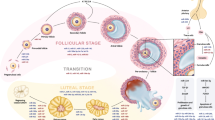
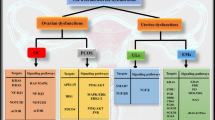
Abstract
MicroRNAs (miRNAs) are a class of short single-stranded, noncoding RNAs that affect the translation of mRNAs by imperfectly binding to homologous 3’UTRs. Research on miRNAs in ovarian diseases is constantly expanding because miRNAs are powerful regulators of gene expression and cellular processes and are promising biomarkers. miRNA mimics, miRNA inhibitors and molecules targeting miRNAs (antimiRs) have shown promise as novel therapeutic agents in preclinical development. Granulosa cells (GCs) are supporting cells for developing oocytes in the ovary. GCs regulate female reproductive health by producing sex hormones and LH receptors. Increasing research has reported the relevance of miRNAs in GC pathophysiology. With in-depth studies of disease mechanisms, there are an increasing number of studies on the biomolecular pathways of miRNAs in gynecology and endocrinology. In the present review, we summarize the different functions of GC-related microRNAs in various ovarian disorders, such as polycystic ovary syndrome, premature ovarian insufficiency, premature ovarian failure and ovarian granulosa cell tumors.


Similar content being viewed by others
Data Availability
Not applicable.
References
Lai EC. Micro RNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30(4):363–4.
Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54.
Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–6.
Reinhart BJ, et al. MicroRNAs in plants. Genes Dev. 2002;16(13):1616–26.
Jones L. Revealing micro-RNAs in plants. Trends Plant Sci. 2002;7(11):473–5.
Libri V, et al. Regulation of microRNA biogenesis and turnover by animals and their viruses. Cell Mol Life Sci. 2013;70(19):3525–44.
Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8-13.
Saliminejad K, et al. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–65.
Suzuki HI. Roles of MicroRNAs in Disease Biology. JMA J. 2023;6(2):104–13.
Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–33.
Liang TS, et al. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38(1):97.
Razavi ZS, et al. Gynecologic cancers and non-coding RNAs: Epigenetic regulators with emerging roles. Crit Rev Oncol Hematol. 2021;157:103192.
Javdani H, et al. Review article epithelial to mesenchymal transition-associated microRNAs in breast cancer. Mol Biol Rep. 2022;49(10):9963–73.
Salinas-Vera YM et al. Three-dimensional organotypic cultures reshape the microRNAs transcriptional program in breast cancer cells. Cancers (Basel). 2022;14(10):2490
Moghbeli M. MicroRNAs as the critical regulators of Cisplatin resistance in ovarian cancer cells. J Ovarian Res. 2021;14(1):127.
Hossain MS, et al. MicroRNAs expression analysis shows key affirmation of Synaptopodin-2 as a novel prognostic and therapeutic biomarker for colorectal and cervical cancers. Heliyon. 2021;7(6):e07347.
Ajabnoor G, et al. Computational approaches for discovering significant microRNAs, microRNA-mRNA regulatory pathways, and therapeutic protein targets in endometrial cancer. Front Genet. 2022;13:1105173.
Lu J, et al. Expression of miR-26b in ovarian carcinoma tissues and its correlation with clinicopathology. Oncol Lett. 2019;17(5):4417–22.
Luo J, et al. Role of microRNA-133a in epithelial ovarian cancer pathogenesis and progression. Oncol Lett. 2014;7(4):1043–8.
Wang W, et al. Five serum microRNAs for detection and predicting of ovarian cancer. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100017.
Su L, Liu M. Correlation analysis on the expression levels of microRNA-23a and microRNA-23b and the incidence and prognosis of ovarian cancer. Oncol Lett. 2018;16(1):262–6.
Lv Y, et al. Roles of microRNAs in preeclampsia. J Cell Physiol. 2019;234(2):1052–61.
Hume L, et al. MicroRNAs emerging coordinate with placental mammals alter pathways in endometrial epithelia important for endometrial function. iScience. 2023;26(4):106339.
Bjorkman S, Taylor HS. MicroRNAs in endometriosis: biological function and emerging biomarker candidates†. Biol Reprod. 2019;100(5):1135–46.
