Abstract
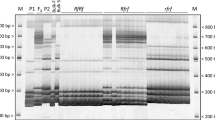
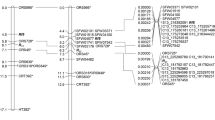
A nuclear male-sterile mutant, NMS 360, induced by streptomycin from an inbred maintainer line HA 89, possesses a single recessive gene, ms9, controlling male sterility. The present study identified DNA markers linked to the ms9 gene in an F2 population derived from the cross of NMS 360 × RHA 271 and maps the ms9 gene to an existing sunflower SSR linkage map. Bulked segregant analysis was performed using the target region amplification polymorphism (TRAP) marker technique and the simple sequence repeats (SSR) technique. From 444 primer combinations, six TRAP markers linked with the ms9 gene were amplified. Two markers, Ts4p03-202 and Tt3p09-529, cosegregated with the ms9 gene. The other four markers, To3d14-310, Tt3p17-390, Ts4p23-300, and Tt3p09-531, linked with ms9 at a distance of 1.2, 3.7, 10.3, and 22.3 cM, respectively. Thirty SSR primers from 17 linkage groups of a PHA × PHB cultivated sunflower linkage map were screened among the two parents and the F2 population. SSR primer ORS 705 of linkage group 10 was tightly linked to ms9 at a distance of 1.2 cM. The ms9 gene was subsequently mapped to linkage group 10 of the public sunflower SSR linkage map. The markers that were tightly linked with the ms9 gene will be useful in marker-assisted selection of male-sterile plants among segregating populations, and will facilitate the isolation of the ms9 gene by map-based cloning.


Similar content being viewed by others
References
Aarts MGM, Dirkse WG, Steikema WJ, Pereira A (1993) Transposon tagging of a male sterility gene in Arabidopsis. Nature 363:715–717
Albertsen MC, Trimnell MR, Fox TW (1993) Tagging, cloning and characterizing a male fertility gene in maize. Am J Bot 80(Suppl):16
Chen J, Ren Z, Kong X, Wu J, Zhou R, Jia J (2005) Isolation, characterization and expression analysis of male sterility gene homology sequence in wheat. Acta Genetica Sinica 32:566–570
Feng J, Vick BA, Lee MK, Zhang H, Jan CC (2006) Construction of BAC and BIBAC libraries from sunflower and identification of linkage group-specific clones by overgo hybridization. Theor Appl Genet (in press)
Gorman SW, Banasiak D, Fairley C, McCormick S (1996) A 610 kb YAC clone harbors 7 cM of tomato (Lycopersicon esculentum) DNA that includes the male sterile 14 gene and a hotspot for recombination. Mol Gen Genet 251:52–59
Gundaev AI (1971) Basic principles of sunflower selection. In: Genetic principles of plant selection. Nauka, Moscown, pp 417–465 (in Russian)
Hu J, Vick BA (2003) TRAP target region amplified polymorphism, a novel marker technique for plant genotyping. Plant Mol Biol Rep 21:289–294
Jan CC (1992) Inheritance and allelism of mitomycin C- and streptomycin-induced recessive genes for male sterility in cultivated sunflower. Crop Sci 32:317–320
Jan CC, Rutger JN (1988) Mitomycin C- and streptomycin-induced male sterility in cultivated sunflower. Crop Sci 28:792–795
Jin W, Palmer RG, Horner HT, Shoemaker RC (1998) Molecular mapping of a male-sterile gene in soybean. Crop Sci 38:1681–1685
Kaul MLH (1988) Male sterility in higher plants. Monographs on theoretical and applied genetics 10. Springer, Berlin Heidelberg New York
Koh HJ, Son YH, Heu MH, Lee HS, McCouch SR (1999) Molecular mapping of a new genic male-sterility gene causing chalky endosperm in rice (Oryza sativa L.). Euphytica 106:57–62
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugenet 12:172–175
Lander ES, Green P, Abrahamson J, Barlow H, Daly M, Lincoln S, Newbury L (1987) MAPMAKER: an interactive computer program for constructing genetic maps of experimental and natural populations. Genomics 1:174–175
Leclercq P (1969) Une stérilité mâle cytoplasmique chez le tournesol. Ann Amélior plantes 19:99–106
