Abstract
Background
Parenteral lipid emulsions in critical care are traditionally based on soybean oil (SO) and rich in pro-inflammatory omega-6 fatty acids (FAs). Parenteral nutrition (PN) strategies with the aim of reducing omega-6 FAs may potentially decrease the morbidity and mortality in critically ill patients.
Methods
A systematic search of MEDLINE, EMBASE, CINAHL and CENTRAL was conducted to identify all randomized controlled trials in critically ill patients published from inception to June 2021, which investigated clinical omega-6 sparing effects. Two independent reviewers extracted bias risk, treatment details, patient characteristics and clinical outcomes. Random effect meta-analysis was performed.
Results
1054 studies were identified in our electronic search, 136 trials were assessed for eligibility and 26 trials with 1733 critically ill patients were included. The median methodologic score was 9 out of 14 points (95% confidence interval [CI] 7, 10). Omega-6 FA sparing PN in comparison with traditional lipid emulsions did not decrease overall mortality (20 studies; risk ratio [RR] 0.91; 95% CI 0.76, 1.10; p = 0.34) but hospital length of stay was substantially reduced (6 studies; weighted mean difference [WMD] − 6.88; 95% CI − 11.27, − 2.49; p = 0.002). Among the different lipid emulsions, fish oil (FO) containing PN reduced the length of intensive care (8 studies; WMD − 3.53; 95% CI − 6.16, − 0.90; p = 0.009) and rate of infectious complications (4 studies; RR 0.65; 95% CI 0.44, 0.95; p = 0.03). When FO was administered as a stand-alone medication outside PN, potential mortality benefits were observed compared to standard care.
Conclusion
Overall, these findings highlight distinctive omega-6 sparing effects attributed to PN. Among the different lipid emulsions, FO in combination with PN or as a stand-alone treatment may have the greatest clinical impact.
Trial registration PROSPERO international prospective database of systematic reviews (CRD42021259238).
Graphical abstract

Similar content being viewed by others
Background
Critical illness is often characterized by an imbalanced immune response, which can lead to excessive cytokine release and accumulation of reactive oxygen species. Systemic inflammation and oxidative stress ultimately result in tissue damage, multi organ failure and high mortality rates [1,2,3,4]. Parenteral lipid emulsions provide fatty acids (FAs) as a source of calories and cellular building blocks [5] and have been under investigation because of their immunomodulating features [6]. Traditionally, parenteral lipid emulsions are derived from plant—and especially soybean oil (SO) to provide the patient with essential long-chain triglycerides (LCTs). However, SO’s ratio of omega-6 polyunsaturated FAs to omega-3 polyunsaturated FAs (7:1) is being regarded critically [6]. In vivo, linoleic acid (omega-6) is converted to arachidonic acid and pro-inflammatory eicosanoids, such as prostaglandins, thromboxanes and leukotrienes. On the other hand, α-linolenic acid (omega-3) is the metabolic precursor of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which both have anti-inflammatory and anti-oxidative properties like inhibitory effects on various innate and adaptive immune cells and the transcription of inflammatory cytokines [7,8,9]. Unfortunately, the pathway of α-linolenic acid conversion to DHA and EPA appears to be highly ineffective in most humans [10] and an abundance of omega-6 FAs will furthermore suppress the balancing effects of omega-3 FA conversion, as both FAs (omega-3 and omega-6) compete for the same enzymes [11]. Therefore, omega-6 FA reducing strategies have been introduced to clinical nutrition. A reduction in omega-6 FAs can be achieved with the addition of medium-chain triglycerides (MCT), olive oil (OO) or fish oil (FO) to SO-based lipid emulsions. FO supplements, as a major source of DHA and EPA, can simultaneously provide adequate levels of omega-3 FAs, which is regarded as an attractive immunomodulatory treatment option with potential clinical benefits [12]. Hitherto, meta-analyses solely focused on the FO aspect, resulting in limited evidence regarding the overall picture of omega-6 FA reduction. After aggregating 10 randomized controlled trials (RCTs) in 2015, Manzanares et al. found that lipid emulsions with a FO component may reduce infectious complications, duration of mechanical ventilation (MV) and hospital length of stay (LOS) in critically ill patients [13]. In the most recent meta-analysis by Pradelli et al., FO containing parenteral nutrition (PN) again reduced the rate of infections and hospital as well as ICU LOS [14]. Current nutrition guidelines state that DHA and EPA (FO dose of 0.1–0.2 g/kg/d) can be provided in patients receiving PN. Ambiguous results regarding other omega-6 sparing strategies are delineated, however without a clear recommendation [15, 16]. In recent years, several new RCTs on omega-6 sparing effects in general and on FO-containing lipid emulsions in particular have been published. This systematic review and meta-analysis aims to give a broad and comprehensive update on the emerging topic.