Fernández-Pérez D, et al. MicroRNA dynamics at the onset of primordial germ and somatic cell sex differentiation during mouse embryonic gonad development. RNA. 2018;24(3):287–303.
Klinge CM. miRNAs and estrogen action. Trends Endocrinol Metab. 2012;23(5):223–33.
Piperigkou Z, et al. Estrogen receptor beta as epigenetic mediator of miR-10b and miR-145 in mammary cancer. Matrix Biol. 2017;64:94–111.
Zierau O, et al. Role of miR-203 in estrogen receptor-mediated signaling in the rat uterus and endometrial carcinoma. J Cell Biochem. 2018;119(7):5359–72.
Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228(1–2):67–78.
Deng Y, et al. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Mol Immunol. 2019;112:266–73.
Findlay JK, et al. Production and actions of inhibin and activin during folliculogenesis in the rat. Mol Cell Endocrinol. 2001;180(1–2):139–44.
Hsueh AJ, et al. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5(1):76–127.
Taghizabet N, et al. In vitro growth of the ovarian follicle: taking stock of advances in research. JBRA Assist Reprod. 2022;26(3):508–21.
Tu J, et al. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol (Lausanne). 2019;10:174.
Li Y, et al. MicroRNAs in ovarian function and disorders. J Ovarian Res. 2015;8:51.
McGinnis LK, Luense LJ, Christenson LK. MicroRNA in ovarian biology and disease. Cold Spring Harbor Perspect Med. 2015;5(9):a022962
Ilie IR, Georgescu CE. Polycystic ovary syndrome-epigenetic mechanisms and aberrant microRNA. Adv Clin Chem. 2015;71:25–45.
Guo Y, Sun J, Lai D. Role of microRNAs in premature ovarian insufficiency. Reprod Biol Endocrinol. 2017;15(1):38.
Nouri N, et al. Role of miRNAs interference on ovarian functions and premature ovarian failure. Cell Commun Signal. 2022;20(1):198.
Zhang B, et al. MicroRNA mediating networks in granulosa cells associated with ovarian follicular development. Biomed Res Int. 2017;2017:4585213.
Donadeu FX, Schauer SN, Sontakke SD. Involvement of miRNAs in ovarian follicular and luteal development. J Endocrinol. 2012;215(3):323–34.
Kim YJ, et al. MicroRNA profile of granulosa cells after ovarian stimulation differs according to maturity of retrieved oocytes. Geburtshilfe Frauenheilkd. 2016;76(6):704–8.
Maruo T, et al. Regulation of granulosa cell proliferation and apoptosis during follicular development. Gynecol Endocrinol. 1999;13(6):410–9.
Yao G, et al. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24(3):540–51.
Andreas E, et al. MicroRNA 17–92 cluster regulates proliferation and differentiation of bovine granulosa cells by targeting PTEN and BMPR2 genes. Cell Tissue Res. 2016;366(1):219–30.
Gebremedhn S, et al. MicroRNA-183-96-182 cluster regulates bovine granulosa cell proliferation and cell cycle transition by coordinately targeting FOXO1. Biol Reprod. 2016;94(6):127.
Pande HO, et al. MicroRNA-424/503 cluster members regulate bovine granulosa cell proliferation and cell cycle progression by targeting SMAD7 gene through activin signalling pathway. J Ovarian Res. 2018;11(1):34.
Yan G, et al. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett. 2012;586(19):3263–70.
Peng JY, et al. MicroRNA-10b suppresses goat granulosa cell proliferation by targeting brain-derived neurotropic factor. Domest Anim Endocrinol. 2016;54:60–7.
Tao H, et al. MicroRNA-27a-3p targeting Vangl1 and Vangl2 inhibits cell proliferation in mouse granulosa cells. Biochim Biophys Acta Gene Regul Mech. 2023;1866(1):194885.
Hilker RE, et al. MicroRNA-21 enhances estradiol production by inhibiting WT1 expression in granulosa cells. J Mol Endocrinol. 2021;68(1):11–22.
Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83(2):286–95.
Guo L, et al. microRNA-10b promotes the apoptosis of bovine ovarian granulosa cells by targeting plasminogen activator inhibitor-1. Theriogenology. 2021;176:206–16.
