Abstract
Purpose of Review
This review summarizes recent advances in the assessment of bone quality using non-X-ray techniques.
Recent Findings
Quantitative ultrasound (QUS) provides multiple measurements of bone characteristics based on the propagation of sound through bone, the attenuation of that sound, and different processing techniques. QUS parameters and model predictions based on backscattered signals can discriminate non-fracture from fracture cases with accuracy comparable to standard bone mineral density (BMD). With advances in magnetic resonance imaging (MRI), bound water and pore water, or a porosity index, can be quantified in several long bones in vivo. Since such imaging-derived measurements correlate with the fracture resistance of bone, they potentially provide new BMD-independent predictors of fracture risk. While numerous measurements of mineral, organic matrix, and bound water by Raman spectroscopy correlate with the strength and toughness of cortical bone, the clinical assessment of person’s bone quality using spatially offset Raman spectroscopy (SORS) requires advanced spectral processing techniques that minimize contaminating signals from fat, skin, and blood.
Summary
Limiting exposure of patients to ionizing radiation, QUS, MRI, and SORS has the potential to improve the assessment of fracture risk and track changes of new therapies that target bone matrix and micro-structure.

Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lester G. Bone quality: summary of NIH/ASBMR meeting. J Musculoskel Neuronal Interact. 2005;5:309.
Siris ES, Chen Y-T, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–12.
Shevroja E, Cafarelli FP, Guglielmi G, Hans D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine. 2021;74:20–8.
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518–30.
Whittier DE, Boyd SK, Burghardt AJ, Paccou J, Ghasem-Zadeh A, Chapurlat R, et al. Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporosis Int. 2020;31:1607–27.
Mikolajewicz N, Bishop N, Burghardt AJ, Folkestad L, Hall A, Kozloff KM, et al. HR-pQCT measures of bone microarchitecture predict fracture: systematic review and meta-analysis. J Bone Miner Res. 2020;35:446–59.
Cappelle SI, Moreau M, Karmali R, Iconaru L, Baleanu F, Kinnard V, et al. Discriminating value of HR-pQCT for fractures in women with similar FRAX scores: a substudy of the FRISBEE cohort. Bone. 2021;143: 115613.
Unal M, Creecy A, Nyman JS. The role of matrix composition in the mechanical behavior of bone. Curr Osteoporos Rep. 2018;16:205–15.
Burr DB. Changes in bone matrix properties with aging. Bone. 2019;120:85–93.
Burstein AH, Reilly DT, Martens M. Aging of bone tissue: mechanical properties. J Bone Joint Surg Am. 1976;58:82–6.
Zioupos P, Currey JD. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone. 1998;22:57–66.
Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthopaed Res. 2007;25:646–55.
Guglielmi G, Adams J, Link TM. Quantitative ultrasound in the assessment of skeletal status. Eur Radiol. 2009;19:1837–48.
Nicholson PHF, Strelitzki R, Cleveland RO, Bouxsein ML. Scattering of ultrasound in cancellous bone: predictions from a theoretical model. J Biomech. 2000;33:503–6.
Hans D, Wu C, Njeh CF, Zhao S, Augat P, Newitt D, et al. Ultrasound velocity of trabecular cubes reflects mainly bone density and elasticity. Calcif Tissue Int. 1999;64:18–23.
Minh HN, Du J, Raum K. Estimation of thickness and speed of sound in cortical bone using multifocus pulse-echo ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67:568–79.
Langton CM, Palmer SB, Porter RW. The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med. 1984;13:89–91.
Swinton PA, Elliott-Sale KJ, Sale C. Comparative analysis of bone outcomes between quantitative ultrasound and dual-energy x-ray absorptiometry from the UK Biobank cohort. Arch Osteoporos. 2023;18:77.
McCloskey EV, Kanis JA, Odén A, Harvey NC, Bauer D, González-Macias J, et al. Predictive ability of heel quantitative ultrasound for incident fractures: an individual-level meta-analysis. Osteoporos Int. 2015;26:1979–87.
Fu Y, Li C, Luo W, Chen Z, Liu Z, Ding Y. Fragility fracture discriminative ability of radius quantitative ultrasound: a systematic review and meta-analysis. Osteoporos Int. 2021;32:23–38.