Methods
Information sources
We searched MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews (CENTRAL) for all relevant RCTs published between January 1980 and June 2021 with the following keywords: “fat emulsion”, “lipid emulsion”, “lipid injectable emulsion”, “lipids”, “triglycerides”, “medium chain triglycerides”, “long chain triglycerides”, “polyunsaturated fatty acids”, “omega-3 fatty acids”, “omega-6 fatty acids”, “fish oil”, “olive oil”, “soybean oil”, “linoleic acid”, “linolenic acid”, “eicosapentaenoic acid”, “EPA”, “docosahexaenoic acid” and “DHA”. Our data acquisition was not limited to articles written in English.
In case of missing data, we contacted the authors and requested additional information. The literature research, selection and methodologic assessment of trials was performed independently by two researchers. Consensus was required to include the respective study in the meta-analysis.
Eligibility criteria
Inclusion criteria were defined as follows:
-
1.
Study design: RCT with a parallel group.
-
2.
Study population: adults, ≥ 18 years of age. Critical illness was defined as admission to the intensive care unit (ICU), or in unclear cases, a mortality rate of ≥ 5% in the control group. Studies that mainly or exclusively enrolled patients admitted for elective and cancer surgery were excluded, even if they were admitted to the ICU for postoperative surveillance.
-
3.
Intervention and control: Studies were classified in two categories and analysed independently. The first group only comprised PN trials. Omega-6 FA reduced formulations were compared with standard care lipid emulsions containing higher amounts of omega-6 FAs.
In the second group, patients received standard parenteral and / or enteral nutrition. Here, intravenous FO was administered as a stand-alone intervention. For control, patients either received no additional treatment or normal saline solution.
-
4.
Outcomes: The primary outcome of our meta-analysis was overall mortality. When multiple mortality endpoints were reported in a trial, we included the data in the following order of preference: 28-day mortality > hospital mortality > ICU mortality > other mortality.
Secondary outcomes included 28-day mortality, ICU LOS, hospital LOS, days on MV and infectious complications. Trials without at least one of these clinical endpoints were excluded.
Subgroup analysis
Among PN trials investigating omega-6 FA reduction, we predefined three subgroups according to the interventional strategy: SO/MCT, SO/OO and FO containing PN. To further reduce the heterogeneity of different FO formulations, we additionally aggregated all FO studies, which specifically tested Omegaven® (Fresenius Kabi, Bad Homburg, Germany), a 10% FO lipid emulsion with broad clinical use, and compared them to other FO solutions.
Assessment of bias and methodologic quality
This systematic review adhered to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17]. As with our established practice in previous meta-analyses, studies were assigned to two categories based on the following criteria for methodologic quality: (1) concealed randomization, (2) blinded outcome adjudication and (3) intention-to-treat analysis. If all three characteristics applied, the study was considered “level I”, if one was unfulfilled we labeled the study as “level II”. In addition, the quality of each study was scored by two independent reviewers as described before [13, 18]. Nine items were considered: concealed randomization, intention-to-treat analysis, double blinding, consecutive patient selection, comparability at baseline, extent of follow-up, description of treatment protocols, description and equality of co-interventions and objective definition of outcomes. The maximum score was 14, the minimum score was 0 points (Additional file 1). The protocol of our meta-analysis was registered a priori at the PROSPERO international prospective database of systematic reviews (CRD42021259238).
Data synthesis
All analyses were performed with a random effects model using RevMan 5.4 (Cochrane IMS, Oxford, UK). To estimate the pooled risk ratio (RR) for dichotomous data and the weighted mean difference (WMD) for continuous variables, data were aggregated from all studies and presented with a 95% confidence interval (CI). For continuous variables, we contacted the authors to provide the mean and standard deviation (SD), in case the original publication presented the median and interquartile range. If we were unable to obtain the mean and SD, the dataset was excluded from our analyses.
The χ2 test and the I2 statistics were used to assess heterogeneity. To address the risk of publication bias, a funnel plot was generated for the primary outcome and tested for asymmetry with the Egger regression test. As the overall purpose of this systematic review and meta-analysis was hypothesis generating, we considered a p value < 0.05 as statistically significant and a p value < 0.20 as a trend [19].
