Summary
Objective
We assessed the effects of a novel lipid emulsion with reduced content of n–6 fatty acids (FA), increased share of MUFA and n–3 FA and supplemental vitamin E on fatty acid and leukotriene pattern in surgical patients.
Methods
In a double–blind, randomized study 33 patients received isonitrogenous, isocaloric TPN over 5 postoperative days following major abdominal surgery. 19 patients received the new SMOFlipid® 20% and 14 patients a standard soybean oil emulsion (Lipovenoes® 20%, both Fresenius Kabi), each 1.5 g fat/kg body weight (BW)/d. Routine lipid biochemistry, plasma tocopherol, fatty acid pattern in plasma phospholipids, as well as leukotriene (LT) release in leukocytes were assessed. Additionally, fatty acid pattern in leukocyte and platelet phospholipids were analysed, but results are not presented.
Results
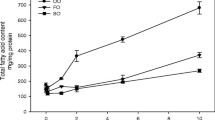
On day 6, plasma α–tocopherol (34.2 ± 10.3 vs. 17.6 ± 2.9 µmol/L) and, in plasma PL, total n–3 FA were higher (11.1 ± 1.9 vs. 4.9 ± 0.9 mol%; p < 0.05) and total n–6 FA lower (23.8 ± 2.2 vs. 31.8 ± 1.7 mol%; P < 0.05); the ratio n–3/n–6 FA being elevated (0.5 ± 0.1 vs. 0.2 ± 0.0 p < 0.05) with SMOFlipid compared to the soybean oil emulsion. The shares of EPA (3.3±1.0 vs. 0.4±0.2 mol%; p<0.05) and DHA (6.9 ± 1.8 vs. 3.7 ± 0.8 mol%; p < 0.05) were highly increased but that of arachidonic acid (AA) was unchanged with SMOFlipid while the ratio EPA/AA was increased (0.7 ± 0.2 vs. 0.1 ± 0.0 p < 0.05). LTB5 release was enhanced on day 6 (8.1 ± 5.3 vs. 1.8 ± 3.8 pmol/107 PMN, p < 0.05) and liberation of LTB4 was lowered, yet not significantly with SMOFlipid (124.0 ± 51.2 vs. 152.1 ± 68.8 pmol/107 PMN). Length of hospital stay was significantly shorter with SMOFlipid (13.4 ± 2.0 vs. 20.4 ± 10.0 days, p < 0.05).
Conclusion
Treatment with the new emulsion SMOFlipid is well tolerated and modulates FA and leukotriene pattern suggesting favourable anti–inflammatory effects and further clinical benefits.
Similar content being viewed by others
References
Dupont IE, Carpentier YA (1999) Clinical use of lipid emulsions. Curr Opin Clin Nutr Metab Care 2:139–145
Calder PC, Deckelbaum RJ (1998) New metabolic pathways for lipids and lipid emulsions. Curr Opin Clin Nutr Metab Care 1:139–141
Giordano C, Gallina C, Consalvi V, Scandurra R (1990) Irreversible inactivation of papain and cathepsin B by epoxidic substrate analogues. Eur J Med Chem 25:479–487
Calder PC, Newsholme EA (1992) Polyunsaturated fatty acids suppress human peripheral blood lymphocyte proliferation and interleukin–2 production. Clin Sci (Colch) 82:695–700
Soyland E, Nenseter MS, Braathen L, Drevon CA (1993) Very long chain n–3 and n–6 polyunsaturated fatty acids inhibit proliferation of human T–lymphocytes in vitro. Eur J Clin Invest 23:112–121
Calder PC, Yaquoob P, Thies F, Wallace FA, Miles EA (2002) Fatty acids and lymphocyte functions. Br J Nutr 87:S31–S48
Grimble RF (1998) Dietary lipids and the inflammatory response. Proc Nutr Soc 57:535–542
Simopoulos AP, Leaf A, Salem N (1999) Essentiality of and recommended dietary intakes of w–6 and w–3 fatty acids. Ann Nutr Metab 43:127–130
Wu D, Meydani SN (1998) n–3 Polyunsaturated fatty acids and immune function. Proc Nutr Soc 57:503–509
Steeger PJK, Mühlebach SF (1998) Lipid peroxidation of IV lipid emulsions in TPN bags: the influence of tocopherols. Nutrition 14:179–185
Morlion BJ, Torwesten E, Lessire A, Sturm G, Peskar BM, Fürst P, et al. (1996) The effect of parenteral fish oil on leukocyte membrane fatty acid composition and leukotriene–synthesizing capacity in postoperative trauma. Metabolism 45:1208–1213
Hjorth R, Jonsson A–K, Vretblad P (1981) A rapid method for purification of human granulocytes using percoll: a comparison with dextran sedimentation. J Immunol Methods 43:95–99
Grimminger F, Becker G, Seeger W (1988) High yield enzymatic conversion of intravascular leukotriene A4 in blood–free perfused lungs. J Immunol 141:2431–2436
Engelhart K, Jentzsch AM, Fürst P, Biesalski H–K (1998) Short–term parenteral application of α–tocopherol leads to increased concentrations in plasma and tissues of the rat. Free Radic Res 29:421–426