Imashuku Y, Takada M, Murata K. Comparisons of bone mass measurements on various skeletal sites including quantitative ultrasonography of the calcaneus for assessing age-related losses, their correlations, and diagnostic agreement using the Japanese and WHO criteria for osteoporosis. Radiat Med. 2007;25:148–54.
Métrailler A, Hans D, Lamy O, Rodriguez EG, Shevroja E. Heel quantitative ultrasound (QUS) predicts incident fractures independently of trabecular bone score (TBS), bone mineral density (BMD), and FRAX: the OsteoLaus Study. Osteoporos Int. 2023;34:1401–9. The study found that Heel-QUS could predict major osteoporotic fractures independently of FRAX, BMD, and the trabecular bone score. This underscores its potential as a pre-screening tool for osteoporosis management.
Strässle M, Grossmann J, Eppenberger P, Faas A, Jerkovic I, Floris J, et al. Short-termed changes in quantitative ultrasound estimated bone density among young men in an 18-weeks follow-up during their basic training for the Swiss Armed Forces. PeerJ. 2023;11: e15205.
Sahota O, San P, Cawte SA, Pearson D, Hosking DJ. A Comparison of the longitudinal changes in quantitative ultrasound with dual-energy X-ray absorptiometry: the four-year effects of hormone replacement therapy. Osteoporos Int. 2000;11:52–8.
Gonnelli S, Cepollaro C, Montagnani A, Martini S, Gennari. L, Mangeri M, et al. Heel ultrasonography in monitoring alendronate therapy: a four-year longitudinal study. Osteoporos Int. 2002;13:415–21.
Hans D, Métrailler A, Rodriguez EG, Lamy O, Shevroja E. Bone quantitative ultrasound, new horizons. Adv Exp Med Biol. 2022;1364:7–34.
Moris M, Peretz A, Tjeka R, Negaban N, Wouters M, Bergmann P. Quantitative ultrasound bone measurements: normal values and comparison with bone mineral density by dual X-ray absorptiometry. Calcif Tissue Int. 1995;57:6–10.
Rosenthall L, Caminis J, Tenehouse A. Calcaneal Ultrasonometry: Response to Treatment in Comparison with Dual X-ray Absorptiometry Measurements of the Lumbar Spine and Femur. Calcif Tissue Int. 1999;64:200–4.
Töyräs J, Nieminen MT, Kröger H, Jurvelin JS. Bone mineral density, ultrasound velocity, and broadband attenuation predict mechanical properties of trabecular bone differently. Bone. 2002;31:503–7.
Hans D, Fuerst T, Uffmann M. Bone density and quality measurement using ultrasound. Curr Opin Rheumatol. 1996;8:370–5.
ABENDSCHEIN W, HYATT GW. 33 Ultrasonics and selected physical properties of bone. Clin Orthop Relat Res. 1970;69:294–301.
Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech. 1993;26:111–9.
Bouxsein ML, Radloff SE. Quantitative ultrasound of the calcaneus reflects the mechanical properties of calcaneal trabecular bone. J Bone Miner Res. 1997;12:839–46.
Bouxsein ML, Coan BS, Lee SC. Prediction of the strength of the elderly proximal femur by bone mineral density and quantitative ultrasound measurements of the heel and tibia. Bone. 1999;25:49–54.
Peralta L, Redin JDM, Fan F, Cai X, Laugier P, Schneider J, et al. Bulk wave velocities in cortical bone reflect porosity and compression strength. Ultrasound Med Biol. 2021;47:799–808.
Hernandez CJ, van der Meulen MC. Understanding bone strength is not enough. J Bone Miner Res. 2017;32:1157–62.
Cook RB, Curwen C, Tasker T, Zioupos P. Fracture toughness and compressive properties of cancellous bone at the head of the femur and relationships to non-invasive skeletal assessment measurements. Méd Eng Phys. 2010;32:991–7.
Rufus-Membere P, Holloway-Kew KL, Diez-Perez A, Kotowicz MA, Pasco JA. Associations between bone material strength index, calcaneal quantitative ultrasound and bone mineral density in men. J Endocr Soc. 2020;5:bvaa179-.
Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. Rev Sci Instrum. 2012;83: 044301.
Abraham AC, Agarwalla A, Yadavalli A, Liu JY, Tang SY. Microstructural and compositional contributions towards the mechanical behavior of aging human bone measured by cyclic and impact reference point indentation. Bone. 2016;87:37–43.
