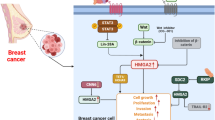
Abstract
Background
Acquired resistance to drug involves multilayered genetic and epigenetic regulation. Inhibition of EZH2 has proven to reverse the tamoxifen resistance back to the sensitive state in breast cancer. However, the molecular players involved in EZH2-mediated effects on tamoxifen-resistant MCF-7 cells are unknown. This study was conducted to understand the global change in proteome profile of tamoxifen-resistant MCF-7 breast cancer cells upon EZH2 knockdown.
Methods
Tamoxifen resistance MCF-7 breast cancer cells were established using increasing concentrations of 4-hydroxy tamoxifen. Using label free proteomics approach, we studied the alteration in total proteome in resistant cells as well as cells transfected with siEZH2 in comparison to sensitive and cells transfected with non-targeting siRNA.
Results
Here, we report list of proteins that were previously not recognized for their role in tamoxifen resistance and hold a close association with breast cancer patient survival. Proteins Annexin A2, CD44, nucleosome assembly protein 1, and lamin A/C were among the most upregulated protein in tamoxifen-resistant cells that were found to be abrogated upon EZH2 knockdown. The study suggests the involvement for various proteins in acquiring resistance towards tamoxifen and anticipates further research for investigating their therapeutic potentials.
Conclusion
Overall, we propose that targeting EZH2 or the molecules down the cascade might be helpful in reacquiring sensitivity to tamoxifen in breast cancer.





Similar content being viewed by others
Availability of data and materials
The mass spectrometry proteomics data generated during the study have been deposited to the ProteomeXchange Consortium via the PRIDE with an accession id PXD012609.
References
Chang M. Tamoxifen resistance in breast cancer. Biomol Ther. 2012;20(3):256–67. https://doi.org/10.4062/biomolther.2012.20.3.256.
Hu Q, Baeg GH. Role of epigenome in tumorigenesis and drug resistance. Food Chem Toxicol. 2017;109(Pt 1):663–8. https://doi.org/10.1016/j.fct.2017.07.022.
Balch C, Huang TH, Brown R, Nephew KP. The epigenetics of ovarian cancer drug resistance and resensitization. Am J Obstet Gynecol. 2004;191(5):1552–72. https://doi.org/10.1016/j.ajog.2004.05.025.
Askarian-Amiri ME, Leung E, Finlay G, Baguley BC. The regulatory role of long noncoding RNAs in cancer drug resistance. Methods Mol Biol. 2016;1395:207–27. https://doi.org/10.1007/978-1-4939-3347-1_12.
Rueff J, Rodrigues AS. Cancer drug resistance: a brief overview from a genetic viewpoint. Methods Mol Biol. 2016;1395:1–18. https://doi.org/10.1007/978-1-4939-3347-1_1.
Issa ME, Takhsha FS, Chirumamilla CS, Perez-Novo C, Vanden Berghe W, Cuendet M. Epigenetic strategies to reverse drug resistance in heterogeneous multiple myeloma. Clin Epigenet. 2017;9:17. https://doi.org/10.1186/s13148-017-0319-5.
Chen S, Yao F, Xiao Q, Liu Q, Yang Y, Li X, Jiang G, Kuno T, Fang Y. EZH2 inhibition sensitizes tamoxifenresistant breast cancer cells through cell cycle regulation. Mol Med Rep. 2018;17(2):2642–50. https://doi.org/10.3892/mmr.2017.8160.
Tamm-Rosenstein K, Simm J, Suhorutshenko M, Salumets A, Metsis M. Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS ONE. 2013;8(7):e68907. https://doi.org/10.1371/journal.pone.0068907.
Wu Y, Zhang Z, Cenciarini ME, Proietti CJ, Amasino M, Hong T, Yang M, Liao Y, Chiang HC, Kaklamani VG, Jeselsohn R, Vadlamudi RK, Huang TH, Li R, De Angelis C, Fu X, Elizalde PV, Schiff R, Brown M, Xu K. Tamoxifen resistance in breast cancer is regulated by the EZH2-ERalpha-GREB1 transcriptional axis. Can Res. 2018;78(3):671–84. https://doi.org/10.1158/0008-5472.CAN-17-1327.