Results
Included trials
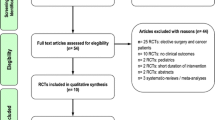
From the 1054 studies that were identified through our systematic searching, a total of 136 potential studies were sought for retrieval (Fig. 1). 40 trials covered non-ICU, elective surgery and cancer patients and 31 trials did not report on our clinical outcomes. 23 studies were not RCTs, including systematic reviews, meta- or sub-analyses. In 15 studies, the treatment paradigm did not fit the research question of our meta-analysis, mostly due to sole enteral nutrition. In one case a full text of the article could not be retrieved (Additional file 2). In the end, 26 trials with a total number of 1733 patients were included [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Five studies were labeled as “level I” and 21 studies as “level II”. The median methodologic score was 9 (95% CI 7, 10) for all included studies, 10 (95% CI 9, 14) for “Level I” trials and 9 (95% CI 6, 9) for “Level II” trials (Additional file 3). Eggers' test did not indicate the presence of a funnel plot asymmetry for our primary outcome (p = 0.77).
Omega-6 fatty acid reducing strategies
There was no effect of omega-6 FA sparing PN in comparison to LCT or LCT/MCT on overall mortality (20 studies, n = 1366; RR 0.91; 95% CI 0.76, 1.10; p = 0.34; I2 = 0%; Fig. 2). However, we observed a trend towards lower 28-day mortality (8 studies, n = 733; RR 0.79; 95% CI 0.61, 1.02; p = 0.07; I2 = 0%; Additional file 4) and shorter ICU LOS (12 studies, n = 825; WMD − 1.94; 95% CI − 4.41, 0.52; p = 0.12; I2 = 83%; Fig. 3).
Compared to LCT or LCT/MCT, omega-6 FA sparing strategies significantly reduced the hospital LOS (6 studies, n = 390; WMD − 6.88; 95% CI − 11.27, − 2.49; p = 0.002; I2 = 20%; Fig. 4) and tended toward a shorter length of MV (9 studies, n = 511; WMD − 0.87; 95% CI − 1.82, 0.07; p = 0.07; I2 = 52%; Additional file 5). No benefits on the development of infectious complications were observed (7 studies, n = 721; RR 0.94; 95% CI 0.7, 1.26; p = 0.68; I2 = 32%; Fig. 5).
Subgroup analysis of omega-6 fatty acid reducing strategies
Omega-6 FA reducing strategies were further analysed in three subgroups, SO/MCT, SO/OO and FO containing PN. None of the strategies affected overall mortality in comparison with LCT or LCT/MCT (test for subgroup differences, p = 0.99; Fig. 2). For FO containing PN, aggregated data suggested a trend toward reduced 28-day mortality (7 studies, n = 529; RR 0.74; 95% CI 0.54, 1.01; p = 0.06; I2 = 0%; Additional file 4). While SO/MCT and SO/OO did not affect the ICU LOS, FO containing PN significantly reduced the duration of intensive care (8 studies, n = 611; WMD − 3.53; 95% CI − 6.16, − 0.90; p = 0.009; I2 = 82%; test for subgroup differences, p = 0.08; Fig. 3). We also observed a trend towards shorter hospital LOS for FO containing PN (4 studies, n = 268; WMD − 5.93; 95% CI − 13.13, 1.27; p = 0.11; I2 = 51%; Fig. 4). In terms of MV, a reduction was reported for SO/MCT compared to SO (2 studies, n = 34; WMD − 3.30; 95% CI − 5.39, − 1.21; p = 0.002; I2 = 0%). Data on MV were furthermore reported in one trial with a SO/OO strategy and in six studies investigating FO containing PN, however, without clear benefits (test for subgroup differences, p = 0.03; Additional file 5). While SO/OO strategies were potentially associated with a trend toward more infectious complications (3 studies, n = 326; RR 1.23; 95% CI 0.92, 1.63; p = 0.16; I2 = 0%), FO containing PN significantly decreased nosocomial infections (4 studies, n = 395; RR 0.65; 95% CI 0.44, 0.95; p = 0.03; I2 = 0%; test for subgroup differences, p = 0.008; Fig. 5).
Among FO-containing PN, we further identified two strategies based on the usage of Omegaven and non-Omegaven lipid emulsions. We observed an overall mortality benefit for Omegaven (6 studies, n = 433; RR 0.68; 95% CI 0.48, 0.95; p = 0.03; I2 = 0%), which was not present in trials using other FO emulsions (test for subgroup differences, p = 0.02; Additional file 6). There were no clear signals regarding other clinical outcomes neither for Omegaven nor other FO lipid emulsions.
