Abstract
Purpose
Parenteral lipid emulsions (LEs) are commonly rich in long-chain triglycerides derived from soybean oil (SO). SO-containing emulsions may promote systemic inflammation and therefore may adversely affect clinical outcomes. We hypothesized that alternative oil-based LEs (SO-sparing strategies) may improve clinical outcomes in critically ill adult patients compared to products containing SO emulsion only. The purpose of this systematic review was to evaluate the effect of parenteral SO-sparing strategies on clinical outcomes in intensive care unit (ICU) patients.
Methods
We searched computerized databases from 1980 to 2013. We included randomized controlled trials (RCTs) conducted in critically ill adult patients that evaluated SO-sparing strategies versus SO-based LEs in the context of parenteral nutrition.
Results
A total of 12 RCTs met the inclusion criteria. When the results of these RCTs were statistically aggregated, SO-sparing strategies were associated with clinically important reductions in mortality (risk ratio, RR 0.83; 95 % confidence intervals, CI 0.62, 1.11; P = 0.20), in duration of ventilation (weighted mean difference, WMD −2.57; 95 % CI −5.51, 0.37; P = 0.09), and in ICU length of stay (LOS) (WMD −2.31; 95 % CI −5.28, 0.66; P = 0.13) but none of these differences were statistically significant. SO-sparing strategies had no effect on infectious complications (RR 1.13; 95 % CI 0.87, 1.46; P = 0.35).
Conclusion
Alternative oil-based LEs may be associated with clinically important reductions in mortality, duration of ventilation, and ICU LOS but lack of statistical precision precludes any clinical recommendations at this time. Further research is warranted to confirm these potential positive treatment effects.
Similar content being viewed by others
Introduction
Lipid emulsions (LEs) are an essential constituent of parenteral nutrition (PN) [1] and are considered an important source of energy, essential fatty acids (FA), and vitamins E and K [2–4]. However, the current literature suggests that soybean oil (SO) and safflower-based LEs which are rich in the ω-6 fatty acid linoleic acid might promote production of pro-inflammatory prostanoids and leukotrienes resulting in increased oxidative stress and systemic inflammation [5, 6] and may be associated with worse clinical outcomes [7].
Over the past three decades, different generations of alternative oil-based LEs have been developed, which could have less pro-inflammatory effects, less immune suppression, and more antioxidant effects than the standard SO-based LEs [8–10]. These SO-sparing strategies consist of different formulations of SO combined with medium-chain triglycerides (MCTs), olive oil (OO) which contains the ω-9 monounsaturated FA (MUFA) oleic acid, and fish oil (FO) which contains ω-3 FA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The purpose of the current study was to provide an up-to-date systematic review and meta-analysis of all randomized clinical trials (RCTs) of alternative oil-based LEs, compared to SO emulsion products, evaluating clinically relevant outcomes in the critically ill. Preliminary results of this systematic review were previously published in abstract form [11].
Methods
Study identification
We conducted a systematic review of the published literature to identify all relevant clinical trials using text word or MeSH headings containing the following: ω-6 sparing, ω-6 reducing, alternative fat emulsions, fish oil lipid emulsions, ω-3, ω-9, olive oil lipid emulsions, MCT lipid emulsions, randomized, blind, clinical trial, nutritional support, parenteral nutrition, lipid emulsions, critical illness, and critically ill. Our comprehensive search strategy included non-English articles.
We included studies if they met all the following eligibility criteria:
-
1.
Study design: randomized controlled trials (RCTs).
-
2.
Population: critically ill adult patients (>18 years old).
-
3.
Intervention: parenteral strategies to reduce the overall load of ω-6 FA (alternative ω-6-sparing LEs) versus ω-6 oil-based LEs (LCT in the control group).
-
4.