Bai Y et al. MicroRNA 195–5p targets Foxo3 promoter region to regulate its expression in granulosa cells. Int J Mol Sci. 2021;22(13):6721
Huo S, et al. MicroRNA 26a targets Ezh2 to regulate apoptosis in mouse ovarian granulosa cells. Syst Biol Reprod Med. 2021;67(3):221–9.
Zhou R, et al. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene. Theriogenology. 2019;127:66–71.
Zhang P, et al. MicroRNA-205 affects mouse granulosa cell apoptosis and estradiol synthesis by targeting CREB1. J Cell Biochem. 2019;120(5):8466–74.
Yao Y, et al. microRNA-125b regulates apoptosis by targeting bone morphogenetic protein receptor 1B in yak granulosa cells. DNA Cell Biol. 2018;37(11):878–87.
Xu L, et al. MicroRNA-145 protects follicular granulosa cells against oxidative stress-induced apoptosis by targeting Krüppel-like factor 4. Mol Cell Endocrinol. 2017;452:138–47.
Zhou J, et al. MicroRNA let-7g regulates mouse granulosa cell autophagy by targeting insulin-like growth factor 1 receptor. Int J Biochem Cell Biol. 2016;78:130–40.
Zhou J, et al. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-β type 1 receptor. Mol Cell Endocrinol. 2015;409:103–12.
Wang L, et al. MicroRNA-764-3p regulates 17β-estradiol synthesis of mouse ovarian granulosa cells by targeting steroidogenic factor-1. In Vitro Cell Dev Biol Anim. 2016;52(3):365–73.
Grossman H, et al. A novel regulatory pathway in granulosa cells, the LH/human chorionic gonadotropin-microRNA-125a-3p-Fyn pathway, is required for ovulation. Faseb j. 2015;29(8):3206–16.
Iwamune M, et al. MicroRNA-376a regulates 78-kilodalton glucose-regulated protein expression in rat granulosa cells. PLoS ONE. 2014;9(10):e108997.
Toms D, et al. Progesterone receptor expression in granulosa cells is suppressed by microRNA-378-3p. Mol Cell Endocrinol. 2015;399:95–102.
Troppmann B, et al. MicroRNA miR-513a-3p acts as a co-regulator of luteinizing hormone/chorionic gonadotropin receptor gene expression in human granulosa cells. Mol Cell Endocrinol. 2014;390(1–2):65–72.
Yao G, et al. MicroRNA-224 is involved in the regulation of mouse cumulus expansion by targeting Ptx3. Mol Cell Endocrinol. 2014;382(1):244–53.
Liang M, et al. Transcriptional cooperation between p53 and NF-κB p65 regulates microRNA-224 transcription in mouse ovarian granulosa cells. Mol Cell Endocrinol. 2013;370(1–2):119–29.
Gebremedhn S et al. Dynamics of extracellular vesicle-coupled microRNAs in equine follicular fluid associated with follicle selection and ovulation. Mol Hum Reprod. 2023;29(4):gaad009
Liu J, et al. MicroRNA-26b functions as a proapoptotic factor in porcine follicular Granulosa cells by targeting Sma-and Mad-related protein 4. Biol Reprod. 2014;91(6):146.
Zhou J, Peng X, Mei S. Autophagy in ovarian follicular development and atresia. Int J Biol Sci. 2019;15(4):726–37.
D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–92.
Bhardwaj JK, et al. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. J Cell Physiol. 2022;237(2):1157–70.
Zhang Y, et al. Autophagy-related lncRNAs in tumor progression and drug resistance: A double-edged sword. Genes Dis. 2024;11(1):367–81.
Hennebold JD. Preventing granulosa cell apoptosis through the action of a single microRNA. Biol Reprod. 2010;83(2):165–7.
Toms D, Pan B, Li J. endocrine regulation in the ovary by MicroRNA during the estrous cycle. Front Endocrinol (Lausanne). 2017;8:378.
Sirotkin AV, et al. Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. Microrna. 2014;3(1):29–36.
Fabová Z, et al. Involvement of microRNA miR-125b in the control of porcine ovarian cell functions. Gen Comp Endocrinol. 2023;334:114215.
