Abstract
Rheumatoid arthritis (RA) is a systemic immune disease characterized by synovial inflammation. Patients with RA commonly experience significant damage to their hand and foot joints, which can lead to joint deformities and even disability. Traditional treatments have several clinical drawbacks, including unclear pharmacological mechanisms and serious side effects. However, the emergence of antibody drugs offers a promising approach to overcome these limitations by specifically targeting interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and other cytokines that are closely related to the onset of RA. This approach reduces the incidence of adverse effects and contributes to significant therapeutic outcomes. Furthermore, combining these antibody drugs with drug delivery nanosystems (DDSs) can improve their tissue accumulation and bioavailability.Herein, we provide a summary of the pathogenesis of RA, the available antibody drugs and DDSs that improve the efficacy of these drugs. However, several challenges need to be addressed in their clinical applications, including patient compliance, stability, immunogenicity, immunosupression, target and synergistic effects. We propose strategies to overcome these limitations. In summary, we are optimistic about the prospects of treating RA with antibody drugs, given their specific targeting mechanisms and the potential benefits of combining them with DDSs.
Similar content being viewed by others
Introduction
Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease, characterized by chronic synovitis and joint injury. In severe cases, bone erosion can occur, leading to loss of joint function and even disability. This condition can seriously affect the patient’s quality of life and longevity. The main clinical symptoms in patients with RA are morning stiffness, joint swelling, and pain, which can also affect extra-articular organs. In addition, patients typically exhibit elevated indices, such as rheumatoid factor (RF), anti-citrullinated protein/peptide antibody (ACPA), and other characteristic indicators [1]. Globally, the incidence of RA in the population is approximately 0.5%, and it is related to sex. The morbidity rate is approximately 3.6% in adult women and 1.7% in men, with women being affected 2–3 times more often than men [2,3,4].
Recently, the pathogenesis of RA remains inconclusive. It is generally agreed that multifactor, such as genetic and environmental factors, synergistically disturbs the immune system, resulting in unnecessary immune responses. Autoreactive T lymphocytes and B lymphocytes promote the immune response against autoantigens, which is considered the central driving factor of the disease. T lymphocytes can differentiate into a variety of helper T-cell (Th cell) subsets and secrete abundant inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-17 (IL-17), which infiltrate, aggregate, and invade the synovium of joints, resulting in inflammation [5, 6].
B lymphocytes produce antibodies known as RFs, which also mediate the occurrence of inflammation. Fibroblast-like synoviocytes (FLSs), the primary cells of the synovium, can release cytokines and chemokines and exhibit obvious invasion in the synovium. Therefore, FLSs are considered the fundamental participants in synovitis. In addition, FLSs can produce matrix metalloproteinases (MMPS), which can disrupt cartilage or joint damage in patients [7, 8, 12].
Macrophages, which are involved in the body’s nonspecific immunity, produce related inflammatory factors such as IL-1, IL-6, and TNF-α. These cytokines will stimulate FLSs and further activate osteoclasts (OCs), leading to bone damage [9,10,11]. Reports have shown that receptor activator for nuclear factor-κ B ligand (RANKL) is indispensable in osteoclast differentiation and activation, and the number and activity of osteoclasts are key factors in bone destruction [13, 14]. (Fig. 1)
Pathogenesis of rheumatoid arthritis. The occurrence of rheumatoid arthritis (RA) is attributed to the activation of immune cells such as T cells, B cells, macrophages, and dendritic cells. B cells release rheumatoid factor (RF), and dendritic cells differentiate into osteoclasts, leading to bone erosion. T cells secrete receptor activator for nuclear factor-κ B ligand (RANKL) and activate osteoclasts, resulting in cartilage destruction. The overproduction of matrix metalloproteinases (MMPs) by fibroblast-like synoviocytes (FLSs) is also a critical factor in cartilage damage. Excessive immune complex activates the complement system and mediates the invasion process of inflammation. Additionally, interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) not only cause the accumulation of inflammation at joints but also systemic inflammation. Abbreviation:TNF: tumor necrosis factor; IL-1: interleukin 1; IL-6: interleukin 6; FLSs: fibroblast-like synoviocytes; MMPS: matrix metalloproteinases; RF: rheumatoid factor; RANKL: receptor activator for nuclear factor-κ B ligand;M-CSF:macrophage-stimulating factor;ACPA:Anti-citrullinated peptide antibodies
Therapeutic approachs
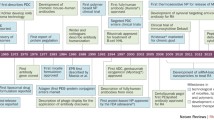
Clinically, the treatment of RA typically involves surgery and drug therapy aimed at eliminating excessive immune complexes, alleviating pain, delaying inflammation, and maintaining bone and joint function [15, 16]. However, surgery can easily result in secondary joint cavity injury, and removal of the synovial membrane can completely impede synovial fluid synthesis and further compromise joint function. In comparison, drug treatment is less invasive and more versatile, offering an expanding range of options for clinical treatment [17]. The drugs used to treat RA include disease-modifying anti-rheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), and antibody drugs [18, 25] (Fig. 2). Despite their clinical benefits, DMARDs have poor specificity and low bioavailability, which make it difficult to deliver these drugs to their target in vivo [19]. Furthermore, the toxicity and side effects associated with DMARDs pose a significant risk to patient health. For example, methotrexate (MTX), the most widely used DMARD, can cause ulcerative stomatitis at high doses and liver and kidney damage after long-term use [20]. NSAIDs, although useful as transitional treatment, have limited effectiveness in treating chronic inflammation and carry a high risk of serious side effects [21, 22]. GCs play a critical role in the treatment of inflammatory diseases and exhibit good immunosuppressive effects, but their long-term use raises safety concerns [23]. In contrast, antibody drugs have several advantages, including fewer side effects and better safety profiles, superior efficacy, and high specificity for their target sites [24]. Additionally, their biological pharmacological mechanism is clear, and they have broad applications in treating RA.
Antibody drugs can fundamentally eliminate high-level immune complexes, inhibit inflammatory factors, and alleviate damage to cartilage and synovium, which effectively improve symptoms and relieve pain. Consequently, antibody drugs represent a safe, reliable, and effective treatment method for RA.
Current drugs used to treat rheumatoid arthritis. There are several treatment strategies available for rheumatoid arthritis, including non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), disease-modifying anti-rheumatic drugs (DMARDs), and antibody drugs. While NSAIDs can effectively reduce pain in patients, they have no influence on the progress of the disease and do not reduce cartilage damage. Glucocorticoids can rapidly reduce pain, but their usage is limited due to serious side effects, particularly in elderly and pediatric patients. Antibody drugs have clear targets and can reduce inflammation at the lesion, reducing bone and joint injury, and clearing immune complexes and cytokines. However, long-term usage may cause drug tolerance problems. Abbreviation: NASIDS: Non-steroidal anti-inflammatory drugs; GCs: Glucocorticoids; DMARDs: Disease-modifying anti-rheumatic drugs
Antibody drugs for the treatment of RA
With the continuous development of molecular biology, we have gained a deeper understanding of the pathogenesis and etiology of RA. In addition, the continuous progress and maturity of biochemistry and nanotechnology have greatly increased the potential application of antibody drugs. Currently, approved antibody drugs are classified into several types based on their mechanisms of action, including TNF-α inhibitors, interleukin 1 inhibitors, interleukin 6 inhibitors, CD80/86-CD 28 inhibitors, and B-cell eliminating antibodies. Antibody drugs that are used for the treatment of RA are shown in Table 1.
TNF-α inhibitors
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine produced primarily by activated macrophages, T lymphocytes, and natural killer cells [26]. Initially believed to be solely responsible for causing tumor necrosis, TNF-α was later found to be a pathological component of autoimmune diseases and play an essential role in the etiology of RA. Abnormal production of TNF-α mediates synovial hyperplasia and generates other proinflammatory factors, such as prostaglandin (PG) and matrix metalloproteinases (MMPS) [31]. In addition, TNF-α can stimulate bone cells to secrete the receptor activator for nuclear factor-κ B ligand (RANKL), indirectly promoting the formation of osteoclasts, and synergize with various factors to induce RA [27, 28]. Therefore, a broad consensus has been reached to alleviate the disease by eliminating the abundant TNF-α in the inflammatory site. TNF-α inhibitors are the most widely used biological drugs to treat RA. Currently, five TNF-α inhibitors have been approved worldwide for the treatment of RA, including etanercept, infliximab, adalimumab, certolizumab, and golimumab [29]. Although all anti-TNF-α drugs can competitively bind to TNF-α receptors on the cell surface, inhibiting TNF-α biological activity and blocking TNF-mediated cellular responses, the drugs differ in their molecular structures and administration schemes [30].
Etanercept was the first TNF-α inhibitor discovered and the first specific anti-cytokine therapy developed for RA. Its clinical efficacy and safety have been confirmed in early clinical trials [31]. As a TNF-α blocker, etanercept not only inhibits tumor necrosis but also has FC effector activity, which can induce antibody-dependent cell-mediated cytotoxicity (ADCC) and trigger the complement pathway to produce complement-dependent cytotoxicity (CDC) and target immune cell apoptosis [32]. Studies have shown that in cases where patients with RA are inadequate responders to MTX, combining MTX with etanercept leads to significant improvement [33]. In addition, before etanercept was used, immunomodulators were utilized to effectively inhibit antibody production and reduce immunogenicity. However, of particular note, long-term injection of etanercept may induce targeted toxicity, including severe infection, and increase the risk of malignancy and tuberculosis during the course of RA treatment [34].
Infliximab is a chimeric monoclonal antibody that binds to the FC region of human immunoglobulin G 1 (IgG1) and contains a variable region (Fab) of mouse anti-TNF-α. Infliximab can bind to both free types and TNF-α on the cell membrane and competitively inhibit the binding of cytokines and related receptors, with its ADC effect and CDC effect superior to those of etanercept [35].