Study outcomes: mortality was the primary outcome for this meta-analysis. Secondary outcomes were intensive care unit (ICU) and hospital length of stay (LOS), infections, and mechanical ventilation (MV) days. We excluded the clinical studies that reported only biochemical, metabolic, immunologic, or nutritional outcomes. Critically ill patients were defined as patients admitted to an ICU who had an urgent or life-threatening complication (high baseline mortality rate ≥5 %) to distinguish them from patients with elective surgery who are also cared for in some ICUs but have a low baseline mortality rate (<5 %).
Data extraction and risk of bias assessment
Two reviewers independently extracted data using a data abstraction form with a scoring system [7]. We scored the methodological quality of individual trials considering the following key features of high-quality studies: (a) extent to which randomization was concealed, (b) blinding, (c) analysis was based on the intention-to-treat (ITT) principle, (d) comparability of groups at baseline, (e) extent of follow-up, (f) description of treatment protocol and co-interventions, and (g) definition of clinical outcomes. Each individual study was scored from 0 to 14. Disagreement was resolved by consensus between both reviewers. We attempted to contact the authors of included trials and requested missing or unclear information. We designated a study as level 1 if all of the following criteria were fulfilled: concealed randomization, blinded outcome adjudication, and an ITT analysis. A study was considered a level 2 study if any one of the above characteristics was unfulfilled.
Data synthesis
The primary outcome of the systematic review was overall mortality. From all trials, we combined hospital mortality where reported. If hospital mortality was not reported, we used ICU mortality or 28-day mortality. Secondary outcomes included infections, MV days, and ICU LOS. We used definitions of infections as defined by the authors in their original papers. We analyzed data using RevMan 5.1 with a random effects model. We calculated pooled relative risks using the Mantel–Haenszel estimator for dichotomous outcomes and weighted mean differences (WMDs) were estimated by the inverse variance approach for continuous outcomes, with associated 95 % CIs. The random effects model of DerSimonian and Laird was used to estimate variances for the Mantel–Haenszel and inverse variance estimators [12]. The possibility of publication bias was assessed by generating funnel plots and testing asymmetry of outcomes using methods proposed by Rucker et al. [13]. Statistical heterogeneity was assessed by the I 2 statistic [14]. We considered P < 0.05 to be statistically significant and P < 0.20 as an indicator of trend.
Hypotheses testing
Given the different ω-6 FA-sparing strategies and the heterogeneity of trial design, we performed pre-specified, hypothesis-generating subgroup analyses to attempt to elucidate potentially more beneficial treatment strategies. We compared the results of trials that provided (a) long-chain triglycerides (LCTs) plus MCT to an LCT emulsion; (b) ω-3 oil-based LEs to an LCT or LCT/MCT mixture, and (c) ω-9 oil-based LEs to an LCT or LCT + MCT mixture.
Post hoc, we determined that the control group solutions included both LCT and an LCT + MCT mixture. To evaluate the influence of this heterogeneity, we conducted a sensitivity analysis removing the RCTs that utilized an LCT plus an MCT-based strategy in the control group.
Results
Study identification and selection
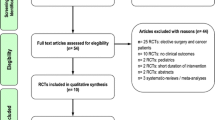
A total of 51 potentially eligible RCTs were identified. Of these, we excluded 39 trials due to the following reasons: 22 trials [15–36] trials did not include ICU patients (mostly elective surgery and cancer patients), 11 trials [31, 37–46] did not evaluate clinically important outcomes; 2 trials [47, 48] did not include SO-based LE in the control group; 1 trial [49] compared LCT versus another LCT emulsion without reduction in SO; 1 trial [50] was conducted in a pediatric population; 1 trial [51] had a short duration of intervention (12 h of lipid emulsion infusion during the first day); 1 trial included patients with poisoning and not representative of ICU patients [52]. In the end, 12 RCTs [53–64] enrolling a total of 806 patients met the inclusion criteria and were included in this systematic review (see Tables 1, 2). The authors reached 100 % agreement for inclusion of relevant trials in this review. The mean methodological score of all trials was 9.8 (6–14). Randomization was concealed in 8/12 (67 %) trials, ITT analysis was performed in 11/12 (92 %) trials, and 8/12 (67 %) trials were double blinded. There were five level 1 studies and seven level 2 studies. The details of the methodological quality of the individual trials are shown in Table 1.
