Abstract
Background
Nodulation and symbiotic nitrogen fixation are mediated by several genes, both of the host legume and of the bacterium. The rhizobial regulatory nodD gene plays a critical role, orchestrating the transcription of the other nodulation genes. Rhizobium tropici strain CIAT 899 is an effective symbiont of several legumes—with an emphasis on common bean (Phaseolus vulgaris)—and is unusual in carrying multiple copies of nodD, the roles of which remain to be elucidated.
Results
Phenotypes, Nod factors and gene expression of nodD1 and nodD2 mutants of CIAT 899 were compared with those of the wild type strain, both in the presence and in the absence of the nod-gene-inducing molecules apigenin and salt (NaCl). Differences between the wild type and mutants were observed in swimming motility and IAA (indole acetic acid) synthesis. In the presence of both apigenin and salt, large numbers of Nod factors were detected in CIAT 899, with fewer detected in the mutants. nodC expression was lower in both mutants; differences in nodD1 and nodD2 expression were observed between the wild type and the mutants, with variation according to the inducing molecule, and with a major role of apigenin with nodD1 and of salt with nodD2. In the nodD1 mutant, nodulation was markedly reduced in common bean and abolished in leucaena (Leucaena leucocephala) and siratro (Macroptilium atropurpureum), whereas a mutation in nodD2 reduced nodulation in common bean, but not in the other two legumes.
Conclusion
Our proposed model considers that full nodulation of common bean by R. tropici requires both nodD1 and nodD2, whereas, in other legume species that might represent the original host, nodD1 plays the major role. In general, nodD2 is an activator of nod-gene transcription, but, in specific conditions, it can slightly repress nodD1. nodD1 and nodD2 play other roles beyond nodulation, such as swimming motility and IAA synthesis.
Similar content being viewed by others
Background
Bacteria commonly known as rhizobia are capable of establishing symbioses with several leguminous species, forming specific structures, the root nodules, where the process of biological fixation of atmospheric nitrogen takes place, bringing important contributions to agriculture and to the environment [1-3]. Legume nodulation requires a cascade of molecular signals exchanged between the host plant and the rhizobium. This molecular dialogue begins with the exudation of flavonoids from the legume, which are recognized by the bacterium. When induced by these plant molecules, rhizobia synthesize lipochitooligosaccharides (LCOs), also known as Nod factors, responsible for launching the nodulation process [3-8]. It is noteworthy that an increasing number of reports show that Nod factors may play roles beyond the nodulation process, including stimulation of photosynthesis, improvements in plant growth and grain yield and changes in immune responses in both legumes and non-legumes [9-12].
We consider nodD as the most intriguing regulatory nodulation gene; it belongs to the LysR family of transcriptor regulators, and it is constitutively expressed and responsible for the transcription of other nodulation genes in the presence of suitable plant inducers, usually flavonoids, thus initiating the nodulation process [8,13,14]. Furthermore, many other symbiosis-related phenotypes, such as polysaccharide production, phytohormone synthesis, motility, quorum-sensing and the activation of the type-III secretion system are directly or indirectly regulated by means of inducing flavonoids via NodD in rhizobia [15-20]. Studies of genomes of rhizobia indicate that, depending on the rhizobial species, there are one to five copies of nodD. In the species that possess only one copy of this gene, such as Rhizobium leguminosarum bv. trifolii, a mutation usually results in loss of nodulation, whereas, in the presence of multiple copies, as in Sinorhizobium (=Ensifer) meliloti, Rhizobium leguminosarum bv. phaseoli and Bradyrhizobium japonicum, an intricate interaction between the nodD genes seems to occur and the nodulation is not completely suppressed [21-23].
Rhizobiun tropici strain CIAT 899 is an effective microsymbiont of common bean (Phaseolus vulgaris L.) in the tropical acid soils of South America. Notable properties of this strain are its high tolerance of environmental stresses and its broad legume host-range [24-26]. Another intriguing feature of CIAT 899 is its capacity for producing a large variety of Nod factors [27,28]. Interestingly, this bacterium is able to produce these key symbiotic molecules under abiotic stresses, such as acid and saline conditions, in the absence of plant-molecular signals [28-30]. In a pioneering study, five distinct nodD-hybridizing DNA regions were identified in CIAT 899 [31], later confirmed as five nodD genes in the sequenced genome of the strain [32]. The nodD1 gene preceding the nodABC operon seems to play the major role in nodulation [31], but a more precise study of the regulatory functions of nodD1 in R. tropici is lacking. In addition, the role of nodD2, present in some rhizobial species, is unclear. It has been reported to be a repressor of the nodABC operon, leading to a negative effect on Nod-factor production in Sinorhizobium (=Ensifer) fredii strain NGR 234 [33]. A suppressive role has also been observed in B. japonicum [23] and a negative regulation by NodD2 products was reported in Bradyrhizobium (Arachis) [34].
Here we report a study in which phenotypes, Nod factors and gene expression of mutants of nodulation genes nodD1 and nodD2 of strain CIAT 899 were compared with those of the wild type strain, to help to elucidate the roles of these regulatory genes.
Results and discussion
Phenotypic characterization of wild type and mutant strains
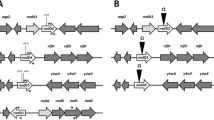
As defined in the genome of R. tropici strain CIAT 899 [32], nodD1 precedes the nodABC operon, while nodD2, corresponding to nodD5 described by van Rhijn et al. [31], is adjacent to the nodA2 and hsnT genes (Additional file 1: Figure S1). R. tropici CIAT 899 nodD1 mutant was obtained in a previous work by insertion of a KmR cassette into a unique XhoI restriction site located on the gene [30]. As described in the Material and Methods section, the nodD2 mutant was obtained after deletion of a 0.6 kb PstI fragment of the gene and the insertion in its place of the Ω interposon (Additional file 1: Figure S1).
