Abstract
Background
Folliculogenesis is an intricate process that involves the development and maturation of ovarian follicles in females. During folliculogenesis, multiple factors including hormones, growth factors, and signaling pathways regulate the growth and maturation of follicles. In recent years, microRNA, short non-coding RNA molecules, has gained attention due to its roles in the physiology and pathophysiology of various diseases in humans. It is known to have an important part in ovarian health and illness and its functions extend to several cellular processes.
Main body
In this overview, we look at the importance of microRNAs in ovarian illnesses and how they function during follicle growth in the ovaries. Short RNA molecules (22 nucleotides) called microRNAs may influence several mRNA targets in different biological processes. The expression patterns of these small non-coding RNAs undergo dynamic changes during the several phases of follicular development; they play a function in post-transcriptional gene regulation. Follicle development, follicular atresia (regression of the follicles), and ovulation are all intricately regulated by the dynamic expression of distinct miRNAs throughout the various phases of folliculogenesis.
The role of microRNAs (miRNAs), which are known to regulate gene expression, has recently come to light as crucial in the development and advancement of a number of ovarian diseases. Abnormalities of the human ovary, such as ovarian cancer, polycystic ovary syndrome (PCOS), and endometriosis, have prompted extensive research into the dysregulation of microRNAs. Endometriosis is associated with miRNAs that are known to have a role in processes such as invasion, cell growth, cell adhesion, angiogenesis, and epithelial-mesenchymal transition. The disturbance of target gene expression resulting from abnormal miRNA production is a potential factor contributing to cancer development. Some microRNAs (miRNAs) differ in expression levels between women with polycystic ovary syndrome and healthy controls, indicating that miRNAs may play a role in the development of PCOS.
Conclusion
Extensive research carried out over the last 20 years has illuminated the roles of microRNAs (miRNAs), demonstrating their critical importance in controlling gene expression and the cell cycle. Changes in the quantities of microRNAs (miRNAs) may affect the aggressiveness of cancer and contribute to a variety of gynecological disorders. It appears that microRNAs hold potential as diagnostic biomarkers and treatment potential for various ovarian diseases.
Similar content being viewed by others
Background
In many cellular functions and illnesses, noncoding RNAs, such as microRNAs (miRNAs), are very important [1]. MiRNAs, consisting of 22 nucleotides, attach to target mRNAs’ complementary sequences, disrupting translation or stability [2]. Discovered by Ambros and Ruvkun in 1993, they are present in all animal lineages and have over 2600 distinct mature miRNAs in humans [3]. Their functions include developmental timing, cell death, fat metabolism, and leaf development [4,5,6,7,8,9].
MicroRNAs, found in cells and bloodstreams, show significant variation in expression profiles between healthy individuals and those with various diseases [10]. Researchers Lawrie et al. found microRNAs (miRNAs) in blood as a non-invasive way to diagnose DLBCL [10]. Alterations in miRNA levels have been observed across various diseases, including cancer, inflammation, reproductive, metabolic, and cardiovascular disorders [11]. Currently, microRNAs are being suggested as diagnosis and monitoring tools for cancers and other conditions [11]. Dysregulation of miRNA expression has been extensively investigated in female reproductive system diseases [11,12,13,14,15,16].
The female reproductive system’s core function is folliculogenesis, a crucial process that shapes ovarian dynamics [17]. The process begins with primordial germ cells migrating towards the embryonic genital ridge, differentiation, and activation of primordial follicles [17]. A significant reduction of these follicles forms the ovarian follicle reserve, which recruits growing follicles for further development [17]. As follicles undergo apoptosis, only a minority become primary follicles, and the process continues with the transformation of primary follicles into pre-antral, antral, and Graafian follicles [17]. The intricate orchestration of events is influenced by localized signals from both oocytes and somatic cells [17].
Follicular granulosa cells (FGCs) are crucial for supporting oocytes by releasing growth factors and growth hormones, and controlling oocyte development [18]. Regulating follicular growth and FGC apoptosis is a crucial role for microRNAs [18]. Understanding these processes is essential for advancing knowledge of ovarian development and related disorders [18]. MicroRNAs are pivotal in overseeing a range of mammalian FGC functions like proliferation, differentiation, and cumulus expansion [18]. Their significance in oocyte and fetal development in mammals is widely acknowledged [18]. Nonetheless, the exact impacts and mechanisms of miRNA regulation, particularly regarding target genes and pathways, are not fully comprehended [19]. Ovarian illnesses and disorders are greatly impacted by microRNAs, a network of genes that regulate gene expression and are essential in the development and advancement of these conditions [19].
Ten percent of reproductive-age women suffer from endometriosis, a benign chronic illness [11]. Finding endometrial glands and stroma outside of the uterus that are functioning and responsive to estrogen signaling is the hallmark of endometriosis [11]. Endometriosis showcases miRNA signatures within lesions, which mediate processes like angiogenesis, inflammation, and cell proliferation, affecting the establishment and perpetuation of the condition [11]. Both ovarian cancer and polycystic ovarian syndrome (PCOS) are endocrine illnesses that are affected by microRNAs [20]. PCOS affects 8–13% of reproductive-aged women globally, while ovarian cancer risk affects 1 in 78 women during their lifetime [20]. The altered miRNAs in PCOS disrupt hormonal balance, follicular development, and insulin resistance, while in ovarian cancer, it leads to oncogenic shifts and metastatic cell spread [21]. In the battle against ovarian cancer, miRNAs may act as indicators for diagnosis and as targets for treatment [22].
The purpose of this study is to provide a synopsis of the most current findings on microRNAs (miRNAs) and their impact on human follicular development and folliculogenesis. This review focuses on findings regarding miRNA roles in follicular development and ovarian diseases (PCOS, endometriosis, and ovarian cancer).
MicroRNA biogenesis
Processing RNA polymerase II/III transcripts, either before or during transcription, initiates microRNA biogenesis [23]. Two separate mechanisms, known as the canonical and non-canonical pathways, are involved in miRNA synthesis [24].
Beginning miRNA biogenesis in the canonical route is the production of the principal miRNA transcript (pri-miRNA) [24]. In order to create the precursor miRNA, the microprocessor complex cleaves this pri-miRNA [24]. Depending on Exportin5/RanGTP, the process of pre-miRNA export to the cytoplasm follows transcription in the nucleus [24]. A mature miRNA duplex undergoes further processing in the cytoplasm; a miRNA-induced silencing complex (miRISC) is formed when one strand of the duplex is integrated with the Argonaute (AGO) proteins [24].
