Mergaert P, Ferguson GP (2013) Molecular insights into bacteroid development during Rhizobium-legume symbiosis. FEMS Microbiol Rev 37:364–383
Herrbach V, Chirinos X, Rengel D, Agbevenou K, Vincent R, Pateyron S, Huguet S, Balzergue S, Pasha A, Provart N, Gough C, Bensmihen S (2017) Nod factors potentiate auxin signaling for transcriptional regulation and lateral root formation in Medicago truncatula. J Exp Bot 68:569–583
Hungria M, Phillips DA (1993) Effects of a seed color mutation on rhizobial nod-gene-inducing flavonoids and nodulation in common bean. Mol Plant Microbe Interact 6:418–422
Hungria M, Johnston AWB, Phillips DA (1992) Effects of flavonoids released naturally from bean (Phaseolus vulgaris) on nodD-regulated gene transcription in Rhizobium leguminosarum bv. phaseoli. Mol Plant Microbe Interact 5:199–203
Hungria M, Andrade DS, Chueire LMO, Probanza A, Gutiérrez-Mañero FJ, Megías M (2000) Isolation and characterization of new efficient and competitive bean (Phaseolus vulgaris L.) rhizobia from Brazil. Soil Biol Biochem 32:1515–1528
Hungria M, O’Hara GW, Zilli JE, Araujo RS, Deaker R, Howieson JG (2016) Isolation and growth of rhizobia. In: Howieson JG, Dilworth JG (eds) Working with rhizobia. ACIAR, Canberra, pp 39–60
Imada EL, Oliveira ALM, Hungria M, Rodrigues EP (2017) Indole-3-acetic acid production via the indole-3-pyruvate pathway by plant growth promoter Rhizobium tropici CIAT 899 is strongly inhibited by ammonium. Res Microbiol 168:283–292
Janczarek M (2011) Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int J Mol Sci 12:7898–7933
Janczarek M, Rachwał K, Marzec A, Grządziel J, Palusińska-Szysz M (2015) Signal molecules and cell-surface components involved in early stages of the legume–rhizobium interactions. Appl Soil Ecol 85:94–113
Jijón-Moreno S, Marcos-Jiménez C, Pedraza RO, Ramírez-Mata A, García de Salamone I, Fernández-Scavino A, Vásquez-Hernández CA, Soto-Urzúa L, Baca BA (2015) The y4wG, hisC1 and hisC2 genes involved in indole-3-acetic production used as alternative phylogenetic markers in Azospirillum brasilense. Antonie Leeuwenhoek 107:1501–1517
Juhas M, Crook DW, Hood DW (2008) Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell Microbiol 10:2377–2386
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophan in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microbe Interact 19(3):250–256
Kearns DB (2010) A field guide to bacterial swarming motility. Nat Rev Microbiol 8:634–644
Liu Y, Jiang X, Guan D, Zhou W, Ma M, Zhao B, Cao F, Li L, Li J (2017) Transcriptional analysis of genes involved in competitive nodulation in Bradyrhizobium diazoefficiens at the presence of soybean root exudates. Sci Rep 7(1):10946
Mathesius U (2008) Goldacre paper: Auxin: at the root of nodule development? Funct Plant Biol 35:651–668
Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14:23–34
Morris DL (1948) Quantitative determination of carbohydrates with dreywood’s anthrone reagent. Science 107:254–255
Oldroyd GE (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263
Ormeño-Orrillo E, Menna P, Almeida LGP, Ollero FJ, Nicolás MF, Rodrigues EP, Nakatani AS, Batista JSS, Chueire LMO, Souza RC, Vasconcelos ATR, Megías M, Hungria M, Martínez-Romero E (2012) Genomic basis of broad host range and environmental adaptability of Rhizobium tropici CIAT 899 and Rhizobium sp. PRF 81 which are used in inoculants for common bean (Phaseolus vulgaris L.). BMC Genom 13:735
Patten CL, Blakney AJ, Coulson TJ (2013) Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit Rev Microbiol 39:395–415
Pérez-Montaño F, Del Cerro P, Jiménez-Guerrero I, López-Baena FJ, Cubo MT, Hungria M, Megías M, Ollero FJ (2016a) RNA-seq analysis of the Rhizobium tropici CIAT 899 transcriptome shows similarities in the activation patterns of symbiotic genes in the presence of apigenin and salt. BMC Genom 17:198
Pérez-Montaño F, Jiménez-Guerrero I, Acosta-Jurado S, Navarro-Gómez P, Ollero FJ, Ruiz-Sainz JE, López-Baena FJ, Vinardell JM (2016b) A transcriptomic analysis of the effect of genistein on Sinorhizobium fredii HH103 reveals novel rhizobial genes putatively involved in symbiosis. Sci Rep 6:31592
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36–e36
Pii Y, Crimi M, Cremonese G, Spena A, Pandolfini T (2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 7:21
Pramanik K, Soren T, Mitra S, Maiti TK (2017) In silico structural and functional analysis of Mesorhizobium ACC deaminase. Comput Biol Chem 68:12–21
Prentki P, Krisch HM (1984) In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313
Prinsen E, Chauvaux N, Schmidt J, John M, Wieneke U, Degreef J, Schell J, Van Onckelen H (1991) Stimulation of indole acetic acid production in Rhizobium by flavonoids. FEBS Lett 282:53–55
Prinsen E, Costacurta A, Michiels K, Vanderleyden J, Van Onckelen H (1993) Azospirillum brasilense indole acetic acid biosynthesis: evidence for a non-tryptophan dependent pathway. Mol Plant Microb Interact 6:609–615