Adalimumab is the first fully humanized TNF-α monoclonal antibody and the third TNF-α inhibitor after etanercept and infliximab. The Fab fragment of adalimumab binds to TNF-α through a large, highly complementary, strong and stable interface (including the formation of hydrogen bonds and salt bridges) [36]. Adalimumab can also mediate the ADCC effect and exhibits good tolerance and effectiveness in treating RA.
Certolizumab is a pegylated recombinant Fab fragment of a humanized anti-TNF-α monoclonal antibody that binds TNF-α with high affinity and selectivity. Unlike other anti-TNF-α drugs, certolizumab lacks a crystallizable IgG fragment (FC) region and therefore does not mediate CDC and ADCC in vitro. Moreover, certolizumab does not induce granulocyte degranulation or apoptosis of peripheral blood lymphocytes and monocytes in vitro, but it appears to induce the death of nonapoptotic cells [37]. Common adverse reactions related to certolizumab include upper respiratory tract infections, rashes, and urinary tract infections [38].
Golimumab, a human IgG1 monoclonal antibody, is produced by mouse hybridoma cell lines using recombinant techniques and functions by targeting and neutralizing TNF-α. After a single subcutaneous injection, the average time to reach the maximum plasma concentration was 2–7 days. After 12 weeks of injection, a steady-state concentration of blood is reached, with an average absolute bioavailability of approximately 50%. Despite good therapeutic outcome via alone application, its effect is better when combined with MTX. When combined with MTX, the average steady-state valley concentration of the drug is approximately 30% higher than that of patients taking golimumab alone, and the apparent plot ratio is approximately 35% lower [39]. Relevant studies have shown that obese patients exhibit decreased sensitivity to the efficacy of TNF-α inhibitors and have poor efficacy [40].
Interleukin 1 inhibitor
Interleukin-1 (IL-1) is not a single cytokine but a group of cytokines that can exert local or systemic effects, and it is associated with inflammation and the innate immune response [41]. Furthermore, cytokines from different IL-1 families exhibit diverse physiological effects. For example, cytokines such as IL-1α, IL-1β, and IL-33 exhibit proinflammatory activity, whereas cytokines such as IL-1Ra, IL-37, and IL-38 demonstrate anti-inflammatory activity [42]. Generally, IL-1 and IL-1Ra are in a relatively dynamic balance in healthy organisms to maintain normal vital activities. However, when the amount of IL-1 in the body increases, this balance is disrupted, resulting in inflammation and disease. Therefore, IL-1-mediated proinflammatory responses can be inhibited by blocking IL-1 signaling, which involves competitively inhibiting the specific binding of IL-1 to its receptor by IL-1 receptor antagonists.
Anakinra is a recombinant human IL-1 receptor antagonist with a short half-life and must be injected subcutaneously daily. Common adverse reactions to anakinra include local injection infection, high-dose infection, and immunogenicity [43]. Compared to TNF-α inhibitors, anakinra is less effective in treating RA and is therefore not a primary treatment for RA [44]. However, anakinra has shown promise in treating other diseases, and recent studies have demonstrated its efficacy in treating recurrent pericarditis and adult-onset Still’s disease [45,46,47,48].
Interleukin 6 inhibitor
Interleukin-6 (IL-6) is a multifunctional cytokine that is secreted by various cells, such as B cells, T cells, and phagocytes, and specifically binds to corresponding receptors to exert biological effects. IL-6 participates in numerous biological processes and plays a significant role in pathological processes such as synovitis, bone erosion, and inflammation, including B-cell proliferation, antibody production, and T-cell differentiation. IL-6 also promotes hepatocytes to produce acute-phase proteins, induce leukocytosis and angiogenesis, and activates synovial fibroblasts to express matrix metalloproteinase, causing cartilage damage [49, 50]. Patients with RA usually have elevated IL-6 levels. Therefore, IL-6 is also considered a key cytokine in the pathogenesis of RA.
Currently, IL-6 inhibitors can be divided into the two categories based on their different targets: (1) specifically bind to IL-6 receptors to block IL-6 signaling, including tocilizumab and sarilumab, (2) directly bind to IL-6 to perform a function, including sirukumab and olokizumab.
Tocilizumab is the first IL-6 inhibitor discovered and can be administered intravenously or subcutaneously as a humanized monoclonal antibody for treating moderate to severe RA [51]. Studies have shown that tocilizumab reduces IL-6 levels in the body and lowers the level of cyclic citrullinated peptide antibodies (CCPs) [52]. Moreover, tocilizumab’s cardiovascular risk is not significantly different from abatacept or etanercept [53, 54]. The most common adverse effects of tocilizumab are skin infections, neutropenia, thrombocytopenia, and dyslipidemia[55, 56].
Salizumab is a humanized monoclonal antibody that binds to the IL-6 receptor to function and is generally administered via subcutaneous injection. Compared to tocilizumab, salizumab exhibits better affinity and a longer half-life [57, 58]. Furthermore, salizumab is more effective in improving body function than adalimumab. Besides, salizumab may be useful in patients who are not sensitive to conventional anti-rheumatoid drugs or who do not respond adequately to TNF-α inhibitors [59,60,61]. The safety of salizumab is similar to that of tocilizumab, with common adverse reactions including infection, erythema at the injection site, and neutropenia. In addition, to reduce the risk of infection, it is recommended that an active vaccine should be avoided while the drug is being administered[62]. Other studies have proven that salizumab exerts a positive effect in reducing pain, regulating mood, and reducing fatigue in patients[63].
CD80/86-CD 28 inhibitor
T cells are essential in the immune system, but an excessive T-cell response can contribute to RA. Cluster of differentiation (CD) proteins, which are located on the surface of cell membrane, play a crucial role in T-cell activation. CD4 + T cells are the primary T cells involved in synovial infiltration and inflammation. To activate T cells, two signals are required: specific binding of T-cell receptor to major histocompatibility complex (MHC) molecules on antigen-presenting cells (APC cells) and costimulation signaling (binding of CD80 or CD86 on APC cells to CD28 ligands on the surface of T cells). Costimulation signaling is vital in the T-cell activation process. Cytotoxic T-lymphocyte-associated protein 4 (CTLA 4) is a membrane protein that activates T-cell expression, and its structure is similar to that of CD28. Therefore, CTLA-4 inhibitors can prohibit T-cell activation by competing with CD28 and interfering with its binding to CD80 or CD86 [1].
Abatacept is a whole-human recombinant protein composed of the extracellular domain of CTLA 4 and the Fc portion of IG1. Therefore, abatacept can bind to CD80 and CD86 on the surface of APCs and competitively inhibit CD80 and CD86 costimulatory signals. Abatacept can be used for subcutaneous or intravenous administration (both of which have shown similar safety and efficacy properties). Abatacept exerts beneficial effects on clinical symptoms, structural damage, and physical functioning in patients, including those with inadequate responses to TNF-α inhibitors or those for whom MTX is ineffective. Studies have shown that after 12 months of abatacept treatment (10 mL/kg), serum levels of IL-6, C-reactive protein (CRP), and soluble IL-2 receptors were significantly reduced compared with those in the placebo group, and the proportion of memory B cells was correspondingly reduced. Combination therapy with abatacept and MTX has better efficacy than MTX monotherapy, with comparable safety profiles [64, 67, 68]. However, RA patients with interstitial lung disease (ILD) may face the risk of ILD exacerbation when treated with abatacept in combination with MTX. Therefore, if ILD exacerbation occurs with associated complications, MTX should be discontinued immediately [69]. Abatacept is also well tolerated when used in combination with non-MTX anti-rheumatoid drugs that are chemically modified and exhibit similar clinical efficacy to MTX. Abatacept has no significant adverse effects, and the most common adverse reactions are upper respiratory tract infections, nausea, and headache. Owing to the increased risk of serious infections [27, 65], abatacept should not be used simultaneously with TNF-α inhibitors [70].
B-Cell depleting antibodies
The overabundance of autoantibodies in the body constitutes a crucial factor in the development of autoimmune diseases. In the past few decades, diminishing the number of B cells and their related antibodies has been considered a crucial approach to treating autoimmune diseases [71, 72]. B cells are intimately associated with the pathogenesis of RA. The antigen presentation of B cells is involved in the autoreactive T-cell activation process, and B cells disorderly undergo apoptosis and secrete an excessive number of pathogenic antibodies (RF, CCP, chemokines, etc.), which form immune complexes. Moreover, activating the complement system eventually leads to cell damage. B cell-related autoantibodies may result in infection through the development of inflammatory cells and stimulation of the apoptosis pathway [1].
CD20 is a B-cell differentiation antigen that exists on the surface of B cells at all stages of development and differentiation but is not expressed on plasma cells. It plays a vital role in the proliferation and differentiation of B cells by regulating the flow of synovial calcium ions. Rituximab (RTX), a chimeric human-mouse monoclonal antibody, binds to the CD20 membrane receptor on the surface of B cells and directly induces B-cell apoptosis, eventually leading to B-cell depletion through host-effect mechanisms, such as mediating ADCC and CDC [73]. The combination of RTX and MTX was superior to MTX and placebo, and there was no significant difference in safety. RTX can be used in patients with moderate to severe RA and is also effective in some patients who do not respond adequately to DMARDs or at least one TNF-α inhibitor [27]. Additionally, regardless of the dosage, when RTX is used for RA, MRI found that at week 24, the imaging progress was significantly reduced [74, 75], which met the 20% improvement criteria of American College of Rheumatology’s (ACR20).
Better treatment results were obtained for patients with RF- and CCP-positive RA when using RTX. Studies have shown that after failure of one TNF-α inhibitor, switching to RTX may be a better approach than switching to another TNF-α inhibitor [75]. The safety of RTX treatment for RA is controllable, and the most common adverse reactions during the first infusion include headache, fever, rash, dyspnea, hypotension, nausea, and mild angioedema. Up to 30%~45% of patients will experience these adverse reactions, but they can be relieved by reducing the rate of drug instillation and taking glucose corticosteroids and antihistamines [16, 78]. Compared with non-RTX drugs, patients using RTX have no additional risk of infection, and there is no significant effect on immunoglobulin levels in patients [76]. Furthermore, long-term use of RTX did not cause significant cumulative side effects and was well-tolerated [77].
Means to improve the efficacy of monoclonal antibody drugs
Compared to chemically synthesized drugs, antibody drugs have more explicit targets and lower incidence of severe adverse reactions. However, antibody drugs also exhibit various limitations. For instance, the stability of biological protein drugs may not meet the expected standards, and delivery to target sites in a timely manner can be challenging. Furthermore, many antibody drugs require injection, which can be inconvenient for patients to self-administer. To resolve these problems and improve drug efficacy, different nanocarriers (Fig. 3; Table 2) have garnered increasing attention.
Nanocarriers applied to load drugs. Some nanocarriers can be utilized to overcome the limitations of antibody drugs and enhance their therapeutic efficacy. For instance, micelles are easily to functionalize, which can improve drug targeting. Dendrimers have a large specific surface area, which enhances drug loading capacity and promotes effective drug distribution. Gold nanoparticles are capable of tunable size, photothermal conversion and high biocompatibility, making them useful in combination therapy. Nanogels have revolutionized the way of administration of conventional antibody drugs, since they can be applied directly to the skin surface, thereby improving patient compliance. Albumin, an endonegous protein, dispalys high safety and compatibility. Loading antibody drugs with it can enhance their affinity and reduce adverse reactions
Gold nanoparticles (GNPs)
In contrast to conventional nanoparticles, gold nanoparticles (GNPs) are increasingly favored by researchers for their effectiveness and stability in drug delivery. GNPs exhibit good biocompatibility, can be prepared in various sizes and shapes according to practical needs, are relatively easy to obtain, and exhibit modifiable and adjustable optical properties, as well as a high drug loading capability. In addition, their noncytotoxicity and lack of serious side effects make them safe for use. In short, due to their excellent performance, the application of GNPs in the biomedical field holds great potential [111,112,113]. For example, high-atomic-number GNPs can preferentially absorb X-rays to enhance the effect of radiation therapy. In addition, GNPs can be used as nanoprobes and contrast agents for diagnosing RA [113, 114]. GNPs not only exhibit good targeting performance but also have a positive effect on treatment. Studies have shown that GNPs can be combined with vascular endothelial growth factor (VEGF) to exhibit anti-angiogenic effects, which is the maor pathological condition of RA. Zeng et al. [112] found that GNPs are also an important antioxidant that promotes osteogenesis and stem cell proliferation, inhibits RANKL-induced osteoclast production, reduces inflammation levels, and reduces bone erosion or cartilage destruction. Lee H et al. [104] designed a new hyaluronic acid-GNP-tocilizumab (termed as HA-GNP-TCZ) drug delivery systems Firstly, HA was modified with cystamine via reductive amination to synthesize end-group thiolated HA. AuNPs were prepared by reducing and stabilizing HAuCl4 with sodium citrate under boiling conditions. The binding of tocilizumab and thiolated HA onto GNPs increases the stability of GNPs and reduces specific binding to serum proteins in vivo. Most importantly, HA-GNP-TCZ can target both IL-6 and VEGF (Fig. 4). After HA-GNP-TCZ treatment, the level of inflammatory cell infiltration, cartilage destruction and bone erosion decreased significantly. Especially, the interface between cartilage and bone was similar to that of the normal control group. It is noteworthy that no synovial hypertrophy was observed in the HA-GNP-TCZ complex treatment group, in contrast to the synovial hypertrophy with cell infiltration in the TCZ treatment group. Besides, the expression levels of IL-6 and CD68 were significantly decreased after treatment with the HA-GNP-TCZ complex, while they were significantly increased in the negative control group. Shahen et al. [115] showed that targeted treatment of TCZ delivered by GNPs alleviated the narrowing of the joint space and bone erosion, as well as the inflammation. Although GNPs accumulate in different organs, they do not cause any toxicity or cell damage and inhibit the expression of inflammation and angiogenesis mediators, effectively delaying the progression of RA.
HA-GNP-TCZ targets both VEGF and IL-6R [104].Reprinted from Lee H, Lee MY, Bhang SH, et al. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. The dual-targeted HA-GNP-TCZ complex was developed to simul-taneously bind VEGF and IL-6R to treat RA. The combination between AuNP and VEGF demonstrated brilliant antiangiogenic effect on RA. TCZ, an immunosuppressive drug, interferes with IL-6 during the pathogenesis of RA. Hyaluronic acid is widely used for cartilage protection and lubrication. This compound alleviates the immune disorder at the joint, and ultimately achieves the therapeutic response of reduced excessive cytokines and repaired cartilage. Abbreviation:TCZ: Tocilizumab ; VEGF:vascular endothelial growth factor; AuNPs:Gold nanoparticles; IL-6:interleukin 6.ACS Nano. 2014;8(5):4790–4798. https://creativecommons.org/licenses/by/4.0/0.44.
Albumin nanoparticles
Albumin is mainly produced by hepatocytes and is the most abundant protein in human plasma, accounting for approximately 50% of the total plasma protein. It has important physiological functions in vivo, such as maintaining the stability of plasma osmotic pressure, ensuring communication between intracellular fluid, extracellular fluid and tissue fluid. Additionally, it serves as a natural detoxifier by binding to heavy metal ions in the body. Albumin also acts as an important nutrient. It possesses several advantages, including a simple molecular structure, high stability, high affinity, increased permeability at the joint inflammation site, natural biodegradation in vivo and no toxic effect [111].
Furthermore, studies by Liu et al. [118] confirmed that the level of the secreted protein acidic and rich in cysteine (SPARC) in inflammatory joints increases with the invasion of inflammatory cells, angiogenesis, and bone erosion. SPARC has a high affinity for albumin, and its overexpression contributes to the active targeting of albumin nanoparticles. Due to the increased synovial metabolism in RA patients, the joints require more energy and nitrogen sources, thus increasing the demand for albumin. This characteristic facilitates the targeting ability of albumin nanoparticles [116,117,118,119]. In summary, albumin is an effective drug carrier that can deliver drugs to the inflammatory joints, prolong the duration of drug action, and improve pharmacokinetic properties and efficacy.
Liu et al. [102] developed a recombinant protein by fusing human serum albumin (HSA) to the carboxyl terminus of IL-1ra, which was produced in Pichia pastoris. The direct fusion of albumin with IL-1Ra had a positive effect, and the fusion protein retained the biological activity of IL-1Ra and exhibited a longer serum half-life. In contrast with the use of IL-1Ra alone, the fusion protein accumulated for an extended period during joint inflammation, with a lower distribution rate in the liver, kidney, lung and other parts, which demonstrated that the fusion protein had excellent targeting performance and a significant therapeutic effect [120].
Dendrimers
Dendrimers are innovative synthetic polymer with a dendritic structure that have the ability to bind to antibodies due to their large surface structure. They possess massive advantages, such as good uniformity, high biocompatibility, and a well-defined structure that enhaces cellular uptake [111]. The synthesized dendrimers have various skeletons that are directly related to their physico-chemical properties. Common skeletons include polyamidoamine dendrimers (PAMAM), polypropylene imine (PPI), polyesters, and scaffolds containing phosphorus and silicon atoms in the structure [121, 126]. In addition to the internal structure, the peripheral functional groups determine their use. For example, dendrimers with cationic groups are employed as antibacterial agents, and anionic groups are used as antiviral drugs.
The application of dendrimers in the biomedical field has aroused great interest. Nowadays, dendrimers have been used as nanoplatforms for drugs, nucleic acid transporters, contrast agents, etc. [88]. PAMAM is a commercial dendrimer that can be classified into 0–10 generations according to its molecular size. Its surface includes different terminal functional groups, allowing it to covalently attach to the active target molecule. In addition, PAMAM with amine or hydroxyl groups on the surface also exhibits anti-inflammatory activity, making it possible to prepare new drugs [111].
Combining PAMAM with synthetic or natural biodegradable polymers facilitates its interaction with living cells and improves its biological performance. Since chondroitin sulfate (CS) is abundant in tissues, Oliveira et al. [122] modified PAMAM with CS and anti-TNF α antibodies (Abs) to increase the affinity with cartilage. The system can be used for controlled and continuous drug delivery and does not cause harmful effects on the metabolic activity and proliferation of the cells subjected, showing good cytocompatibility and hemocompatibility.
Besides the PAMAM skeleton, the carbon silane skeleton has also attracted considerable attention. According to another study, the carbosilane dendrimer skeleton shows excellent hydrophobicity due to its special structure, which enables it to bind with antibodies and interact with cell membrane more effectively [88].
Nanogels
Nano-gel is a type of polymeric gel that exists in the form of nanoparticles, with typical network structures of molecular cross-linking. It can disperse into nanoscale hydrogel particles in aqueous solution. Nanogels can respond to different environmental stimuli, such as chemical signal stimulation (pH, chemical or biological substances) and physical signal stimulation (temperature, light intensity, electromagnetic field). Nanogels have several advantages, including a small particle size, high stability of chemical structure, good biocompatibility, good permeability and good water retention. In addition, chemically modified nanogels exhibit good targeting properties and extended circulation time. Thus, nanogels have attracted wide attention in the field of drug carriers [123].
As a topical administration method, nanogels can alleviate adverse reactions caused by systemic administration and reduce the invasive trauma of injection administration, making them more patient-friendly. Studies have proven that nanogels can penetrate the skin and migrate to the epidermis with good permeability. Notably, nanogels possess several characteristics, including high loading capacity for protein drugs, good stability, and controllable protein release, making them ideal carriers for protein drugs [124].
Samah et al. [125] demonstrated that nanogels can effectively deliver drugs to the viable epidermis (VE) and produce a definite anti-inflammatory effect without causing immunogenic or toxic effects. Nguyen et al. [127] reported a new nanogel that depends on agarose-curdlan to load etanercept. The diameter of dispersed nanogel was measured at 30–100 nm. This nanosystem enhanced the permeability and retention effects, assisting in large accumulation at inflammatory sites. Likewise, it protected etanercept from immune clearance and improved its biological half-life.
Others
Temperature-sensitive drug delivery systems have also gained significant attention from researchers. Jung et al. [79] developed a novel type of temperature-modulated noncovalent (TMN) interaction controllable complex. This TMN complex exhibited mutual electrostatic interactions with positively charged etanercept at a temperature lower than the polymer clouding temperature (CT) of 4 °C. When the temperature reaches physiological conditions (37.5 °C), a new polymer-protein complex is formed by double noncovalent interactions. This significantly improves serum stability and prolongs the pharmacokinetic parameters of etanercept in vivo. Pathological analysis of joint tissue revealed that the TMN complex significantly improved inflammatory cell infiltration without obvious vascular and synovitis formation or cartilage destruction. In addition, bone erosion was alleviated, indicating an augmented therapeutic effect.
Since RA is a chronic inflammatory disease, frequent injections may result in discomfort and reduced patient compliance. The emergence of transdermal drug delivery using microneedles has created new opportunities to achieve long-term patient compliance. Cao et al. [80] developed a hyaluronic acid crosslinked microneedle (MN) system as a carrier to deliver etanercept (EN). This system was easy to self-administer after application on the skin, and the drug could be released without additional procedures, reducing the pain from injection. After treatment, the paw swelling ratio of EN treated using MN mice decreased from 1.68 to 1.44 within 10 days, showing a good anti-inflammatory effect. Moreover, the concentration of TNF-α and IL-6 decreased in serum. Pathological sections showed that the joint structure of the saline-treated mice (SA) group was poor. In contrast, EN treated using SC mice (eSC) and eMN effectively protected the joint from erosion. In conclusion, compared with the eSC group, eMN shows similar efficacy in foot swelling, clinical score, cytokines, and joint erosion with classic SC administration. Moreover, MN exhibits higher biocompatibility and compliance, offering great prospects for carrying etanercept (Fig. 5).
After MNs arrive at the body, etanercept blocks the TNF-α-to-TNF receptor [80]. The microneedle system is applied to the skin on the back of mice, and etanercept (EN) is released from the system and absorbed by the capillaries in the surrounding tissue. In arthritic tissue, EN combines with TNF-α receptors and blocks TNF-α-mediated pathway to exhibit therapeutic potential. Reprinted from Cao J, Zhang N, Wang Z, et al. Microneedle-Assisted Transdermal Delivery of Etanercept for Rheumatoid Arthritis Treatment. Pharmaceutics. 2019;11(5):235.https://creativecommons.org/licenses/by/4.0/0.44.
Summary and outlook
Rheumatoid arthritis (RA) is an autoimmune disease that results in the infiltration of inflammatory cytokines and erosion of cartilage and bones, leading to joint swelling, pain, and bone damage, ultimately affecting the survival status and lifespan of patients [130]. Surgery cannot fundamentally solve the excessive immune status of patients, and it causes secondary injury to the patient as the joint synovium is removed. Therefore, drug therapy remains the routine method for the treatment of rheumatoid arthritis. At present, NSAIDs and DMARDs are still used as first-line treatment drugs. However, the lack of clear therapeutic targets, cumulative side effects, and drug resistance caused by long-term use seriously restrict their clinical application [134].
Antibody drugs represent a new choice for the treatment of RA. Antibody drugs have a strong immunosuppressive ability and can clear excessive immune complexes in vivo, contributing to an enhanced therapeutic response [132, 133]. However, there are still some challenges that need to be resolved in their clinical applications. One such challenge is patient compliance as the lifetime maintenance of antibody drugs is generally required. Long-term intravenous administration of antibodies leads to a lack of patient compliance. Therefore, exploring a convenient delivery system for RA treatment is crucial.
The advent of transdermal delivery nanosystems has improved patient compliance by avoiding the inconvenience of injections and the associated pain. Walsh et al. [128] designed nanotopography-based microneedles to enhance the transdermal delivery of etanercept. The nanotopography can combine with integrin to cause tight junction protein remodeling, induce clustering of focal adhesion proteins, and increase paracellular permeability. The prepared microneedles do not penetrate the dermis, thereby alleviating the pain and improving patient compliance caused by intravenous administration. Thus, transdermal drug delivery systems may be a profound way to improve patient compliance for RA patients.
RA is an autoimmune disease. Its pathological manifestations are infiltration of inflammatory cytokines and erosion of cartilage and bones, leading to joint swelling, pain and bone damage, seriously affecting the survival status and life span of patients. Surgery cannot fundamentally solve the excessive immune status of patients but causes secondary injury to the patient because the joint synovium is removed. Therefore, drug therapy is still a routine method for the treatment of rheumatoid arthritis. At present, NSAIDs and DMARDs are still used as first-line treatment drugs. However, the lack of clear therapeutic targets, cumulative side effects, and drug resistance caused by long-term use seriously restrict its clinical application. Antibody drugs represent a new choice for the treatment of RA. Antibody drugs have a strong immunosuppressive ability and can clear excessive immune complexes in vivo, contributing to an enhanced therapeutic response. However, there are still some challenges that need to be resolved in their clinical applications.
1) Patient compliance. Lifetime maintenance of antibody drugs are generally required. Long-term intravenous administration of antibodies leads to a lack of patient compliance. Therefore, exploring a convenient delivery system for RA treatment is crucial. The advent of transdermal delivery nanosystems potentiates improved patient compliance, reflected by avoiding the inconvenience of injections and the associated pain. Walsh et al. [128] designed nanotopography-based microneedles to enhance the transdermal delivery of etanercept. The nanotopography can combine with integrin to cause tight junction protein remodeling, induce clustering of focal adhesion proteins and increase paracellular permeability. The prepared microneedles do not penetrate the dermis; thus, they alleviate the pain and improve patient compliance caused by intravenous administration. Thus, transdermal drug delivery systems may be a profound way to improve patient compliance for RA patients.
2) Immunogenicity. Despite great advances in treatment outcome, antibody drugs still faced secondary failure in extensive patients, featured by adverse events and loss of effectiveness after the secondary application. Emerging evidences indicated that the secondary failure and adverse events were tightly associated with the development of anti-drug antibodies (ADA) in terms of systemic exposure [135]. Chimeric monoclonal antibodies, especially fully human antibodies, were generated to reduce the immunogenicity. Recently, the humanized efficiency was extremely high, up to 99.1% in Golimumab [136]. However, the immunogenicity still cannot be eliminated completely. It was reported that the incidence of ADA from etanercept, golimumab infliximab, adalimumab and certolizumab reach 1.2%, 3.8%, 25.3%, 14.1% and 6.9%, respectively [137]. The occurrence of ADAs may lead to rapid clearance and loss of therapeutic response. Furthermore, PEGylation is wildly used in bioconjugation of antibodies or their fractions to improve solubility and prolong circulation in blood. Whereas, PEGylated antibodies increasingly induce the formation of ADA that specifically recognize and bind to PEG (termed as accelerated blood clearance (ABC) phenomenon). Nanoparticles capable of carrying a payload was regarded as powerful tools to avoid systemic exposure of antigenic epitope in blood circulation, thereby reducing immunogenicity and subsequent clearance [138].
3) Immunosuppression. Systemic exposure of antibody drugs nonspecifically neutralizes cytokines, inhibit antigen presentation or deplete activated B cell, inevitably suppressing the immune system and resulting in potential infection and cancer, especially tuberculosis [34, 36]. Active targeted nanodevices provide a profound strategy to avoid systemic exposure of antibody drugs and aggregate drugs in the lesion. The targeting moiety of existing nanosystems includes a small molecule compound (curdlan) and albumin. It is worth noting that the targeting efficiency of the nanosystems in arthritic joints was not on-demand recently, reflected by the lower fluorescence intensity in paws when compared with that in livers. Increasing evidence indicates that membrane/exosome-coated nanoparticles can significantly improve drug accumulation in the synovium of arthritic joints. Yu et al. [129] prepared hybrid membrane-coated Prussian blue nanoparticles to encapsulate the anti-RA compound schisanlactone E. This multifunctional nanoparticle showed preferential accumulation in paws with respect to that in the liver, creating a possible alternative for improving the biodistribution of antibody-based drugs.
4) Stability. As a protein, the physiochemical properties of antibody drugs can be easily affected by the complicated environment in vitro and in vivo, resulting in increased immunogenicity, reduced half-life and eventually invalidity. Hence, it is critical to stabilize antibody drugs in the storage and administration process. When the antibody drug is encapsulated in the core of nanocarriers (micelles, liposomes, nanocages, vehicles), it can resist external stimuli, contributing to augmented stability. Nevertheless, every coin has two sides. The targeting ability of the antibody itself may be changed without exposure to the microenvironment. Moreover, when antibody drugs are covalently conjugated to the surface of dendrimer/Au nanoparticles, their stability will be influenced by external triggers; however, the targeting ability remains.
5) Target. Majority of antibodies was water-soluble, preventing it from penetrating through cell membrane and interplay with cellular target [139]. Even if internalization into cytoplasm, antibody drugs will be immediately degraded under lysosome conditions with acidic environment (pH 4.0–6.0) and hydrolytic enzymes. To data, various funcational nanodevices have been developped for assisting drugs to escape from endo/lysosomes [140], redirecting down a new way towards effective cellular delivery and further construction of antibody with cellular target.
6) Synergistic effect. Owing to the diminished response over time, monoclonal antibody drugs was suggested to combine with other DMARDs. Increasing evidences revealed that concomitant administration of MTX was invloved in reduced immunogenicity and ADAs [141], thus significantly improved efficacy. Benefiting fron carrying payloads, co-delivery of monoclonal antibody adrugs and other DMARDs can be fabricated in one nanosystem to achieve synergistic effect.
Furthermore, after nanosystems finish the delivery task, the residual nanocarriers can also have a positive effect on the treatment of RA rather than waste. To this end, Zhou et al. [9, 10] prepared tannic acid-based MOF and folic acid-anchored silver nanoparticles. Both of these two nanosystems primarily deliver anti-rheumatic drugs to the joints. Furthermore, the residual nanoplatforms exerted anti-oxidantive effect, pro-apoptosis and re-polarization of macrophages, respectively, contributing to synergistic effect between nanocarriers and drugs.
In addition to functionalize in inflammatory cells, Pandey et al. [131] reported that a hydroxyapatite nanoparticle carrying Teflon and methotrexate successfully transported teriflunomide and methotrexate to the joint, achieving enhanced therapeutic benefits and reduced hepatoxicity. Moreover, the residual hydroxyapatite is a kind of human natural bone component that can promote the proliferation, differentiation and mineralization of osteoblasts. The combination of hydroxyapatite and antibody drugs may not only serve as a good targeted transporter but also play a beneficial role in the process of bone remodeling.
It is a pity that antibody drug loaded nanoparticles barely reached the market. Only some antibody-functionalized nanoparticles have entered clinical trials. For instance, Kadcyla is a nanoscale antibody-drug conjugate (diameter in 15 nm) that consists of the chemotherapy drug DM1 (emtansine) and monoclonal antibody trastuzumab. Clinical trials have shown that Kadcyla can significantly improve the progression-free survival and overall survival of patients with HER2-positive breast cancer compared to other treatments [142]. Kadcyla has also been shown to have fewer side effects than traditional chemotherapy drugs, which can improve patients’ quality of life during treatment. Sgt-94 is an anti-transferrin receptor antibody-engineered liposome encapsulated with a Rb94 plasmid DNA, representing a clinical perspective in phase I in patients with neoplasm (NCT01517464) [143]. Lipovaxin-MM incorporating a specific antibody fragment in the liposomal surface was regarded as a vaccine for malignant melanoma, entering phase I trial.[144]The clinical results demonstrated that Lipovaxin-MM exhibited partial response, well tolerance and absence of severe adverse events .
Accordingly, transformation of antibody drug loaded nanoparticles from laboratory to clinical trials remains an immense challenge, attributing to low drug-loading efficiency, poor reproducibility of nanoparticles, unknown pharmacokinetics property and long-term toxicity [145]. To facilitate the clinical transformation, the property of nanoparticles involved in their performance should be well summarized. Particle size was tightly associated with biodistribution [146, 147], immune response [148] and loading efficiency [149] of monoclonal drugs. Small nanoparticles (less than 10 nm) can rapidly extravasate from inflammatory endothelial windows, while they may be rapidly cleared from the body. Larger nanoparticles (greater than 100 nm) may prolong blood circulation time and achieve desirable accumulation in lesions, but they may be less efficient in penetrating into the deep layers of synovium and cartilage. Similarly, the surface charge of nanoparticles affects their interactions with cells and tissues. Positively charged nanoparticles was favorable to interplay with negatively charged cell membranes and enhance cellular uptake, while they may be hijacked in blood circulation to form “protein crown”. Generally, nanoparticles with a neutral or slightly negative surface charge are preferred for drug delivery to avoid immune recognition and clearance. The drug encapsulation efficiency and release manner can be controlled by adjusting the material properties, size, and surface characteristics of the nanoparticle. Controlled release of the drug can improve drug bioavailability and reduce side effects; however, it may also require more complex nanoparticle designs and manufacturing processes. Therefore, the construction of antibody-based was required to consider a variety of factors comprehensively.
Taken together, as an effective drug to treat rheumatoid arthritis, antibody drugs have broad application prospects and development space in combination with DDSs.
Data Availability
Not applicable.
References
Shams S, Martinez JM, Dawson JRD, et al. The Therapeutic Landscape of Rheumatoid Arthritis: current state and future directions. Front Pharmacol. 2021;12:680043. https://doi.org/10.3389/fphar.2021.680043. Published 2021 May 28.
McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328–37. https://doi.org/10.1016/S0140-6736(17)31472-1
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72. https://doi.org/10.1001/jama.2018.13103
Alam J, Jantan I, Bukhari SNA. Rheumatoid arthritis: recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother. 2017;92:615–33. https://doi.org/10.1016/j.biopha.2017.05.055
Wang Q, Qin X, Fang J, Sun X. Nanomedicines for the treatment of rheumatoid arthritis: state of art and potential therapeutic strategies. Acta Pharm Sin B. 2021;11(5):1158–74. https://doi.org/10.1016/j.apsb.2021.03.013
Kondo Y, Yokosawa M, Kaneko S, et al. Review: transcriptional regulation of CD4 + T-Cell differentiation in experimentally Induced Arthritis and Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70(5):653–61. https://doi.org/10.1002/art.40398
Matsuo Y, Saito T, Yamamoto A, Kohsaka H. Origins of fibroblasts in rheumatoid synovial tissues: implications from organ fibrotic models. Mod Rheumatol. 2018;28(4):579–82. https://doi.org/10.1080/14397595.2017.1386837
Chuang SY, Lin CH, Huang TH, Fang JY. Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials (Basel). 2018;8(1):42. Published 2018 Jan 15. doi:https://doi.org/10.3390/nano8010042
Guo L, Zhong S, Liu P, Guo M, Ding J, Zhou W. Radicals scavenging MOFs enabling targeting delivery of siRNA for rheumatoid arthritis therapy. Small. 2022;18(27):e2202604. https://doi.org/10.1002/smll.202202604
Yang Y, Guo L, Wang Z, et al. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and repolarization. Biomaterials. 2021;264:120390. https://doi.org/10.1016/j.biomaterials.2020.120390
Chen SJ, Lin GJ, Chen JW et al. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int J Mol Sci. 2019;20(6):1332. Published 2019 Mar 16. doi:https://doi.org/10.3390/ijms20061332
Araki Y, Mimura T. Matrix Metalloproteinase Gene Activation resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int J Mol Sci. 2017;18(5):905. https://doi.org/10.3390/ijms18050905. Published 2017 Apr 25.
Arioka M, Takahashi-Yanaga F. Glycogen synthase kinase-3 inhibitor as a multitargeting anti-rheumatoid drug. Biochem Pharmacol. 2019;165:207–13. https://doi.org/10.1016/j.bcp.2019.02.020
Tanaka S, Tanaka Y, Ishiguro N, Yamanaka H, Takeuchi T. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol. 2018;28(1):9–16. https://doi.org/10.1080/14397595.2017.1369491
Küçükdeveci AA. Nonpharmacological treatment in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33(5):101482. https://doi.org/10.1016/j.berh.2019.101482
Köhler BM, Günther J, Kaudewitz D, Lorenz HM. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8(7):938. https://doi.org/10.3390/jcm8070938. Published 2019 Jun 28.
Xu XL, Lu KJ, Yao XQ, Ying XY, Du YZ. Stimuli-responsive drug Delivery Systems as an emerging platform for treatment of rheumatoid arthritis. Curr Pharm Des. 2019;25(2):155–65. https://doi.org/10.2174/1381612825666190321104424
Zhou S, Zou H, Chen G, Huang G. Synthesis and Biological Activities of Chemical drugs for the treatment of rheumatoid arthritis. Top Curr Chem (Cham). 2019;377(5):28. https://doi.org/10.1007/s41061-019-0252-5. Published 2019 Sep 28.
Bedoui Y, Guillot X, Sélambarom J et al. Methotrexate an Old Drug with New Tricks. Int J Mol Sci. 2019;20(20):5023. Published 2019 Oct 10. doi:https://doi.org/10.3390/ijms20205023
Guo L, Chen Y, Wang T, et al. Rational design of metal-organic frameworks to deliver methotrexate for targeted rheumatoid arthritis therapy. J Control Release. 2021;330:119–31. https://doi.org/10.1016/j.jconrel.2020.10.069
Dogra A, Sharma A, Kumar Mandal U, Kotwal P, Bhatt S, Nandi U. Liquid chromatography based methods for analysis of Disease-Modifying antirheumatic drugs (DMARDs) in Biological Matrices. Crit Rev Anal Chem. 2019;49(3):224–42. https://doi.org/10.1080/10408347.2018.1503943
Braun J, Baraliakos X, Westhoff T. Nonsteroidal anti-inflammatory drugs and cardiovascular risk - a matter of indication. Semin Arthritis Rheum. 2020;50(2):285–8. https://doi.org/10.1016/j.semarthrit.2019.07.012
Buttgereit F. Views on glucocorticoid therapy in rheumatology: the age of convergence. Nat Rev Rheumatol. 2020;16(4):239–46. https://doi.org/10.1038/s41584-020-0370-z
Xiao Q, Li X, Li Y, et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2021;11(4):941–60. https://doi.org/10.1016/j.apsb.2020.12.018
Kesharwani D, Paliwal R, Satapathy T, Das Paul S. Rheumatiod Arthritis: an updated overview of latest therapy and drug delivery. J Pharmacopunct. 2019;22(4):210–24. https://doi.org/10.3831/KPI.2019.22.029
Jang DI, Lee AH, Shin HY, et al. The role of Tumor necrosis factor alpha (TNF-α) in Autoimmune Disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021;22(5):2719. https://doi.org/10.3390/ijms22052719. Published 2021 Mar 8.
Lin YJ, Anzaghe M, Schülke S. Update on the Pathomechanism, diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9(4):880. https://doi.org/10.3390/cells9040880. Published 2020 Apr 3.
Marahleh A, Kitaura H, Ohori F, et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front Immunol. 2019;10:2925. https://doi.org/10.3389/fimmu.2019.02925. Published 2019 Dec 13.
Lim H, Lee SH, Lee HT et al. Structural Biology of the TNFα Antagonists Used in the Treatment of Rheumatoid Arthritis. Int J Mol Sci. 2018;19(3):768. Published 2018 Mar 7. doi:https://doi.org/10.3390/ijms19030768
Köhler BM, Günther J, Kaudewitz D, Lorenz HM. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8(7):938. https://doi.org/10.3390/jcm8070938. Published 2019 Jun 28.
Zhao S, Mysler E, Moots RJ. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy. 2018;10(6):433–45. https://doi.org/10.2217/imt-2017-0155
Chen SJ, Lin GJ, Chen JW et al. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int J Mol Sci. 2019;20(6):1332. Published 2019 Mar 16. doi:https://doi.org/10.3390/ijms20061332
Dervieux T, Kremer JM, Weinblatt ME. Differing contribution of methotrexate polyglutamates to adalimumab blood levels as compared with etanercept. Ann Rheum Dis. 2019;78(9):1285–6. https://doi.org/10.1136/annrheumdis-2018-214860
Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006;43(6):717–22. https://doi.org/10.1086/506935
Abbasi M, Mousavi MJ, Jamalzehi S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019;234(7):10018–31. https://doi.org/10.1002/jcp.27860
Watanabe A, Matsumoto T, Igari H, Sawa J, Yamaguchi Y, Sakatani M. Risk of developing active tuberculosis in rheumatoid arthritis patients on adalimumab in Japan. Int J Tuberc Lung Dis. 2016;20(1):101–8. https://doi.org/10.5588/ijtld.15.0283
Deeks ED. Certolizumab Pegol: A Review in Inflammatory Autoimmune Diseases. BioDrugs. 2016;30(6):607–17. https://doi.org/10.1007/s40259-016-0197-y
Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open. 2019;5(1):e000942. https://doi.org/10.1136/rmdopen-2019-000942. Published 2019 May 31.
Pelechas E, Voulgari PV, Drosos AA. Golimumab for Rheumatoid Arthritis. J Clin Med. 2019;8(3):387. https://doi.org/10.3390/jcm8030387. Published 2019 Mar 20.
Singh S, Facciorusso A, Singh AG et al. Obesity and response to antitumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis [published correction appears in PLoS One. 2018 Aug 29;13(8):e0203499]. PLoS One. 2018;13(5):e0195123. Published 2018 May 17. doi:https://doi.org/10.1371/journal.pone.0195123
Striz I. Cytokines of the IL-1 family: recognized targets in chronic inflammation underrated in organ transplantations. Clin Sci (Lond). 2017;131(17):2241–56. https://doi.org/10.1042/CS20170098. Published 2017 Aug 10.
Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50(4):778–95. https://doi.org/10.1016/j.immuni.2019.03.012
Ramírez J, Cañete JD. Anakinra for the treatment of rheumatoid arthritis: a safety evaluation. Expert Opin Drug Saf. 2018;17(7):727–32. https://doi.org/10.1080/14740338.2018.1486819
Tarp S, Furst DE, Dossing A, et al. Defining the optimal biological monotherapy in rheumatoid arthritis: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2017;46(6):699–708. https://doi.org/10.1016/j.semarthrit.2016.09.003
Lopalco G, Rigante D, Cantarini L, et al. The autoinflammatory side of recurrent pericarditis: enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc Med. 2021;31(5):265–74. https://doi.org/10.1016/j.tcm.2020.04.006
Khayata M, Shah NP, Verma BR, et al. Usefulness of Interleukin-1 receptor antagonists in patients with recurrent Pericarditis. Am J Cardiol. 2020;127:184–90. https://doi.org/10.1016/j.amjcard.2020.03.041
Vastert SJ, Jamilloux Y, Quartier P, et al. Anakinra in children and adults with still’s disease. Rheumatology (Oxford). 2019;58(Suppl 6):vi9–vi22. https://doi.org/10.1093/rheumatology/kez350
Zhou S, Qiao J, Bai J, Wu Y, Fang H. Biological therapy of traditional therapy-resistant adult-onset still’s disease: an evidence-based review. Ther Clin Risk Manag. 2018;14:167–71. https://doi.org/10.2147/TCRM.S155488. Published 2018 Jan 24.
Chatzidionysiou K, Circiumaru A, Rethi B, et al. Tocilizumab decreases T cells but not macrophages in the synovium of patients with rheumatoid arthritis while it increases the levels of serum interleukin-6 and RANKL. RMD Open. 2021;7(2):e001662. https://doi.org/10.1136/rmdopen-2021-001662
Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8(7):538–42. https://doi.org/10.1016/j.autrev.2009.01.012
Scott LJ, Tocilizumab. A Review in Rheumatoid Arthritis [published correction appears in Drugs. 2017 Dec 19;:]. Drugs. 2017;77(17):1865–1879. doi:https://doi.org/10.1007/s40265-017-0829-7
Cappelli LC, Palmer JL, Kremer J, Bingham CO 3. Tocilizumab treatment leads to improvement in disease activity regardless of CCP status in rheumatoid arthritis. Semin Arthritis Rheum. 2017;47(2):165–9. https://doi.org/10.1016/j.semarthrit.2017.03.024
Morel J, Constantin A, Baron G, et al. Risk factors for serious infections in patients with rheumatoid arthritis treated with tocilizumab in the French Registry REGATE. Rheumatology (Oxford). 2017;56(10):1746–54. https://doi.org/10.1093/rheumatology/kex238
Kim SC, Solomon DH, Rogers JR, et al. No difference in cardiovascular risk of tocilizumab versus abatacept for rheumatoid arthritis: a multidatabase cohort study. Semin Arthritis Rheum. 2018;48(3):399–405. https://doi.org/10.1016/j.semarthrit.2018.03.012
Ogata A, Kato Y, Higa S, Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol. 2019;29(2):258–67. https://doi.org/10.1080/14397595.2018.1546357
Ahmadzadeh A, Farahmand AN, Gachkar L. Evaluation of safety, efficacy and postcessation efficacy durability of tocilizumab in patients with active rheumatoid arthritis. Int J Rheum Dis. 2017;20(2):231–7. https://doi.org/10.1111/1756-185X.12686
Lamb YN, Deeks ED, Sarilumab. A review in moderate to severe rheumatoid arthritis. Drugs. 2018;78(9):929–40. https://doi.org/10.1007/s40265-018-0929-z
Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593–603. https://doi.org/10.2147/DDDT.S100302. Published 2017 May 24.
Aly AM, Furst DE. Update of sarilumb to treat rheumatoid arthritis based on randomized clinical trials: a systematic review. Expert Rev Clin Immunol. 2017;13(8):741–52. https://doi.org/10.1080/1744666X.2017.1351297
June RR, Olsen NJ. Room for more IL-6 blockade? Sarilumab for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2016;16(10):1303–9. https://doi.org/10.1080/14712598.2016.1217988
Scott LJ, Sarilumab. First Global approval. Drugs. 2017;77(6):705–12. https://doi.org/10.1007/s40265-017-0724-2
McCarty D, Robinson A. Efficacy and safety of sarilumab in patients with active rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10(3):61–7. https://doi.org/10.1177/1759720X17752037
Atzeni F, Nucera V, Masala IF, Sarzi-Puttini P, Bonitta G. Il-6 involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab. Pharmacol Res. 2019;149:104402. https://doi.org/10.1016/j.phrs.2019.104402
Pombo-Suarez M, Gomez-Reino JJ. Abatacept for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2019;15(4):319–26. https://doi.org/10.1080/1744666X.2019.1579642
Blair HA, Deeks ED, Abatacept. A review in rheumatoid arthritis. Drugs. 2017;77(11):1221–33. https://doi.org/10.1007/s40265-017-0775-4
Scarsi M, Paolini L, Ricotta D, et al. Abatacept reduces levels of switched memory B cells, autoantibodies, and immunoglobulins in patients with rheumatoid arthritis. J Rheumatol. 2014;41(4):666–72. https://doi.org/10.3899/jrheum.130905
Correction. Abatacept in combination with methotrexate in Japanese biologic-naive patients with active rheumatoid arthritis: a randomized placebo-controlled phase IV study. RMD Open. 2019;5(2):e000813corr1. Published 2019 Dec 5. doi:https://doi.org/10.1136/rmdopen-2018-000813corr1
Abbasi M, Mousavi MJ, Jamalzehi S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019;234(7):10018–31. https://doi.org/10.1002/jcp.27860
Mochizuki T, Ikari K, Yano K, Sato M, Okazaki K. Long-term deterioration of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept. Mod Rheumatol. 2019;29(3):413–7. https://doi.org/10.1080/14397595.2018.1481566
Ozen G, Pedro S, Schumacher R, Simon TA, Michaud K. Safety of abatacept compared with other biologic and conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: data from an observational study. Arthritis Res Ther. 2019;21(1):141. https://doi.org/10.1186/s13075-019-1921-z. Published 2019 Jun 7.
Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in Autoimmune Diseases. Front Immunol. 2018;9:835. https://doi.org/10.3389/fimmu.2018.00835. Published 2018 Apr 23.
Singh A, Behl T, Sehgal A, et al. Mechanistic insights into the role of B cells in rheumatoid arthritis. Int Immunopharmacol. 2021;99:108078. https://doi.org/10.1016/j.intimp.2021.108078
Bryl E. B cells as target for immunotherapy in rheumatic diseases - current status. Immunol Lett. 2021;236:12–9. https://doi.org/10.1016/j.imlet.2021.05.006
Kaegi C, Wuest B, Schreiner J, et al. Systematic Review of Safety and Efficacy of Rituximab in Treating Immune-Mediated Disorders. Front Immunol. 2019;10. Published 2019 Sep 6. https://doi.org/10.3389/fimmu.2019.01990
Rath E, Zwerina J, Oppl B, Nell-Duxneuner V. Efficacy and safety of rituximab in rheumatic diseases. Wien Med Wochenschr. 2015;165(1–2):28–35. https://doi.org/10.1007/s10354-014-0331-8
Shi Y, Wu Y, Ren Y, Jiang Y, Chen Y. Infection risks of rituximab versus nonrituximab treatment for rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. 2019;22(8):1361–70. https://doi.org/10.1111/1756-185X.13596
Vikse J, Jonsdottir K, Kvaløy JT, Wildhagen K, Omdal R. Tolerability and safety of long-term rituximab treatment in systemic inflammatory and autoimmune diseases. Rheumatol Int. 2019;39(6):1083–90. https://doi.org/10.1007/s00296-019-04272-1
Wang KC, Liao HT, Chen WS, et al. Real-world effectiveness and safety of rituximab in the treatment of rheumatoid arthritis: a single-center experience in Taiwan. Int J Rheum Dis. 2019;22(5):860–8. https://doi.org/10.1111/1756-185X.13511
Jung YS, Park W, Na K. Temperature-modulated noncovalent interaction controllable complex for the long-term delivery of etanercept to treat rheumatoid arthritis. J Control Release. 2013;171(2):143–51. https://doi.org/10.1016/j.jconrel.2013.07.012
Cao J, Zhang N, Wang Z, et al. Microneedle-Assisted transdermal delivery of Etanercept for Rheumatoid Arthritis Treatment. Pharmaceutics. 2019;11(5):235. https://doi.org/10.3390/pharmaceutics11050235. Published 2019 May 15.
Giulbudagian M, Yealland G, Hönzke S et al. Breaking the Barrier - Potent Anti-Inflammatory Activity following Efficient Topical Delivery of Etanercept using Thermoresponsive Nanogels. Theranostics. 2018;8(2):450–463. Published 2018 Jan 1. doi:https://doi.org/10.7150/thno.21668
Erdemli Ö, Özen S, Keskin D, et al. In vitro evaluation of effects of sustained anti-TNF release from MPEG-PCL-MPEG and PCL microspheres on human rheumatoid arthritis synoviocytes. J Biomater Appl. 2014;29(4):524–42. https://doi.org/10.1177/0885328214535958
Shen K, Sun G, Chan L, et al. Anti-inflammatory nanotherapeutics by targeting Matrix Metalloproteinases for Immunotherapy of spinal cord Injury. Small. 2021;17(41):e2102102. https://doi.org/10.1002/smll.202102102
Campos Y, Fuentes G, Almirall A et al. The Incorporation of Etanercept into a Porous Tri-Layer Scaffold for Restoring and Repairing Cartilage Tissue. Pharmaceutics. 2022;14(2):282. Published 2022 Jan 26. doi:https://doi.org/10.3390/pharmaceutics14020282
Ferreira M, Barreiros L, Segundo MA, et al. Topical codelivery of methotrexate and etanercept using lipid nanoparticles: a targeted approach for psoriasis management. Colloids Surf B Biointerfaces. 2017;159:23–9. https://doi.org/10.1016/j.colsurfb.2017.07.080
Sun G, Yang S, Cai H, et al. Molybdenum disulfide nanoflowers mediated anti-inflammation macrophage modulation for spinal cord injury treatment. J Colloid Interface Sci. 2019;549:50–62. https://doi.org/10.1016/j.jcis.2019.04.047
Pabari RM, Tambuwala MM, Lajczak-McGinley N, et al. Novel polyurethane based particulate formulations of infliximab reduce inflammation in DSS induced murine model of colitis - A preliminary study. Int J Pharm. 2021;604:120717. https://doi.org/10.1016/j.ijpharm.2021.120717
Rodríguez-Prieto T, Hernández-Breijo B, Ortega MA, Gómez R, Sánchez-Nieves J, Guijarro LG. Dendritic nanotheranostic for the delivery of Infliximab: a potential carrier in Rheumatoid Arthritis Therapy. Int J Mol Sci. 2020;21(23):9101. https://doi.org/10.3390/ijms21239101. Published 2020 Nov 30.
Li X, Yu M, Zhu Z, et al. Oral delivery of infliximab using nanoin-microparticles for the treatment of inflammatory bowel disease. Carbohydr Polym. 2021;273:118556. https://doi.org/10.1016/j.carbpol.2021.118556
Chen W, Li Z, Wang Z, Gao H, Ding J, He Z. Intraarticular Injection of Infliximab-Loaded Thermosensitive Hydrogel alleviates Pain and protects cartilage in rheumatoid arthritis. J Pain Res. 2020;13:3315–29. https://doi.org/10.2147/JPR.S283518. Published 2020 Dec 7.
Likhitpanichkul M, Kim Y, Torre OM, et al. Fibrin-genipin annulus fibrosus sealant as a delivery system for anti-TNFα drug. Spine J. 2015;15(9):2045–54. https://doi.org/10.1016/j.spinee.2015.04.026
Gokhale KS, Jonnalagadda S. Preparation and evaluation of sustained release infliximab microspheres. PDA J Pharm Sci Technol. 2013;67(3):255–66. https://doi.org/10.5731/pdajpst.2013.00919
Kim JM, Kim DH, Park HJ et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J Nanobiotechnology. 2020;18(1):133. Published 2020 Sep 15. doi:https://doi.org/10.1186/s12951-020-00693-4
Zhang R, Qian J, Li X, Yuan Y. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of infliximab encapsulated in liposomes. Br J Ophthalmol. 2017;101(12):1731–8. https://doi.org/10.1136/bjophthalmol-2016-310044
Wang X, Yan J, Wang L, et al. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics. 2020;10(23):10808–22. https://doi.org/10.7150/thno.47601. Published 2020 Aug 29.
Ries M, Moulari B, Shetab Boushehri MA et al. Adalimumab Decorated Nanoparticles Enhance Antibody Stability and Therapeutic Outcome in Epithelial Colitis Targeting. Pharmaceutics. 2022;14(2):352. Published 2022 Feb 2. doi:https://doi.org/10.3390/pharmaceutics14020352
Barnes T, Moots R. Targeting nanomedicines in the treatment of rheumatoid arthritis: focus on certolizumab pegol. Int J Nanomedicine. 2007;2(1):3–7. https://doi.org/10.2147/nano.2007.2.1.3
Fernandes JC, Wang H, Jreyssaty C, et al. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol Ther. 2008;16(7):1243–51. https://doi.org/10.1038/mt.2008.99
Cao J, Cheng J, Xi S, Qi X, Shen S, Ge Y. Alginate/chitosan microcapsules for in situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur J Pharm Biopharm. 2019;137:112–21. https://doi.org/10.1016/j.ejpb.2019.02.011
Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, García AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33(30):7665–75. https://doi.org/10.1016/j.biomaterials.2012.06.101
Ren B, Lu J, Li M, et al. Anti-inflammatory effect of IL-1ra-loaded dextran/PLGA microspheres on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages in vitro and in vivo in a rat model of periodontitis. Biomed Pharmacother. 2021;134:111171. https://doi.org/10.1016/j.biopha.2020.111171
Liu M, Huang Y, Hu L et al. Selective delivery of interleukine-1 receptor antagonist to inflamed joint by albumin fusion. BMC Biotechnol. 2012;12:68. Published 2012 Sep 25. doi:https://doi.org/10.1186/1472-6750-12-68
Clements AEB, Groves ER, Chamberlain CS, Vanderby R, Murphy WL. Microparticles locally deliver active Interleukin-1 receptor antagonist in vivo. Adv Healthc Mater. 2018;7(16):e1800263. https://doi.org/10.1002/adhm.201800263
Lee H, Lee MY, Bhang SH, et al. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano. 2014;8(5):4790–8. https://doi.org/10.1021/nn500685h
Wang Q, Sun X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater Sci. 2017;5(8):1407–20. https://doi.org/10.1039/c7bm00254h
Bisker G, Yeheskely Hayon D, Minai L, Yelin D. Controlled release of Rituximab from gold nanoparticles for phototherapy of malignant cells. J Control Release. 2012;162(2):303–9. https://doi.org/10.1016/j.jconrel.2012.06.030
Kim Y, Youn YS, Oh KT, Kim D, Lee ES. Tumor-targeting liposomes with transient holes allowing Intact Rituximab internally. Biomacromolecules. 2021;22(2):723–31. https://doi.org/10.1021/acs.biomac.0c01514
Li H, Guo K, Wu C, et al. Controlled and targeted drug delivery by a UV-responsive Liposome for overcoming chemo-resistance in Non-Hodgkin Lymphoma. Chem Biol Drug Des. 2015;86(4):783–94. https://doi.org/10.1111/cbdd.12551
Saesoo S, Sathornsumetee S, Anekwiang P, et al. Characterization of liposome-containing SPIONs conjugated with anti-CD20 developed as a novel theranostic agent for central nervous system lymphoma. Colloids Surf B Biointerfaces. 2018;161:497–507. https://doi.org/10.1016/j.colsurfb.2017.11.003
Yin H, Meng T, Shu L, et al. Novel reduction-sensitive micellar nanoparticles assembled from Rituximab-doxorubicin conjugates as smart and intuitive drug delivery systems for the treatment of non-hodgkin’s lymphoma. Chem Biol Drug Des. 2017;90(5):892–9. https://doi.org/10.1111/cbdd.13010
Feng X, Chen Y. Drug delivery targets and systems for targeted treatment of rheumatoid arthritis. J Drug Target. 2018;26(10):845–57. https://doi.org/10.1080/1061186X.2018.1433680
Zeng L, Geng H, Gu W, et al. Au nanoparticles attenuate RANKL-Induced Osteoclastogenesis by suppressing Pre-Osteoclast Fusion. J Nanosci Nanotechnol. 2019;19(4):2166–73. https://doi.org/10.1166/jnn.2019.15764
Pirmardvand Chegini S, Varshosaz J, Taymouri S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif Cells Nanomed Biotechnol. 2018;46(sup2):502–14. https://doi.org/10.1080/21691401.2018.1460373
Koushki K, Keshavarz Shahbaz S, Keshavarz M, Bezsonov EE, Sathyapalan T, Sahebkar A. Gold nanoparticles: multifaceted roles in the management of Autoimmune Disorders. Biomolecules. 2021;11(9):1289. https://doi.org/10.3390/biom11091289. Published 2021 Aug 30.