One of the non-canonical routes involves the microprocessor complex transferring short hairpin RNA (shRNA) to the cytoplasm via Exportin5/RanGTP after cleaving it [24]. Extra processing happens via Dicer-independent AGO2-dependent cleavage [24]. Although their nuclear and cytoplasmic transit is different, both Mirtrons and m7G-pre-miRNA need Dicer for cytoplasmic maturation. Mirtrons are exported by Exportin5/RanGTP, in contrast to m7G-pre-miRNA, which is exported through Exportin [24].
Regardless of the pathway, the end result is the formation of a functional miRISC complex [24]. When miRISC attaches to target mRNAs, it will interfere with the eIF4F complex, leading to translational inhibition [24]. The decapping complex is able to remove the m7G cap from the target mRNA because deadenylation begins with PAN2/3 and ends with the CCR4-NOT complex [24]. At long last, the decapped messenger RNA is assisted in its 5′-3′ degradation by the exoribonuclease XRN1 [24].
Folliculogenesis: an overview
Folliculogenesis is the intricate process by which a woman’s ovarian follicles grow and mature [25]. The process starts with the recruitment of primordial follicles, which are then transformed into primary follicles after a sequence of growth and differentiation phases [25]. Follicle development continues with the formation of secondary follicles, which are identified by the presence of an antrum, a hollow filled with fluid, and antral follicles [25]. The final stage of folliculogenesis focuses on selecting a dominant follicle, responsible for further growth and eventually ovulation [25]. Throughout folliculogenesis, numerous factors such as hormones, growth factors, and signaling pathways work together to coordinate the enlargement and maturation of the follicles [25]. Particularly important are the granulosa cells that surround the oocyte, playing a vital role in supporting its maturation [25]. The development of the oocyte depends on the nutrition and growth substances provided by these cells. The orchestration of folliculogenesis involves precise regulation and coordination, with intricate interactions among the oocyte, granulosa cells, and surrounding ovarian tissues [25]. Any disruptions or abnormalities in this process can lead to various reproductive disorders and fertility issues [25].
MiRNA regulation in folliculogenesis
Identification of miRNAs involved in folliculogenesis
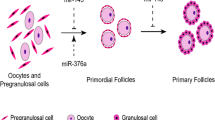
All stages of ovarian follicle development—from growth to regression (atresia) to ovulation—rely on microRNAs (miRNAs) [19]. Figure 1 shows a summary of the folliculogenesis process and the miRNAs that play roles in each stage of follicular development. These tiny non-coding RNA molecules are involved in post-transcriptional gene regulation and undergo dynamic changes in their expression patterns during the various phases of follicular development in Fig. 1 [19]. Multiple studies have revealed miRNA expression patterns during folliculogenesis (Table 1).
microRNA regulates the folliculogenesis. Blue text represents the upregulated miRNA and red text represents the downregulated miRNA arranged key steps in the follicular development process. This includes the development of primordial follicles from pre-granulosal cells, and progression through the stages of primary, secondary, and antral follicle development, with some follicles experiencing atresia. The pre-ovulatory follicle, influenced by luteinizing hormone (LH) and follicle-stimulating hormone (FSH), undergoes ovulation and transitions into the luteal stage
The following basic phases of follicle development are associated with specific microRNA (miRNA) expression profiles from primordial, to primary, pre-antral, tiny antral, and large antral follicles, continues to the pre-ovulatory follicles, then early and late corpus luteum, and corpus albicans [26]. Out of all the stages, let-7a, let-7b, miR-125b, and miR-21 had the highest levels of expression of microRNAs. While miR-199a-3p, miR-145, and miR-31 were all overexpressed during follicular development, their expression dropped dramatically during follicular-luteal transition [27].
Follicle development, also known as folliculogenesis, initiates with the breakdown of germ cell clusters and progresses with the formation of primordial follicles [27]. A prior investigation discovered the expression of miR-143 within pre-granulosa cells through the application of in situ hybridization. This miRNA reportedly suppresses the expression of genes associated with the cell cycle and reduces the proliferation of pre-granulosa cells, hence inhibiting the formation of primordial follicles [50]. More than ninety-nine percent of ovarian follicles undergo atresia degeneration during folliculogenesis [50].
Mature oocytes and follicular development (FD) take place in a woman’s ovary [21]. However, during these processes, a complex and natural phenomenon known as atresia occurs [21]. The apoptosis of the granulosa cells (GCs) surrounding the oocytes is a hallmark of atresia, the spontaneous death of ovarian follicles [21]. Ovulation occurs in less than one percent of mammalian ovarian follicles, whereas atresia affects more than ninety-nine percent [21]. This phenomenon of atresia impacts follicular growth and development at all stages [21]. A recent study found that follicular atresia and its development are influenced by certain miRNA clusters and families. Which miRNA cluster(s) linked to each stage of follicular development, however, remained undetermined [18].
Mechanisms of miRNAs in folliculogenesis regulation
Multiple variables, including Smads, ligand activation of type I receptors (also called activin receptor-like kinases, or ALKs), and members of the TGF-β superfamily, impact the complex process of follicle formation [27]. The effect of microRNAs on these components is crucial to the control of follicle maturation [27]. Research found that the TGF-β/Smad signaling pathway controls miRNA-224 expression [27]. It has been discovered that elevated levels of miR-224, which target Smad4, enhance the proliferation of granulosa cells triggered by TGF-β [27]. On the other hand, granulosa cell proliferation triggered by TGF-β1 is somewhat reduced when the endogenous miR-224 is suppressed [27].
Furthermore, miRNAs also influence ovulation indirectly [27]. Research conducted by Hasuwa et al. on the effects of miR-200b and miR-429 on female mouse infertility found that blocking the production of luteinizing hormone (LH) was hindered by inactivating these miRNAs [27]. This finding indicated that these miRNAs indirectly contribute to ovulation by playing a role in the hypothalamus-pituitary-ovarian axis [27].
Researchers studying ovarian development have now recognized that follicular atresia, the process of degeneration and regression of ovarian follicles, involves granulosa cell (GC) apoptosis as a fundamental physical mechanism [51]. This process initiates with the observation of pyknotic nuclei in GCs. Subsequently, the GC layer detaches, leading to basal membrane fragmentation [51]. These changes eventually lead to the hypertrophy of thecal cells, disrupting thecal integration and thecal vessels [51]. It is noteworthy that oocyte degeneration may happen at any stage of atresia [51]. As a result of these findings, research on follicular atresia has shifted its focus toward investigating the molecular regulation of GC apoptosis [51].