Reijm EA, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, van Gelder ME, Sieuwerts AM, Sleijfer S, Foekens JA, Berns EM. Decreased expression of EZH2 is associated with upregulation of ER and favorable outcome to tamoxifen in advanced breast cancer. Breast Cancer Res Treat. 2011;125(2):387–94. https://doi.org/10.1007/s10549-010-0836-9.
Reijm EA, Timmermans AM, Look MP, Meijer-van Gelder ME, Stobbe CK, van Deurzen CH, Martens JW, Sleijfer S, Foekens JA, Berns PM, Jansen MP. High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol. 2014;25(11):2185–90. https://doi.org/10.1093/annonc/mdu391.
Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, Ebbs SR, Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Can Res. 1995;55(15):3331–8.
Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–44. https://doi.org/10.1210/en.2002-220620.
Zhou C, Zhong Q, Rhodes LV, Townley I, Bratton MR, Zhang Q, Martin EC, Elliott S, Collins-Burow BM, Burow ME, Wang G. Proteomic analysis of acquired tamoxifen resistance in MCF-7 cells reveals expression signatures associated with enhanced migration. Breast Cancer Res. 2012;14(2):R45. https://doi.org/10.1186/bcr3144.
Jene-Sanz A, Varaljai R, Vilkova AV, Khramtsova GF, Khramtsov AI, Olopade OI, Lopez-Bigas N, Benevolenskaya EV. Expression of polycomb targets predicts breast cancer prognosis. Mol Cell Biol. 2013;33(19):3951–61. https://doi.org/10.1128/MCB.00426-13.
van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45. https://doi.org/10.1007/978-1-61779-080-5_20.
Kumari K, Das B, Adhya A, Chaudhary S, Senapati S, Mishra SK. Nicotine associated breast cancer in smokers is mediated through high level of EZH2 expression which can be reversed by methyltransferase inhibitor DZNepA. Cell Death Dis. 2018;9(2):152. https://doi.org/10.1038/s41419-017-0224-z.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–31. https://doi.org/10.1007/s10549-009-0674-9.
Shaul YD, Yuan B, Thiru P, Nutter-Upham A, McCallum S, Lanzkron C, Bell GW, Sabatini DM. MERAV: a tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2016;44(D1):D560–566. https://doi.org/10.1093/nar/gkv1337.
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–35.
Kumar R, Mandal M, Lipton A, Harvey H, Thompson CB. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin Cancer Res. 1996;2(7):1215–9.
Ali S, Rasool M, Chaoudhry H, Pushparaj NP, Jha P, Hafiz A, Mahfooz M, Abdus Sami G, Azhar Kamal M, Bashir S, Ali A, Sarwar Jamal M. Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation. 2016;12(3):135–9. https://doi.org/10.6026/97320630012135.
Klinge CM, Riggs KA, Wickramasinghe NS, Emberts CG, McConda DB, Barry PN, Magnusen JE. Estrogen receptor alpha 46 is reduced in tamoxifen resistant breast cancer cells and re-expression inhibits cell proliferation and estrogen receptor alpha 66-regulated target gene transcription. Mol Cell Endocrinol. 2010;323(2):268–76. https://doi.org/10.1016/j.mce.2010.03.013.
Sobolewski C, Abegg D, Berthou F, Dolicka D, Calo N, Sempoux C, Fournier M, Maeder C, Ay AS, Clavien PA, Humar B, Dufour JF, Adibekian A, Foti M. S100A11/ANXA2 belongs to a tumour suppressor/oncogene network deregulated early with steatosis and involved in inflammation and hepatocellular carcinoma development. Gut. 2020. https://doi.org/10.1136/gutjnl-2019-319019.
Rehman I, Azzouzi AR, Cross SS, Deloulme JC, Catto JW, Wylde N, Larre S, Champigneuille J, Hamdy FC. Dysregulated expression of S100A11 (calgizzarin) in prostate cancer and precursor lesions. Hum Pathol. 2004;35(11):1385–91. https://doi.org/10.1016/j.humpath.2004.07.015.