Meta-analysis of primary outcome
When the results of the 12 RCTs [53–64] that evaluated mortality were statistically aggregated, ω-6-sparing strategies were associated with a reduction in mortality that was not statistically significant [risk ratio (RR) 0.83; 95 % confidence intervals (CI) 0.62, 1.11; P = 0.20, heterogeneity I 2 = 0 % see Fig. 1]. In addition, when a sensitivity analysis was done excluding five RCTs that supplemented LCT + MCT in the control group [57, 58, 60, 61, 63, 64], ω-6-sparing strategies had no effect on mortality (RR 0.72; 95 % CI 0.43, 1.21; P = 0.21, heterogeneity I 2 = 0 %, see Fig. 2).
Secondary outcomes
Compared to LCT, when the RCTs reporting ventilator days were aggregated [57, 58, 60, 61, 63], overall ω-6 FA-sparing strategies were consistent with a reduction in duration of MV but differences were not statistically significant (WMD −2.57; 95 % CI −5.51, 0.37; P = 0.09, heterogeneity I 2 = 25 %) (Fig. 3). There was a trend towards a reduction in ICU LOS associated with the use of ω-6-sparing strategies when compared to LCT [53, 55, 57–61, 63] (WMD −2.31; 95 % CI −5.28, 0.66; P = 0.13, heterogeneity I 2 = 68 % (Fig. 4). When the data from five RCTs [57, 59, 61, 62, 64] that reported ICU-acquired infections were aggregated, ω-6-sparing strategy had no effect (RR 1.13, heterogeneity 95 % CI 0.87, 1.46; P = 0.35, heterogeneity I 2 = 0 %).
Subgroup analysis
LCTs plus MCT versus LCT emulsion
Four RCTs [53–56] compared LCTs plus MCT to an LCT emulsion. When statistically aggregated, these studies showed no difference in mortality (RR 0.84; 95 % CI 0.43, 1.61; P = 0.59, heterogeneity I 2 = 0 %) (Fig. 1). Only one trial [56] compared LCT + MCT to LCT that reported duration of ventilation and no significant differences were seen between the two groups. When the data from the two trials [53, 55] that report ICU LOS were aggregated, there were no differences in ICU LOS (WMD −1.46; 95 % CI −5.77, 2.85; P = 0.51, heterogeneity I 2 = 78 % (Fig. 4).
Fish oil-containing emulsions versus LCT or LCT + MCT
Four RCTs [60–63] comparing ω-3 oil-based LEs to an LCT or LCT + MCT reported mortality. When these data were aggregated, this strategy was not associated with a reduction in mortality (RR 0.76; 95 % CI 0.48, 1.21; P = 0.25 heterogeneity I 2 = 0 %) (Fig. 1). We found a trend towards a reduction in the duration of MV (WMD −1.81; 95 % CI −3.98, 0.36; P = 0.10, heterogeneity I 2 = 0 %) (Fig. 3). There were no differences between the groups in ICU LOS (WMD −1.13; 95 % CI −8.96, 6.69; P = 0.78; heterogeneity I 2 = 78 %) (Fig. 3) and infections (RR 0.79; 95 % CI 0.43, 1.43; P = 0.43, heterogeneity I 2 = 0 %).