Karbalaeisadegh Y, Yousefian O, Iori G, Raum K, Muller M. Acoustic diffusion constant of cortical bone: numerical simulation study of the effect of pore size and pore density on multiple scattering. J Acoust Soc Am. 2019;146:1015–23.
Karbalaeisadegh Y, Yao S, Zhu Y, Grimal Q, Muller M. Ultrasound characterization of cortical bone using Shannon entropy. Ultrasound Med Biol. 2023;49:1824–9.
Gräsel M, Glüer C-C, Barkmann R. Characterization of a new ultrasound device designed for measuring cortical porosity at the human tibia: a phantom study. Ultrasonics. 2017;76:183–91.
Iori G, Du J, Hackenbeck J, Kilappa V, Raum K. Estimation of cortical bone microstructure from ultrasound backscatter. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68:1081–95.
Armbrecht G, Minh HN, Massmann J, Raum K. Pore-size distribution and frequency-dependent attenuation in human cortical tibia bone discriminate fragility fractures in postmenopausal women with low bone mineral density. JBMR Plus. 2021;5: e10536.
Minonzio J, Bochud N, Vallet Q, Ramiandrisoa D, Etcheto A, Briot K, et al. ultrasound-based estimates of cortical bone thickness and porosity are associated with nontraumatic fractures in postmenopausal women: a pilot study. J Bone Miner Res. 2019;34:1585–96.
Cheng S, Tylavsky FA, Orwoll ES, Rho J-Y, Carbone LD. The role of collagen abnormalities in ultrasound and densitometry assessment. In VivoEvidence Calcif Tissue Int. 1999;64:470–6.
Hoffmeister BK, Whitten SA, Kaste SC, Rho JY. Effect of collagen and mineral content on the high-frequency ultrasonic properties of human cancellous bone. Osteoporos Int. 2002;13:26–32.
Kann P, Bergink AP, Fang Y, van Daele PLA, Hofman A, van Leeuwen JPTM, et al. The collagen Ia1 SP1 polymorphism is associated with differences in ultrasound transmission velocity in the calcaneus in postmenopausal women. Calcif Tissue Int. 2002;70:450–6.
Lalli P, Mautino C, Busso C, Bardesono F, Monaco MD, Lippi L, et al. Reproducibility and accuracy of the radiofrequency echographic multi-spectrometry for femoral mineral density estimation and discriminative power of the femoral fragility score in patients with primary and disuse-related osteoporosis. J Clin Med. 2022;11:3761.
Messina C, Gitto S, Colombo R, Fusco S, Guagliardo G, Piazza M, Poli JC, Albano D, Sconfienza LM. Short-term precision and repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on lumbar spine and proximal femur: an in vivo study. J Imaging. 2023;9(6):118.
Conversano F, Franchini R, Greco A, Soloperto G, Chiriacò F, Casciaro E, et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med Biol. 2015;41:281–300.
Casciaro S, Peccarisi M, Pisani P, Franchini R, Greco A, Marco TD, et al. An advanced quantitative echosound methodology for femoral neck densitometry. Ultrasound Med Biol. 2016;42:1337–56.
Sergio R-O, Nayelli RGE. Evaluation of the bone mineral density in the Mexican female population using the Radiofrequency Echographic Multi Spectrometry (REMS) technology. Arch Osteoporos. 2022;17:43.
Cortet B, Dennison E, Diez-Perez A, Locquet M, Muratore M, Nogués X, et al. Radiofrequency Echographic Multi Spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone. 2021;143: 115786.
Quarta E, Ciardo D, Ciccarese M, Conversano F, Paola MD, Forcignanò R, et al. SAT0461 Short-term monitoring of denosumab effect in breast cancer patients receiving aromatase inhibitors using rems technology on lumbar spine. Ann Rheum Dis. 2020;79:1187.2–1188.
Greco A, Pisani P, Conversano F, Soloperto G, Renna MD, Muratore M, et al. Ultrasound fragility score: an innovative approach for the assessment of bone fragility. Measurement. 2017;101:236–42.
Diez-Perez A, Brandi ML, Al-Daghri N, Branco JC, Bruyère O, Cavalli L, et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: state of the art—outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin Exp Res. 2019;31:1375–89.
