Abstract
Background
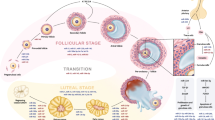
One of the main health issues that can affect women's health is reproductive diseases, such as polycystic ovary syndrome (PCOS), endometriosis (EMs), uterine leiomyomas (ULs), and ovarian cancer (OC). Although these diseases are very common, we do not have a complete understanding of their underlying cellular and molecular mechanisms. It is important to mention that the majority of patients are diagnosed with these diseases at later stages because of the absence of early diagnostic techniques and dependable molecular indicators. Hence, it is crucial to discover novel and non-invasive biomarkers that have prognostic, diagnostic and therapeutic capabilities. MiRNAs, also known as microRNAs, are small non-coding RNAs that play a crucial role in regulating gene expression at the post-transcriptional level. They are short in length, typically consisting of around 22 nucleotides, and are highly conserved across species. Numerous studies have shown that miRNAs are expressed differently in various diseases and can act as either oncogenes or tumor suppressors.
Methods
The author conducted a comprehensive review of all the pertinent papers available in web of science, PubMed, Google Scholar, and Scopus databases.
Results
We achieved three goals: providing readers with better information, enhancing search results, and making peer review easier.
Conclusions
This review focuses on the investigation of miRNAs and their involvement in various reproductive disorders in women, including their molecular targets. Additionally, it explores the role of miRNAs in the development and progression of these disorders.


Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- MiRNAs:
-
MicroRNAs
- PCOS:
-
Polycystic ovarian syndrome
- IR:
-
Insulin resistance
- KGN:
-
Human granulosa tumor cells
- CDKI:
-
Cyclin-dependent kinase inhibitor
- GC:
-
Granulosa cell
- PDCD4:
-
Programmed cell death protein 4
- TNF-α:
-
Tumor necrosis factor α
- ESR2:
-
Estrogen receptor 2
- CYP11A1:
-
Cytochrome P450 family 11A1
- OD:
-
Oocyte donor
- VDR:
-
Vitamin D receptors
- ET-1:
-
Endothelin-1
- Foxa1:
-
Forkhead box A1
- GLUT4:
-
Glucose transporter type 4
- IRS1:
-
Insulin receptor substrate 1
- IGF-1:
-
Insulin-like growth factor 1
- EMs:
-
Endometriosis
- ESCs:
-
Endometrial stromal cells
- TLR-4:
-
Toll-like receptor 4
- LAMC2:
-
Laminin-2
- TFAP2C:
-
Transcription factor AP-2
- UPK1B:
-
Uroplakin1B
- FRAS1:
-
Fraser syndrome 1
- COL3A:
-
Collagen type III A
- ECSCs:
-
Endometriotic cyst stromal cells
- SMARCD1:
-
Chromatin subfamily D member 1
- MMP1:
-
Matrix metallopeptidase 1
- MMP-2:
-
Matrix metalloproteinase-2
- MMP-9:
-
Matrix metalloproteinase-9
- SIRT1:
-
Sirtuin 1
- KLF-12:
-
Kruppel-like factor 12
- KLF4:
-
Krüppel-like factor 4
- ZEB1:
-
Zinc finger E-box binding homeobox 1
- ZEB2:
-
Zinc finger E-box binding homeobox 2
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- HOXA9:
-
Homeobox A9
- HOXA10:
-
Homeobox A10
- NTN4:
-
Netrin-4
- FGFR1:
-
Fibroblast growth factor receptor
- RTK:
-
Receptor tyrosine kinase
- PGR:
-
Progesterone receptor
- KLF9:
-
Krüppel-like factor 9
- ULs:
-
Uterine leiomyomas
- AUB:
-
Abnormal uterine bleeding
- ECM:
-
Extracellular matrix
- HMGA2:
-
High motility group A2
- OncomiR:
-
Oncogenic miRNA
- PKB:
-
Protease kinase B
- PAI-1:
-
Plasminogen activator inhibitor-1
- TF3:
-
Tissue factor 3
- CTGF:
-
Connective tissue growth factor
- IL-8:
-
Interleukin-8
- E2F1:
-
E2F transcription factor 1
- CCND1:
-
Cyclin D1
- IGFBP5:
-
Insulin-like growth factor binding proteins 5
- EMT:
-
Epithelial-mesenchymal transition
- LPCs:
-
Leiomyoma progenitor cells
- OC:
-
Ovarian cancer
- PARP:
-
Poly ADP-ribose polymerase
- PLK1:
-
Polo-like kinase-1
- RAD51AP1:
-
RAD51-associated protein 1 gene
- EAOC:
-
Endometriosis-associated ovarian cancer
- SOC:
-
Serous ovarian cancer