Growth rate was not affected by mutation in nodD1 or nodD2 genes of R. tropici CIAT 899 (data not shown). However, it is known that some bacterial properties may be regulated via NodD proteins, such as EPS (exopolysacharide) production, LPS (lipopolysaccharide) profiles, swimming and swarming motilities, biofilm formation and IAA (indole acetic acid) synthesis, among others (e.g., [15-20]). We evaluated some these properties in the wild type and mutant strains in the presence or absence of two nod-gene inducing molecules, apigenin (3.7 μM) and salt (NaCl 300 mM). Results showed statistical differences only in swarming motility (Figure 1) and in the production of IAA (Figure 2).
Swarming motility phenotype of the R. tropici CIAT 899 wild type and nodD1 and nodD2 mutants. Quantified swarm ring diameters of wild type strain (continuous line), the nodD1 mutant (striped line) and the nodD2 mutant (dotted line). Values are the averages of three swarm plates per strain. nodD1 and nodD2 mutant parameters were individually compared with the parental strain CIAT 899 parameters by using the Mann–Whitney non-parametric test. Values tagged by * are significantly different at the level α = 5%. Swarming motility in: A. TY medium, B. TY medium supplemented with 3.7 μM of apigenin, and C. TY medium supplemented with 300 mM of NaCl.
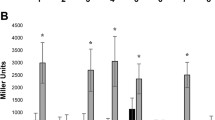
Indole-3-acetic acid (IAA) relative production by R. tropici CIAT 899 wild type, and by the nodD1 and nodD2 mutants. Bacteria were grown in TY medium containing tryptophan in absence and presence of apigenin (3.7 μM) or NaCl (300 mM). Supernatants were taken 96 h after the addition of flavonoid or salt. IAA production was calculated relative to the production without inducing molecules of the wild type strain by using the Mann–Whitney non-parametrical test. The asterisks indicate a significant different at the level α = 5%. Black bars: CIAT 899. Light gray bars: nodD1 mutant. Dark gray bars: nodD2 mutant.
Swarming motility is caused by rotation of single or multiple flagellae along wetted surfaces [35] and, in certain rhizobia, is altered in the presence of legume root exudates that are rich in flavonoids [36]. Our experiments showed that, under control conditions, both nodD mutants showed more swarming motility than did the wild type strain. Interestingly, these differences were even stronger when the medium was augmented with apigenin for the nodD1 mutant and with NaCl for the nodD2 mutant (Figure 1). Therefore, the results suggest a constitutive suppression of swarming by NodD1 and NodD2 proteins.
IAA is an essential plant hormone that promotes growth, including lateral-root proliferation. Previous work has demonstrated that synthesis of this molecule is regulated by NodD1 and NodD2 in S. fredii strain NGR234 [16]. In CIAT 899, our experiments showed an increase in the production of IAA in the presence of apigenin and NaCl (Figure 2), suggesting that both inducing molecules promote the synthesis of this phytohormone. This finding is supported by the presence of a nod-box upstream of the IAA operon in the genome of CIAT 899 [32]. In addition, NodD1 seems to be the main regulator in the presence of apigenin, since, in this mutant, the production of IAA was significantly lower than in the presence of NaCl. The production of IAA in the nodD2 mutant was strongly reduced when the medium was supplemented with NaCl (Figure 2), suggesting that this regulator may be mainly implied in the activation of the IAA operon in the presence of salt. Altogether, the results indicate a predominant role of NodD1 in activation of the IAA gene by apigenin and a predominant role of NodD2 when the inducing molecule is NaCl.
The nodulation phenotype in common bean was first evaluated in pouches bags, where it was possible to observe that a mutation in nodD1 caused a significant decrease in nodule number of common bean; to a lesser extent, a decrease was also observed with a mutation in nodD2 (Additional file 2: Figure S2). In both leucaena [Leucaena leucocephala (Lam.) de Wit] and siratro [Macroptilium atropurpureum (DC.) Urb.], no nodules were observed when plants were inoculated with the nodD1 mutant, but apparently no differences were observed when plants of both species were inoculated with the nodD2 mutant in comparison with plants inoculated with the wild type strain (data not shown).
Nodulation of the type and mutants was confirmed by growing plants in larger pots, in Leonard jars containing sterile substrate. In common bean, a mutation in nodD1 did not suppress nodulation, but caused a reduction of 82% in nodule number (Table 1). The absence of nodulation in both leucaena and siratro when inoculated with the nodD1 mutant was confirmed. Vis-à-vis the nodD2 mutant, nodulation of common bean was reduced by 55%, and no statistical differences in relation to the wild type strain were observed in the nodulation of either leucaena or siratro. However, shoot dry weight of the leucaena plants inoculated with the nodD2 mutant was lower than with the wild type (α = 10%) (Table 1).
In rhizobial species with more than one copy of the regulatory nodD gene, nodD1 preceding the nodABC operon has been recognized as the main gene regulating nodulation e.g. [23,33,34,37-39]. However, reports show that the role of each nodD copy, their responses to flavonoids, and the nodulation phenotypes vary on a case-by-case basis with the rhizobium strain and the host-plant species/cultivar. An intricate pattern of responses in nodulation leads to the assumption that S. meliloti utilizes the three copies of nodD to optimize the interaction with each of its legume hosts [37,40]. A mutation in nodD1 of S. meliloti delays but does not eliminate nodulation of both alfalfa (Medicago sativa) and sweet clover (Melilotus alba), and only a triple mutation of nodD1-nodD2-nodD3 results in absence of nodules [37]. Contrarily, in the promiscuous strain S. fredii NGR 234, capable of nodulating more than 110 plant species, a mutation in nodD1 abolishes nodulation in several temperate and tropical species [41]. In addition, in B. japonicum nodD1 is sufficient for nodulation of the putative main host plant, soybean (Glycine max), but the additional genes nodVW are required for the nodulation of mung bean (Vigna radiata), cowpea (Vigna unguiculata) and siratro [39,42]. In our study, a mutation in nodD1 decreased, but did not suppress, nodulation of common bean; however, nodD1 proved to be essential for the nodulation both of leucaena and of siratro (Table 1).