Fabová Z, Loncová B, Sirotkin AV. MicroRNA miR-125b can suppress ovarian granulosa cell functions: Interrelationships with FSH. Cell Biochem Funct. 2023;41(2):177–88.
Pan B, Zhan X, Li J. MicroRNA-574 impacts granulosa cell estradiol production via targeting TIMP3 and ERK1/2 signaling pathway. Front Endocrinol (Lausanne). 2022;13:852127.
Li L, et al. Taurine promotes estrogen synthesis by regulating microRNA-7a2 in mice ovarian granulosa cells. Biochem Biophys Res Commun. 2022;626:129–34.
Dai A, et al. MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett. 2013;587(15):2474–82.
Fiedler SD, et al. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod. 2008;79(6):1030–7.
Yao N, et al. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine. 2010;38(2):158–66.
Xu Y, et al. TGF-β1 resulting in differential microRNA expression in bovine granulosa cells. Gene. 2018;663:88–100.
Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7.
Zeng X, et al. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21.
Khan SH, et al. Dehydroepiandrosterone Sulfate (DHEAS) Levels in Polycystic Ovarian Syndrome (PCOS). J Coll Physicians Surg Pak. 2021;31(3):253–7.
Garg D, Tal R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod Biomed Online. 2016;33(1):15–28.
Li Y, et al. Effect of luteinizing hormone vs follicular stimulating hormone ratio on anti-Müllerian hormone secretion and folliculogenesis in patients with polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi. 2010;45(8):567–70.
Adone A, Fulmali DG. Polycystic Ovarian Syndrome in Adolescents. Cureus. 2023;15(1):e34183.
Chang RJ, Cook-Andersen H. Disordered follicle development. Mol Cell Endocrinol. 2013;373(1–2):51–60.
Motahari Rad H, et al. Characterization of altered microRNAs related to different phenotypes of polycystic ovarian syndrome (PCOS) in serum, follicular fluid, and cumulus cells. Taiwan J Obstet Gynecol. 2022;61(5):768–79.
Xu C, et al. MicroRNA-1298-5p in granulosa cells facilitates cell autophagy in polycystic ovary syndrome by suppressing glutathione-disulfide reductase. Cell Tissue Res. 2023;392(3):763–78.
Shen X, Gong A. The expression of microRNA-197-3p regulates the proliferation of ovarian granulosa cells through CUL3 in polycystic ovarian syndrome. Acta Biochim Pol. 2022;69(3):599–604.
Guo Y, et al. Long non-coding RNA-X-inactive specific transcript inhibits cell viability, and induces apoptosis through the microRNA-30c-5p/Bcl2-like protein 11 signaling axis in human granulosa-like tumor cells. Bioengineered. 2022;13(6):14107–17.
Li X, Zhu L, Luo Y. Long non-coding RNA HLA-F antisense RNA 1 inhibits the maturation of microRNA-613 in polycystic ovary syndrome to promote ovarian granulosa cell proliferation and inhibit cell apoptosis. Bioengineered. 2022;13(5):12289–97.
Xu X, et al. MicroRNA let-7i inhibits granulosa-luteal cell proliferation and oestradiol biosynthesis by directly targeting IMP2. Reprod Biomed Online. 2022;44(5):803–16.
Wan T, et al. Vitamin D deficiency inhibits microRNA-196b-5p which regulates ovarian granulosa cell hormone synthesis, proliferation, and apoptosis by targeting RDX and LRRC17. Ann Transl Med. 2021;9(24):1775.
Wang W et al. MicroRNA-16 represses granulosa cell proliferation in polycystic ovarian syndrome through inhibition of the PI3K/Akt pathway by downregulation of Apelin13. Hum Fertil (Camb). 2023;26(3):611-21
Fu X, et al. MicroRNA-16 promotes ovarian granulosa cell proliferation and suppresses apoptosis through targeting PDCD4 in polycystic ovarian syndrome. Cell Physiol Biochem. 2018;48(2):670–82.
Wu YX, et al. microRNA-194 is increased in polycystic ovary syndrome granulosa cell and induce KGN cells apoptosis by direct targeting heparin-binding EGF-like growth factor. Reprod Biol Endocrinol. 2021;19(1):170.