Fish oil as a stand-alone intervention
Five studies in patients on standard care nutrition compared a stand-alone FO intervention versus no additional supply of lipids. FO tended toward an improvement in overall mortality (4 studies, n = 287; RR 0.76; 95% CI 0.53, 1.10; p = 0.14; I2 = 0%) and significantly reduced 28-day mortality (3 studies, n = 237; RR 0.60; 95% CI 0.36, 0.99; p = 0.04; I2 = 0%; Fig. 6). Stand-alone FO was not associated with a shorter duration of intensive care (4 studies, n = 264; WMD − 1.38; 95% CI − 4.11, 1.34; p = 0.32; I2 = 52%) or hospital stay (3 studies, n = 148; WMD 0.78; 95% CI − 2.89, 4.46; p = 0.68; I2 = 0%). There was no aggregated data on MV and infectious complications available.
Discussion
Overall, our systematic review and meta-analysis covered 26 trials in critically ill patients, of which 16 studies with 1046 patients were newly included in comparison with the previous meta-analysis by Manzanares et al. [13]. We hereby provide an update and additional insights not only on FO but also on general omega-6 FA reducing strategies in intensive care. Omega-6 sparing effects included a significant decrease in hospital LOS and trends towards reduction in 28-day mortality, ICU LOS and mechanical ventilation. Among different omega-6 FA reducing PN regimens, FO containing lipid emulsions reduced the length of intensive care and rate of nosocomial infections. Potential signals on mortality rates were observed with stand-alone use of FO, which encourages further research in this area.
In the past decades, clinical and technological advances promoting physiologic and metabolic resuscitation as well as early diagnosis and optimal treatment have significantly decreased hospital mortality in critical illness. In this context, nutritionally derived compounds exhibiting pharmacological effects contribute to an improved clinical outcome [46]. However, due to low quality of evidence, the potential benefits of anti-inflammatory and immunomodulating effects in critically ill patients remain unproven [47]. While a balance between pro- and anti-inflammatory response mechanisms is crucial for an adequate immune response, critically ill patients often exhibit immune dysregulation [48,49,50]. Imbalance arises from either excessive production of pro-inflammatory cytokines and reactive oxygen species or a lack of their physiological anti-inflammatory and anti-oxidative counterparts. A similar concept applies for omega-6 and omega-3 FAs or rather their metabolites. By reducing prostaglandins, leukotrienes and thromboxanes via MCT, OO or FO on the one hand and increasing DHA and EPA levels by FO on the other, pharmaconutrition might play a role in regaining inflammatory balance in critically ill patients. This basic concept is tempting, even though clinical evidence is scarce and ambiguous. There are—if at all—only minor effects of sole omega-6 FA reduction on inflammatory parameters and markers of oxidative stress [42, 51, 52], which is in line with our results, where the impact of SO/MCT and SO/OO on the clinical course was rather small. However, an RCT, in which OO was significantly associated with reduced ICU LOS and MV, had to be excluded, due to an unusual lipid- versus glucose-based treatment regimen [53]. In the case of FO, omega-6 sparing effects are complemented by a simultaneous increase in omega-3 FAs, which may be even more beneficial for inflammatory control.
In fact, FO has been studied for decades, after realizing that Inuits in Greenland with a fish-based diet had low rates of cardiovascular events [54]. This led to general recommendations for FO supplements in patients with coronary heart disease [55] and multiple promising investigations in autoimmune and inflammatory disorders [56,57,58]. Intravenous FO administration immediately affects physiological parameters, which was demonstrated by a significant increase in DHA and EPA concentrations in blood cell phospholipids within 60 min after infusion [59]. The rapid incorporation into various cellular membranes is crucial for an acute modulation of the immune response and suppression of pro-inflammatory cytokine release [60,61,62]. In addition, it has been recently discovered that DHA- and EPA-derived metabolites like resolvins, protectins, and maresins are required for the resolution of inflammation in the post-acute phase [7], as they regulate neutrophil recruitment and initiate macrophages to clear apoptotic cells and microorganisms [63].