Still considering nodulation phenotype, in S. meliloti the nodD2 gene did not have any apparent effect on nodulation of either alfalfa or sweet clover [37]. Similarly, no detectable effects were observed by inoculating siratro and cowpea with a nodD2 mutant of Bradyrhizobium (Arachis) sp. strain NC92 [34]. Contrarily, in our study, a significant decrease in nodulation of common bean was detected with the mutation in nodD2, but no effects were observed in leucaena and siratro (Table 1).
Nod factor patterns
Rhizobium tropici strain CIAT 899 is known as an interesting strain in relation to its production of a large variety of Nod factors, not only when induced by flavonoids [27,28], but also under high-salinity conditions in the absence of flavonoids [28-30].
A list of all Nod factors detected in the wild type strain in comparison to the nodD1 and nodD2 mutants is shown in Tables 2, 3 and 4. Unexpectedly, Nod factors were found in the B− medium [43], even in the absence of inducer molecules. In this condition, around ten Nod factors were synthesized, with no significant differences among wild type CIAT 899, nodD1 and nodD2 mutants (Table 2). When induced by 3.7 μM apigenin, the synthesis of a variety of Nod factors was confirmed in all strains, such that numerically, 29 Nod factors were detected in the wild type CIAT 899 and 25 in the nodD2 mutant; a slight reduction was observed with the nodD1 mutant, but, even then, 20 Nod factors were observed (Table 3). This number is higher than in other wild type rhizobial species, e.g.the four Nod factors identified in B. japonicum strain USDA 138 [44]. Up to 36 Nod factors were found in CIAT 899 under saline conditions (Table 4), and in the nodD1 and nodD2 mutants the numbers were lower, of 20 and 18 Nod factors, respectively. These results indicate that NaCl has a stronger nod-induction capacity than apigenin does, and that it is affected by nodD2 but not nodD1. However, one might also consider that it deserves further studies to investigate the possibility that Nod factors are more stable in a 300 mM NaCl supplemented medium.
The production of a large number of Nod factors in all conditions tested might be related to broad host promiscuity and abiotic-stress tolerance of R. tropici [24-26]. The promiscuous S. fredii strain NGR 234 also produces a larger number of Nod factors (≥18) [41], and the composition of Nod factors produced by this strain varies with the activity of host-specific nodulation genes [45]. Furthermore, one interesting feature observed in our study was that the Nod factors with structure III (C18:1, NMe), IV (C18:1, NMe), IV (C18:0, NMe), V (C18:0, NMe) and V (C16:0, NMe, S) were present in the wild type and in the nodD2 mutant, but not in the nodD1 mutant; therefore, this structure might be implicated in host-specific nodulation, and could explain why the mutant in the nodD1 gene is unable to induce nodules on leucaena or siratro. It is also worth mentioning that Folch-Mallol et al. [46] described that in CIAT 899 the sulfation of the LCOs, mediated by the nodHPQ genes are important for nodulation efficiency on L. leucocephala. A mutant in the nodH gene induced about half of nodules than those induced by the wild type strain [46]. Interestingly, one of the five LCOs not synthesized by the CIAT 899 nodD1 mutant is sulphated [V (C16: 0, NMe, S)] (Tables 3 and 4) and may be important for nodulation on leucaena. However, because the nodD1 mutant is unable to nodulate leucaena, other LCOs not secreted by this mutant must be important to explain its symbiotic phenotype.
In R. tropici, the amount and diversity of Nod factors produced are directly influenced by the conditions of bacterial growth. Our results are consistent with the report that CIAT 899 produces of a high number of Nod factors in the presence of nod-gene-inducing molecules [27-30], which provides a better understanding of the control of Nod-factor biosynthesis, and which, in R. tropici, does not follow the classical pathway mediated by flavonoids.
Gene expression
In various strains of rhizobia, the nodD1 gene is the chief regulator of Nod-factor biosynthesis and symbiotic phenotype e.g. [34,37,38,47]. Contrarily, nodD2 has been proposed as a repressor of nod-gene expression [33,39,48,49], affecting the bacterial Nod-factor profile. We performed gene expression studies with the wild type and nodD1 and nodD2 mutants, to improve our understanding of the roles of these two genes (Figure 3).
RT-qPCR analysis of the expression of several nod genes from wild type R. tropici CIAT 899 and nodD1 and nodD2 mutants grown in absence and presence of apigenin (3.7 μM) or NaCl (300 mM). Expression data shown are the mean of three biological replicates. Data were normalized in relation to the endogenous control (16S rRNA). The asterisks indicate a statistically significant expression at the level α = 5%, determined by REST2009 software. Light gray bars: nodD1 mutant, dark gray bars: nodD2 mutant, black bars: wild type strain. A. nodC expression. B. nodD1 expression. C. nodD2 expression.
We evaluated the relative expression of the nodC gene (Figure 3A), which controls the elongation of the oligosaccharide chain of Nod factors and is transcribed with the activation of nod genes. The relative expression of nodC was lower for both mutants in comparison to the wild type strain, both in the apigenin and in the salt treatments (Figure 3A).
Significant expressions of the nodD1 gene was observed in the WT strain both with salt and apigenin, while for the nodD2 mutant it was statistically significant only when induced with apigenin, and higher than in the WT (Figure 3B).
In relation to the expression of nodD2, CIAT 899 WT strain significantly expressed the gene both with salt and apigenin. Contrarily, no statistically significant expression was observed for the nodD1 mutant in none of the conditions evaluated (Figure 3C). However, we must consider that the expression levels of nodD2 were all very low, and numerically even higher for the nodD1 mutant in the presence of apigenin, therefore no strong conclusion can be taken from this assay at this moment (Figure 3C).
All together, these results indicate that the nodD1 is a positive regulator gene, while nodD2 may positively or negatively regulate the expression of the nodD1 gene. Supporting these results is evidence of the involvement of nodD2 in the regulation of the expression of nodD1 by binding to nod box-like sequences located upstream of its coding region [33,49].