Through altering the target genes’ expression levels, miRNAs influence how follicular granulosa cells (FGCs) function [52]. One such miRNA family, known as the miR-let-7 family, exhibits a high degree of sequence conservation across various animal species [52]. There is a wide range of functions performed by members of the let-7 family, including regulating the differentiation and proliferation of cells, tissue development, and inhibition of tumor development [52]. This miR-let-7 family demonstrates differential expression patterns during follicular atresia [52]. MiR-let-7a, let-7b, let-7c, and let-7i gene expression levels were specifically observed to be lower in early and progressed stages of follicular atresia compared to healthy follicles [53, 54]. Premature ovarian failure (POF) is distinguished by lower levels of let-7c compared to healthy women, according to the research [54]. This implies that let-7c likely helps in promoting normal follicular development. On the other hand, let-7g is highly expressed during atresia, unlike its family members, indicating a different role in follicles [54].
The regulation of apoptosis in follicular granulosa cell (FGC) apoptosis involves a complex mechanism wherein miR-let-7g suppresses mitogen-activated protein kinase 1 (MAP3K1), resulting in the transcription factor forkhead O1’s (FOXO1) expression and dephosphorylation [53, 54]. This, in turn, triggers FGC apoptosis. By overexpressing miR-let-7g, the apoptotic rate of FGCs in mice increases, along with an elevation in FOXO1 expression within FGCs [53, 54]. As a result, dephosphorylated FOXO1 eventually builds up in the nucleus. In addition, caspase 3, BES1-interacting Myc-like protein (BIM), and BCL2-Associated X (BAX) are among the apoptosis-related genes that are markedly upregulated after transfection of porcine FGCs with miR-let-7g mimics [53, 54]. Alternatively, there is a significant decrease in anti-apoptotic gene expression, as shown in B cell lymphoma-2 (Bcl-2) and myeloid cell leukemia-1 (Bcl-1). The results indicate that the miR-let-7 family has promising future applications in controlling FGC apoptosis [53, 54].
MiR-21, among the three miRNAs strongly stimulated in murine follicular granulosa cells (FGCs) by luteinizing hormone (LH), functions as an antiapoptotic factor within granulosa cells (GCs) [6]. When miR-21 is absent in vivo, it results in a decrease in the rates of ovulation [6]. Cumulus oocyte complexes (COCs) exhibited significantly increased amounts of mature miR-21 and its parent transcript (pri-miR-21) throughout maturation [6].
Blocking the expression of pri-miR-21 directly influences the expression of miR-21 in bovine oocytes and cumulus cells (CCs) [55]. Enhancing the expression of miR-21 had a notable impact on decreasing apoptosis in cumulus cells (CCs) [55]. Oocyte-secreted factors (OSFs) initiate the activation of the PI3K/Akt signaling pathway, resulting in increased miR-21 levels and the suppression of apoptosis in CCs [56]. It has been demonstrated that oocytes and CCs withstand apoptosis more than other antral follicle components [56].
MiRNA and steroidogenesis
miRNAs act as regulators of ovarian steroid hormones by aiming at hormone receptors and influencing hormone biosynthesis and release [57]. A specific example is the regulation of estradiol (E2), which is crucial for ovarian follicle development and primarily controlled by the aromatase enzyme [57]. A post-transcriptional mechanism that limits estradiol production in granulosa cells and downregulates aromatase expression was uncovered by Xu et al. as miR-378 [42]. In contrast, miR-133b enhances the synthesis of ovarian estradiol by targeting Foxl2, a transcriptional regulator that represses the expression of StAR and CYP19A1 [58]. By targeting Foxl2, miR-133b promotes the biosynthesis of estradiol, thereby facilitating the production of this hormone in the ovary [58].
Apart from controlling estradiol production, miRNAs also play a role in regulating the release of estradiol [59]. In ovarian GCs, enhancement of estradiol happened by the release of miR-383 that inhibits RBMS1 [59]. How this is accomplished by regulating RBMS1 mRNA stability, which in turn affects granulosa cell steroidogenesis by rendering c-Myc inactive [59]. In addition, miR-378 and miR-423-5p target the CYP19A1 mRNA and are important in controlling estradiol production [59]. They decrease the protein content and enzyme activity of CYP19A1, thereby exerting an inhibitory effect on estradiol synthesis. These regulatory mechanisms have been observed in newborn piglets [59].
Sirotkin et al. conducted a study that revealed several miRNAs’ role in controlling reproductive functions [38]. They identified a set of 36 miRNAs that inhibited the release of progesterone in granulosa cells [38]. Conversely, they found that 16 miRNAs promoted progesterone release [38]. Additionally, the research found that the following microRNAs were implicated in the inhibition of testosterone production in granulosa cells: mir-108, mir-122, let-7a, let-7b, let-7c, miR-16, miR-17-3p, miR-24, miR-25, and miR-26a [38]. These findings emphasize the diverse regulatory effects of particular miRNAs on the release of reproductive hormones in the ovary [59].
MiRNA in ovarian diseases
Endometriosis
A benign inflammatory condition known as endometriosis affects 10% of reproductive-aged women. Hormonal, immunological, and genetic factors contribute to its etiology [51]. Endometriosis is diagnosed when stroma and functioning endometrial glands are discovered in locations other than the uterus, such as the ovaries, pelvis, and rectovaginal septum [60]. Current evidence has shown molecular defects in endometrial cells, with miRNA potentially acting as an endometriosis biomarker. Several dysregulated miRNAs have been directly linked to the disease’s pathogenesis (Table 2) [6].
Haikalis et al. assessed six distinct miRNAs in endometriosis lesions, revealing distinct expression profiles for each type of lesion [52]. There were noticeable differences in the expression patterns of the microRNAs miR-10a, miR-10b, miR-21, miR-9, miR-204, and miR-424 according to the various types of lesions. The most often downregulated miRNA in endometriosis was miR-200b [52]. An essential step in endometriosis, the epithelial-mesenchymal transition (EMT) involves this microRNA [53, 54]. Other miRNAs were up- or downregulated in endometriotic lesions, and also involved in endometriotic lesion processes, angiogenesis, cell proliferation, adhesion, and invasion [53].
Endometriosis can cause diminished fertility and a shortened reproductive window through factors like disrupted folliculogenesis, poor oocyte quality, abnormal follicular development, and increased ROS levels [55]. Endometriosis-related infertility is associated with changes in microRNAs that affect genes such as HOXA10, aromatase, progesterone receptors, matrix metalloproteinases (MMPs), and alphaV beta 3-integrin [56]. Upregulation of miR-135a/b in endometriosis-affected women leads to repression of HOXA10, a transcription factor crucial for endometrial receptivity. This illustrates an early instance where dysregulated miRNA in endometriosis correlates with implantation failure [56].