- OCCC:
-
Ovarian clear cell cancer
- ATG7:
-
Autophagy-related protein 7
- IGT:
-
Impaired glucose tolerance
- AR:
-
Antiandrogenic
- HEECs:
-
Human endometrial epithelial cells
- FOXP3:
-
Forkhead box P3
References
Wang J, Chen J, Sen S (2016) MicroRNA as biomarkers and diagnostics. J Cell Physiol 231(1):25–30
Friedman RC et al (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19(1):92–105
Berezikov E et al (2005) Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120(1):21–24
O’Brien J et al (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402
Nagaraja AK et al (2010) A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 24(2):447–463
Azimi T et al (2021) Pap Smear miR-92a-5p and miR-155-5p as potential diagnostic biomarkers of squamous intraepithelial cervical cancer. Asian Pac J Cancer Prev APJCP 22(4):1271
Mansouri S et al (2020) Alteration in expression of miR-32 and FBXW7 tumor suppressor in plasma samples of patients with T-cell acute lymphoblastic leukemia. Cancer Manag Res 12:1253–1259
Tafti A et al (2023) A systems biology approach and in vitro experiment indicated Rapamycin targets key cancer and cell cycle-related genes and miRNAs in triple-negative breast cancer cells. Mol Carcinog. https://doi.org/10.1002/mc.23628
Bagheri M, Sarabi PZ, Mondanizadeh M (2022) The role of miRNAs as a big master regulator of signaling pathways involved in lymphoblastic leukemia. J Cell Physiol 237(4):2128–2139
Mouillet JF et al (2015) MicroRNAs in placental health and disease. Am J Obstet Gynecol 213(4):S163–S172
Condrat CE et al (2020) miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells 9(2):276
Pan Q, Chegini N (2008) MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med. https://doi.org/10.1055/s-0028-1096128
Vannuccini S et al (2016) Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update 22(1):104–115
Gebremedhn S et al (2021) MicroRNA-mediated gene regulatory mechanisms in mammalian female reproductive health. Int J Mol Sci 22(2):938
Teng Y et al (2019) Effect of microRNA-409 on the pathogenesis of polycystic ovary syndrome. Eur Rev Med Pharmacol Sci 23(5):1874–1881
Ohlsson Teague EMC, Print CG, Hull ML (2010) The role of microRNAs in endometriosis and associated reproductive conditions. Human Reprod Update 16(2):142–165
Filigheddu N et al (2010) Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010:1–29
McWilliams MM, Chennathukuzhi VM (2017) Recent advances in uterine fibroid etiology. Semin Reprod Med 35:181
Zhang L et al (2008) Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci 105(19):7004–7009
Zaman MS et al (2012) Current status and implications of microRNAs in ovarian cancer diagnosis and therapy. J Ovarian Res 5(1):1–11
Hunter MP et al (2008) Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE 3(11):e3694
Azziz R (2016) Introduction: determinants of polycystic ovary syndrome. Fertil Steril 106(1):4–5
Liu AL et al (2016) New insights into mTOR signal pathways in ovarian-related diseases: polycystic ovary syndrome and ovarian cancer. Asian Pac J Cancer Prev APJCP 17(12):5087
Khan MJ, Ullah A, Basit S (2019) Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet 12:249
Yildiz BO (2015) Approach to the patient: contraception in women with polycystic ovary syndrome. J Clin Endocrinol Metab 100(3):794–802
Butler AE et al (2020) microRNA expression in women with and without polycystic ovarian syndrome matched for body mass index. Front Endocrinol 11:206
Long W et al (2014) Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell Physiol Biochem 33(5):1304–1315
Shen H et al (2017) Pathway and network-based analysis of genome-wide association studies and RT-PCR validation in polycystic ovary syndrome. Int J Mol Med 40(5):1385–1396
Huang X et al (2019) MiR-222 promotes the progression of polycystic ovary syndrome by targeting p27 Kip1. Pathol Res Pract 215(5):918–923