Liu Z, Anderson JA, Hu J, Friesen TL, Rasmussen JB, Faris JD (2005) A wheat intervarietal genetic linkage map based on microsatellite and target region amplified polymorphism markers and its utility for detecting quantitative trait loci. Theor Appl Genet 111:792–794
Martínez-Rivas JM, Sperling P, Lühs W, Heinz E (2001) Spatial and temporal regulation of three different microsomal oleate desaturase genes (FAD2) from normal-type and high-oleic varieties of sunflower (Helianthus annuus L.). Mol Breed 8:159–168
McCormick S (1993) Male gametophyte development. Plant Cell 5:1265–1275
Miao Y, Dreyer F, Cai A, Jung C (2003) Molecular markers for genic male sterility in Chinese cabbage. Euphytica 132:227–234
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Moffatt BA, Somerville C (1988) Positive selection for male-sterile mutants of Arabidopsis lacking adenine phosphoribosyl transferase activity. Plant Physiol 86:1150–1154
Muehlbauer GJ, Specht JE, Thomas-Comton MA, Staswick PE, Bernard RL (1988) Near-isogenic lines—a potential source in the integration of conventional and molecular marker linkage maps. Crop Sci 28:729–735
Pérez-Vich B, Berry ST, Velasco L, Fernández-Martínez JM, Gandhi S, Freeman C, Heesacker A, Knapp SJ, Leon AJ (2005) Molecular mapping of nuclear male sterility genes in sunflower. Crop Sci 54:1851–1857
Rubinelli P, Hu Y, Ma H (1998) Identification, sequence analysis and expression studies of novel anther-specific genes of Arabidopsis thaliana. Plant Mol Biol 37:607–619
Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97:10625–10630
Subudhi PK, Borkakati RP, Virmani SS, Huang N (1997) Molecular mapping of a thermo-sensitive genetic male sterility gene in rice using bulked segregant analysis. Genome 40:188–194
Tang S, Yu JK, Slabaugh MB, Shintani DK, Knapp SJ (2002) Simple sequence repeat map of the sunflower genome. Theor Appl Genet 105:1124–1136
Tanksley SD, Ganal MW, Martin GB (1995) Chromosome landing: a paradigm for map-based gene cloning in plants with large genomes. Trends Genet 11:63–68
Voorrips RE (2002) MapChart, software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Vos P, Hogers R, Bleeker M, Reijans M, Van der Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acids Res 23:4407–4414
Vranceanu V (1970) Advances in sunflower breeding in Romania. In: International Sunflower Association (eds) Proceedings of the 4th international sunflower conference, Memphis, TN, 23–25 Jun, pp 136–148
Wang B, Xu WW, Wang JZ, Wu W, Zheng HG, Yang ZY, Ray JD, Nguyen HT (1995) Tagging and mapping the thermo-sensitive genic male-sterile gene in rice (Oryza sativa L.) with molecular markers. Theor Appl Genet 91:1111–1114
Wang YG, Xing QH, Deng QY, Liang FS , Yuan LP, Weng ML, Wang B (2003) Fine mapping of the rice thermo-sensitive genic male-sterile gene tms5. Theor Appl Genet 107:917–921
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res 18:6231–6235
Yu JK, Tang S, Slabaugh MB, Heesacker A, Cole G, Herring M, Soper J, Han F, Chu WC, Webb DM, Thompson L, Edwards KJ, Berry ST, Leon AJ, Grondona M, Olungu C, Maes N, Knapp SJ (2003) Towards a saturated molecular genetic linkage map for cultivated sunflower. Crop Sci 43:367–387
Zhang Q, Shen BZ, Dai XK, Mei MH, Saghai Maroof MA, Li ZB (1994) Using bulked extremes and recessive class to map genes for photoperiod sensitive genic male sterility in rice. Proc Natl Acad Sci USA 91:8675–8679
Acknowledgments
The authors thank Angelia Hogness and Lisa Brown for their technical assistance. The authors also wish to thank L. G. Campbell and G. J. Seiler for thoughtful discussion and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Xu
Rights and permissions
About this article
Cite this article
Chen, J., Hu, J., Vick, B.A. et al. Molecular mapping of a nuclear male-sterility gene in sunflower (Helianthus annuus L.) using TRAP and SSR markers. Theor Appl Genet 113, 122–127 (2006). https://doi.org/10.1007/s00122-006-0278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-006-0278-2