One hallmark of endometriosis is progesterone resistance, which is aided by microRNAs such as the let-7 family, miR-29c, miR-125b, miR-135a/b, miR-194, and miR-196a [6]. This resistance disrupts essential mechanisms like endometrial cell decidualization, affecting targets like FKBP4, PGR, and MMP26, thereby impairing fertility potential [6]. As a result of its negative regulation of Ras oncogenes, the Let-7 family—the first human miRNA to be discovered—regulates cell differentiation and acts as a tumor suppressor. Endometriosis and several malignancies are associated with its downregulation [77]. In severe endometriosis, polymorphisms at the KRAS gene’s let-7 binding site increase, leading to higher KRAS mRNA and protein levels [42, 78]. Let-7 family is involved in estrogen biosynthesis and can be inhibited by aromatase inhibitors [79].
Enhancement of angiogenesis and anti-apoptotic process have been suggested as potential links between the pathophysiology and progression of endometriosis [80]. During embryonic development in zebrafish, MiR-126 controls the response of endothelial cells to VEGF, leading to a decrease in vascular integrity and bleeding [79]. It represses negative VEGF pathway regulators, potentially influencing vascular integrity and function [79]. Mir-126 is also thought to affect the EGFL7 function which limits the endothelial cells’ spatial distribution to control their migration [79]. Endometriotic cyst stromal cells also showed disruption in the angiogenesis process through DNA hypermethylation in miR-503, which interacts with cyclin D1, BCL-2, Ras homology A, and VEFG-A, among others, and contributes to ECM contractility, angiogenesis, resistance to apoptosis, and proliferation [79].
Ovarian cancer
Dysregulation of miRNAs can disrupt their target genes’ expression, thereby contributing to the onset of cancer development [81] (Table 3). Genetic abnormalities like chromosomal deletions, rearrangements, and mutations, together with epigenetic modifications, are among the pathways that might lead to this misexpression [81]. Furthermore, abnormalities in transcription and post-transcription also play a major role in the development and advancement of ovarian cancer [81]. MiRNA deregulation is influenced by factors like epigenetic changes, chromosome rearrangements, and genomic copy number alteration [82].
There is great promise for miRNAs as clinical indicators for the early detection of OC. If miRNA is to be used as a biomarker for the early diagnosis of ovarian cancer, it has to have a specificity of 99.6%, a sensitivity of 75%, and a positive predictive value of 10% [104]. A research that looked at the diagnostic accuracy of miRNAs in patients with stage I high-grade serous ovarian cancer discovered that some miRNAs were far more accurate than the standard marker, CA-125, with an area under the curve (AUC) of 0.99 [105]. The model showed effectiveness across different disease stages with higher sensitivity in borderline tumors [105]. Song et al. [106] discovered that ovarian cancer patients had lower serum miR-26b expression and greater miR-21 expression, which is important for diagnosing OC and substantially correlates with clinical stage, lymph node metastases, and a 3-year survival rate. The three microRNAs (miR-200a, miR-200b, and miR-200c) were identified in a comprehensive study of ovarian cancer biomarkers by Cui et al. [107]. Likewise, Halvorsen et al. [108] also showed miR-200a-3p and 200c-3p as a biomarker for epithelial OC detection and suggested six more miRs that substantially showed a link between prognosis and survival.
Alterations in the levels of miRNA expression can affect the cancer aggressivity by affecting aspects like migration, chemoresistance, and metastasis [109]. The expression patterns of microRNAs (miRNAs) in normal and malignant samples are different [109]. As ovarian cancer progresses, miRNA dysregulation changes the expression of certain genes; for example, miR-141 levels rise with advanced illness, but miR-200c levels fall; this suggests that higher levels of miR-200c indicate longer survival times and lower levels of miR-141 indicate better survival rates [109, 110].
Dysregulation of miRNAs in blood (exosomes) could improve early ovarian cancer diagnosis and prognosis. Both bodily fluids and tissue specimens contain miRNAs; however, tissue samples are only valuable after the early diagnosis of OC [109]. Blood-based circulatory miRNA is less invasive for diagnosis but has low abundance [109]. Prior to their clinical use, tissue-based miRNA and serum/plasma-based miRNA must be distinguished. To completely comprehend their roles, therapeutic potential, and usefulness as diagnostic or prognostic biomarkers in OC, additional investigation is required, despite the identification of numerous miRNAs with dysregulated patterns [109].
PCOS
Androgen excess and ovarian dysfunction define polycystic ovarian syndrome (PCOS), being the most prevalent endocrine disorder in reproductive-aged women globally [20]. Diagnosis requires at least two criteria: chronic anovulation, hyperandrogenism, and polycystic ovaries, noting that other diagnoses mimicking PCOS features must be excluded [111]. On a global scale, 8–13% of women of childbearing age have this illness, with an additional 70% going unidentified [112]. Insulin resistance, excessive hair growth, difficulty conceiving, irregular ovulation, weight gain, high blood pressure, cancer, and depressive symptoms are some of the additional health conditions that may arise due to this disorder [112]. Consequently, in order to minimize potential long-term health consequences, it is vital that women diagnosed with polycystic ovary syndrome (PCOS) get appropriate treatment measures as soon as possible.
New evidence suggests that specific microRNA expression levels differ between healthy persons and women with polycystic ovary syndrome [113, 114] (Table 4). These observations suggest that miRNAs could potentially have significant involvement in the onset and progression of PCOS [113, 114]. Granulosa cells have been shown to have both elevated proliferation and apoptotic rates in relation to a large number of miRNAs that exhibit variable expression. These results may be rationally explained, even if they seem to be inconsistent at first. Potentially, the transformation of primordial follicles into primary follicles is responsible for the surge in primary follicles [115]. Table 4 shows that aberrant miRNA expression may affect cell proliferation, apoptosis, steroidogenesis, folliculogenesis, glucose metabolism, and insulin sensitivity, all of which may play a role in the pathogenesis of polycystic ovary syndrome [115]. Furthermore, circulating microRNAs may serve as potential biomarkers for distinguishing PCOS patients from healthy women [115].
As a modulator of the insulin-IGF-1, Wnt, and Akt signaling pathways, the klotho protein has recently emerged as a promising therapeutic target for polycystic ovary syndrome (PCOS). Researchers discovered that granulosa cell miR-129a-5p and miR-126-5p expression were substantially downregulated in PCOS patients and DHEA-induced PCOS animals [115]. It is believed that aberrant folliculogenesis and metabolic problems in polycystic ovary syndrome (PCOS) are caused by granulosa cell death, and this discovery suggests that klotho plays a role in this process [115]. Reducing klotho gene expression in PCOS GCs increased cell proliferation and mitigated insulin’s anti-apoptotic effects [115].