Lloyd RV et al (1999) p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154(2):313–323
Fu X et al (2018) MicroRNA-16 promotes ovarian granulosa cell proliferation and suppresses apoptosis through targeting PDCD4 in polycystic ovarian syndrome. Cell Physiol Biochem 48(2):670–682
Wang W et al (2023) MicroRNA-16 represses granulosa cell proliferation in polycystic ovarian syndrome through inhibition of the PI3K/Akt pathway by downregulation of Apelin13. Hum Fertil 26(3):611–621
Song Y et al (2019) Altered miR-186 and miR-135a contribute to granulosa cell dysfunction by targeting ESR2: a possible role in polycystic ovary syndrome. Mol Cell Endocrinol 494:110478
Chan MC et al (2010) Molecular basis for antagonism between PDGF and the TGFβ family of signalling pathways by control of miR-24 expression. EMBO J 29(3):559–573
Sørensen AE et al (2016) MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. J Clin Endocrinol Metab 101(4):1579–1589
Nanda D et al (2020) Evaluation of serum miRNA-24, miRNA-29a and miRNA-502-3p expression in PCOS subjects: correlation with biochemical parameters related to PCOS and insulin resistance. Indian J Clin Biochem 35(2):169–178
Kong F, Du C, Wang Y (2019) MicroRNA-9 affects isolated ovarian granulosa cells proliferation and apoptosis via targeting vitamin D receptor. Mol Cell Endocrinol 486:18–24
Rashad NM et al (2019) Association of miRNA−320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndrome. J Ovarian Res 12(1):1–10
Flammer J, Konieczka K (2015) Retinal venous pressure: the role of endothelin. EPMA J 6(1):1–12
Li C, Sun Y, Liu B (2003) The relationship between endothelin and tumor. Foreign Med Oncol Branch 30:360–362
Yuan W-N, Tan L (2017) MicroRNA-320 inhibits insulin resistance in patients with PCOS through regulating ERK1/2 signaling pathway. Biomed Res 28(11):4946–4949
Sang Q et al (2013) Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab 98(7):3068–3079
Cui X et al (2020) miR-132 is upregulated in polycystic ovarian syndrome and inhibits granulosa cells viability by targeting Foxa1. Mol Med Rep 22(6):5155–5162
Fu X et al (2019) FOXA1 upregulation promotes enhancer and transcriptional reprogramming in endocrine-resistant breast cancer. Proc Natl Acad Sci 116(52):26823–26834
Gou L, Zou H, Li B (2019) Long noncoding RNA MALAT1 knockdown inhibits progression of anaplastic thyroid carcinoma by regulating miR-200a-3p/FOXA1. Cancer Biol Ther 20(11):1355–1365
Chen YH et al (2013) miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62(7):2278–2286
Shepherd PR, Kahn BB (1999) Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med 341(4):248–257
Cai G et al (2017) MicroRNA-145 negatively regulates cell proliferation through targeting IRS1 in isolated ovarian granulosa cells from patients with polycystic ovary syndrome. Reprod Sci 24(6):902–910
Xia H, Zhao Y (2020) miR-155 is high-expressed in polycystic ovarian syndrome and promotes cell proliferation and migration through targeting PDCD4 in KGN cells. Artif Cells Nanomed Biotechnol 48(1):197–205
Tam W (2001) Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene 274(1–2):157–167
Arancio W et al (2018) Serum miRNAs in women affected by hyperandrogenic polycystic ovary syndrome: the potential role of miR-155 as a biomarker for monitoring the estroprogestinic treatment. Gynecol Endocrinol 34(8):704–708
Yang H-S et al (2004) A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol 24(9):3894–3906
Yang H-S et al (2003) The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 23(1):26–37
Ding L et al (2016) Higher PDCD4 expression is associated with obesity, insulin resistance, lipid metabolism disorders, and granulosa cell apoptosis in polycystic ovary syndrome. Fertil Steril 105(5):1330-1337.e3
Geng Y et al (2019) MiRNA-99a can regulate proliferation and apoptosis of human granulosa cells via targeting IGF-1R in polycystic ovary syndrome. J Assist Reprod Genet 36(2):211–221