Liu XG, Wang XP, Li WF, Yang S, Zhou X, Li SJ, Li XJ, Hao DY, Fan ZM. Ca2+-binding protein S100A11: a novel diagnostic marker for breast carcinoma. Oncol Rep. 2010;23(5):1301–8. https://doi.org/10.3892/or_00000764.
Zhang S, Wang Z, Liu W, Lei R, Shan J, Li L, Wang X. Distinct prognostic values of S100 mRNA expression in breast cancer. Sci Rep. 2017;7:39786. https://doi.org/10.1038/srep39786.
Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. https://doi.org/10.1016/S0140-6736(05)66544-0.
Tabb DL, Vega-Montoto L, Rudnick PA, Variyath AM, Ham AJ, Bunk DM, Kilpatrick LE, Billheimer DD, Blackman RK, Cardasis HL, Carr SA, Clauser KR, Jaffe JD, Kowalski KA, Neubert TA, Regnier FE, Schilling B, Tegeler TJ, Wang M, Wang P, Whiteaker JR, Zimmerman LJ, Fisher SJ, Gibson BW, Kinsinger CR, Mesri M, Rodriguez H, Stein SE, Tempst P, Paulovich AG, Liebler DC, Spiegelman C. Repeatability and reproducibility in proteomic identifications by liquid chromatography-tandem mass spectrometry. J Proteome Res. 2010;9(2):761–76. https://doi.org/10.1021/pr9006365.
Bian Y, Zheng R, Bayer FP, Wong C, Chang YC, Meng C, Zolg DP, Reinecke M, Zecha J, Wiechmann S, Heinzlmeir S, Scherr J, Hemmer B, Baynham M, Gingras AC, Boychenko O, Kuster B. Robust, reproducible and quantitative analysis of thousands of proteomes by micro-flow LC-MS/MS. Nat Commun. 2020;11(1):157. https://doi.org/10.1038/s41467-019-13973-x.
Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, Uhlen M, Nilsson P. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics. 2009;10:365. https://doi.org/10.1186/1471-2164-10-365.
Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep. 2015;5:10775. https://doi.org/10.1038/srep10775.
Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4(9):117. https://doi.org/10.1186/gb-2003-4-9-117.
Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165(3):535–50. https://doi.org/10.1016/j.cell.2016.03.014.
Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033–46. https://doi.org/10.1007/s00018-011-0735-1.
Kim JA, Choi DK, Min JS, Kang I, Kim JC, Kim S, Ahn JK. VBP1 represses cancer metastasis by enhancing HIF-1alpha degradation induced by pVHL. FEBS J. 2018;285(1):115–26. https://doi.org/10.1111/febs.14322.
Malouf GG, Su X, Yao H, Gao J, Xiong L, He Q, Comperat E, Couturier J, Molinie V, Escudier B, Camparo P, Doss DJ, Thompson EJ, Khayat D, Wood CG, Yu W, Teh BT, Weinstein J, Tannir NM. Next-generation sequencing of translocation renal cell carcinoma reveals novel RNA splicing partners and frequent mutations of chromatin-remodeling genes. Clin Cancer Res. 2014;20(15):4129–40. https://doi.org/10.1158/1078-0432.CCR-13-3036.
Ura B, Monasta L, Arrigoni G, Franchin C, Radillo O, Peterlunger I, Ricci G, Scrimin F. A proteomic approach for the identification of biomarkers in endometrial cancer uterine aspirate. Oncotarget. 2017;8(65):109536–45. https://doi.org/10.18632/oncotarget.22725.
Wang D, Liu H, Ren C, Wang L. High expression of ABRACL is associated with tumorigenesis and affects clinical outcome in gastric cancer. Genet Test Mol Biomarkers. 2019;23(2):91–7. https://doi.org/10.1089/gtmb.2018.0195.
Guo F, Li Y, Liu Y, Wang J, Li G. ARL6IP1 mediates cisplatin-induced apoptosis in CaSki cervical cancer cells. Oncol Rep. 2010;23(5):1449–555.
Guo F, Liu Y, Li Y, Li G. Inhibition of ADP-ribosylation factor-like 6 interacting protein 1 suppresses proliferation and reduces tumor cell invasion in CaSki human cervical cancer cells. Mol Biol Rep. 2010;37(8):3819–25. https://doi.org/10.1007/s11033-010-0037-y.