Proposal of a regulatory model for nodD1 and nodD2 genes of R. tropici
A graphic summary of the main features of the wild type CIAT 899 and nodD1 and nodD2 mutants is shown in Additional file 3: Figure S3. In our study a major role of nodD1 in R. tropici CIAT 899 was confirmed. In the presence of the nod-gene-inducer apigenin, nodD1 greatly increased the expression of nodC (42-fold), decreasing to 15-fold when the gene was mutated. Similar responses, but lower in magnitude, were observed under saline conditions (Figure 3A). Although confirming a major role of nodD1, the results also indicate that other nodD genes are involved in the activation of nodC, in the presence both of flavonoids and of salts.
Still in relation to nodD1, a mutation abolished nodulation in leucaena and siratro, but not in common bean. As nodD1 gene is the chief regulator of Nod-factor biosynthesis and thus nodulation of the host plant e.g. [5,8,43,44], our results suggest that common bean might not be the main host of R. tropici, although it has been largely isolated from this host legume in acid soils of South America [24,26,50-52]. Indeed, doubts about common bean as the main host of R. tropici have been raised, giving support to the hypothesis that the species might be an original symbiont of another indigenous legume, further “adapting” to common bean [50]. R. tropici has been isolated from common bean and other indigenous legumes in Europe, Africa, Australia, and North America [50], and results of some studies suggest the following as original host candidates for R. tropici: Gliricidia spp., from which the strain has been isolated in Mexico [53] and Brazil [54]; Acaciella angustissima in Mexico [55]; and Mimosa spp. in Brazil [54].
Understanding the relation between Nod factors and host specificity has been a goal of several studies, but without full success. In our research, we found that Nod factors of the following structures, III (C18:1, NMe), IV (C18:1, NMe), IV (C18:0, NMe),V (C18:0, NMe) and V (C16:0, NMe, S) (Tables 3 and 4) might be related—to a greater or lesser extent—to the nodulation of the original host plant, as they are absent in the nodD1 mutant. We have also confirmed the great variety of Nod factors produced by R. tropici, as reported before [27-30], even in the absence of nod-gene inducers [29,30] (Additional file 3: Figure S3). We propose a new, constitutive mechanism of Nod-factor synthesis that is highly enhanced when environmental conditions are stressful, such as strongly acidic pH or salinity. Some transcriptional regulators may be activated in these conditions and they could be responsible for the regulation of nod-gene expression via nodD regulators.
In various rhizobial strains, nodD2 has been described as a repressor of the expression of nod genes e.g. [23,33,39,49]. For example, in B. japonicum, induction of nodC by flavonoids is virtually suppressed by elevated levels of NodD2 [23], and in S. fredii extra plasmid copies of nodD2 reduced the level of nodD1 transcripts to below the limits of detection [49]. However, there is still no evidence that the suppression by NodD2 is mediated by nodD1. In our study, we found that nodD2 activated nodC at similar levels as those observed for nodD1 with both apigenin and salt. However, a slight repression of nodD1 by nodD2 was observed in the presence of apigenin (Figure 3B). Accordingly, we hypothesize that nodD2 is usually an activator of nod-gene transcription, although, in the presence of some flavonoids it may slightly repress nodD1. Nevertheless, if this repression is biologically significant, it remains to be determined, as no differences in nodulation were observed for leucaena or siratro in the absence of nodD2, whereas nodulation was decreased in common bean.
Our model contends that full nodulation of common bean by R. tropici requires both nodD1 and nodD2, while, in other plant species that might represent the original host, nodD1 plays the major role. nodD2 is not a strong repressor as described in other rhizobial species, and, in general, plays a role as an activator of nod-gene transcription, but, in specific conditions, it may slightly repress nodD1. The nodD regulation in R. tropici CIAT 899 resembles the pattern observed in S. meliloti—need for three copies of nodD to optimize the interaction with each of its legume hosts [40]. The biological significance of producing an abundance of Nod factors is not completely understood yet, but we hypothesize that represents an evolutionary strategy to avoid abiotic stresses by nodulating a range of legume species. Reports show that nod genes may also control other functions that contribute to nodulation, as described for nodD2 in the exopolysaccharide synthesis of S. fredii [38], and chaperones and other genes by nodD1 in S. meliloti [56], inter alia. Our results demonstrate extra roles for nodD1 and nodD2 of R. tropici in swarming motility and IAA synthesis.
Conclusions
Our model proposes that full nodulation of common bean by R. tropici requires both nodD1 and nodD2, while in other plant species that might represent the original host nodD1 plays the major role. Contrarily to other rhizobial species, nodD2 of R. tropici is usually not a strong repressor of nod-gene transcription. R. tropici synthesizes a variety of Nod factors that might be related to the ability of nodulating a variety of legume species, representing an evolutionary strategy of the symbiosis under abiotic stressful conditions. nodD1 and nodD2 of R. tropici also play roles in swarming motility and IAA synthesis.
Methods
Bacterial strains, plasmids, media, and growth conditions
Rhizobium tropici CIAT 899 and derivative strains were grown at 28°C on tryptone yeast (TY) medium [57], B− minimal medium [43] or yeast-extract mannitol (YM) medium [58], supplemented when necessary with apigenin to a final concentration of 3.7 μM or with NaCl at 300 mM. Escherichia coli strains were cultured on Luria-Bertani (LB) medium [59] at 37°C. When required, the media were supplemented with the appropriate antibiotics as described by Lamrabet et al. [60]. R. tropici RSP82 [30] was used as a nodD1 mutant derivative of R. tropici CIAT899 (KmR 30 μg mL−1).