In a comparative study of miRNAs, MiR-29a-5p, a recently discovered miRNA, has been found to be a superior diagnostic biomarker, demonstrating a significantly higher AUC value of 0.95, and is associated with metabolic disorders and cancers, involved in regulating cell growth, differentiation, and proliferation [132]. Therefore, assessing the expression level of miR-29a-5p holds greater clinical significance compared to other miRNAs, making it a more valuable tool for diagnostic purposes [132].
Conclusion and future direction
MicroRNA, a subset of small RNAs, accounts for various biological pathways involved in folliculogenesis and related diseases. One way it works is by blocking the translation of certain messenger RNAs. A number of biological processes, including angiogenesis, cell adhesion, invasion, apoptosis, and proliferation, have been shown to rely on microRNAs (miRNAs). Atresia and follicular development are both thought to be impacted by certain miRNA clusters and families. In the early phases of follicular atresia, relative to healthy follicles, the expression of this family of miR-lets is decreased, indicating differential expression during follicular atresia. Through their targeting of hormone receptors and their influence on hormone production and release, microRNAs (miRNAs) regulate ovarian steroid hormones.
Over the last 20 years, scientists have learned a great deal about miRNAs and their roles in gene expression and cell cycle control. Alterations in miRNA expression levels can impact cancer aggressivity and contribute to various gynecological disorders such as PCOS and endometriosis, impacting various molecular processes. To conclude, It appears that microRNAs hold potential as a diagnostic biomarker and enable more effective treatment potential as the future therapeutic targets for the diseases. Further exploration of functional studies on miRNA and its role in targeting specific mRNA will be needed with several notes.
Recent studies regarding microRNAs in ovarian disorders are still conducted on a relatively small scale. The limited sample sizes compromise the generalizability of findings, hindering the ability to extrapolate results to the broader population of individuals with ovarian disorders. Selection bias and insufficient statistical power further challenge the reliability of these studies, potentially leading to overlooked associations and biased conclusions. Confounding variables, publication bias, and technical variability in laboratory methodologies add layers of complexity, requiring researchers to approach findings with a critical lens. Recruitment bias may happen in this case and may affect the external validity of the findings and limit the applicability to different patient groups.
Several contradictory findings in the recent studies can be due to various factors. There are several potential sources of discrepancies, including study design, patient diversity, and microRNA measurement techniques. Additionally, the limited exploration of miRNA networks and interactions among multiple miRNAs, temporal variability, and validation challenges emphasize the need for more comprehensive and well-powered research.
To address these limitations, future research endeavors should prioritize larger sample sizes, diverse participant cohorts, and standardized methodologies. Collaborative efforts within the scientific community can facilitate the validation of findings across independent cohorts, improving the robustness and reliability of identified miRNA associations in ovarian disorders compared to independent cohorts alone. Last but not least, creating useful apps for the detection and treatment of ovarian diseases will depend on resolving these obstacles.
Availability of data and materials
This manuscript has no associated data.
References
Kozomara A, Birgaoanu M, Griffiths-Jones S (2019) miRBase: from microRNA sequences to function. Nucleic Acids Res 47(D1):D155–D162. https://doi.org/10.1093/nar/gky1141
Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B et al (2000) Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408(6808):86–89
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75(5):843–854
Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ (2006) miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34(Database issue):140–144
Gonzalez Dos Anjos L, de Almeida BC, Gomes de Almeida T, MourãoLavorato Rocha A, De NardoMaffazioli G, Soares FA et al (2018) Could miRNA signatures be useful for predicting uterine sarcoma and carcinosarcoma prognosis and treatment? Cancers (Basel) 10(9):315
van Beijnum JR, Giovannetti E, Poel D, Nowak-Sliwinska P, Griffioen AW (2017) miRNAs: micro-managers of anticancer combination therapies. Angiogenesis 20(2):269–285
Chatterjee N, Rana S, Espinosa-Diez C, Anand S (2017) MicroRNAs in Cancer: challenges and opportunities in early detection, disease monitoring, and therapeutic agents. Curr Pathobiol Rep 5(1):35–42
Wu XB, Fan GX, Gu X, Shen TG, Guan XF, Hu AN et al (2016) Learning curves of percutaneous endoscopic lumbar discectomy in transforaminal approach at the L4/5 and L5/S1 levels: a comparative study. J Zhejiang Univ Sci B 17(7):553–560
Guo Y, Yan K, Fang J, Qu Q, Zhou M, Chen F (2013) Let-7b expression determines response to chemotherapy through the regulation of cyclin D1 in glioblastoma. J Exp Clin Cancer Res 32(1):41
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K et al (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141(5):672–675
Bjorkman S, Taylor HS (2019) MicroRNAs in endometriosis: biological function and emerging biomarker candidates†. Biol Reprod 100(5):1135–1146
Torres A, Torres K, Maciejewski R, Harvey WH (2011) MicroRNAs and their role in gynecological tumors. Med Res Rev 31(6):895–923
de Almeida BC, Dos Anjos LG, Uno M, da Cunha IW, Soares FA, Baiocchi G et al (2019) Let-7 miRNA’s expression profile and its potential prognostic role in uterine leiomyosarcoma. Cells 8(11):1452
Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE (2009) The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112(1):55–59
Traver S, Assou S, Scalici E, Haouzi D, Al-Edani T, Belloc S et al (2014) Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update 20(6):905–923
Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS (2016) Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril 106(2):402–409
Gershon E, Dekel N (2020) Newly identified regulators of ovarian folliculogenesis and ovulation. Int J Mol Sci 21(12):4565
Gong Z, Yang J, Bai S, Wei S (2020) MicroRNAs regulate granulosa cells apoptosis and follicular development - a review. Asian-Australas J Anim Sci 33(11):1714–1724
McGinnis LK, Luense LJ, Christenson LK (2015) MicroRNA in ovarian biology and disease. Cold Spring Harb Perspect Med 5(9):a022962
Reid BM, Permuth JB, Sellers TA (2017) Epidemiology of ovarian cancer: a review. Cancer Biol Med 14(1):9–32
Jiao J, Shi B, Wang T, Fang Y, Cao T, Zhou Y et al (2018) Characterization of long non-coding RNA and messenger RNA profiles in follicular fluid from mature and immature ovarian follicles of healthy women and women with polycystic ovary syndrome. Hum Reprod 33(9):1735–1748
Alshamrani AA (2020) Roles of microRNAs in ovarian cancer tumorigenesis: two decades later, what have we learned? Front Oncol 10:1084