Shahen SM, Mohamed MR, Ali MRK, Samaka RM, Hamdy GM, Talaat RM. Therapeutic potential of targeted-gold nanospheres on collagen-induced arthritis in rats. Clin Exp Pharmacol Physiol. 2021;48(10):1346–57. https://doi.org/10.1111/1440-1681.13531
Zhao P, Wang Y, Wu A, Rao Y, Huang Y. Roles of albumin-binding proteins in Cancer Progression and Biomimetic targeted drug delivery. ChemBioChem. 2018;19(17):1796–805. https://doi.org/10.1002/cbic.201800201
Zhong J, Zhang Q, Zhang Z et al. Albumin mediated reactive oxygen species scavenging and targeted delivery of methotrexate for rheumatoid arthritis therapy.Nano Research.2021,15(1):153–161. doi:https://doi.org/10.1007/s12274-021-3449-1
Liu L, Hu F, Wang H, et al. Secreted protein acidic and rich in cysteine mediated Biomimetic Delivery of Methotrexate by Albumin-Based nanomedicines for rheumatoid arthritis therapy. ACS Nano. 2019;13(5):5036–48. https://doi.org/10.1021/acsnano.9b01710
Zheng X, Yu X, Wang C, et al. Targeted codelivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Deliv. 2022;29(1):1025–37. https://doi.org/10.1080/10717544.2022.2057616
Yan F, Li H, Zhong Z, et al. Co-Delivery of Prednisolone and Curcumin in Human serum albumin nanoparticles for effective treatment of rheumatoid arthritis. Int J Nanomedicine. 2019;14:9113–25. https://doi.org/10.2147/IJN.S219413. Published 2019 Nov 22.
Martinho N, Florindo H, Silva L, Brocchini S, Zloh M, Barata T. Molecular modeling to study dendrimers for Biomedical Applications. Molecules. 2014;19(12):20424–67. https://doi.org/10.3390/molecules191220424. Published 2014 Dec 8.
Oliveira IM, Gonçalves C, Oliveira EP, et al. PAMAM dendrimers functionalised with an anti-TNF α antibody and chondroitin sulphate for treatment of rheumatoid arthritis. Mater Sci Eng C Mater Biol Appl. 2021;121:111845. https://doi.org/10.1016/j.msec.2020.111845
Hajebi S, Rabiee N, Bagherzadeh M, et al. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1–18. https://doi.org/10.1016/j.actbio.2019.05.018
Preman NK, Barki RR, Vijayan A, Sanjeeva SG, Johnson RP. Recent developments in stimuli-responsive polymer nanogels for drug delivery and diagnostics: a review. Eur J Pharm Biopharm. 2020;157:121–53. https://doi.org/10.1016/j.ejpb.2020.10.009
Samah NA, Williams N, Heard CM. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int J Pharm. 2010;401(1–2):72–8. https://doi.org/10.1016/j.ijpharm.2010.08.011
Wang Q, Sun X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater Sci. 2017;5(8):1407–20. https://doi.org/10.1039/c7bm00254h
Nguyen Dung HT, Nguyen Ngoc B, Nguyen Linh TP et al. An Agarose–Curdlan Nanogel that Carries Etanercept to Target and Neutralizes TNF-α Produced by Dectin-1-Expressing Immune Cells. J Elec Mater. 2019;48(10):6570–6582. https://doi.org/10.1007/s11664-019-07458-2
Walsh L, Ryu J, Bock S, et al. Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism. Nano Lett. 2015;15(4):2434–41. https://doi.org/10.1021/nl504829f
Yu HH, Fan JL, Shehla NZ, et al. Biomimetic Hybrid membrane-coated Xuetongsu assisted with laser irradiation for efficient rheumatoid arthritis therapy. ACS Nano. 2022;16(1):502–21. https://doi.org/10.1021/acsnano.1c07556
Qiu S, Wu XN, Li Z, et al. A Smart Nanoreactor based on an O2-Economized Dual Energy Inhibition Strategy Armed with Dual Multistimuli-Responsive “Doorkeepers” for enhanced CDT/PTT of Rheumatoid Arthritis. ACS Nano. 2022;16(10):17062–79. https://doi.org/10.1021/acsnano.2c07338
Pandey S, Kumar V, Leekha A, et al. Co-Delivery of Teriflunomide and Methotrexate from Hydroxyapatite Nanoparticles for the treatment of rheumatoid arthritis: in Vitro characterization, pharmacodynamic and biochemical investigations. Pharm Res. 2018;35(11):201. https://doi.org/10.1007/s11095-018-2478-2
Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomized, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):58–64. https://doi.org/10.1136/annrheumdis-2015-207764
Emery P, Vencovský J, Sylwestrzak A, et al. A phase III randomized, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):51–7. https://doi.org/10.1136/annrheumdis-2015-207588
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. https://doi.org/10.1136/annrheumdis-2019-216655
Trotta MC, Alfano R, Cuomo G, et al. Comparison of timing to develop anti-drug antibodies to Infliximab and Adalimumab between Adult and Pediatric Age Groups, Males and Females. J Pediatr Pharmacol Ther. 2022;27(1):63–71. https://doi.org/10.5863/1551-6776-27.1.63
Melo AT, Campanilho-Marques R, Fonseca JE. Golimumab (anti-TNF monoclonal antibody): where we stand today. Hum Vaccin Immunother. 2021;17(6):1586–98. https://doi.org/10.1080/21645515.2020.1836919
Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of Autoimmune Diseases. A systematic review and Meta-analysis. BioDrugs. 2015;29(4):241–58. https://doi.org/10.1007/s40259-015-0134-5
Kishimoto TK, Maldonado RA. Nanoparticles for the induction of Antigen-Specific Immunological Tolerance. Front Immunol. 2018;9:230. https://doi.org/10.3389/fimmu.2018.00230. Published 2018 Feb 20.
Chen SY, Xu XX, Li X, et al. Recent advances in the intracellular delivery of macromolecule therapeutics. Biomater Sci. 2022;10(23):6642–55. https://doi.org/10.1039/d2bm01348g. Published 2022 Nov 22.
Patel S, Kim J, Herrera M, Mukherjee A, Kabanov AV, Sahay G. Brief update on endocytosis of nanomedicines. Adv Drug Deliv Rev. 2019;144:90–111. https://doi.org/10.1016/j.addr.2019.08.004
Farhangian ME, Feldman SR. Immunogenicity of biologic treatments for psoriasis: therapeutic consequences and the potential value of concomitant methotrexate. Am J Clin Dermatol. 2015;16(4):285–94. https://doi.org/10.1007/s40257-015-0131-y
Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in previously treated HER2-Positive breast Cancer. N Engl J Med. 2020;382(7):610–21. https://doi.org/10.1056/NEJMoa1914510
Eskandari Z, Bahadori F, Celik B, Onyuksel H. Targeted Nanomedicines for Cancer Therapy, from basics to clinical trials. J Pharm Pharm Sci. 2020;23(1):132–57. https://doi.org/10.18433/jpps30583
Gargett T, Abbas MN, Rolan P, et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol Immunother. 2018;67(9):1461–72. https://doi.org/10.1007/s00262-018-2207-z
Sikder S, Gote V, Alshamrani M, Sicotte J, Pal D. Long-term delivery of protein and peptide therapeutics for cancer therapies. Expert Opin Drug Deliv. 2019;16(10):1113–31. https://doi.org/10.1080/17425247.2019.1662785
Mellor RD, Uchegbu IF. Ultrasmall-in-Nano: why size matters. Nanomaterials (Basel). 2022;12(14):2476. https://doi.org/10.3390/nano12142476. Published 2022 Jul 19.
Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9(9–10):1521–32. https://doi.org/10.1002/smll.201201390
Genito CJ, Batty CJ, Bachelder EM, Ainslie KM. Considerations for size, Surface Charge, Polymer Degradation, Co-Delivery, and Manufacturability in the development of Polymeric particle vaccines for infectious Diseases. Adv Nanobiomed Res. 2021;1(3):2000041. https://doi.org/10.1002/anbr.202000041
Liu Y, Yang G, Jin S, Xu L, Zhao CX. Development of high-drug-loading nanoparticles. ChemPlusChem. 2020;85(9):2143–57. https://doi.org/10.1002/cplu.202000496
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (81874302) and the university level scientific research project of Zhejiang Shuren University (2022R005), Training plan for leading talents from Universities in Zhejiang Province.
Author information
Authors and Affiliations
Contributions
Xiao-Kai Chi and Xiao-Ling Xu wrote the original draft. Bang-Yao Chen prepared Figs. 2 and 3. Jin Su and Yong-Zhong Du, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chi, XK., Xu, XL., Chen, BY. et al. Combining nanotechnology with monoclonal antibody drugs for rheumatoid arthritis treatments. J Nanobiotechnol 21, 105 (2023). https://doi.org/10.1186/s12951-023-01857-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-01857-8