Pisani P, Greco A, Conversano F, Renna MD, Casciaro E, Quarta L, et al. A quantitative ultrasound approach to estimate bone fragility: a first comparison with dual X-ray absorptiometry. Measurement. 2017;101:243–9.
Caffarelli C, Pitinca MDT, Refaie AA, Vita MD, Catapano S, Gonnelli S. Could radiofrequency echographic multispectrometry (REMS) overcome the overestimation in BMD by dual-energy X-ray absorptiometry (DXA) at the lumbar spine? BMC Musculoskelet Disord. 2022;23:469.
Pisani P, Conversano F, Muratore M, Adami G, Brandi ML, Caffarelli C, et al. Fragility score: a REMS-based indicator for the prediction of incident fragility fractures at 5 years. Aging Clin Exp Res. 2023;35:763–73. The Fragility Score from REMS outperformed DXA BMD in identifying individuals at risk for fragility fracture for both males and females at both sites. This indicates that backscattered echo signals in ultrasound likely asses bone quality.
Schacter GI, Leslie WD. DXA-based measurements in diabetes: can they predict fracture risk? Calcif Tissue Int. 2017;100:150–64. While women with type 2 diabetes exhibited an expected higher DXA-derived BMD value compared to control women, the REMS-estimated BMD was lower in the women with diabets. This allowed for more women with T2DM to be classified as 'osteoporotic' when using REMS, highlighting its potential value as a diagnostic tool in diseases where DXA-derived BMD falls short.
Caffarelli C, Pitinca MDT, Refaie AA, Ceccarelli E, Gonnelli S. Ability of radiofrequency echographic multispectrometry to identify osteoporosis status in elderly women with type 2 diabetes. Aging Clin Exp Res. 2022;34:121–7.
Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, et al. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47:458–66.
Workman C, Blalock DV, Mehler PS. Bone density status in a large population of patients with anorexia nervosa. Bone. 2020;131: 115161.
Caffarelli C, Refaie AA, Vita MD, Pitinca MDT, Goracci A, Fagiolini A, et al. Radiofrequency echographic multispectrometry (REMS): an innovative technique for the assessment of bone status in young women with anorexia nervosa. Eat Weight Disord Stud Anorex Bulim Obes. 2022;27:3207–13.
Fassio A, Andreola S, Gatti D, Bianco B, Gatti M, Gambaro G, et al. Radiofrequency echographic multi-spectrometry and DXA for the evaluation of bone mineral density in a peritoneal dialysis setting. Aging Clin Exp Res. 2023;35:185–92.
Sollmann N, Löffler MT, Kronthaler S, Böhm C, Dieckmeyer M, Ruschke S, et al. MRI-based quantitative osteoporosis imaging at the spine and femur. J Magn Reson Imaging. 2021;54:12–35.
Jerban S, Alenezi S, Afsahi AM, Ma Y, Du J, Chung CB, et al. MRI-based mechanical competence assessment of bone using micro finite element analysis (micro-FEA): Review. Magn Reson Imaging. 2022;88:9–19.
Nyman JS, Does MD. Bound water and pore water in osteoporosis. In: Du J, Bydder GM, editors. MRI of Short and Ultrashort-T2 Tissues: Making the Invisible Visible. Spinger; 2023.
Majumdar S, Genant HK, Grampp S, Newitt DC, Truong V-H, Lin JC, et al. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res. 1997;12:111–8.
Wehrli FW, Hwang SN, Ma J, Song HK, Ford JC, Haddad JG. Cancellous bone volume and structure in the forearm: noninvasive assessment with MR microimaging and image processing. Radiology. 1998;206:347–57.
Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y, et al. In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res. 1998;13:1175–82.
Benito M, Gomberg B, Wehrli FW, Weening RH, Zemel B, Wright AC, et al. Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab. 2003;88:1497–502.
Krug R, Banerjee S, Han ET, Newitt DC, Link TM, Majumdar S. Feasibility of in vivo structural analysis of high-resolution magnetic resonance images of the proximal femur. Osteoporos Int. 2005;16:1307–14.
Chang G, Deniz CM, Honig S, Rajapakse CS, Egol K, Regatte RR, et al. Feasibility of three-dimensional MRI of proximal femur microarchitecture at 3 tesla using 26 receive elements without and with parallel imaging. J Magn Reson Imaging. 2014;40:229–38.