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15(8):509–524
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 9:402
Monniaux D, Cadoret V, Clément F, Dalbies-Tran R, Elis S, Fabre S, et al (2019) Folliculogenesis. In: Huhtaniemi I, Martini LBT-E of ED (Second E, editors. Academic Press, Oxford, p 377–98. Available from: https://www.sciencedirect.com/science/article/pii/B9780128012383645506
McBride D, Carré W, Sontakke SD, Hogg CO, Law A, Donadeu FX et al (2012) Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction 144(2):221–233
Li Y, Fang Y, Liu Y, Yang X (2015) MicroRNAs in ovarian function and disorders. J Ovarian Res 8(1):1–8. https://doi.org/10.1186/s13048-015-0162-2
Tu F, Pan ZX, Yao Y, Liu HL, Liu SR, Xie Z et al (2014) miR-34a targets the inhibin beta B gene, promoting granulosa cell apoptosis in the porcine ovary. Genet Mol Res 13(2):2504–2512
Xiao G, Xia C, Yang J, Liu J, Du H, Kang X et al (2014) MiR-133b regulates the expression of the Actin protein TAGLN2 during oocyte growth and maturation: a potential target for infertility therapy. PLoS ONE 9(6):e100751
Hasuwa H, Ueda J, Ikawa M, Okabe M (2013) miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 341(6141):71–73
Carletti MZ, Fiedler SD, Christenson LK (2010) MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells1. Biol Reprod 83(2):286–295. https://doi.org/10.1095/biolreprod.109.081448
Sinha PB, Tesfaye D, Rings F, Hossien M, Hoelker M, Held E et al (2017) MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation. J Ovarian Res 10(1):37
Yuan C, Li N, Mao X, Liu Z, Ou W, Wang SY (2017) Elevated pretreatment neutrophil/white blood cell ratio and monocyte/lymphocyte ratio predict poor survival in patients with curatively resected non-small cell lung cancer: results from a large cohort. Thorac Cancer 8(4):350–358
Liu J, Du X, Zhou J, Pan Z, Liu H, Li Q (2014) MicroRNA-26b functions as a proapoptotic factor in porcine follicular granulosa cells by targeting Sma-and Mad-related protein 41. Biol Reprod 91(6):146. https://doi.org/10.1095/biolreprod.114.122788
Liu J, Yao W, Yao Y, Du X, Zhou J, Ma B et al (2014) MiR-92a inhibits porcine ovarian granulosa cell apoptosis by targeting Smad7 gene. FEBS Lett 588(23):4497–4503
Zhou J, Liu J, Pan Z, Du X, Li X, Ma B et al (2015) The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-β type 1 receptor. Mol Cell Endocrinol 409:103–112
Cao R, Wu WJ, Zhou XL, Xiao P, Wang Y, Liu HL (2015) Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol Cells 38(4):304–311
Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlyncek M (2010) Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol 223(1):49–56
Wang J, Xu B, Tian GG, Sun T, Wu J (2018) Ablation of the MiR-17-92 MicroRNA cluster in germ cells causes subfertility in female mice. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 45(2):491–504
Andrei D, Nagy RA, van Montfoort A, Tietge U, Terpstra M, Kok K et al (2019) Differential miRNA expression profiles in cumulus and mural granulosa cells from human pre-ovulatory follicles. MicroRNA 8(1):61–67
Du X, Li Q, Pan Z, Li Q (2016) Androgen receptor and miRNA-126* axis controls follicle-stimulating hormone receptor expression in porcine ovarian granulosa cells. Reproduction 152(2):161–169
Xu S, Linher-Melville K, Yang BB, Wu D, Li J (2011) Micro-RNA378 (miR-378) regulates ovarian estradiol production by targeting aromatase. Endocrinology 152(10):3941–3951
Shin C, Nam J-W, Farh KK-H, Chiang HR, Shkumatava A, Bartel DP (2010) Expanding the microRNA targeting code: functional sites with centered pairing. Mol Cell 38(6):789–802
Chen X, Xie M, Liu D, Shi K (2015) Downregulation of microRNA-146a inhibits ovarian granulosa cell apoptosis by simultaneously targeting interleukin-1 receptor-associated kinase and tumor necrosis factor receptor-associated factor 6. Mol Med Rep 12(4):5155–5162
Liu J, Li X, Yao Y, Li Q, Pan Z, Li Q (2018) miR-1275 controls granulosa cell apoptosis and estradiol synthesis by impairing LRH-1/CYP19A1 axis. Biochim Biophys Acta Gene Regul Mech 1861(3):246–257
Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK (2010) The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315(1):63–73. Available from: https://www.sciencedirect.com/science/article/pii/S0303720709005048
Xiong F, Hu L, Zhang Y, Xiao X, Xiao J (2016) miR-22 inhibits mouse ovarian granulosa cell apoptosis by targeting SIRT1. Biol Open 5(3):367–371
Li X, Jin Y, Mu Z, Chen W, Jiang S (2017) MicroRNA-146a-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes. Int J Oncol 51(1):327–335
Naji M, Aleyasin A, Nekoonam S, Arefian E, Mahdian R, Amidi F (2017) Differential expression of miR-93 and miR-21 in granulosa cells and follicular fluid of polycystic ovary syndrome associating with different phenotypes. Sci Rep 7(1):14671
Zhang J, Xu Y, Liu H, Pan Z (2019) MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod Biol Endocrinol 17(1):1–11
Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L et al (2010) Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010:369549
Haikalis ME, Wessels JM, Leyland NA, Agarwal SK, Foster WG (2018) MicroRNA expression pattern differs depending on endometriosis lesion type. Biol Reprod 98(5):623–633
Saare M, Rekker K, Laisk-Podar T, Rahmioglu N, Zondervan K, Salumets A et al (2017) Challenges in endometriosis miRNA studies - from tissue heterogeneity to disease specific miRNAs. Biochim Biophys Acta Mol Basis Dis 1863(9):2282–2292
Yang Y-M, Yang W-X (2017) Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 8(25):41679–41689
Falcone T, Flyckt R (2018) Clinical management of endometriosis. Obstet Gynecol 131(3):557–571
Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS (2011) MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab 96(12):E1925–E1933
Grechukhina O, Petracco R, Popkhadze S, Massasa E, Paranjape T, Chan E et al (2012) A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med 4(3):206–217
Yoo J-Y, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C et al (2017) KRAS Activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep 7(1):6765
Cho S, Mutlu L, Zhou Y, Taylor HS (2016) Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil Steril 106(3):673–680
Macer ML, Taylor HS (2012) Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 39(4):535–549
Zhang H, Wang X, Chen Z, Wang W (2015) MicroRNA-424 suppresses estradiol-induced cell proliferation via targeting GPER in endometrial cancer cells. Cell Mol Biol (Noisy-le-grand) 61(7):96–101
Ohlsson Teague EMC, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA et al (2009) MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23(2):265–275
Kiba A, Kouji B, Yanokura M, Asada M, Nakayama Y, Aoki D et al (2016) Differential micro ribonucleic acid expression profiling in ovarian endometrioma with leuprolide acetate treatment. J Obstet Gynaecol Res 42:1734–1743
Chang CY-Y, Chen Y, Lai M-T, Chang H-W, Cheng J, Chan C et al (2013) BMPR1B up-regulation via a miRNA binding site variation defines endometriosis susceptibility and CA125 levels. PLoS One 8(12):e80630
Yang RQ, Teng H, Xu XH, Liu SY, Wang YH, Guo FJ, Liu XJ (2016) Microarray analysis of microRNA deregulation and angiogenesis-related proteins in endometriosis. Genet Mol Res. 15(2):gmr 7826 10.4238/gmr.15027826. https://doi.org/10.4238/gmr.15027826.