Yu Y, et al. MicroRNA-21 regulate the cell apoptosis and cell proliferation of polycystic ovary syndrome (PCOS) granulosa cells through target toll like receptor TLR8. Bioengineered. 2021;12(1):5789–96.
Aldakheel FM, et al. MicroRNA-21 inhibits ovarian granulosa cell proliferation by targeting SNHG7 in premature ovarian failure with polycystic ovary syndrome. J Reprod Immunol. 2021;146:103328.
Yang T, et al. MicroRNA-451a plays a role in polycystic ovary syndrome by regulating ovarian granulosa cell proliferation and apoptosis. Exp Ther Med. 2021;21(6):583.
Wei Y, et al. MicroRNA-874-3p promotes testosterone-induced granulosa cell apoptosis by suppressing HDAC1-mediated p53 deacetylation. Exp Ther Med. 2021;21(4):359.
Wei Y, et al. MicroRNA-135a regulates VEGFC expression and promotes luteinized granulosa cell apoptosis in polycystic ovary syndrome. Reprod Sci. 2020;27(7):1436–42.
Jiang B, et al. Down-regulated lncRNA HOTAIR alleviates polycystic ovaries syndrome in rats by reducing expression of insulin-like growth factor 1 via microRNA-130a. J Cell Mol Med. 2020;24(1):451–64.
Han XM, Tian PY, Zhang JL. MicroRNA-486-5p inhibits ovarian granulosa cell proliferation and participates in the development of PCOS via targeting MST4. Eur Rev Med Pharmacol Sci. 2019;23(17):7217–23.
Deng J, et al. MicroRNA-125b controls growth of ovarian granulosa cells in polycystic ovarian syndrome by modulating cyclin B1 expression. Arch Med Sci. 2022;18(3):746–52.
Sen A, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A. 2014;111(8):3008–13.
Zhong Z, et al. Inhibition of microRNA-19b promotes ovarian granulosa cell proliferation by targeting IGF-1 in polycystic ovary syndrome. Mol Med Rep. 2018;17(4):4889–98.
Li D, et al. MicroRNA-141-3p targets DAPK1 and inhibits apoptosis in rat ovarian granulosa cells. Cell Biochem Funct. 2017;35(4):197–201.
Cai G, et al. MicroRNA-145 negatively regulates cell proliferation through targeting irs1 in isolated ovarian granulosa cells from patients with polycystic ovary syndrome. Reprod Sci. 2017;24(6):902–10.
Jiang L, et al. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2015;100(5):E729–38.
He T, et al. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod Biol Endocrinol. 2019;17(1):68.
Yin M, et al. Transactivation of micrornA-320 by microRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 proteins. J Biol Chem. 2014;289(26):18239–57.
Chen H, et al. Defective CFTR-regulated granulosa cell proliferation in polycystic ovarian syndrome. Reproduction. 2015;149(5):393–401.
Yu M, Liu J. MicroRNA-30d-5p promotes ovarian granulosa cell apoptosis by targeting Smad2. Exp Ther Med. 2020;19(1):53–60.
Das M, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3):881–7.
Almahbobi G, et al. Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol (Oxf). 1996;44(5):571–80.
Torrealday S, Kodaman P, Pal L. Premature Ovarian Insufficiency - an update on recent advances in understanding and management. F1000Res. 2017;6:2069.
Huhtaniemi I, et al. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol Metab. 2018;29(6):400–19.
Wesevich V, Kellen AN, Pal L. Recent advances in understanding primary ovarian insufficiency. F1000Res. 2020;9:F1000 Faculty Rev-1101
Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68(4):499–509.
McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–14.
Song J, et al. Exposure to multiple pyrethroid insecticides affects ovarian follicular development via modifying microRNA expression. Sci Total Environ. 2022;828:154384.
Zhang L, et al. Translation regulatory long non-coding RNA 1 (TRERNA1) sponges microRNA-23a to suppress granulosa cell apoptosis in premature ovarian failure. Bioengineered. 2022;13(2):2173–80.
Wang C, et al. MicroRNA-125a-5p induces mouse granulosa cell apoptosis by targeting signal transducer and activator of transcription 3. Menopause. 2016;23(1):100–7.