Moustafa S et al (2020) Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstetrics Gynecol 223(4):557.e1-557.e11
Zhou CF et al (2019) miR-205-5p inhibits human endometriosis progression by targeting ANGPT2 in endometrial stromal cells. Stem Cell Res Ther 10(1):1–13
Matalliotakis M et al (2017) The familial risk of endometriosis among the female relatives of patients with endometriosis in Greece. J Endometriosis Pelvic Pain Disorders 9(3):184–187
Nouri K et al (2010) Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol 8(1):1–7
Kennedy S (1999) The genetics of endometriosis. Eur J Obstet Gynecol Reprod Biol 82(2):129–133
Moen MH, Magnus P (1993) The familial risk of endometriosis. Acta Obstet Gynecol Scand 72(7):560–564
Husby GK, Haugen RS, Moen MH (2003) Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand 82(7):649–653
Giudice LC, Kao LC (2004) Endometriosis. Lancet 364(9447):1789–1799
Ohlsson Teague EMC et al (2009) MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23(2):265–275
Zhang J et al (2019) Endometriosis pathoetiology: the role of microRNAs in the dysregulation of endometrial functions. Reprod Develop Med 3(04):247–251
Cho S et al (2015) Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 103(5):1252-1260.e1
Cosar E et al (2016) Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril 106(2):402–409
Bjorkman S, Taylor HS (2019) MicroRNAs in endometriosis: biological function and emerging biomarker candidates. Biol Reprod 101(6):1167–1178
Wang W-T et al (2013) Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab 98(1):281–289
Zhang D et al (2020) MicroRNA-202 inhibits endometrial stromal cell migration and invasion by suppressing the K-Ras/Raf1/MEK/ERK signaling pathway. Int J Mol Med 46(6):2078–2088
Nematian SE et al (2018) Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b–5p and Let-7b-5p regulate macrophage cytokine production. J Clin Endocrinol Metab 103(1):64–74
Sahin C et al (2018) micro RNA Let-7b: a novel treatment for endometriosis. J Cell Mol Med 22(11):5346–5353
Grechukhina O et al (2012) A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med 4(3):206–217
Hawkins SM et al (2011) Functional microRNA involved in endometriosis. Mol Endocrinol 25(5):821–832
Long M et al (2015) miR-29c is downregulated in the ectopic endometrium and exerts its effects on endometrial cell proliferation, apoptosis and invasion by targeting c-Jun. Int J Mol Med 35(4):1119–1125
Takebayashi K et al (2020) hsa-miR-100-5p, an overexpressed miRNA in human ovarian endometriotic stromal cells, promotes invasion through attenuation of SMARCD1 expression. Reprod Biol Endocrinol 18(1):1–10
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci 105(36):13421–13426
Rezk NA, Lashin MB, Sabbah NA (2021) MiRNA 34-a regulate SIRT-1 and Foxo-1 expression in endometriosis. Non-coding RNA Res 6(1):35–41
Potente M et al (2007) SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21(20):2644–2658
Potente M, Dimmeler S (2008) Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 7(14):2117–2122
Rekker K et al (2015) Circulating miR-200–family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil Steril 104(4):938-946.e2
Suske G, Bruford E, Philipsen S (2005) Mammalian SP/KLF transcription factors: bring in the family. Genomics 85(5):551–556
Zhang Y, Yan J, Pan X (2019) miR-141-3p affects apoptosis and migration of endometrial stromal cells by targeting KLF-12. Eur J Physiol 471(8):1055–1063
Eggers JC et al (2016) microRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod Biomed Online 32(4):434–445
Liang Z et al (2017) miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res Ther 8(1):1–11
Rekker K et al (2018) Differentially-expressed miRNAs in ectopic stromal cells contribute to endometriosis development: the plausible role of miR-139-5p and miR-375. Int J Mol Sci 19(12):3789