Rodrigues EP, Soares CP, Galvão PG, Imada EL, Simões-Araújo JL, Rouws L (2016) Identification of genes involved in indole acetic acid biosynthesis by Gluconacetobacter diazotrophicus PAL5 strain using transposon mutagenesis. Front Microbiol 7:1572
Rodríguez-Navarro DN, Rodríguez-Carvajal MA, Acosta-Jurado S, Soto MJ, Margaret I, Crespo-Rivas JC, Sanjuan J, Temprano F, Gil-Serrano A, Ruiz-Sainz JE, Vinardell JM (2014) Structure and biological roles of Sinorhizobium fredii HH103 exopolysaccharide. PLoS One 9:e115391
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Simon R (1984) High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet 196:413–420
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:a001438
Spaepen S, Vanderleyden J, Remans R (2007a) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Spaepen S, Versées W, Gocke D, Pohl M, Steyaert J, Vanderleyden J (2007b) Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J Bacteriol 180:7626–7763
Spaink HP, Aarts A, Stacey G, Bloemberg GV, Lugtenberg BJ, Kennedy EP (1992) Detection and separation of Rhizobium and Bradyrhizobium Nod metabolites using thin-layer chromatography. Mol Plant Microbe Interact 5:72–80
Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139:3997–4006
Swiecicki JM, Sliusarenko O, Weibel DB (2013) From swimming to swarming: Escherichia coli cell motility in two-dimensions. Integr Biol 5(12):1490–1494
Tambalo DD, Vanderlinde EM, Robinson S, Halmillawewa A, Hynes MF, Yost CK (2013) Legume seed exudates and Physcomitrella patens extracts influence swarming behavior in Rhizobium leguminosarum. Can J Microbiol 60:15–24
Theunis M, Kobayashi H, Broughton WJ, Prinsen E (2004) Flavonoids, NodD1, NodD2, and nod-box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Mol Plant Microbe Interact 17:1153–1161
Tittabutr P, Awaya JD, Li QX, Borthakur D (2008) The cloned 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene from Sinorhizobium sp. strain BL3 in Rhizobium sp. strain TAL1145 promotes nodulation and growth of Leucaena leucocephala. Syst Appl Microbiol 31:141–150
Tomlinson AD, Ramey-Hartung B, Day TW, Merritt PM, Fuqua C (2010) Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiology 156:2670–2681
Turner M, Nizampatnam NR, Baron M, Coppin S, Damodaran S, Adhikari S, Arunachalam SP, Yu O, Subramanian S (2013) Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiol 162:2042–2055
van Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Mathesius U (2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol 144:1115–1131
Vinardell JM, Ollero FJ, Hidalgo Á, López-Baena FJ, Medina C, Ivanov-Vangelov K, Parada M, Madinabeitia N, Espuny MR, Bellogín RA, Camacho M, Rodríguez-Navarro DN, Soria-Díaz ME, Gil-Serrano AM, Ruiz-Sainz JE (2004) NolR regulates diverse symbiotic signals of Sinorhizobium fredii HH103. Mol Plant Microbe Interact 17:676–685
Vinardell JM, Acosta-Jurado S, Zehaner S, Göttfert M, Becker A, Baena I et al (2015) The Sinorhizobium fredii HH103 genome: a comparative analysis with S. fredii strains differing in their symbiotic behavior with soybean. Mol Plant Microbe Interact 28:811–824
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell, Oxford
Wielbo J, Kuske J, Marek-Kozaczuk M, Skorupska A (2010) The competition between Rhizobium leguminosarum bv. viciae strains progresses until late stages of symbiosis. Plant Soil 337:125–135
Acknowledgements
Authors thank João Alves Filho, Dr. Estela de Oliveira Nunes and Dr. Clara Beatriz Hoffman-Campo (Embrapa Soja) for help in the UPLC analysis, and to Dr. Allan R. J. Eaglesham for English review. L.D. Tullio acknowledges a PhD fellow and D.F. Gomes a post-doc fellow from CAPES-Embrapa (Edital 15/2014); A.S. Nakatani acknowledges a post-doc fellowship from Fundação Araucária (Edital 14/2012), F.J. Ollero a research project of the Spanish Government (AGL2016-77163-R), and M. Hungria a research fellow from CNPq (300878/2015-0).
Funding
Funded by INCT-Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq 465133/2014-2, Fundação Araucária-STI, CAPES), Embrapa (02.13.08.001.00.00), CNPq-Universal (400468/2016-6), and Ministerio de Economía y Competitividad (MINECO, AGL2016-77163-R).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: All authors. Performed the experiments: LDT, ASN, DFG. Analyzed the data: All authors. Contributed reagents/materials/analysis tools: FJO, MM, MH. Wrote the paper: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare no ethical conflicts; authors declare that they have consented to contribute to the manuscript.
Consent to publish
The authors declare consent to publish the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tullio, L.D., Nakatani, A.S., Gomes, D.F. et al. Revealing the roles of y4wF and tidC genes in Rhizobium tropici CIAT 899: biosynthesis of indolic compounds and impact on symbiotic properties. Arch Microbiol 201, 171–183 (2019). https://doi.org/10.1007/s00203-018-1607-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-018-1607-y