Chen X, et al. Downregulation of microRNA-146a inhibits ovarian granulosa cell apoptosis by simultaneously targeting interleukin-1 receptor-associated kinase and tumor necrosis factor receptor-associated factor 6. Mol Med Rep. 2015;12(4):5155–62.
Zhang C, et al. MicroRNA-181a promotes follicular granulosa cell apoptosis via sphingosine-1-phosphate receptor 1 expression downregulationdagger. Biol Reprod. 2019;101(5):975–85.
Gao T et al. MicroRNA-22–3p in human umbilical cord mesenchymal stem cell-secreted exosomes inhibits granulosa cell apoptosis by targeting KLF6 and ATF4-ATF3-CHOP pathway in POF mice. Apoptosis. 2023;28(7-8):997-1011.
Gao T, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles carrying microRNA-29a improves ovarian function of mice with primary ovarian insufficiency by targeting HMG-Box transcription factor/Wnt/β-catenin signaling. Dis Markers. 2022;2022:5045873.
Zhang Q, et al. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS ONE. 2013;8(3):e59667.
Zhang X, et al. MicroRNA-127-5p impairs function of granulosa cells via HMGB2 gene in premature ovarian insufficiency. J Cell Physiol. 2020;235(11):8826–38.
Dang Y, et al. MicroRNA-379-5p is associate with biochemical premature ovarian insufficiency through PARP1 and XRCC6. Cell Death Dis. 2018;9(2):106.
Koukourakis GV, et al. Granulosa cell tumor of the ovary: tumor review. Integr Cancer Ther. 2008;7(3):204–15.
Li X, et al. Adult-type granulosa cell tumor of the ovary. Am J Cancer Res. 2022;12(8):3495–511.
Baillard P, et al. Rare DICER1 and Absent FOXL2 mutations characterize ovarian juvenile granulosa cell tumors. Am J Surg Pathol. 2021;45(2):223–9.
Guerrieri C, Hudacko R, Anderson P. Composite FOXL2 mutation-positive adult granulosa cell tumor and serous borderline tumor of the ovary. Int J Gynecol Pathol. 2023;42(5):500-507.
Pierini S et al. Ovarian granulosa cell tumor characterization identifies FOXL2 as an immunotherapeutic target. JCI Insight. 2020;5(16):e136773.
Pilsworth JA, et al. Adult-type granulosa cell tumor of the ovary: a FOXL2-centric disease. J Pathol Clin Res. 2021;7(3):243–52.
Li J, et al. Progress in the management of ovarian granulosa cell tumor: A review. Acta Obstet Gynecol Scand. 2021;100(10):1771–8.
Rosario R, Blenkiron C, Shelling AN. Comparative study of microRNA regulation on FOXL2 between adult-type and juvenile-type granulosa cell tumours in vitro. Gynecol Oncol. 2013;129(1):209–15.
Cheng WT, et al. MicroRNA profiling of ovarian granulosa cell tumours reveals novel diagnostic and prognostic markers. Clin Epigenetics. 2017;9:72.
Tu J, et al. MicroRNA-10a promotes granulosa cells tumor development via PTEN-AKT/Wnt regulatory axis. Cell Death Dis. 2018;9(11):1076.
Brandmaier A, Hou SQ, Shen WH. Cell cycle control by PTEN. J Mol Biol. 2017;429(15):2265–77.
Laguë MN, et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29(11):2062–72.
Tu J, et al. microRNA-126 is a tumor suppressor of granulosa cell tumor mediated by its host gene EGFL7. Front Oncol. 2019;9:486.
Diez-Fraile A, et al. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil (Camb). 2014;17(2):90–8.
Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–50.
Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993;133(5):2204–12.
Kaneko T, et al. Effects of controlled ovarian hyperstimulation on oocyte quality in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet. 2000;17(10):580–5.
Funding
This work was supported by the Natural Science Foundation of China (82305011).
Author information
Authors and Affiliations
Contributions
SX and LS designed the research and were responsible for the project conception. SX drafted the manuscript, together with LS and XL. SX revised the manuscript together with JD and GY. All of the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Patient Consent for Publication
Not applicable.
Conflict of Interest
All the authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, S., Du, J., Yuan, G. et al. Granulosa Cells-Related MicroRNAs in Ovarian Diseases: Mechanism, Facts and Perspectives. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01523-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01523-w