Bashti O et al (2018) miR-31 and miR-145 as potential non-invasive regulatory biomarkers in patients with endometriosis. Cell J (Yakhteh) 20(1):84
Adammek M et al (2013) MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril 99(5):1346-1355.e5
Liu S et al (2012) Expression of miR-126 and Crk in endometriosis: miR-126 may affect the progression of endometriosis by regulating Crk expression. Arch Gynecol Obstet 285(4):1065–1072
Feng R et al (2010) miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett 298(1):50–63
Jia SZ et al (2013) Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod 28(2):322–330
Zhao M et al (2014) miR-20a contributes to endometriosis by regulating NTN4 expression. Mol Biol Rep 41(9):5793–5797
Liu Y et al (2004) Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol 14(10):897–905
Hoang S et al (2009) Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J Cereb Blood Flow Metab 29(2):385–397
Yang W et al (2017) MiR-424-5p regulates proliferation and apoptosis by targeting FGFR1 in endometriosis cells. Int J Clin Exp Med 10(1):666–674
Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10(2):116–129
Ma L et al (2019) MicroRNA-142-3p suppresses endometriosis by regulating KLF9-mediated autophagy in vitro and in vivo. RNA Biol 16(12):1733–1748
Pabona JM et al (2012) Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab 97(3):E376–E392
Simmen RC et al (2004) Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Krüppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem 279(28):29286–29294
Zhou M et al (2016) miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum Reprod 31(11):2598–2608
Karmon AE et al (2014) MicroRNAs in the development and pathobiology of uterine leiomyomata: does evidence support future strategies for clinical intervention? Hum Reprod Update 20(5):670–687
Giuliani E, As-Sanie S, Marsh EE (2020) Epidemiology and management of uterine fibroids. Int J Gynecol Obstet 149(1):3–9
Stewart EA et al (2017) Epidemiology of uterine fibroids: a systematic review. BJOG Int J Obstet Gynaecol 124(10):1501–1512
Wise LA, Laughlin-Tommaso SK (2016) Epidemiology of uterine fibroids–from menarche to menopause. Clin Obstet Gynecol 59(1):2
Ciavattini A et al (2013) Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int 2013:1–11
Wang T et al (2007) A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosom Cancer 46(4):336–347
Marsh EE et al (2008) Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril 89(6):1771–1776
Zota AR et al (2020) Phthalate exposures and MicroRNA expression in uterine fibroids: the FORGE study. Epigen Insights 13:2516865720904057
Peng Y et al (2008) Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res 6(4):663–673
Klemke M et al (2010) Loss of let-7 binding sites resulting from truncations of the 3′ untranslated region of HMGA2 mRNA in uterine leiomyomas. Cancer Genet Cytogenet 196(2):119–123
Fitzgerald JB et al (2012) Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil Steril 98(3):726-734.e2
Noetel A et al (2012) microRNA are central players in anti-and profibrotic gene regulation during liver fibrosis. Front Physiol 3:49
Cardozo ER et al (2018) MicroRNA 21a–5p overexpression impacts mediators of extracellular matrix formation in uterine leiomyoma. Reprod Biol Endocrinol 16(1):1–11
Kriegel AJ et al (2012) The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 44(4):237–244
Marsh EE et al (2016) Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril 106(3):766–772
Qiang W et al (2014) Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology 155(3):663–669
Xu X et al (2018) Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma. J Mol Med 96(10):1095–1106
Chuang T-D, Khorram O (2019) Regulation of cell cycle regulatory proteins by microRNAs in uterine leiomyoma. Reprod Sci 26(2):250–258
Ciebiera M et al (2020) The role of miRNA and related pathways in pathophysiology of uterine fibroids—from bench to bedside. Int J Mol Sci 21(8):3016