Chang G, Rajapakse CS, Regatte RR, Babb J, Saxena A, Belmont HM, et al. 3 Tesla MRI detects deterioration in proximal femur microarchitecture and strength in long-term glucocorticoid users compared with controls. J Magn Reson Imaging. 2015;42:1489–96.
Kazakia GJ, Carballido-Gamio J, Lai A, Nardo L, Facchetti L, Pasco C, et al. Trabecular bone microstructure is impaired in the proximal femur of human immunodeficiency virus-infected men with normal bone mineral density. Quant Imaging Med Surg. 2017;8:5–13.
Vu B-TD, Jones BC, Lee H, Kamona N, Deshpande RS, Wehrli FW, et al. Six-minute, in vivo MRI quantification of proximal femur trabecular bone 3D microstructure. Bone. 2023;177:116900. This study provides the current state-of-the art for clinical evaluation of trabecular microarchitecture in the proximal femur.
Chang G, Honig S, Brown R, Deniz CM, Egol KA, Babb JS, et al. Finite element analysis applied to 3-T MR imaging of proximal femur microarchitecture: lower bone strength in patients with fragility fractures compared with control subjects. Radiology. 2014;272(464):474.
Fernández-Seara MA, Wehrli SL, Takahashi M, Wehrli FW. Water content measured by proton-deuteron exchange NMR predicts bone mineral density and mechanical properties. J Bone Miner Res [Internet]. 2004;19:289–96.
Fantazzini P, Brown RJS, Borgia GC. Bone tissue and porous media: common features and differences studied by NMR relaxation. Magn Reson Imaging. 2003;21:227–34.
Wang X, Ni Q. Determination of cortical bone porosity and pore size distribution using a low field pulsed NMR approach. J Orthopaed Res. 2003;21:312–9.
Ni Q, King JD, Wang X. The characterization of human compact bone structure changes by low-field nuclear magnetic resonance. Meas Sci Technol. 2004;15:58.
Horch RA, Nyman JS, Gochberg DF, Dortch RD, Does MD. Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging. Magnet Reson Med. 2010;64:680–7.
Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS. Identifying novel clinical surrogates to assess human bone fracture toughness. J Bone Miner Res. 2015;30:1290–300.
Manhard MK, Uppuganti S, Granke M, Gochberg DF, Nyman JS, Does MD. MRI-derived bound and pore water concentrations as predictors of fracture resistance. Bone. 2016;87:1–10.
Jerban S, Lu X, Dorthe EW, Alenezi S, Ma Y, Kakos L, et al. Correlations of cortical bone microstructural and mechanical properties with water proton fractions obtained from ultrashort echo time (UTE) MRI tricomponent T2* model. Nmr Biomed. 2020;33: e4233.
Nyman JS, Roy A, Shen X, Acuna RL, Tyler JH, Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39:931–8.
Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–16.
Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):7.
Robson MD, Bydder GM. Clinical ultrashort echo time imaging of bone and other connective tissues. Nmr Biomed. 2006;19(765):780.
Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging1. Radiology. 2008;248:824–33.
Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson [Internet]. 2010;207:304–11.
Horch RA, Gochberg DF, Nyman JS, Does MD. Clinically compatible MRI strategies for discriminating bound and pore water in cortical bone. Magnet Reson Med. 2012;68:1774–84.
Manhard MK, Horch RA, Harkins KD, Gochberg DF, Nyman JS, Does MD. Validation of quantitative bound- and pore-water imaging in cortical bone. Magnet Reson Med. 2014;71:2166–71.
Manhard MK, Horch RA, Gochberg DF, Nyman JS, Does MD. In vivo quantitative MR imaging of bound and pore water in cortical bone. Radiology. 2015;277:221–9.
Ketsiri T, Uppuganti S, Harkins KD, Gochberg DF, Nyman JS, Does MD. T1 relaxation of bound and pore water in cortical bone. Nmr Biomed. 2023;36: e4878.
Zhao X, Song HK, Seifert AC, Li C, Wehrli FW. Feasibility of assessing bone matrix and mineral properties in vivo by combined solid-state 1H and 31P MRI. Vashishth D, editor. Plos ONE. 2017;12:e0173995.