To obtain the nodD2 mutant, primer pairs nodD2-F (5′ – GTA GGC CAT AAT GTC CAG A) and nodD2-R (5′ – GCG GCT TTA TAC TCA CCA) were used for amplifying the nodD2 gene. The 1450-bp PCR product was cloned into pGEM®-T Easy (Promega) (AmpR 100 μg mL−1). The PCR-amplified nodD2 fragment was then excised from the plasmid obtained with the endonuclease EcoRI and cloned into the vector pK18mob [61], which is suicide in rhizobia, confers resistance to kanamycin (kmR 30 μg mL−1) and was previously digested also with EcoRI. This new plasmid was digested with the enzyme PstI, which cuts the nodD2 gene in two sites, releasing a fragment of approximately 600 pb. The rest of the plasmid was treated with the Klenow enzyme to convert the cohesive ends to blunt ends. This treated plasmid was ligated with a 2-Kb DNA fragment containing the Ω interposon [carrying the spectinomycin resistance gene (spcR 100 μg mL−1)], which was obtained from a previous digestion of the pHP45Ω plasmid [62] with the SmaI enzyme (blunt end). The resulting plasmid was transformed into the E. coli strain DH5α. Plasmids were transferred from E. coli to Rhizobium strains by conjugation, as described by Simon [63], using plasmid pRK2013 [64] as helper. The plasmid generated was used for the homogenization of the mutated version of the nodD2 gene in R. tropici CIAT 899 by using the methodology previously described [65]. The homogenization was confirmed by DNA-DNA hybridization. For this purpose, DNA was blotted to Hybond-N nylon membranes (Amersham, UK), and the DigDNA method of Roche (Switzerland) was employed according to the manufacturer’s instructions. Additional file 1: Figure S1 displays the type of mutation realized to obtain the nodD2 mutant.
The parental and mutant strains are deposited in the culture collection of the Department of Biology of the Universidad de Sevilla and at the Diazotrophic and Plant Growth Promoting Bacteria Culture Collection of Embrapa Soja (WFCC Collection # 1213, WDCC Collection # 1054).
Identification of Nod factors
Purification and LC-MS/MS analyses of Nod factors produced by R. tropici CIAT 899 and derivative strains grown in B− minimal medium [43] (supplemented when required with NaCl 300 mM or apigenin 3.7 μM) were performed as described previously [30].
RNA isolation, cDNA synthesis and quantitative RT-PCR
Wild type CIAT 899 and nodD1 and nodD2 mutants were pre-cultured in 10-mL aliquots of TY medium at 100 rpm and 28°C in the dark. After 48 h, the three strains pre-inoculated were transferred to new media and subjected to the following conditions: control (without induction), 300 mM NaCl and apigenin 3.7 μM. These new cultures were performed in triplicate under the same conditions as for the pre-cultures, 100 rpm and 28°C in the dark, except that were grown into the exponential phase (O.D. at 600 nm of 0.5 to 0.6).
Total RNA was extracted using Trizol® reagent (Life Technologies) as previously described [66]. The total concentrations were estimated in a NanoDrop ND 1000 spectrophotometer (NanoDrop-Technologies, Inc., City etc. here and elsewhere) and the integrity was assessed by gel electrophoresis. Extracted RNA samples were submitted to DNAseI treatment (Invitrogen/Life Technologies, Grand Island, NY, USA) and the first stand of cDNA was synthesized using SuperscriptIII™ reverse transcriptase (Invitrogen™), according to manufacturer’s protocol.
Primers for the RT-qPCR targets, genes nodD1, nodD2 and nodC, were designed using Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/), to obtain amplicons of 50–150 bp. With the same software, a primer to 16S rRNA was obtained and applied to normalize the relative expression of the targets. To avoid unspecific alignments, the primer sequences were searched against the R. tropici CIAT 899 genome (http://www.ncbi.nlm.nih.gov/nuccore/440224888?report=genbank). The primer sequences and sizes of the amplified fragments are available in Additional file 4: Table S1.
RT-qPCR reactions were performed in a 7500 RT-qPCR Thermocycler (Applied Biosystems/Life Technologies). The reactions were performed in triplicate for each of the three biological replicates. The Platinum® SYBR Green® Master Mix kit (Applied Biosystems) was used according to the manufacturer’s instructions. Cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, 45 cycles at 95°C for 2 min, 60°C for 30 s and 72°C for 30 s, in 45 cycles. Rest2009 software package [67] was used to evaluate the data by providing a robust statistical analysis (p < 0.05). The normalization of cycle threshold (Ct) of RT-qPCR amplifications was performed based on the selected endogenous gene (16S rRNA).
Studies of external exopolysaccharides
The anthrone-H2SO4 method, which measures the total reducing sugar content in a given sample [68] was used to determine the total carbohydrate amounts of exopolysaccharide (EPS) contained in supernatants from bacterial cultures. For this purpose, R. tropici CIAT 899 and derivatives were grown in 5 mL of TY liquid medium on an orbital shaker (180 rpm) for 96 h at 28°C. When required, the media were supplemented with NaCl (300 mM) or apigenin (3.7 μM). Samples of 1 mL were centrifuged to remove cells. Cell-free culture supernatants were assayed for EPS content via H2SO4 hydrolysis in the presence of the colorimetric indicator anthrone. Every experiment was performed three times with three replicates each time. Lipopolysaccharide (LPS) extraction, separation on SDS-PAGE, and silver staining were performed as previously described using the same bacteria, medium and conditions [17].
Motility assays
Swimming and swarming phenotypes were tested on TY medium [57] (supplemented when necessary with NaCl 300 mM or apigenin 3.7 μM) agar plates containing 0.28% or 0.4%, respectively, of Bacto Agar. The strains to be assayed (wild type and mutants) were grown in 5 mL of TY medium on an orbital shaker (180 rpm) for 96 h at 28°C. Aliquots (2 μL) of culture suspensions were drop-inoculated (swarming assay) or sink-inoculated (swimming assay) onto plates and air-dried in a laminar-flow cabinet. The inoculated plates were wrapped with parafilm and incubated for the required time at 28°C in an upright position. Every experiment was performed three times with three replicates each time.
Biofilm formation assay
The biofilm formation assay on polystyrene surfaces was performed using the method described by O’Toole and Kolter [69] with modifications [20]. CIAT 899 and mutant strains were grown on TY medium [57] (supplemented with NaCl 300 mM or apigenin 3.7 μM when required) for 7 days with gentle rocking at 28°C. Every experiment was performed three times with eight replicates each time.