Mello J et al (2018) MicroRNAs involved in the HMGA2 deregulation and its co-occurrence with MED12 mutation in uterine leiomyoma. MHR Basic Sci Reprod Med 24(11):556–563
Panda H et al (2012) Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKβ, KLF9, and FBLN5. Reprod Sci 19(8):786–796
Kim YJ et al (2018) Variation in microRNA expression profile of uterine leiomyoma with endometrial cavity distortion and endometrial cavity non-distortion. Int J Mol Sci 19(9):2524
Wu X et al (2015) Effects of miRNA-197 overexpression on proliferation, apoptosis and migration in levonorgestrel treated uterine leiomyoma cells. Biomed Pharmacother 71:1–6
Ling J et al (2015) Upregulation of miR-197 inhibits cell proliferation by directly targeting IGFBP5 in human uterine leiomyoma cells. In Vitro Cell Dev Biol Animal 51(8):835–842
Chuang T-D, Khorram O (2017) Tranilast inhibits genes functionally involved in cell proliferation, fibrosis, and epigenetic regulation and epigenetically induces miR-29c expression in leiomyoma cells. Reprod Sci 24(9):1253–1263
Momenimovahed Z et al (2019) Ovarian cancer in the world: epidemiology and risk factors. Int J Women’s Health 11:287
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Falzone L et al (2021) A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer. Int J Oncol 59(1):1–14
Dockery LE et al (2017) Tolerance and toxicity of the PARP inhibitor olaparib in older women with epithelial ovarian cancer. Gynecol Oncol 147(3):509–513
Foo T, George A, Banerjee S (2021) PARP inhibitors in ovarian cancer: an overview of the practice-changing trials. Genes Chromosom Cancer 60(5):385–397
Jiang X et al (2019) Current status and future prospects of PARP inhibitor clinical trials in ovarian cancer. Cancer Manag Res 11:4371
Aletti GD et al (2017) Multidisciplinary approach in the management of advanced ovarian cancer patients: a personalized approach. Results from a specialized ovarian cancer unit. Gynecol Oncol 144(3):468–473
Bengrine L et al (2022) Multi-disciplinary care planning of ovarian cancer in older patients: general statement—a position paper from SOFOG-GINECO-FRANCOGYN-SFPO. Cancers 14(5):1295
Koutsaki M, Spandidos DA, Zaravinos A (2014) Epithelial–mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: Prognostic value and prospective role in ovarian cancer therapeutics. Cancer Lett 351(2):173–181
Korpal M et al (2008) The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283(22):14910–14914
Leskelä S et al (2011) The miR-200 family controls β-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer 18(1):85–95
Person F et al (2017) Prevalence of βIII-tubulin (TUBB3) expression in human normal tissues and cancers. Tumor Biol 39(10):1010428317712166
Ferrandina G et al (2006) Class III β-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res 12(9):2774–2779
Yang N et al (2008) MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Can Res 68(24):10307–10314
Johnson SM et al (2005) RAS is regulated by the let-7 microRNA family. Cell 120(5):635–647
Büssing I, Slack FJ, Großhans H (2008) Let-7 microRNAs in development, stem cells and cancer. Trends Mol Med 14(9):400–409
Johnson CD et al (2007) The let-7 microRNA represses cell proliferation pathways in human cells. Can Res 67(16):7713–7722
Schultz J et al (2008) MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res 18(5):549–557
Corney DC et al (2010) Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res 16(4):1119–1128
Suzuki HI et al (2009) Modulation of microRNA processing by p53. Nature 460(7254):529–533
Peng D-X et al (2012) Prognostic implications of microRNA-100 and its functional roles in human epithelial ovarian cancer. Oncol Rep 27(4):1238–1244
Mabuchi S et al (2009) mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res 15(17):5404–5413
Guo LM et al (2009) MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-κB1. FEBS J 276(19):5537–5546