Kanojia D, Zhou W, Zhang J, Jie C, Lo PK, Wang Q, Chen H. Proteomic profiling of cancer stem cells derived from primary tumors of HER2/Neu transgenic mice. Proteomics. 2012;12(22):3407–15. https://doi.org/10.1002/pmic.201200103.
Tripathi SC, Matta A, Kaur J, Grigull J, Chauhan SS, Thakar A, Shukla NK, Duggal R, Choudhary AR, Dattagupta S, Sharma MC, Ralhan R, Siu KW. Overexpression of prothymosin alpha predicts poor disease outcome in head and neck cancer. PLoS ONE. 2011;6(5):e19213. https://doi.org/10.1371/journal.pone.0019213.
Ha SY, Song DH, Hwang SH, Cho SY, Park CK. Expression of prothymosin alpha predicts early recurrence and poor prognosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14(2):171–7.
Abdel-Hafiz HA, Horwitz KB. Role of epigenetic modifications in luminal breast cancer. Epigenomics. 2015;7(5):847–62. https://doi.org/10.2217/epi.15.10.
Abdel-Hafiz HA. Epigenetic mechanisms of tamoxifen resistance in luminal breast cancer. Diseases. 2017. https://doi.org/10.3390/diseases5030016.
Lanzetti L, Di Fiore PP. Behind the scenes: endo/exocytosis in the acquisition of metastatic traits. Can Res. 2017;77(8):1813–7. https://doi.org/10.1158/0008-5472.CAN-16-3403.
Hunter MC, O'Hagan KL, Kenyon A, Dhanani KC, Prinsloo E, Edkins AL. Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells. PLoS ONE. 2014;9(1):e86842. https://doi.org/10.1371/journal.pone.0086842.
Holmstrom MO, Martinenaite E, Ahmad SM, Met O, Friese C, Kjaer L, Riley CH, Thor Straten P, Svane IM, Hasselbalch HC, Andersen MH. The calreticulin (CALR) exon 9 mutations are promising targets for cancer immune therapy. Leukemia. 2018;32(2):429–37. https://doi.org/10.1038/leu.2017.214.
Hsu KS, Kao HY. Alpha-actinin 4 and tumorigenesis of breast cancer. Vitam Horm. 2013;93:323–51. https://doi.org/10.1016/B978-0-12-416673-8.00005-8.
Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, Der Nigoghossian C, Chuich T, Nouri SN, Dreyfus I, Driggin E, Sethi S, Sehgal K, Chatterjee S, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Bertoletti L, Giri J, Cushman M, Quere I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Tafur AJ, Francese DP, Batra J, Falanga A, Clerkin KJ, Uriel N, Kirtane A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Leon MB, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH, Global C-TCG. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020. https://doi.org/10.1055/s-0040-1713152.
Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65(11):904–10. https://doi.org/10.1002/iub.1216.
Liu Y, Han X, Gao B. Knockdown of S100A11 expression suppresses ovarian cancer cell growth and invasion. Exp Ther Med. 2015;9(4):1460–4. https://doi.org/10.3892/etm.2015.2257.
Xie R, Liu J, Yu X, Li C, Wang Y, Yang W, Hu J, Liu P, Sui H, Liang P, Huang X, Wang L, Bai Y, Xue Y, Zhu J, Fang T. ANXA2 silencing inhibits proliferation, invasion, and migration in gastric cancer cells. J Oncol. 2019;2019:4035460. https://doi.org/10.1155/2019/4035460.
Chen CY, Lin YS, Chen CH, Chen YJ. Annexin A2-mediated cancer progression and therapeutic resistance in nasopharyngeal carcinoma. J Biomed Sci. 2018;25(1):30. https://doi.org/10.1186/s12929-018-0430-8.
Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med J. 2015;36(3):273–9. https://doi.org/10.15537/smj.2015.3.9622.
Le Y, Kan A, Li QJ, He MK, Chen HL, Shi M. NAP1L1 is a prognostic biomarker and contribute to doxorubicin chemotherapy resistance in human hepatocellular carcinoma. Cancer Cell Int. 2019;19:228. https://doi.org/10.1186/s12935-019-0949-0.
