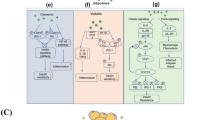
Abstract
Purpose of Review
Chronic inflammation, adipokines, and hepatokines have been identified as basis of insulin resistance and β cell failure in animal models. We present our current knowledge concerning the potential relationship between these cytokines, inflammation, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM) in the pediatric population.
Recent Findings
Pro-inflammatory cytokines related to insulin resistance and MetS in children are tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, interferon gamma, pigment epithelium-derived factor, chemerin, vaspin, and fetuin A. Anti-inflammatory cytokines associated with insulin resistance and MetS in children are leptin, adiponectin, omentin, fibroblast growth factor (FGF)-21, osteocalcin, and irisin. These anti-inflammatory cytokines are decreased (adiponectin, omentin, and osteocalcin) or increased (leptin, FGF-21, and irisin) in obesity suggesting a resistance state. TNF-α, fetuin A, and FGF-21 are altered in obese children with T2DM suggesting an involvement in β cell failure.
Summary
These cytokines, adipokines, and hepatokines may be able to predict development of MetS and T2DM and have a potential therapeutic target ameliorating insulin resistance.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28.
Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–93.
Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90(1):10–4.
Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8(5):299–306.
Reinehr T. Metabolic syndrome in children and adolescents: a critical approach considering the interaction between pubertal stage and insulin resistance. Curr Diab Rep. 2016;16(1):8.
Magge SN, Goodman E, Armstrong SC. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140:e20171603.
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46.
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46(1):3–19.
•• Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107 This paper summarizes our knowledge concerning the relationship between inflammation and type 2 diabetes.
Rempel JD, Packiasamy J, Dean HJ, McGavock J, Janke A, Collister M, et al. Preliminary analysis of immune activation in early onset type 2 diabetes. Int J Circumpolar Health. 2013;72.
Lucas R, Parikh SJ, Sridhar S, Guo DH, Bhagatwala J, Dong Y, et al. Cytokine profiling of young overweight and obese female African American adults with prediabetes. Cytokine. 2013;64(1):310–5.
Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6(1):99.
• Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60(4):445–52 This paper demonstrates the change of adipokines in obese children with weight loss.
Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28(5):1219–21.
Reinehr T, Karges B, Meissner T, Wiegand S, Stoffel-Wagner B, Holl RW, et al. Inflammatory markers in obese adolescents with type 2 diabetes and their relationship to hepatokines and adipokines. J Pediatr. 2016;173:131–5.
•• Bluher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3(3):230–40 This paper shows the potential of modifying adipokines as a treatment option in obesity and diabetes.
• Roth CL, Reinehr T. Roles of gastrointestinal and adipose tissue peptides in childhood obesity and changes after weight loss due to lifestyle intervention. Arch Pediatr Adolesc Med. 2010;164(2):131–8 This review summarizes the changes of adipokine and GI hormones in obese children with successful weight loss.
Smitka K, Maresova D. Adipose tissue as an endocrine organ: an update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med Rep. 2015;116(2):87–111.
Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–8.
Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9(6):753–62.
Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–26.
Ibarra UA, Friberg J, Christensen DP, Lund CG, Billestrup N. Inflammatory cytokines stimulate bone morphogenetic protein-2 expression and release from pancreatic beta cells. J Interf Cytokine Res. 2015.
Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58(11):727–36.
Vanderford NL. Defining the regulation of IL-1beta- and CHOP-mediated beta-cell apoptosis. Islets. 2010;2(5):334–6.
Fogeda M, Gallart L, Gutierrez C, Vendrell J, Simón I, García-España A, et al. High expression of tumor necrosis factor alpha receptors in peripheral blood mononuclear cells of obese type 2 diabetic women. Eur Cytokine Netw. 2004;15(1):60–6.
Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–17.
Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3.
Sjoholm A, Nystrom T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev. 2006;22(1):4–10.
Moss RB, Moll T, El-Kalay M, et al. Th1/Th2 cells in inflammatory disease states: therapeutic implications. Expert Opin Biol Ther. 2004;4(12):1887–96.
Rajkovic N, Zamaklar M, Lalic K, Jotic A, Lukic L, Milicic T, et al. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: relevance for cardiovascular risk prevention. