Abstract
BACKGROUND:
Rheumatoid arthritis (RA) is characterized by chronic inflammation and joint damage. Methotrexate (MTX), a commonly used disease-modifying anti-rheumatic drug (DMARD) used in RA treatment. However, the continued use of DMARDs can cause adverse effects and result in limited therapeutic efficacy. Cartilage extracellular matrix (CECM) has anti-inflammatory and anti-vascular effects and promotes stem cell migration, adhesion, and differentiation into cartilage cells.
METHODS:
CECM was assessed the dsDNA, glycosaminoglycan, collagen contents and FT-IR spectrum of CECM. Furthermore, we determined the effects of CECM and MTX on cytocompatibility in the SW 982 cells and RAW 264.7 cells. The anti-inflammatory effects of CECM and MTX were assessed using macrophage cells. Finally, we examined the in vivo effects of CECM in combination with MTX on anti-inflammation control and cartilage degradation in collagen-induced arthritis model. Anti-inflammation control and cartilage degradation were assessed by measuring the serum levels of RA-related cytokines and histology.
RESULTS:
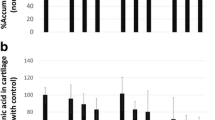
CECM in combination with MTX had no effect on SW 982, effectively suppressing only RAW 264.7 activity. Moreover, anti-inflammatory effects were enhanced when low-dose MTX was combined with CECM. In a collagen-induced arthritis model, low-dose MTX combined with CECM remarkably reduced RA-related and pro-inflammatory cytokine levels in the blood. Additionally, low-dose MTX combined with CECM exerted the best cartilage-preservation effects compared to those observed in the other therapy groups.
CONCLUSION:
Using CECM as an adjuvant in RA treatment can augment the therapeutic effects of MTX, reduce existing drug adverse effects, and promote joint tissue regeneration.








Similar content being viewed by others
Data availability
The data presented in this study are available on request from all the authors.
References
Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. 2021;10:2857.
Dijkshoorn B, Raadsen R, Nurmohamed MT. Cardiovascular disease risk in rheumatoid arthritis anno 2022. J Clin Med. 2022;11:2704.
Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica (Bucur). 2010;5:286.
Chi XK, Xu XL, Chen BY, Su J, Du YZ. Combining nanotechnology with monoclonal antibody drugs for rheumatoid arthritis treatments. J Nanobiotechnology. 2023;21:105.
Fraenkel L, Bathon JM, England BR, St. Clair EW Arayssi T Carandang K, et al. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;2021:1108–23.
Yang N, Li M, Wu L, Song Y, Yu S, Wan Y, et al. Peptide-anchored neutrophil membrane-coated biomimetic nanodrug for targeted treatment of rheumatoid arthritis. J Nanobiotechnology. 2023;21:1–20.
Caplan L, Wolfe F, Russell AS, Michaud K. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J Rheumatol. 2007;34:696–705.
Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15:S2.
Mahmoudi Z, Karamali N, Roghani SA, Assar S, Pournazari M, Soufivand P, et al. Efficacy of DMARDs and methylprednisolone treatment on the gene expression levels of HSPA5, MMD, and non-coding RNAs MALAT1, H19, miR-199a-5p, and miR-1-3p, in patients with rheumatoid arthritis. Int Immunopharmacol. 2022;108:108878.
Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 2019;86:301–7.
García-González CM, Baker J. Treatment of early rheumatoid arthritis: Methotrexate and beyond. Curr Opin Pharmacol. 2022;64:102227.
Nagafuchi H, Goto Y, Kiyokawa T, Ooka S, Kawahata K. Pregnancy outcomes in patients with rheumatoid arthritis who discontinue methotrexate treatment to conceive. Clin Rheumatol. 2022;41:669–75.
Niknahad H, Heidari R, Mohammadzadeh R, Ommati MM, Khodaei F, Azarpira N, et al. Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren Fail. 2017;39:745–53.
Abdollahi AR, Firouzian F, Haddadi R, Nourian A. Indomethacin loaded dextran stearate polymeric micelles improve adjuvant-induced arthritis in rats: Design and in vivo evaluation. Inflammopharmacology. 2021;29:107–21.
Yin N, Tan X, Liu H, He F, Ding N, Gou J, et al. A novel indomethacin/methotrexate/MMP-9 siRNA in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale. 2020;12:8546–62.
Zhao W, Zheng L, Yang J, Ma Z, Tao X, Wang Q. Dissolving microneedle patch-assisted transdermal delivery of methotrexate improve the therapeutic efficacy of rheumatoid arthritis. Drug Deliv. 2023;30:121–32.
Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J Cell Physiol. 2014;229:984–9.
Oh HJ, Kim SH, Cho JH, Park SH, Min BH. Mechanically reinforced extracellular matrix scaffold for application of cartilage tissue engineering. Tissue Eng Regen Med. 2018;15:287–99.
Chua ILS, Kim HW, Lee JH. Signaling of extracellular matrices for tissue regeneration and therapeutics. Tissue Eng Regen Med. 2016;13:1–12.
Lin W, Klein J. Recent progress in cartilage lubrication. Adv Mater. 2021;33:2005513.
Yao Q, Zheng YW, Lan QH, Kou L, Xu HL, Zhao YZ. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater Sci Eng C. 2019;104:109942.
Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials. 2014;35:8605–12.
Tian G, Jiang S, Li J, Wei F, Li X, Ding Y, et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: In vitro and in vivo preclinical study. Acta Biomater. 2021;127:131–45.
Chen ZJ, Zhang Y, Zheng L, Zhang H, Shi HH, Zhang XC, et al. Mineralized self-assembled silk fibroin/cellulose interpenetrating network aerogel for bone tissue engineering. Biomater Adv. 2022;134:112549.
Cheng S, Liu X, Qian Y, Maitusong M, Yu K, Cao N, et al. Double-network hydrogel armored decellularized porcine pericardium as durable bioprosthetic heart valves. Adv Healthc Mater. 2022;11:2102059.
Lih E, Park W, Park KW, Chun SY, Kim H, Joung YK, et al. A bioinspired scaffold with anti-inflammatory magnesium hydroxide and decellularized extracellular matrix for renal tissue regeneration. ACS Cent Sci. 2019;5:458–67.
Luo Y, Fan L, Liu C, Wen H, Wang S, Guan P, et al. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact Mater. 2022;7:98–111.
Park JY, Park SH, Ju HJ, Ji YB, Yun HW, Min BH, et al. Preparation of a cross-linked cartilage acellular matrix-poly (caprolactone-ran-lactide-ran-glycolide) film and testing its feasibility as an anti-adhesive film. Mater Sci Eng C. 2020;117:111283.
Gulfam M, Jo SH, Jo SW, Vu TT, Park SH, Lim KT. Highly porous and injectable hydrogels derived from cartilage acellularized matrix exhibit reduction and NIR light dual-responsive drug release properties for application in antitumor therapy. NPG Asia Mater. 2022;14:8.
Jung CS, Kim BK, Lee J, Min BH, Park SH. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng Regen Med. 2018;15:155–62.
Visscher DO, Lee H, van Zuijlen PPM, Helder MN, Atala A, Yoo JJ, et al. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2021;121:193–203.
Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–87.
Lee SH, Jo SH, Kim SH, Kim CS, Park SH. Anti-osteoarthritic effects of cartilage-derived extracellular matrix in a rat osteoarthritis model. Tissue Eng Regen Med. 2022;20:83–92.
Kim HJ, Lee S, Yun HW, Yin XY, Kim SH, Choi BH, et al. In vivo degradation profile of porcine cartilage-derived extracellular matrix powder scaffolds using a non-invasive fluorescence imaging method. J Biomater Sci Polym Ed. 2016;27:177–90.
Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–75.
Hama S, Kirimura N, Obara A, Takatsu H, Kogure K. Tocopheryl phosphate inhibits rheumatoid arthritis-related gene expression in vitro and ameliorates arthritic symptoms in mice. Molecules. 2022;27:1425.
Giollo A, Fuzzi E, Doria A. Methotrexate in early rheumatoid arthritis: Is the anchor drug still holding? Autoimmun Rev. 2022;21:103031.
Ren S, Liu H, Wang X, Bi J, Lu S, Zhu C, et al. Acupoint nanocomposite hydrogel for simulation of acupuncture and targeted delivery of triptolide against rheumatoid arthritis. J Nanobiotechnology. 2021;19:409.
Rivellese F, Surace AEA, Goldmann K, Sciacca E, Çubuk C, Giorli G, et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat Med. 2022;28:1256–68.
Lin YJ, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9:880.
Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77.
Benders KEM, Weeren PRV, Badylak SF, Saris DBF, Dhert WJA, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–76.
Rowley AT, Nagalla RR, Wang SW, Liu WF. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv Healthc Mater. 2019;8:1801578.
Capella-Monsonís H, Tilbury M, Wall J, Zeugolis D. Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater Today Bio. 2020;7:100057.
Yin H, Lu Q, Wang X, Majumdar S, Jun AS, Stark WJ, et al. Tissue-derived microparticles reduce inflammation and fibrosis in cornea wounds. Acta Biomater. 2019;85:192–202.
Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int. 2017;2017:9831534.
Vidal BdC, Mello MLS. FT-IR microspectroscopy of rat ear cartilage. PLoS ONE. 2016;11:e0151989.
Prekasan D, Saju KK. Review of the tribological characteristics of synovial fluid. Proc Technol. 2016;25:1170–4.
Kim EA, Kim SY, Ye BR, Kim J, Ko SC, Lee WW, et al. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int Immunopharmacol. 2018;59:339–46.
Nguyen VT, Ko SC, Oh GW, Heo SY, Jeon YJ, Park WS, et al. Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells. Int J Biol Macromol. 2016;93:1620–32.
Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci. 2020;21:379.
Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. 2011;122:143–59.
Takeuchi T, Yoshida H, Tanaka S. Role of interleukin-6 in bone destruction and bone repair in rheumatoid arthritis. Autoimmun Rev. 2021;20:102884.
Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F, et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front Immunol. 2021;12:637829.
Lubberts E. The IL-23–IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:415–29.
Skrzypczak-Wiercioch A, Sałat K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules. 2022;27:5481.
Wang Y, Li Y, Xu Y. Pyroptosis in kidney disease. J Mol Biol. 2022;434:167290.
Acknowledgement
This research was supported by the Pukyong National University Research Fund in 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical statement
The animal studies were approved by the Institutional Review Board of Pukyong National University (PKNUIACUC-2022–06).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, JW., Jo, SH., Kim, SH. et al. Application of Cartilage Extracellular Matrix to Enhance Therapeutic Efficacy of Methotrexate. Tissue Eng Regen Med 21, 209–221 (2024). https://doi.org/10.1007/s13770-023-00587-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00587-0