Jones BC, Lee H, Cheng C-C, Mukaddam M al, Song HK, Snyder PJ, et al. MRI quantification of cortical bone porosity, mineralization, and morphologic structure in postmenopausal osteoporosis. Radiology. 2023;307:e221810.
Bae WC, Chen PC, Chung CB, Masuda K, D’Lima D, Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27:848–57.
Biswas R, Bae W, Diaz E, Masuda K, Chung CB, Bydder GM, et al. Ultrashort echo time (UTE) imaging with bi-component analysis: bound and free water evaluation of bovine cortical bone subject to sequential drying. Bone. 2012;50:749–55.
Chen J, Carl M, Ma Y, Shao H, Lu X, Chen B, et al. Fast volumetric imaging of bound and pore water in cortical bone using three-dimensional ultrashort-TE (UTE) and inversion recovery UTE sequences. Nmr Biomed. 2016;29:1373–80.
Lu X, Jerban S, Wan L, Ma Y, Jang H, Le N, et al. Three-dimensional ultrashort echo time imaging with tricomponent analysis for human cortical bone. Magnet Reson Med. 2019;82:348–55.
Seifert AC, Wehrli SL, Wehrli FW. Bi-component T2* analysis of bound and pore bone water fractions fails at high field strengths. Nmr Biomed. 2015;28:861–72.
Li C, Seifert AC, Rad HS, Bhagat YA, Rajapakse CS, Sun W, et al. Cortical bone water concentration: dependence of MR imaging measures on age and pore volume fraction. Radiology. 2014;272:796–806.
Rajapakse CS, Bashoor-Zadeh M, Li C, Sun W, Wright AC, Wehrli FW. Volumetric cortical bone porosity assessment with MR imaging: validation and clinical feasibility. Radiology. 2015;276:526–35.
Jones BC, Jia S, Lee H, Feng A, Shetye SS, Batzdorf A, et al. MRI-derived porosity index is associated with whole-bone stiffness and mineral density in human cadaveric femora. Bone. 2021;143: 115774.
Abbasi-Rad S, Rad HS. Quantification of human cortical bone bound and free water in vivo with ultrashort echo time MR imaging: a model-based approach. Radiology. 2017;283: 160780.
Xiong Y, He T, Wang Y, Liu WV, Hu S, Zhang Y, et al. CKD Stages, Bone metabolism markers, and cortical porosity index: associations and mediation effects analysis. Front Endocrinol. 2021;12:775066. The is the first in vivo patient study involving MRI measures of cortical bone material properties.
Jerban S, Ma Y, Moazamian D, Athertya J, Dwek S, Jang H, et al. MRI-based porosity index (PI) and suppression ratio (SR) in the tibial cortex show significant differences between normal, osteopenic, and osteoporotic female subjects. Front Endocrinol. 2023;14:1148345.
Nyman JS, Ketsiri T, Louie EA, Harkins KD, Manhard MK, Gochberg DF, et al. Toward the use of MRI measurements of bound and pore water in fracture risk assessment. Bone. 2023;176:116863. In this case-control study, the combination of bound and pore water concentrations in the radius significantly predicted patients with a fragility fracture and subjects without a history of osteoporosis.
Taylor EA, Donnelly E. Raman and Fourier transform infrared imaging for characterization of bone material properties. Bone. 2020;139: 115490.
Unal M, Ahmed R, Mahadevan-Jansen A, Nyman JS. Compositional assessment of bone by Raman spectroscopy. Analyst. 2021;146:7464–90.
Roschger A, Gamsjaeger S, Hofstetter B, Masic A, Blouin S, Messmer P, et al. Relationship between the v2PO4/amide III ratio assessed by Raman spectroscopy and the calcium content measured by quantitative backscattered electron microscopy in healthy human osteonal bone. J Biomed Opt. 2014;19:065002–065002.
Mandair GS, Akhter MP, Esmonde-White FWL, Lappe JM, Bare SP, Lloyd WR, et al. Altered collagen chemical compositional structure in osteopenic women with past fractures: a case-control Raman spectroscopic study. Bone. 2021;148: 115962.
Unal M, Yang S, Akkus O. Molecular spectroscopic identification of the water compartments in bone. Bone. 2014;67:228–36.
Unal M, Akkus O. Raman spectral classification of mineral- and collagen-bound water’s associations to elastic and post-yield mechanical properties of cortical bone. Bone. 2015;81:315–26.