ω-9 oil-based LEs versus an LCT + MCT mixture
Four RCTs [57–59, 64] compared an ω-9 oil-based LE to an LCT + MCT mixture. We did not find any difference between the groups in mortality (RR 0.90; 95 % CI 0.58, 1.39; P = 0.62, heterogeneity I 2 = 0 %) (Fig. 1); however, we found a significant reduction in the duration of MV (WMD −6.47; 95 % CI −11.41, −1.53; P = 0.01, heterogeneity I 2 = 0 %) (Fig. 2) but no effect on ICU LOS (WMD −4.08; 95 % CI −10.97, 2.81; P = 0.25, heterogeneity I 2 = 59 %) (Fig. 4). When three RCTs [57, 59, 64] that reported on ICU-acquired infections were aggregated, this strategy showed a tendency towards an increase in infections (RR 1.23; 95 % CI 0.92, 1.63; P = 0.16, heterogeneity I 2 = 0 %).
Risk of publication bias
There was no indication that publication bias influenced the observed aggregated results. Funnel plots were created for each study outcome (data not shown) and the tests of asymmetry were not significant for any outcome measure (mortality, P = 0.48; ICU LOS, P = 0.88; MV days, P = 0.78; and infections, P = 0.29).
Discussion
Our systematic review and meta-analysis is the first to evaluate the overall effects of parenteral ω-6-reducing strategies in the critically ill. When 12 eligible trials were statistically aggregated, we did not find statistically significant effects. However, the magnitude of the potential treatment effect, in terms of a reduction in mortality (relative risk reduction 17 %) and reduction in ICU LOS (more than 2 days less), if realized, would be consistent with a large and clinically and economically important difference. Furthermore, after removing the RCTs that utilized an LCT plus MCT-based strategy in the control group, we found that the magnitude of the effect increased with a 28 % relative risk reduction in mortality without achieving statistical significance. The lack of statistical precision is likely due to the small number of studies and the small sample size of each study. Given the heterogeneous population of ICU patients included in this systematic review (sepsis, severe sepsis/septic shock, surgery, trauma, burns, and SIRS), the conclusions of our systematic review could be applied to a broad group of ICU patients. However, given the heterogeneity of alternative LEs, we explored several subgroups to evaluate if the treatment effect was different across different commercial preparations. There are no head-to-head comparisons of these different alternative LEs strategies. Indirectly, by examining the risk ratios of the different alternatives, there does not appear to be any difference in the treatment effects. Therefore, we are unable to define the best ω-6-sparing strategy in the critically ill as available evidence on the differential effects of LEs in ICU patients remains limited after our meta-analysis.
Recently, two meta-analyses on parenteral FO have been published. In summary, all three reviews agree there is inadequate evidence to recommend the routine use of FO-containing emulsions in PN in the critically ill. Pradelli et al. [65] summarized 23 trials in elective surgery and critically ill patients and demonstrated that parenteral FO-enriched LEs were associated with a statistically and clinically significant reduction in infections (RR 0.61; 95 % CI, 0.45–0.84; P = 0.002) and the LOS, both in the ICU (MWD, −1.92; −3.27 to −0.58; P = 0.005) and in hospital (MWD, −3.29; −5.13 to −1.45; P = 0.0005), but no effect on overall mortality was shown (RR 0.89; 95 % CI 0.59, 1.33; P = NS). More recently, Palmer et al. [66] statistically aggregated nine randomized trials of parenteral FO and showed no significant effect on mortality (RR 0.83; 95 % CI 0.57, 1.20; P = 0.32), infectious complications (RR 0.78; 95 % CI 0.43, 1.41; P = 0.41), and ICU LOS (MWD, 0.57; 95 % CI –5.05, 3.90; P = 0.80) in comparison with standard PN. These latter results are similar to our subgroup findings but in addition, we found a tendency toward a reduction in MV days associated with FO administration (WMD −1.81; 95 % CI −3.98, 0.36; P = 0.10). We believe that the difference between these two reviews and our subgroup analysis of FO administration was largely due to the difference in the papers included in the different reviews. Pradelli et al. [65] included ten trials in patients undergoing elective major abdominal surgery and not admitted to ICU (N = 740). Palmer et al. [66] included both papers published by Wang et al. in 2008 [29] and 2009 [62]. However, we excluded the 2008 Wang trial [29] because it did not include ICU patients and did not report on relevant clinical outcomes. In addition, we excluded two unpublished trials by Leiderman et al. [67] and Ignatenko et al. [68]. Both of these trials were included in the prior meta-analyses but are only published as abstracts and we were not able to obtain the data from the investigators necessary to have these trials included in our review.