Despite significant bias risk and rather small patient populations in the underlying studies [64,65,66,67], ESPEN guidelines state that parenteral FO can be provided for critically ill patients, as it likely decreases LOS and infections [16]. These conclusions align nicely with our results, including potential benefits on 28-day mortality and duration of MV. Hence, omega-6 FA reduction seems to hasten the recovery of patients requiring PN, which might justify the higher cost of these lipid emulsions in an economic evaluation. Pradelli et al. have recently conducted a systematic review and cost-effectiveness analysis on the use of omega-3 containing parenteral nutrition in ICU patients, which clearly demonstrated considerable cost savings due to a significant reduction in the risk of infections, and length of hospital or ICU stay. One main difference to our meta-analysis is that the authors included surgical patients without critical illness, for example postoperative surveillance after elective and cancer surgery [14]. Patterns of inflammation and the extent of organ injury might be very different in surgical patients with a defined tissue trauma, compared to systemic inflammation due to infection and sepsis. Our approach potentially provides a more homogenous collective of critically ill patients with a strong focus on sepsis and acute respiratory distress syndrome as well as additional insights into further omega-6 FA reducing strategies beyond FO. In comparison with Manzanares et al., we also excluded enteral nutrition protocols to further reduce heterogeneity [13]. Despite the distinctive differences between the meta-analyses, it is reassuring, that the results compare well.
We observe a trend towards improved 28-day survival, both for overall omega-6 FA reducing strategies as well as for FO containing PN alone. However, this potential signal could not be confirmed in terms of overall mortality. It has to be said, that multiple other factors and treatments beyond PN influence survival in critically ill patients, so that it is often difficult to interpret observed mortality benefits of a single intervention in modern ICU settings [68, 69]. In this light, the significant mortality benefits suggested for Omegaven and FO as a stand-alone medication have to be considered carefully.
We acknowledge several limitations of our meta-analysis. First, only five trials fulfilled the criteria of a “level I” study, which are concealed randomization, blinded outcome adjudication and intention-to-treat analysis. The median methodological score of all included studies was 9 out of 14 points, highlighting a considerable bias risk and mirroring the mediocre quality of most trials in the field of immunonutrition. This is a well-known problem in ICU research, as authors are faced with challenges of resources, recruitment and early randomization [70]. Second, the number of included studies and overall sample sizes for certain endpoints are too small to draw strong conclusions. Patient populations are still rather heterogeneous, despite our efforts to reduce variety. Third, overall mortality is an unsharp endpoint. Aggregating as many trials as possible comes at the price of including the whole variety of reported mortalities. Four studies for example only reported “other mortality” with a range from 15 to 109 days. Last, all included studies predominantly focused on “hard outcomes” and neglected the patient centered perspective. This requires more attention in future research.
On the other hand, this systematic review also has distinctive strengths. In contrast to previous meta-analyses, which merely focused on FO, we integrated additional omega-6 FA reducing PN strategies like SO/MCT and SO/OO [13, 14]. We only included RCTs with a relative specific patient population of critically ill ICU patients to reduce heterogeneity. We were not limited to any language barriers so that all internationally available evidence could be considered. Published abstracts, which did not undergo peer-review, were considered for inclusion after careful assessment. This approach might potentially reduce publication bias, we do, however, acknowledge the ongoing controversy on this topic [71,72,73]. We report the largest and the most current aggregation of PN lipid trials conducted in the critical care setting. To guarantee the reproducibility of this systematic review and meta-analysis, study eligibility decisions are provided in detail and data were abstracted using two independent experienced reviewers and referees.
Conclusion
Taken together, the present meta-analysis in critically ill patients suggests omega-6 sparing effects attributed to parenteral immunonutrition. Among different lipid emulsions, clinical benefits were most pronounced for FO, either in combination with PN or as a stand-alone treatment.
Availability of data and materials
Data generated or analysed during this study are included in this published article and its Additional files. Further information is available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- FO:
-
Fish oil
- ICU:
-
Intensive care unit
- LCT:
-
Long-chain triglycerides
- LOS:
-
Length of stay
- MCT:
-
Medium-chain triglycerides
- MV:
-
Mechanical ventilation
- OO:
-
Olive oil
- PN:
-
Parenteral nutrition
- RCT:
-
Randomized controlled trial
- RR:
-
Risk ratio
- SD:
-
Standard deviation
- SO:
-
Soybean oil
- WMD:
-
Weighted mean difference
References
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet (London, England). 2018;392(10141):75–87.
Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19(4):327–41.
Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–43.
Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317(3):290–300.
de Carvalho C, Caramujo MJ. The various roles of fatty acids. Molecules. 2018;23(10):2583.
Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85(5):1171–84.
Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20(20):528.
Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009;24(4):487–99.
Calder PC. Intravenous lipid emulsions to deliver bioactive omega-3 fatty acids for improved patient outcomes. Mar Drugs. 2019;17(5):274.
Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467s-s1476.
Calder PC, Waitzberg DL, Klek S, Martindale RG. Lipids in parenteral nutrition: biological aspects. JPEN J Parenter Enteral Nutr. 2020;44(Suppl 1):S21–7.
Edmunds CE, Brody RA, Parrott JS, Stankorb SM, Heyland DK. The effects of different IV fat emulsions on clinical outcomes in critically ill patients. Crit Care Med. 2014;42(5):1168–77.
Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care (London, England). 2015;19(1):167.
Pradelli L, Klek S, Mayer K, Omar Alsaleh AJ, Rosenthal MD, Heller AR, et al. Omega-3 fatty acid-containing parenteral nutrition in ICU patients: systematic review with meta-analysis and cost-effectiveness analysis. Crit Care (London, England). 2020;24(1):634.
Calder PC, Adolph M, Deutz NE, Grau T, Innes JK, Klek S, et al. Lipids in the intensive care unit: recommendations from the ESPEN Expert Group. Clin Nutr (Edinburgh, Scotland). 2018;37(1):1–18.
Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr (Edinburgh, Scotland). 2019;38(1):48–79.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA. 1998;280(23):2013–9.
Wischmeyer PE, Dhaliwal R, McCall M, Ziegler TR, Heyland DK. Parenteral glutamine supplementation in critical illness: a systematic review. Crit Care (London, England). 2014;18(2):R76.
Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care (London, England). 2010;14(1):R5.
Burkhart CS, Dell-Kuster S, Siegemund M, Pargger H, Marsch S, Strebel SP, et al. Effect of n-3 fatty acids on markers of brain injury and incidence of sepsis-associated delirium in septic patients. Acta Anaesthesiol Scand. 2014;58(6):689–700.
Chen H, Wang W, Hong C, Zhang M, Hong Y, Wang S, et al. Omega-3 fish oil reduces mortality due to severe sepsis with acute gastrointestinal injury grade III. Pharmacogn Mag. 2017;13(51):407–12.
Chen H, Wang W, Hong Y, Zhang H, Hong C, Liu X. Single-blinded, randomized, and controlled clinical trial evaluating the effects of Omega-3 fatty acids among septic patients with intestinal dysfunction: a pilot study. Exp Ther Med. 2017;14(2):1505–11.
Donoghue V, Schleicher GK, Spruyt MGL, Malan L, Nel DG, Calder PC, et al. Four-oil intravenous lipid emulsion effect on plasma fatty acid composition, inflammatory markers and clinical outcomes in acutely ill patients: a randomised control trial (Foil fact). Clin Nutr (Edinburgh, Scotland). 2019;38(6):2583–91.
Friesecke S, Lotze C, Köhler J, Heinrich A, Felix SB, Abel P. Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomised controlled trial. Intensive Care Med. 2008;34(8):1411–20.
García-de-Lorenzo A, Denia R, Atlan P, Martinez-Ratero S, Le Brun A, Evard D, et al. Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: a randomised double-blind study of an olive oil-based lipid emulsion v. medium/long-chain triacylglycerols. Br J Nutr. 2005;94(2):221–30.
Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Garcia-Garmendia JL, Jiménez-Jiménez LM, Garnacho-Montero MC, et al. Clinical and metabolic effects of two lipid emulsions on the parenteral nutrition of septic patients. Nutrition. 2002;18(2):134–8.
Grau-Carmona T, Bonet-Saris A, García-de-Lorenzo A, Sánchez-Alvarez C, Rodríguez-Pozo A, Acosta-Escribano J, et al. Influence of n-3 polyunsaturated fatty acids enriched lipid emulsions on nosocomial infections and clinical outcomes in critically ill patients: ICU lipids study. Crit Care Med. 2015;43(1):31–9.
Grecu I, Mirea L, Grintescu I. Parenteral fish oil supplementation in patients with abdominal sepsis. Clin Nutr. 2003;22:S23.
Gultekin G, Sahin H, Inanc N, Uyanik F, Ok E. Impact of Omega-3 and Omega-9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak J Med Sci. 2014;30(2):299–304.
Guo Y. ω-3 duo bu baohe zhifangsuan dui nong du zheng huanzhe linchuang liaoxiao ji yuhou de yingxiang (Effect of omega-3 polyunsaturated fatty acids on clinical efficacy and prognosis in patients with sepsis). Shanxi Med J. 2008;37(15):727–9.