Heath S, Han Y, Hua R, Roy A, Jiang J, Nyman JS, et al. Assessment of glycosaminoglycan content in bone using Raman spectroscopy. Bone. 2023;171: 116751.
Shitole P, Choubey A, Mondal P, Ghosh R. Influence of low dose naltrexone on Raman assisted bone quality, skeletal advanced glycation end-products and nano-mechanical properties in type 2 diabetic mice bone. Mater Sci Eng C. 2021;123: 112011.
Gamsjaeger S, Brozek W, Recker R, Klaushofer K, Paschalis EP. Transmenopausal changes in trabecular bone quality. J Bone Miner Res. 2014;29:608–17.
Matousek P, Clark IP, Draper ERC, Morris MD, Goodship AE, Everall N, et al. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl Spectrosc. 2005;59:393–400.
Mosca S, Conti C, Stone N, Matousek P. Spatially offset Raman spectroscopy. Nat Rev Methods Primers. 2021;1:21.
Nicolson F, Kircher MF, Stone N, Matousek P. Spatially offset Raman spectroscopy for biomedical applications. Chem Soc Rev. 2021;50:556–68.
Matousek P, Draper ERC, Goodship AE, Clark IP, Ronayne KL, Parker AW. Noninvasive Raman spectroscopy of human tissue in vivo. Appl Spectrosc. 2006;60:758–63.
Buckley K, Kerns JG, Vinton J, Gikas PD, Smith C, Parker AW, et al. Towards the in vivo prediction of fragility fractures with Raman spectroscopy. J Raman Spectrosc. 2015;46:610–8.
Maher JR, Inzana JA, Awad HA, Berger AJ. Overconstrained library-based fitting method reveals age- and disease-related differences in transcutaneous Raman spectra of murine bones. J Biomed Opt. 2013;18:077001–077001.
Chen K, Massie C, Berger AJ. Soft‐tissue spectral subtraction improves transcutaneous Raman estimates of murine bone strength in vivo. J Biophotonics. 2020;13:e202000256. This pre-clinical study shows how a spectral library of Raman spectra from different tissues (bone, skin, fat) can be used to derive meaningful spectra from transcutaneous in vivo acquisition of Raman signals from the hindlimb (tibia) of mice between 4 to 23 weeks of age using SORS.
Chen K, Massie C, Awad HA, Berger AJ. Determination of best Raman spectroscopy spatial offsets for transcutaneous bone quality assessments in human hands. Biomed Opt Express. 2021;12:7517. In a translational study involving SORS acquisition of Raman signals from a cadaveric hand (phalanx and metacarpal), increasing the offset distance increased Raman peaks specific to bone at the cost of lower signal-to-noise.
Gautam R, Ahmed R, Haugen E, Unal M, Fitzgerald S, Uppuganti S, et al. Assessment of spatially offset Raman spectroscopy to detect differences in bone matrix quality. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2023;303:123240. In this cadaver study, SORS detected changes in bone composition that were related to a loss in bone strength, but only few Raman bands could be detected when tissue phantom layers with a thickness of 4 mm or higher were placed between the bone and the probe.
Ahmed R, Unal M, Gautam R, Uppuganti S, Derasari S, Mahadevan-Jansen A, et al. Sensitivity of the amide I band to matrix manipulation in bone: a Raman micro-spectroscopy and spatially offset Raman spectroscopy study. Analyst. 2023;148:4799–809.
Acknowledgements
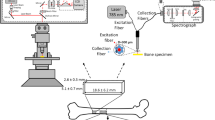
We thank Paola Pisani, PhD, and Rafay Ahmed, PhD, for the REMS image and the SORS image, respectively, in Fig. 1.
Funding
NIH/NIBIB 2R01 EB014308 (MDD), NIH/NIAMS R01 AR063157 and VA/ORD (JSN), NIH/NIDDK 1L30DK130133 and NSF 1952993 (RKS).
Author information
Authors and Affiliations
Contributions
R.S., M.D., and J.N. wrote the main manuscript text. R.S. and J.N. prepared Fig. 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Surowiec, R.K., Does, M.D. & Nyman, J.S. In Vivo Assessment of Bone Quality Without X-rays. Curr Osteoporos Rep 22, 56–68 (2024). https://doi.org/10.1007/s11914-023-00856-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00856-w