The strength of our meta-analysis includes the fact that we used several methods to reduce bias (comprehensive literature search, duplicate data abstraction, specific criteria for searching and analysis) and have focused on clinically important primary outcomes for ICU patients. The major limitation of our meta-analysis was the small number of trials included, which may have resulted in statistical imprecision. Furthermore, the presence of heterogeneity, both clinical and statistical, weakens any inferences we can make from these data.
In spite of these limitations, we have demonstrated that alternative oil-based LEs in the critically ill may be able to reduce overall mortality and shorten ventilation days and ICU LOS. However, our study lacks the statistical precision to confirm these preliminary findings and further research is clearly warranted. Future trials should define the best mixture of lipids, target patient population, best timing, and duration of therapy to optimize the effects on underlying systemic inflammation, immune status, and metabolic processes while at the same time achieving an acceptable safety and tolerance profile.
References
Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, Miles JM, Valentine CJ, Kochevar M, Novel Nutrient Task Force, Intravenous Fat Emulsions Workgroup, and the American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. ASPEN (2012) Position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract 27:150–192
Vinnars E, Wilmore D (2003) Jonathan Roads symposium papers. History of parenteral nutrition. JPEN J Parenter Enteral Nutr 27:225–231
Hippalgaonkar K, Majumdar S, Kansara V (2010) Injectable lipid emulsions: advancements, opportunities and challenges. AAPS Pharm Sci Technol 11:1526–1540
Carpentier YA, Dupont IE (2000) Advances in intravenous lipid emulsions. World J Surg 24:1493–1497
Wanten GJA, Calder P (2007) Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 85:1171–1184
Furukawa K, Yamamori H, Takagi K, Hayashi N, Suzuki R, Nakajima N, Tashiro T (2002) Influences of soybean oil emulsion on stress response and cell-mediated immune function in moderately or severely stressed patients. Nutrition 18:235–240
Heyland DK, MacDonald S, Keefe L, Drover JW (1998) Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 280:2013–2019
Bannenberg G, Serhan CN (2010) Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta 1801:1260–1273
Serhan CN, Yacoubian S, Yang R (2008) Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol 3:279–312
Calder PC, Jensen GL, Koletzko BV, Singer P, Wanten GJA (2010) Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med 36:735–749
Manzanares W, Dhaliwal R, Jurewitsch B, Stapleton RD, Jeejeebhoy KN, Heyland DK (2013) Alternative lipid strategies in the critically ill: a systematic review and meta-analysis. Preliminary data. JPEN J Parenter Enteral Nutr 37:73–74
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Rucker G, Schwarzer G, Carpenter J (2008) Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med 27:746–763
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Wachtler P, König W, Senkal M, Kemen M, Köller M (1997) Influence of a total parenteral nutrition enriched with omega-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J Trauma 42:191–198
Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F (1998) Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer 82:395–402
Furukawa K, Tashiro T, Yamamori H, Takagi K, Morishima Y, Sugiura T, Otsubo Y, Hayashi N, Itabashi T, Sano W, Toyoda Y, Nitta H, Nakajima N (1999) Effects of soybean oil emulsion and eicosapentaenoic acid on stress response and immune function after a severely stressful operation. Ann Surg 229:255–261