Gupta A, Govil D, Bhatnagar S, Gupta S, Goyal J, Patel S, et al. Efficacy and safety of parenteral omega 3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med. 2011;15(2):108–13.
Hall TC, Bilku DK, Al-Leswas D, Neal CP, Horst C, Cooke J, et al. A randomized controlled trial investigating the effects of parenteral fish oil on survival outcomes in critically ill patients with sepsis: a pilot study. JPEN. 2015;39(3):301–12.
Iovinelli G, Marinangeli F, Ciccone A, Ciccozzi A, Leonardis M, Paladini A, et al. Parenteral nutrition in ventilated patients with chronic obstructive pulmonary disease: long chain vs medium chain triglycerides. Minerva Anestesiol. 2007;73(1–2):65–76.
Khor BS, Liaw SJ, Shih HC, Wang LS. Randomized, double blind, placebo-controlled trial of fish-oil-based lipid emulsion infusion for treatment of critically ill patients with severe sepsis. Asian J Surg. 2011;34(1):1–10.
Lindgren BF, Ruokonen E, Magnusson-Borg K, Takala J. Nitrogen sparing effect of structured triglycerides containing both medium-and long-chain fatty acids in critically ill patients; a double blind randomized controlled trial. Clin Nutr (Edinburgh, Scotland). 2001;20(1):43–8.
Nijveldt RJ, Tan AM, Prins HA, de Jong D, van Rij GL, Wesdorp RI, et al. Use of a mixture of medium-chain triglycerides and longchain triglycerides versus long-chain triglycerides in critically ill surgical patients: a randomized prospective double-blind study. Clin Nutr (Edinburgh, Scotland). 1998;17(1):23–9.
Pontes-Arruda A, Dos Santos MC, Martins LF, González ER, Kliger RG, Maia M, et al. Influence of parenteral nutrition delivery system on the development of bloodstream infections in critically ill patients: an international, multicenter, prospective, open-label, controlled study–EPICOS study. JPEN. 2012;36(5):574–86.
Qu A, Xu L. Yuyou zhifang ru dui nong du zheng huanzhe mianyi gongneng de tiaojie zuoyong guancha (Regulation of fish oil fat emulsion on immune function in patients with sepsis). Shandong Med J. 2009;49(6):13–5.
Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects of an omega-3 fatty acid-enriched lipid emulsion on eicosanoid synthesis in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Nutr Metab (Lond). 2011;8(1):22.
Singer P, Bendavid I, Mesilati-Stahy R, Green P, Rigler M, Lev S, et al. Enteral and supplemental parenteral nutrition enriched with omega-3 polyunsaturated fatty acids in intensive care patients—a randomized, controlled, double-blind clinical trial. Clin Nutr (Edinburgh, Scotland). 2021;40(5):2544–54.
Umpierrez GE, Spiegelman R, Zhao V, Smiley DD, Pinzon I, Griffith DP, et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med. 2012;40(6):1792–8.
Wang X, Li W, Zhang F, Pan L, Li N, Li J. Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: a randomized controlled trial. Inflammation. 2009;32(5):304–9.
Wang X, Tong F, Chen H, Huo S, Liu F, Liu Z, et al. Clinical effect of ω-3 polyunsaturated fatty acid on patients with abdominal sepsis. Clin Focus. 2014;29(5):502–8.
Zhao K, Zhou W, Bo C. ω-3 yuyou zhifang ru dui nong du zheng huanzhe de linchuang liaoxiao (Clinical efficacy of omega-3 fish oil fat emulsion in patients with sepsis). Shandong Med J. 2011;51(16):102.
Gajic O, Ahmad SR, Wilson ME, Kaufman DA. Outcomes of critical illness: what is meaningful? Curr Opin Crit Care. 2018;24(5):394–400.
Dushianthan A, Cusack R, Burgess VA, Grocott MP, Calder P. Immunonutrition for adults with ARDS: results from a cochrane systematic review and meta-analysis. Respir Care. 2020;65(1):99–110.
Duggal NA, Snelson C, Shaheen U, Pearce V, Lord JM. Innate and adaptive immune dysregulation in critically ill ICU patients. Sci Rep. 2018;8(1):10186.
van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–20.
Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045.
Siqueira J, Smiley D, Newton C, Le NA, Gosmanov AR, Spiegelman R, et al. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab. 2011;96(10):3207–16.
Jia ZY, Yang J, Xia Y, Tong DN, Zaloga GP, Qin HL. Safety and efficacy of an olive oil-based triple-chamber bag for parenteral nutrition: a prospective, randomized, multi-center clinical trial in China. Nutr J. 2015;14:119.