Brabletz S, Brabletz T (2010) The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 11(9):670–677. https://doi.org/10.1038/embor.2010.117
Li X, Zhang W, Fu J, Xu Y, Gu R, Qu R et al (2019) MicroRNA-451 is downregulated in the follicular fluid of women with endometriosis and influences mouse and human embryonic potential. Reprod Biol Endocrinol 17(1):96
Pan Q, Luo X, Toloubeydokhti T, Chegini N (2007) The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod 13(11):797–806. https://doi.org/10.1093/molehr/gam063
Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S et al (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9(6):582–589. https://doi.org/10.1038/embor.2008.74
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M et al (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102(39):13944–13949
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD et al (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15(2):272–284
Schmidt M, Paes K, De Mazière A, Smyczek T, Yang S, Gray A et al (2007) EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development 134(16):2913–2923
Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK (2007) MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A 104(38):15144–15149
Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC et al (2008) Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol 173(3):700–715
Abe W, Nasu K, Nakada C, Kawano Y, Moriyama M, Narahara H (2013) miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Hum Reprod 28(3):750–761. https://doi.org/10.1093/humrep/des446
Hirakawa T, Nasu K, Abe W, Aoyagi Y, Okamoto M, Kai K et al (2016) miR-503, a microRNA epigenetically repressed in endometriosis, induces apoptosis and cell-cycle arrest and inhibits cell proliferation, angiogenesis, and contractility of human ovarian endometriotic stromal cells. Hum Reprod 31(11):2587–2597. https://doi.org/10.1093/humrep/dew217
Pokrovenko DA, Vozniuk V, Medvediev MV (2021) MicroRNA let-7: a promising non-invasive biomarker for diagnosing and treating external genital endometriosis. Turkish J Obstet Gynecol 18(4):291–297
Dai A, Sun H, Fang T, Zhang Q, Wu S, Jiang Y et al (2013) MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett 587(15):2474–2482
Sirotkin AV, Ovcharenko D, Grossmann R, Lauková M, Mlyncek M (2009) Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol 219(2):415–420
Delbandi A-A, Mahmoudi M, Shervin A, Heidari S, Kolahdouz-Mohammadi R, Zarnani A-H (2020) Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health 20(1):3
Di Leva G, Croce CM (2010) Roles of small RNAs in tumor formation. Trends Mol Med 16(6):257–267
Parikh A, Lee C, Joseph P, Marchini S, Baccarini A, Kolev V et al (2014) microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat Commun 5:2977
Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P et al (2007) MicroRNA signatures in human ovarian cancer. Cancer Res 67(18):8699–8707
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH et al (2008) MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res 14(9):2690–2695
Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK et al (2009) A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol 114(3):457–464
Kim TH, Kim YK, Kwon Y, Heo JH, Kang H, Kim G et al (2010) Deregulation of miR-519a, 153, and 485–5p and its clinicopathological relevance in ovarian epithelial tumours. Histopathology 57(5):734–743
Choi KC, Lee JH, Kim JS, Sabal LA, Lee S, Kim H et al (2015) Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10 228 cases. Neurosurgery 76(4):372–380
Lee H, Park CS, Deftereos G, Morihara J, Stern JE, Hawes SE et al (2012) MicroRNA expression in ovarian carcinoma and its correlation with clinicopathological features. World J Surg Oncol 10(1):174. https://doi.org/10.1186/1477-7819-10-174
Yin G, Chen R, Alvero AB, Fu H-H, Holmberg J, Glackin C et al (2010) TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene 29(24):3545–3553
Ayaz L, Çayan F, Balci Ş, Görür A, Akbayir S, Yıldırım Yaroğlu H et al (2014) Circulating microRNA expression profiles in ovarian cancer. J Obstet Gynaecol J Inst Obstet Gynaecol 34(7):620–624
Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D et al (2007) The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res 67(16):7713–7722
Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A et al (2005) RAS is regulated by the let-7 microRNA family. Cell 120(5):635–647
Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S et al (2013) A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc Natl Acad Sci U S A 110(24):9845–9850
Ji T, Zheng Z-G, Wang F-M, Xu L-J, Li L-F, Cheng Q-H et al (2014) Differential microRNA expression by Solexa sequencing in the sera of ovarian cancer patients. Asian Pac J Cancer Prev 15(4):1739–1743
Xie J, Liu M, Li Y, Nie Y, Mi Q, Zhao S (2014) Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. Cell Mol Immunol 11(5):495–502
Schmid G, Notaro S, Reimer D, Abdel-Azim S, Duggan-Peer M, Holly J et al (2016) Expression and promotor hypermethylation of miR-34a in the various histological subtypes of ovarian cancer. BMC Cancer 16:102
Shapira I, Oswald M, Lovecchio J, Khalili H, Menzin A, Whyte J et al (2014) Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer 110(4):976–983
Chen SF, Liu Z, Chaurasiya S, Dellinger TH, Lu J, Wu X et al (2018) Identification of core aberrantly expressed microRNAs in serous ovarian carcinoma. Oncotarget 9(29):20451–20466
Agostini A, Brunetti M, Davidson B, Tropé CG, Eriksson AGZ, Heim S et al (2018) The microRNA miR-192/215 family is upregulated in mucinous ovarian carcinomas. Sci Rep 8(1):11069
Braga EA, Loginov VI, Burdennyi AM, Filippova EA, Pronina IV, Kurevlev SV et al (2018) Five hypermethylated MicroRNA genes as potential markers of ovarian cancer. Bull Exp Biol Med 164(3):351–355
Su YY, Sun L, Guo ZR, Li JC, Bai TT, Cai XX et al (2019) Upregulated expression of serum exosomal miR-375 and miR-1307 enhance the diagnostic power of CA125 for ovarian cancer. J Ovarian Res 12(1):6. https://doi.org/10.1186/s13048-018-0477-x