Linseisen J, Hoffmann J, Lienhard S, Jauch KW, Wolfram G (2000) Antioxidant status of surgical patients receiving TPN with an omega-3-fatty acid-containing lipid emulsion supplemented with alpha-tocopherol. Clin Nutr 19:177–184
Heller AR, Fischer S, Rossel T, Geiger S, Siegert G, Ragaller M, Zimmermann T, Koch T (2002) Impact of n-3 fatty acid supplemented parenteral nutrition on haemostasis patterns after major abdominal surgery. Br J Nutr 87(Suppl 1):S95–S101
Heller AR, Rössel T, Gottschlich B, Tiebel O, Menschikowski M, Litz RJ, Zimmermann T, Koch T (2004) Omega-3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer 111:611–616
Kłek S, Kulig J, Szczepanik AM, Jedrys J, Kołodziejczyk P (2005) The clinical value of parenteral immunonutrition in surgical patients. Acta Chir Belg 105:175–179
Grimm H, Mertes N, Goeters C, Schlotzer E, Mayer K, Grimminger F, Fürst P (2006) Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr 45:55–60
Mertes N, Grimm H, Fürst P, Stehle P (2006) Safety and efficacy of a new parenteral lipid emulsion (SMOF lipid) in surgical patients: a randomized, double-blind, multicenter study. Ann Nutr Metab 50:253–259
Senkal M, Geier B, Hannemann M, Deska T, Linseisen J, Wolfram G, Adolph M (2007) Supplementation of omega-3 fatty acids in parenteral nutrition beneficially alters phospholipid fatty acid pattern. JPEN J Parenter Enteral Nutr 31:12–17
Wendel M, Rössel T, Bergmann S, Otto S, Ragaller M, Zimmermann T, Konopke R, Koch T, Heller AR (2007) Impact of total parenteral nutrition including omega-3 fatty acids on the regulation of plasma lipoproteins and glycemic control after major abdominal surgery. E-SPEN 2:e103–e110
Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW (2007) Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med 35:700–706
Berger MM, Tappy L, Revelly JP, Koletzko BV, Gepert J, Corpataux JM, Cayeux MC, Chiolero RL (2008) Fish oil after abdominal aorta aneurysm surgery. Eur J Clin Nutr 62:1116–1122
Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, Xie QW, Yin MJ (2008) Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol 14:2434–2439
Wang X, Li W, Li N, Li J (2008) Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J Parenter Enteral Nutr 32:236–241
Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, Vogt PR, Erdogan A (2009) Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg 57:276–280
Piper SN, Schade I, Beschmann R, Maleck W, Boldt J, Rohm KD (2009) Hepatocellular integrity after parenteral nutrition: comparison of a fish-oil-containing lipid emulsion with an olive-soybean oil-based lipid emulsion. Eur J Anaesthesiol 26:1076–1082
Puiggròs C, Sánchez J, Chacón P, Sabín P, Roselló J, Bou R, Planas M (2009) Evolution of lipid profile, liver function, and pattern of plasma fatty acids according to the type of lipid emulsion administered in parenteral nutrition in the early postoperative period after digestive surgery. JPEN J Parenter Enteral Nutr 33:501–512
Badía-Tahull MB, Llop-Talaverón JM, Leiva-Badosa E, Biondo S, Farran-Teixidó L, Ramón-Torrell JM, Jódar-Masanes R (2010) A randomised study on the clinical progress of high-risk elective major gastrointestinal surgery patients treated with olive oil-based parenteral nutrition with or without a fish oil supplement. Br J Nutr 104:737–741
Jiang ZM, Wilmore DW, Wang XR, Wei JM, Zhang ZT, Gu ZY, Wang S, Han SM, Jiang H, Yu K (2010) Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg 97:804–809
Han YY, Lai SL, Ko WJ, Chou CH, Lai HS (2012) Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract 27:91–98