Quantification of indole acetic acid (IAA) production
Quantification of an IAA-like compound from R. tropici strain cultures was carried out by using Salkowski colorimetric assays [70], as described previously by Fierro-Coronado et al. [71]. To measure IAA production, 5 mL of TY medium with tryptophan (0.4 g L−1) (supplemented when required with NaCl 300 mM or apigenin 3.7 μM) were inoculated and incubated during 96 h at 28°C on an orbital shaker (180 rpm) with R. tropici strains. Of these cultures, samples of 1 mL were centrifuged to remove cells. Cell-free culture supernatants were assayed for IAA production. Every experiment was performed three times with eight replicates each time.
Nodulation assays
nodD1 and nodD2 mutants were grown in YM medium until a concentration of 109 cells mL−1 was achieved, to be used as inoculum. Surface-sterilized seeds [58] were used for the assays with common bean (Phaseolus vulgaris L.), leucaena [Leucaena leucocephala (Lam.) de Wit] and siratro [Macroptilium atropurpureum (DC) Urb.]. Pre-germinated seeds (about 2 days after germination) were placed in sterilized pouches or Leonard jars containing N-free nutrient solution [58], with 1 mL of inoculum of each strain added and verified for nodulation capacity after 25 (common bean) or 42 days (leucaena and siratro) with a 16-h 25C°/18°C photoperiod and about 70% relative humidity. Shoots were dried at 65°C until constant weight was achieved, and then weighed. Experiments were performed three times.
References
Peoples MB, Brockwell J, Herridge DF, Rochester IJ, Alves BJR, Urquiaga S, et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis. 2009;48:1–17.
Ormeño-Orrillo E, Hungria M, Martínez-Romero E. Dinitrogen-fixing prokaryotes. In: The prokaryotes - prokaryotic physiology and biochemistry. Berlin-Heidelberg: Springer-Verlag; 2013. p. 427–51.
Van Hameren B, Hayashi S, Gresshoff PM, Ferguson BJ. Advances in the identification of novel factors required in soybean nodulation, a process critical to sustainable agriculture and food security. J Plant Biol Soil Health. 2013;1:6.
Denarié J, Debbelle F, Promé JC. Rhizobium lipo-chitinoligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Ann Rev Biochem. 1996;65:503–35.
Schlaman HRM, Phillips DA, Kondorosi E. Genetic organization and transcriptional regulation of rhizobial nodulation genes. In: Spaink HP, Kondorosi A, Hooykaas PJJ, editors. The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria. Dordrecht: Kluwer Academic Publishers; 1998. p. 361–86.
Geurts R, Bisseling T. Rhizobium Nod factor perception and signalling. Plant Cell. 2002;14:S239–49.
Brencic A, Winans SC. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol Mol Biol Rev. 2005;69:155–94.
Oldroyd GE. Speak, friend, and enter: signaling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol. 2013;11:252–63.
Khan W, Prithiviraj B, Smith DL. Nod factor [Nod Bj V (C18:1, MeFuc)] and lumichrome enhance photosynthesis and growth of corn and soybean. J Plant Physiol. 2008;163:1342–51.
Liang Y, Cao Y, Tanaka K, Thibivilliers S, Wan J, Choi J, et al. Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. Science. 2013;341:1384–7.
Marks BB, Nogueira MA, Hungria M, Megías M. Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp and Azospirillum brasilense inoculants with soybean and maize. AMB Express. 2013;3:21.
Smith S, Habib A, Kang Y, Leggett M, Diaz-Zorita M. LCO applications provide improved responses with legumes and non-legumes. In: De Bruijn F, editor. Biological Nitrogen Fixation. New Jersey: John Wiley & Sons, Inc; 2015. p. 1073–81.
Kondorosi E, Gyuris J, Schmidt J, John M, Duda E, Hoffmann B, et al. Positive and negative control of nod gene expression in Rhizobium meliloti is required for optimal nodulation. Embo J. 1989;8:1331–40.
Spaink HP. Root nodulation and infection factors produced by rhizobial bacteria. Annu Rev Microbiol. 2000;54:257–88.
Krause A, Doerfel A, Gottfert M. Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Mol Plant Microbe Interact. 2002;15:1228–35.
Theunis M, Kobayashi H, Broughton WJ, Prinsen E. Flavonoids, NodD1, NodD2, and Nod-Box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Mol Plant Microbe Interac. 2004;17:1153–61.
Vinardell JM, Lopez-Baena FJ, Hidalgo A, Ollero FJ, Bellogin R, Espuny MR, et al. The effect of FITA mutations on the symbiotic properties of Sinorhizobium fredii varies in a chromosomal-background-dependent manner. Arch Microbiol. 2004;181:144–54.
López-Baena FJ, Vinardell JM, Pérez-Montaño F, Crespo-Rivas JC, Bellogín RA, Espuny Mdel R, et al. Regulation and symbiotic significance of nodulation outer proteins secretion in Sinorhizobium fredii HH103. Microbiology. 2008;154:1825–36.
Pérez-Montaño F, Guasch-Vidal B, González-Barroso S, López-Baena FJ, Cubo T, Ollero FJ, et al. Nodulation-gene-inducing flavonoids increase overall production of autoinducers and expression of N-acyl homoserine lactone synthesis genes in rhizobia. Res Microbiol. 2011;162:715–23.
Pérez-Montaño F, Jiménez-Guerrero I, Del Cerro P, Baena-Ropero I, López-Baena FJ, Ollero FJ, et al. The symbiotic biofilm of Sinorhizobium fredii SMH12, necessary for successful colonization and symbiosis of Glycine max cv Osumi, is regulated by quorum sensing systems and inducing flavonoids via NodD1. PLoS One. 2014;9:e105901.
Hungria M, Johnston AWB, Phillips DA. Effects of flavonoids released naturally from bean (Phaseolus vulgaris) on nodD-regulated gene transcription in Rhizobium leguminosarum bv. phaseoli. Mol Plant Microbe Interac. 1992;5:199–203.
Broughton WJ, Jabbouri S, Perret X. Keys to symbiotic harmony. J Bacteriol. 2000;182:5641–52.
Garcia MLJ, Dunlap J, Loh J, Stacey G. Phenotypic characterization and regulation of the nolA gene of Bradyrhizobium japonicum. Mol Plant Microbe Interact. 1996;9:625–35.