Eaton SB, Konner M (1985) Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 312:283–289
Bang HO, Dyerberg J, Hjorne N (1976) Composition of food consumed by Greenland Eskimos. Acta Med Scand 200:69–73
Arthaud JB (1970) Cause of death in 339 Alaskan natives as determined by autopsy. Arch Pathol 90:433–438
Eaton SB, Eaton SBI, Sinclair AJ, Cordain L, Mann NJ (1998) Dietary intake of long–chain polyunsaturated fatty acids during the paleolithic. Wld Rev Nutr Diet 83:12–23
Deutsche Gesellschaft für Ernährung e. V. (2004) DGE Editor. Ernährungsbericht 2004. DGE–MedienService, Bonn. 36
Black PN, Sharpe S (1997) Dietary fat and asthma: is there a connection? Eur Respir J 10:6–12
Lewis S, Butland B, Strachen D, Bynner J, Richards D, Butler N, et al. (1996) Study of the aetiology of wheezing illness at age 16 in two national British birth cohorts. Thorax 51:670–671
Shoda R, Matsueda K, Yamato S, Umeda N (1996) Epidemiologic analysis of Crohn’s disease in Japan: increased dietary intake of n–6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn’s disease in Japan. Am J Clin Nutr 63:741–745
Grimm H, Tibell A, Norrlind B, Blecher C, Wilker S, Schwemmle K (1994) Immunoregulation by parenteral lipids: impact of the n–3 to n–6 fatty acid ratio. JPEN 18:417–421
Morlion BJ, Torwesten E, Wrenger K, Puchstein C, Fürst P (1997) What is the optimum n–3 to n–6 fatty acid (FA) ratio of parenteral lipid emulsions in postoperative trauma? Clin Nutr 16:49S
Fürst P, Kuhn KS (2000) Fish oil emulsions – what benefits can they bring? Clin Nutr 19:7–14
Grimm H, Kraus A (2001) Immunonutrition– supplementary amino acids and fatty acids ameliorate immune deficiency in critically ill patients. Arch Surg 386:369–376
Adolph M (2001) Lipid emulsions in parenteral nutrition – state of the art and future perspectives. Clin Nutr 20 (Suppl 4):11–14
Mayer K, Seeger W, Grimminger F (1998) Clinical use of lipids to control inflammatory disease. Curr Opin Clin Nutr Met Care 1:179–184
Wander RC, Hall JA, Gradin JL, Du SH, Jewell DE (1997) The ratio of dietary (n– 6) to (n–3) fatty acids influences immune system function, eicosanoid metabolism, lipid peroxidation and vitamin E status in aged dogs. J Nutr 127:1198–1205
Calder PC (1997) N–3 polyunsaturated fatty acids and immune cell function. Adv Enzyme Regul 37:197–237
Linseisen J, Hoffmann J, Lienhard S, Jauch KW, Wolfram G (2000) Antioxidant status of surgical patients receiving TPN with an omega–3–fatty acid–containing lipid emulsion supplemented with alpha–tocopherol. Clin Nutr 19:177–184
Antebi H, Mansoor O, Ferrier C, Tétégan M, Morvan C, Rangaraj J, Alcindor LG (2004) Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: comparison of 2 fat emulsions. JPEN 28:142–148
Ikehata A, Hiwatashi N, Kinouchi Y, Yamazaki H, Kumagai Y, Ito K, et al. (1992) Effect of intravenously infused eicosapentaenoic acid on the leukotriene generation in patients with active Crohn’s disease. Am J Clin Nutr 56:938–942
Roulet M, Frascarolo P, Pilet M, Chapuis G (1997) Effects of intravenously infused fish oil on platelet fatty acid phospholipid composition and on platelet function in postoperative trauma. JPEN 21:296–301
Heller AR, Rössel T, Gottschlich B, Tiebel O, Menschikowski M, Litz RJ, Zimmermann T, Koch T (2004) Omega– 3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer 11:611–616
Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H (2002) Immunonutrition by perioperative administration of n–3 fatty acids. Br J Nutr 87:S89–S94
Linseisen J, Wolfram G (1997) Efficacy of different triglycerides in total parenteral nutrition for preventing atrophy of the gut in traumatized rats. JPEN 21:21–26
Yaqoob P (1998) Monounsaturated fatty acids and immune function. Proc Nutr Soc 57:511–520
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimm, H., Mertes, N., Goeters, C. et al. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr 45, 55–60 (2006). https://doi.org/10.1007/s00394-005-0573-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-005-0573-8