Zhu MW, Tang DN, Hou J, Wei JM, Hua B, Sun JH, Chui HY (2012) Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin Med J 125:178–181
Adams S, Yeh YY, Jensen GL (1993) Changes in plasma and erythrocyte fatty acids in patients fed enteral formulas containing different fats. JPEN J Parenter Enteral Nutr 17:30–34
Schauder P, Rohn U, Schafer G, Korff G, Schenk HD (2002) Impact of fish oil enriched total parenteral nutrition on DNA synthesis, cytokine release and receptor expression by lymphocytes in the postoperative period. Br J Nutr 87(Suppl 1):S103–S110
Koller M, Senkal M, Kemen M, Konig W, Zumtobel V, Muhr G (2003) Impact of omega-3 fatty acid enriched TPN on leukotriene synthesis by leukocytes after major surgery. Clin Nutr 22:59–64
Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, Grimminger F, Seeger W (2003) Short-time infusion of fish oil-based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol 171:4837–4843
Mayer K, Fegbeutel C, Hattar K, Sibelius U, Krämer HJ, Heuer KU, Temmesfeld-Wollbrück B, Gokorsch S, Grimminger F, Seeger W (2003) Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med 29:1472–1481
Mayer K, Gokorsch S, Fegbeutel C, Hattar K, Rosseau S, Walmrath D, Seeger W, Grimminger F (2003) Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J Respir Crit Care Med 15(167):1321–1328
Antébi H, Mansoor O, Ferrier C, Tétégan M, Morvan C, Rangaraj J, Alcindor LG (2004) Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: comparison of 2 fat emulsions. JPEN J Parenter Enteral Nutr 28:142–148
Tappy L, Berger MM, Schwarz JM, Schneiter P, Kim S, Revelly JP, Chioléro R (2006) Metabolic effects of parenteral nutrition enriched with n-3 polyunsaturated fatty acids in critically ill patients. Clin Nutr 25:588–595
Xiong J, Zhu S, Zhou Y, Wu H, Wang C (2009) Regulation of omega-3 fish oil emulsion on the SIRS during the initial stage of severe acute pancreatitis. J Huazhong Univ Sci Technol Med Sci 29:35–38
Sungurtekin H, Değirmenci S, Sungurtekin U, Oguz BE, Sabir N, Kaptanoglu B (2011) Comparison of the effects of different intravenous fat emulsions in patients with systemic inflammatory response syndrome and sepsis. Nutr Clin Pract 26:665–671
Khor BS, Liaw SJ, Shih HC, Wang LS (2011) Randomized, double blind, placebo-controlled trial of fish-oil-based lipid emulsion infusion for treatment of critically ill patients with severe sepsis. Asian J Surg 34:1–10
Gupta A, Govil D, Bhatnagar S, Gupta S, Goyal J, Patel S, Baweja H (2011) Efficacy and safety of parenteral omega-3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med 15:108–113
Kari A, Hersio K, Takala J, Penttila I (1989) Comparison of two long-chain triglyceride fat emulsions in parenteral nutrition of critically ill patients. Curr Ther Res 45:1077–1087
Larsen BM, Goonewardene LA, Joffe AR, Van Aerde JE, Field CJ, Olstad DL, Clandinin MT (2012) Pre-treatment with an intravenous lipid emulsion containing fish oil (eicosapentaenoic and docosahexaenoic acid) decreases inflammatory markers after open-heart surgery in infants: a randomized, controlled trial. Clin Nutr 31:322–329
Sabater J, Masclans JP, Sacanell J, Chacon P, Sabin P, Planas M (2008) Effects on hemodynamics and gas exchange of omega-3 fatty acid-enriched lipid emulsion in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Lipids Health Dis 7:39
Taftachi F, Sanaei-Zadeh H, Sepehrian B, Zamani N (2012) Lipid emulsion improves Glasgow coma scale and decreases blood glucose level in the setting of acute non-local anesthetic drug poisoning—a randomized controlled trial. Eur Rev Med Pharmacol Sci 16(Suppl 1):38–42