Martínez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P, Pardo MA. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol. 1991;41:417–26.
Hernández-Lucas I, Segovia L, Martínez-Romero E, Pueppke SG. Phylogenetic relationships and host range of Rhizobium spp. that nodulates Phaseolus vulgaris L. Appl Environ Microbiol. 1995;61:2775–9.
Hungria M, Andrade DS, Chueire LMO, Probanza A, Guitierrez-Manero FJ, Megías M. Isolation and characterization of new efficient and competitive bean (Phaseolus vulgaris L.) rhizobia from Brazil. Soil Biol Biochem. 2000;21:1515–28.
Poupot R, Martínez-Romero E, Promé JC. Nodulation factors from Rhizobium tropici are sulfated or nonsulfated chitopentasaccharides containing an N-methyl-N-acylglucosaminyl terminus. Biochemistry. 1993;32:10430–5.
Morón B, Soria-Díaz ME, Ault J, Verroios G, Noreen S, Rodríguez-Navarro DN, et al. Low pH changes the profile of nodulation factors produced by Rhizobium tropici CIAT899. Chem Biol. 2005;12:1029–40.
Estevez J, Soria-Diaz ME, De Cordoba FF, Moron B, Manyani H, Gil A, et al. Different and new Nod factors produced by Rhizobium tropici CIAT899 following Na+ stress. FEMS Microbiol Lett. 2009;293:220–31.
Guasch-Vidal B, Estévez J, Dardanelli MS, Soria-Díaz ME, De Córdoba FF, Balog CI, et al. High NaCl concentrations induce the nod genes of Rhizobium tropici CIAT899 in the absence of flavonoid inducers. Mol Plant Microbe Interact. 2013;26:451–60.
van Rhijn PJS, Feys B, Verreth C, Vanderleyden J. Multiple copies of nodD in Rhizobium tropici CIAT899 and BR816. J Bacteriol. 1993;175:438–47.
Ormeño-Orrillo E, Menna P, Gonzaga LA, Ollero FJ, Nicolas MF, Rodrigues EP, et al. Genomic basis of broad host range and environmental adaptability of Rhizobium tropici CIAT 899 and Rhizobium sp PRF 81 which are used in inoculants for common bean (Phaseolus vulgaris L). BMC Genomics. 2012;13:735.
Fellay R, Hanin M, Montorzi G, Frey J, Freiberg C, Golinowski W, et al. nodD2 of Rhizobium sp. NGR234 is involved in the repression of the nodABC operon. Mol Microbiol. 1998;27:1039–50.
Gillette WK, Elkan GH. Bradyrhizobium (Arachis) sp. strain NC92 contains two nodD genes involved in the repression of nodA and a nolA gene required for the efficient nodulation of host plants. J Bacteriol. 1996;178:2757–66.
Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54.
Tambalo DD, Vanderlinde EM, Robinson S, Halmillawewa A, Hynes MF, Yost CK. Legume seed exudates and Physcomitrella patens extracts influence swarming behavior in Rhizobium leguminosarum. Can J Microbiol. 2014;60:15–24.
Honma MA, Ausubel FM. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987;84:8558–62.
Appelbaum ER, Thompson DV, Idler K, Chartrain N. Rhizobium japonicum USDA 191 has two nodD genes that differ in primary structure and function. J Bacteriol. 1998;170:12–20.
Loh J, Stacey G. Nodulation gene regulation in Bradyrhizobium japonicum: a unique integration of global regulatory circuit. Appl Environ Microbiol. 2003;69:10–7.
Honma MA, Asomaning M, Ausubel FM. Rhizobium meliloti nodD genes mediate host-specific activation of nodABC. J Bacteriol. 1990;172:901–11.
Relić B, Fellay R, Lewin A, Perret K, Price NPJ, Rochepeau P, et al. nod genes and Nod Factors of Rhizobium species NGR 234. In: Palacios R, Mora J, Newton WE, editors. New Horizons in Nitrogen Fixation. Dordrecht: Kluwer Academic Publishers; 1993. p. 183–9.
Göttfert M, Groß P, Hennecke H. Proposed regulatory pathway encoded by the nodV and nodW genes, determinants of host specificity in Bradyrhizobium japonicum. Proc Natl Acad Sci. 1990;87:2680–4.
Spaink HP. Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol Biol. 1992;20:977–86.
Muñoz N, Soria-Díaz ME, Manyani H, Sánchez-Matamoros RC, Serrano AG, Megías M, et al. Structure and biological activities of lipochitooligosaccharide nodulation signals produced by Bradyrhizobium japonicum USDA 138 under saline and osmotic stress. Biol Fertil Soils. 2014;50:207–15.
Jabbouri S, Relić B, Hanin M, Kamalaprija P, Burger U, Promé D, et al. nolO and noeI (HsnIII) of Rhizobium sp. NGR234 are involved in 3-O-carbamoylation and 2-O-methylation of Nod factors. J Bio Chem. 1998;273:12047–55.
Folch-Mallol JL, Marroqui S, Sousa C, Manyani H, Lopez-Lara IM, van der Drift KM, et al. Characterization of Rhizobium tropici CIAT899 nodulation factors: the role of nodH and nodPQ genes in their sulfation. Mol Plant Microbe Interact. 1996;9(3):151–63.
Vargas C, Martínez LJ, Megías M, Quinto C. Identification and cloning of nodulation genes and host specificity determinants of the broad host-range Rhizobium leguminosarum biovar phaseoli strain CIAT899. Mol Microbiol. 1990;4:1899–910.
Machado D. Krishnan HB: nodD alleles of Sinorhizobium fredii USDA 191 differentially influence soybean nodulation, nodC expression, and production of exopolysaccharides. Curr Microbiol. 2003;47:134–7.
Machado D, Pueppke SG, Vinardel JM, Ruiz-Sainz JE, Krishnan HB. Expression of nodD1 and nodD2 in Sinorhizobium fredii, a nitrogen-fixing symbiont of soybean and other legumes. Mol Plant Microbe Interac. 1998;11:375–82.