Miles GD et al (2012) Identifying microRNA/mRNA dysregulations in ovarian cancer. BMC Res Notes 5(1):1–10
Wilson CA et al (2005) HER-2 overexpression differentially alters transforming growth factor-β responses in luminal versus mesenchymal human breast cancer cells. Breast Cancer Res 7(6):1–22
Yang H et al (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Can Res 68(2):425–433
Paul A, Sadek ST, Mahesan AM (2019) The role of microRNAs in human embryo implantation: a review. J Assist Reprod Genet 36(2):179–187
Cuman C et al (2015) Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine 2(10):1528–1535
Kuokkanen S et al (2010) Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod 82(4):791–801
Kresowik JD et al (2014) MicroRNA-31 is significantly elevated in both human endometrium and serum during the window of implantation: a potential biomarker for optimum receptivity. Biol Reprod 91(1):1–6
Tochigi H et al (2017) Loss of miR-542-3p enhances IGFBP-1 expression in decidualizing human endometrial stromal cells. Sci Rep 7(1):1–10
Kresowik J et al (2012) microRNA 31 is upregulated in serum during the window of implantation. Fertil Steril 98(3):S187
Fang L et al (2022) MiR-17–5P regulates autophagy of ovarian granulosa cells in patients with polycystic ovary syndrome via targeting ATG7.
Zhao W et al (2022) Effects of miRNA-199a-5p on cell proliferation and apoptosis of uterine leiomyoma by targeting MED12. Open Med 17(1):151–159
Ahn SH et al (2021) MicroRNA-139-5p regulates fibrotic potentials via modulation of collagen type 1 and phosphorylated p38 MAPK in uterine leiomyoma. Yonsei Med J 62(8):726
Wuerkenbieke D et al (2015) miRNA-150 downregulation promotes pertuzumab resistance in ovarian cancer cells via AKT activation. Arch Gynecol Obstet 292(5):1109–1116
Lan H et al (2015) miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed Pharmacother 75:117–122
Chen Z et al (2019) Role of microRNA in the pathogenesis of polycystic ovary syndrome. DNA Cell Biol 38(8):754–762
Echevarría-Vargas IM, Valiyeva F, Vivas-Mejía PE (2014) Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS ONE 9(5):e97094
Kiba A et al (2016) Differential micro ribonucleic acid expression profiling in ovarian endometrioma with leuprolide acetate treatment. J Obstet Gynaecol Res 42(12):1734–1743
Lu L et al (2011) MicroRNA let-7a: a potential marker for selection of paclitaxel in ovarian cancer management. Gynecol Oncol 122(2):366–371
Li G et al (2022) Long non-coding RNA placenta-specific protein 2 regulates micorRNA-19a/tumor necrosis factor α to participate in polycystic ovary syndrome. Bioengineered 13(1):856–862
Roth LW et al (2014) Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet 31:355–362
Pei T et al (2018) miR-194-3p represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Endocrinology 159(7):2554–2562
Acknowledgements
The authors acknowledge the Deputy for research and technology, Arak University of Medical Sciences, Iran for providing spiritual support to accomplish the present study. This study was approved by the Ethics Committee of Arak University of Medical Sciences with the No: IR.ARAKMU.REC.1402.007.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: BK, SA, and MM; Investigation: MB, BK, SA, and MM; Resources: MM, MB, and MA; Writing—original draft preparation: MM and MB; Writing review and editing: MA, MB and MM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no conflicts of interest.
Ethical approval
No applicable.
Informed consent
Not applicable.
Consent for publications
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bagheri, M., Khansarinejad, B., Mondanizadeh, M. et al. MiRNAs related in signaling pathways of women’s reproductive diseases: an overview. Mol Biol Rep 51, 414 (2024). https://doi.org/10.1007/s11033-024-09357-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09357-0