Nijveldt RJ, Tan AM, Prins HA, de Jong D, van Rij GL, Wesdorp RI, van Leeuwen PA (1998) Use of a mixture of medium-chain triglycerides and longchain triglycerides versus long-chain triglycerides in critically ill surgical patients: a randomized prospective double-blind study. Clin Nutr 17:23–29
Lindgren BF, Ruokonen E, Magnusson-Borg K, Takala J (2001) Nitrogen sparing effect of structured triglycerides containing both medium-and long-chain fatty acids in critically ill patients; a double blind randomized controlled trial. Clin Nutr 20:43–48
Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Garcia-Garmendia JL, Jiménez-Jiménez LM, Garnacho-Montero MC, Barrero-Almodóvar A (2002) Clinical and metabolic effects of two lipid emulsions on the parenteral nutrition of septic patients. Nutrition 18:134–138
Iovinelli G, Marinangeli F, Ciccone A, Ciccozzi A, Leonardis M, Paladini A, Varrassi G (2007) Parenteral nutrition in ventilated patients with chronic obstructive pulmonary disease: long chain vs. medium chain triglycerides. Minerva Anestesiol 73:65–76
García-de-Lorenzo A, Denia R, Atlan P, Martinez-Ratero S, Le Brun A, Evard D, Bereziat G (2005) Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: a randomised double-blind study of an olive oil-based lipid emulsion v. medium/long-chain triacylglycerols. Br J Nutr 94:221–230
Huschak G, Zur Nieden K, Hoell T, Riemann D, Mast H, Stuttmann R (2005) Olive oil based nutrition in multiple trauma patients: a pilot study. Intensive Care Med 31:1202–1208
Umpierrez GE, Spiegelman R, Zhao V, Smiley DD, Pinzon I, Griffith DP, Peng L, Morris T, Luo M, Garcia H, Thomas C, Newton CA, Ziegler TR (2012) A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med 40:1792–1798
Grecu I, Mirea L, Grintescu I (2003) Parenteral fish oil supplementation in patients with abdominal sepsis. Clin Nutr 22:S23
Friesecke S, Lotze C, Köhler J, Heinrich A, Felix SB, Abel P (2008) Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomised controlled trial. Intensive Care Med 34:1411–1420
Wang X, Li W, Zhang F, Pan L, Li N, Li J (2009) Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: a randomized controlled trial. Inflammation 32:304–309
Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC (2010) Effects of fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care 14:R5
Pontes-Arruda A, Dos Santos MC, Martins LF, Gonzalez ER, Kliger RG, Maia M, Magnan GB, EPICOS Study Group (2012) Influence of parenteral nutrition delivery system on the development of bloodstream infections in critically ill patients: an international, multicenter, prospective, open-label, controlled study—EPICOS study. JPEN J Parenter Enteral Nutr 36:574–586
Pradelli L, Mayer K, Muscaritoli M, Heller AR (2012) n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care 16:R184
Palmer AJ, Ho CKM, Ajibola O, Avenell A (2013) The role of ω-3 fatty acid supplemented parenteral nutrition in critical illness in adults: a systematic review and meta-analysis. Crit Care Med 41:307–316
Leiderman I, Malkova O, Levit A (2010) Omega 3 enriched lipid emulsion decreases APACHE II and SOFA scores values in abdominal sepsis patients. Clin Nutr Suppl 5:30
Ignatenko O, Yaroshetskiy A, Masolitin S, Protsenko D, Gelfand B (2010) Fish oil treatment in severe trauma patients. Intensive Care Med 36:S316
Conflicts of interest
Daren Heyland received speaking honorarium and research grants from Fresenius Kabi and Baxter. The other authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manzanares, W., Dhaliwal, R., Jurewitsch, B. et al. Alternative lipid emulsions in the critically ill: a systematic review of the evidence. Intensive Care Med 39, 1683–1694 (2013). https://doi.org/10.1007/s00134-013-2999-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2999-4