Kim K, Kim MJ, Kim KH, Ahn SA, Kim JH, Cho JY, Yeo SG. C1QBP is upregulated in colon cancer and binds to apolipoprotein A-I. Exp Ther Med. 2017;13(5):2493–500. https://doi.org/10.3892/etm.2017.4249.
Wang Y, Fu D, Su J, Chen Y, Qi C, Sun Y, Niu Y, Zhang N, Yue D. C1QBP suppresses cell adhesion and metastasis of renal carcinoma cells. Sci Rep. 2017;7(1):999. https://doi.org/10.1038/s41598-017-01084-w.
Guinde J, Frankel D, Perrin S, Delecourt V, Levy N, Barlesi F, Astoul P, Roll P, Kaspi E. Lamins in lung cancer: biomarkers and key factors for disease progression through miR-9 regulation? Cells. 2018. https://doi.org/10.3390/cells7070078.
Irianto J, Pfeifer CR, Ivanovska IL, Swift J, Discher DE. Nuclear lamins in cancer. Cell Mol Bioeng. 2016;9(2):258–67. https://doi.org/10.1007/s12195-016-0437-8.
Acknowledgements
We acknowledge DBT, Govt. of India and DST, Govt. of India, for funding. We also acknowledge the Director, Institute of Life Sciences, for the core grant as well as his support in the performance of this project.
Author information
Authors and Affiliations
Contributions
The conception and design of the study, or acquisition of data, or analysis and interpretation of data: KK, SK, DKP, and SKM. Drafting the article or revising it critically for important intellectual content: KK and SK. Final approval of the version to be submitted: KK, SK, DKP, and SKM.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12282_2020_1166_MOESM1_ESM.pdf
Supplementary file1 Figure S1. Pathway analysis of differentially expressed proteins in tamoxifen resistant MCF-7 cells. Figure shows biological process regulating network analysis of total deferentially expressed proteins in tamoxifen resistant MCF-7 breast cancer cells. Edges (connecting line) describes the kappa score, the thicker the line, higher is the confidence of the kappa score (interaction), the threshold used kappa score for the formation of the network is 0.5 which is already too high for any network. Size of the nodes (circles) depicts the number of genes involved in the process and color of the nodes describes the confidence of the corrected P-value identified. Darker the color, more is the confidence of the identified process in the interaction network. Figure S2. Expression of genes encoding differentially expressed proteins in MTR cells that are significantly associated with overall patient survival. Survival curves show the significant association of genes encoding ANO8, PLP2, ARL6IP1, VBP1 and PTMA with breast cancer patient survival as studied using KM plotter database. HR: Hazard Ratio. Figure S3. Survival curves of genes encoding differentially expressed proteins in MTR cells that are not significantly associated with overall patient survival. Graphs show the non-significant association of genes encoding SH3BGRL3, RHOA, PEA15, AK1, HSPA5, FSCN1, OA48, HSPA1L, CP94 and ABRACL. Figure S4. Correlation graphs for expression of genes encoding ANO8, PLP2, ARL6IP1 and VBP1 with ERα expression in primary breast tumor samples. Using MERAV database, correlation for expression of ANO8, PLP2, ARL6IP1 and VBP1 with ERα was calculated as displayed in the graphs. Figure S5. Protein-protein interaction network for differentially expressed proteins in EZH2 knockdown in MTR cells. Differentially over-expressed and down-regulated proteins were used to identify PPI network for biological processes, molecular functions and cellular component analysis. The connection line-width defines the co-expression the interacting partner proteins in the cellular system. Darker the color, more is the confidence of the identified process in the interaction network. Figure S6. EZH2 knockdown increases the sensitivity of tamoxifen resistant cells towards tamoxifen. Line graph shows the percent cell inhibition in siControl and siEZH2 transfected MTR cells. By taking the log concentrations of tamoxifen and the relative percent absorbance values mentioned below the figure were used to calculate the IC50 value for tamoxifen in both the groups. (PDF 9304 kb)
About this article
Cite this article
Kumari, K., Kumar, S., Parida, D.K. et al. EZH2 knockdown in tamoxifen-resistant MCF-7 cells unravels novel targets for regaining sensitivity towards tamoxifen. Breast Cancer 28, 355–367 (2021). https://doi.org/10.1007/s12282-020-01166-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01166-0