Ebrahimi SO, Reiisi S (2019) Downregulation of miR-4443 and miR-5195-3p in ovarian cancer tissue contributes to metastasis and tumorigenesis. Arch Gynecol Obstet 299(5):1453–1458
Oliveira DNP, Carlsen AL, Heegaard NHH, Prahm KP, Christensen IJ, Høgdall CK et al (2019) Diagnostic plasma miRNA-profiles for ovarian cancer in patients with pelvic mass. PLoS One 14(11):e0225249. https://doi.org/10.1371/journal.pone.0225249
Clarke-Pearson DL (2009) Clinical practice. Screening for ovarian cancer. N Engl J Med 361(2):170–177
Kandimalla R, Wang W, Yu F, Zhou N, Gao F, Spillman M et al (2021) OCaMIR-A noninvasive, diagnostic signature for early-stage ovarian cancer: a multi-cohort retrospective and prospective study. Clin Cancer Res 27(15):4277–4286
Song K-W, Zhang Q-G, Tan W-B, Fang Y-N (2020) Diagnostic significance of serum miR-26b and miR-21 expressions in ovarian cancer and their associations with clinicopathological characteristics and prognosis of patients. Eur Rev Med Pharmacol Sci 24(4):1697–1703
Cui Y, Hong S, Zhu X (2020) The accuracy of single MicroRNAs in peripheral blood to diagnose ovarian cancer: an updated meta-analysis. Dis Markers 2020:1075942
Halvorsen AR, Kristensen G, Embleton A, Adusei C, Barretina-Ginesta MP, Beale P et al (2017) Evaluation of prognostic and predictive significance of circulating MicroRNAs in ovarian cancer patients. Dis Markers 2017:3098542
Kumar V, Gupta S, Varma K, Sachan M (2020) microRNA as biomarker in ovarian cancer management: advantages and challenges. DNA Cell Biol 39(12):2103–2124
Nagaraja AK, Creighton CJ, Yu Z, Zhu H, Gunaratne PH, Reid JG et al (2010) A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 24(2):447–463
Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 14(5):270–284
Ramanand SJ, Ghongane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS (2013) Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab 17(1):138–145
Cai G, Ma X, Chen B, Huang Y, Liu S, Yang H et al (2017) MicroRNA-145 negatively regulates cell proliferation through targeting IRS1 in isolated ovarian granulosa cells from patients with polycystic ovary syndrome. Reprod Sci 24(6):902–910
Liu H-Y, Huang Y-L, Liu J-Q, Huang Q (2016) Transcription factor-microRNA synergistic regulatory network revealing the mechanism of polycystic ovary syndrome. Mol Med Rep 13(5):3920–3928
Chen B, Xu P, Wang J, Zhang C (2019) The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 706:91–96
Mao Z, Fan L, Yu Q, Luo S, Wu X, Tang J et al (2018) Abnormality of Klotho signaling is involved in polycystic ovary syndrome. Reprod Sci 25(3):372–383
Sørensen AE, Wissing ML, Englund ALM, Dalgaard LT (2016) MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. J Clin Endocrinol Metab 101(4):1579–1589
Ding C-F, Chen W-Q, Zhu Y-T, Bo Y-L, Hu H-M, Zheng R-H (2015) Circulating microRNAs in patients with polycystic ovary syndrome. Hum Fertil (Camb) 18(1):22–29
Scalici E, Traver S, Mullet T, Molinari N, Ferrières A, Brunet C et al (2016) Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci Rep 6:24976
Lin L, Du T, Huang J, Huang L-L, Yang D-Z (2015) Identification of differentially expressed microRNAs in the ovary of polycystic ovary syndrome with hyperandrogenism and insulin resistance. Chin Med J (Engl) 128(2):169–174
Xu B, Zhang Y-W, Tong X-H, Liu Y-S (2015) Characterization of microRNA profile in human cumulus granulosa cells: identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol Cell Endocrinol 404:26–36
Wu H-L, Heneidi S, Chuang T-Y, Diamond MP, Layman LC, Azziz R et al (2014) The expression of the miR-25/93/106b family of micro-RNAs in the adipose tissue of women with polycystic ovary syndrome. J Clin Endocrinol Metab 99(12):E2754–E2761. https://doi.org/10.1210/jc.2013-4435
Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X et al (2015) MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab 100(5):E729–E738
Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X et al (2013) Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab 98(7):3068–3079
Zhang C-L, Wang H, Yan C-Y, Gao X-F, Ling X-J (2017) Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem Biophys Res Commun 482(4):1469–1476
Eisenberg I, Nahmias N, Novoselsky Persky M, Greenfield C, Goldman-Wohl D, Hurwitz A et al (2017) Elevated circulating micro-ribonucleic acid (miRNA)-200b and miRNA-429 levels in anovulatory women. Fertil Steril 107(1):269–275
Chuang T-Y, Wu H-L, Chen C-C, Gamboa GM, Layman LC, Diamond MP et al (2015) MicroRNA-223 expression is upregulated in insulin resistant human adipose tissue. J Diabetes Res 2015:943659
Yuan WN, Tan L (2017) MicroRNA-320 inhibits insulin resistance in patients with PCOS through regulating ERK1/2 signaling pathway. Biomed Res 28(11):4946–4949
Yin M, Wang X, Yao G, Lü M, Liang M, Sun Y et al (2014) Transactivation of micrornA-320 by microRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 proteins. J Biol Chem 289(26):18239–18257
Huang X, Liu C, Hao C, Tang Q, Liu R, Lin S et al (2016) Identification of altered microRNAs and mRNAs in the cumulus cells of PCOS patients: miRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction 151(6):643–655
Song J, Luo S, Li S-W (2015) miRNA-592 is downregulated and may target LHCGR in polycystic ovary syndrome patients. Reprod Biol 15(4):229–237
Deswal R, Dang AS (2020) Dissecting the role of micro-RNAs as a diagnostic marker for polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril 113(3):661–669.e2
Acknowledgements
None.
Funding
The authors declare that this study had no external funding resources.
Author information
Authors and Affiliations
Contributions
IK, DD, SM, AHS, MCS, LS: conceptualization; IK, MCS, LS: visualization; IK, DD, SM, AHS, MCS: writing, original draft; IK, DD, SM, AHS, MCS, LS: writing, review, editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kusumaningtyas, I., Dasuki, D., Harjana, S.M. et al. Unraveling the microRNAs, key players in folliculogenesis and ovarian diseases. Middle East Fertil Soc J 29, 13 (2024). https://doi.org/10.1186/s43043-024-00173-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-024-00173-x