Gomes DF, Ormeno-Orrillo E, Hungria M. Biodiversity, symbiotic efficiency and genomics of Rhizobium tropici and related species. In: De Bruijn F, editor. Biological Nitrogen Fixation. New Jersey, Hoboken: Wiley-Blackwell; 2015. p. 747–56.
Grange L, Hungria M, Graham PH, Martínez-Romero E. New insights into the origins and evolution of rhizobia that nodulate common bean (Phaseolus vulgaris) in Brazil. Soil Biol Biochem. 2007;39(4):867–76.
Pinto FGS, Hungria M, Mercante FM. Polyphasic characterization of Brazilian Rhizobium tropici strains effective in fixing N2 with common bean (Phaseolus vulgaris L.). Soil Biol Biochem. 2007;39(8):1851–64.
Acosta-Durán C, Martínez-Romero E. Diversity of rhizobia from nodules of the leguminous tree Gliricidia sepium, a natural host of Rhizobium tropici. Arch Microbiol. 2002;178:161–4.
Menna P, Hungria M, Barcellos FG, Bangel EV, Hess PN, Martínez-Romero E. Molecular phylogeny based on the 16S rRNA gene of elite rhizobial strains used in Brazilian commercial inoculants. Syst Appl Microbiol. 2006;29:315–32.
Rincón-Rosales R, Lloret L, Ponce E, Martínez-Romero E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol Ecol. 2009;67:103–17.
Capela D, Carrere S, Batut J. Transcriptome-based identification of the Sinorhizobium meliloti NodD1 regulon. Appl Environ Microbiol. 2005;71:4910–3.
Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–98.
Vincent JM. The modified Fahraeus slide technique. In: Vincent JM, editor. A manual for the practical study of root nodule bacteria. Oxford, UK: Blackwell Scientific Publications; 1970.
Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1989.
Lamrabet Y, Bellogín RA, Cubo T, Espuny MR, Gil-Serrano A, Krishnan HB, et al. Mutation in GDP-fucose synthesis genes of Sinorhizobium fredii alters Nod factors and significantly decreases competitiveness to nodulate soybeans. Mol Plant-Microbe Interact. 1999;12:207–17.
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73.62.
Prentki P, Krisch HM. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–13.
Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vivo constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196:413–20.
Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979;76:1648–52.
López-Baena FJ, Monreal JA, Pérez-Montaño F, Guasch-Vidal B, Bellogín RA, Vinardell JM, et al. The absence of Nops secretion in Sinorhizobium fredii HH103 increases GmPR1 expression in Williams soybean. Mol Plant Microbe Interac. 2009;22:1445–54.
Gomes DF, Batista JSS, Rolla AAP, Silva LPS, Bloch C, Galli-Terasawa LV, et al. Proteomic analysis of free-living Bradyrhizobium diazoefficiens: highlighting potential determinants of a successful symbiosis. BMC Genomics. 2014;15:643. doi: 10.1186/1471-2164-15-643.
Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36.
Tomlinson AD, Ramey-Hartung B, Day TV, Merrit PM, Fuqua C. Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiology. 2010;156:2670–81.
O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–61.
Glickmann E, Dessaux Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–6.
Fierro-Coronado RA, Quiroz-Figueroa FR, García-Pérez LM, Ramírez-Chávez E, Molina-Torres J, Maldonado-Mendoza IE. IAA-producing rhizobacteria from chickpea (Cicer arietinum L.) induce changes in root architecture and increase root biomass. Can J Microbiol. 2014;60:639–48.
Acknowledgements
The study was partially supported by AGL2012-38831, Embrapa, CNPq (National Council for Scientific and Technological Development), Project Science without Borders (400205/2012-5). Authors acknowledge Dr. Jesiane S.S. Batista for helping in obtaining the nodD2 mutant and Allan R.J. Eaglesham for suggestions on the manuscript and English review. A.A.P.R.-Santos acknowledges a posdoc fellowship from project Science without Borders, D.F.Gomes a PhD fellowship from Project Repensa and A.S.Nakatani a posdoc fellowship from Fundação Araucária. M.H. is also a research fellow from CNPq. We thank the Centro de Investigación, Tecnología e Innovación (CITIUS) of the University of Seville for MS facilities. Approved for publication by the Editorial Board of Embrapa Soja as manuscript 315/2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: MM, FJO, MH Performed the experiments: PC, APR-S, DFG, BBM, FP-M, MAR-C, ASN, AG-S, FJO. Analyzed the data: all authors Contributed reagents/materials/analysis tools: MM, FJO, MH. Wrote the paper: PC, APR-S, DFG, MM, FJO, MH. All authors read and approved the final manuscript.
Pablo del Cerro and Amanda Alves Paiva Rolla-Santos contributed equally to this work.
Additional files
Additional file 1: Figure S1.
Information about the nodD1 and nodD2 genes of R. tropici used in our study. A. Gene neighborhood of nodD1 and nodD2 genes in the genome of R. tropici strain CIAT 899. B. Location of primers (dark arrows) used to perform RTqPCR experiments. C. Schematic representation of the nodD2 mutation.
Additional file 2: Figure S2.
Nodulation phenotype in common bean (Phaseolus vulgaris) inoculated with CIAT 899 and derivative nodD strains assayed in pouch bags. Experiment performed under controlled conditions of growth chamber and plants harvested at 25 days after inoculation. A. wild type strain. B. nodD1 mutant. C. nodD2 mutant. D. Uninoculated.
Additional file 3: Figure S3.
Main properties observed in wild type (WT), nodD1 and nodD2 mutants of R. tropici strain CIAT 899.
Additional file 4: Table S1.
Sequences of the primers used in the RT-qPCR and sizes of the PCR products obtained.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
del Cerro, P., Rolla-Santos, A.A.P., Gomes, D.F. et al. Regulatory nodD1 and nodD2 genes of Rhizobium tropici strain CIAT 899 and their roles in the early stages of molecular signaling and host-legume nodulation. BMC Genomics 16, 251 (2015). https://doi.org/10.1186/s12864-015-1458-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-015-1458-8