Huschak G, Zur Nieden K, Hoell T, Riemann D, Mast H, Stuttmann R. Olive oil based nutrition in multiple trauma patients: a pilot study. Intensive Care Med. 2005;31(9):1202–8.
Bang HO, Dyerberg J, Hjøorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200(1–2):69–73.
Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135(15):e867–84.
AlAmmar WA, Albeesh FH, Ibrahim LM, Algindan YY, Yamani LZ, Khattab RY. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: a systematic review. Nutr Neurosci. 2021;24(7):569–79.
Ajabnoor SM, Thorpe G, Abdelhamid A, Hooper L. Long-term effects of increasing omega-3, omega-6 and total polyunsaturated fats on inflammatory bowel disease and markers of inflammation: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. 2021;60(5):2293–316.
Abdulrazaq M, Innes JK, Calder PC. Effect of ω-3 polyunsaturated fatty acids on arthritic pain: a systematic review. Nutrition. 2017;39–40:57–66.
Carpentier YA, Hacquebard M, Portois L, Dupont IE, Deckelbaum RJ, Malaisse WJ. Rapid cellular enrichment of eicosapentaenoate after a single intravenous injection of a novel medium-chain triacylglycerol:fish-oil emulsion in humans. Am J Clin Nutr. 2010;91(4):875–82.
Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, et al. Short-time infusion of fish oil-based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol (Baltimore, Md : 1950). 2003;171(9):4837–43.
Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, et al. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14(15):2434–9.
Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr. 2002;87(Suppl 1):S89-94.
Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61.
Palmer AJ, Ho CK, Ajibola O, Avenell A. The role of ω-3 fatty acid supplemented parenteral nutrition in critical illness in adults: a systematic review and meta-analysis. Crit Care Med. 2013;41(1):307–16.
Pradelli L, Mayer K, Muscaritoli M, Heller AR. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care (London, England). 2012;16(5):R184.
Chen W, Jiang H, Zhou ZY, Tao YX, Cai B, Liu J, et al. Is omega-3 fatty acids enriched nutrition support safe for critical ill patients? A systematic review and meta-analysis. Nutrients. 2014;6(6):2148–64.
Zhu D, Zhang Y, Li S, Gan L, Feng H, Nie W. Enteral omega-3 fatty acid supplementation in adult patients with acute respiratory distress syndrome: a systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40(4):504–12.
Landoni G, Comis M, Conte M, Finco G, Mucchetti M, Paternoster G, et al. Mortality in multicenter critical care trials: an analysis of interventions with a significant effect. Crit Care Med. 2015;43(8):1559–68.
Ospina-Tascón GA, Büchele GL, Vincent JL. Multicenter, randomized, controlled trials evaluating mortality in intensive care: doomed to fail? Crit Care Med. 2008;36(4):1311–22.
Pattison N, Arulkumaran N, Humphreys S, Walsh T. Exploring obstacles to critical care trials in the UK: a qualitative investigation. J Intensive Care Soc. 2017;18(1):36–46.
Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7(1):1–76.
Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;2:Mr000010.
Korevaar DA, Salameh JP, Vali Y, Cohen JF, McInnes MDF, Spijker R, et al. Searching practices and inclusion of unpublished studies in systematic reviews of diagnostic accuracy. Res Synth Methods. 2020;11(3):343–53.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by QN, ZYL, PM, DKH, CS; methodology was contributed by QN, ZYL, JM, DKH, CS; acquisition and analysis were contributed by QN, ZYL, JM, DKH, CS; interpretation of data was contributed by QN, ZYL, GE, AH, PM, DKH, CS; writing—original draft, was contributed by QN, DKH, CS; writing—review, was contributed by ZYL, JM, GE, AH, PK, DR, CL, PM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Methodological quality scoring system
Additional file 2
. List of excluded studies
Additional file 3
. List of included studies, outcomes and bias risk assessment
Additional file 4
. 28-day mortality in trials using an omega-6 fatty acid reducing strategy
Additional file 5
. Length of mechanical ventilation in trials using an omega-6 fatty acid reducing strategy
Additional file 6
. Overall mortality in trials using either Omegaven or other fish oil lipid emulsions
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Notz, Q., Lee, ZY., Menger, J. et al. Omega-6 sparing effects of parenteral lipid emulsions—an updated systematic review and meta-analysis on clinical outcomes in critically ill patients. Crit Care 26, 23 (2022). https://doi.org/10.1186/s13054-022-03896-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-03896-3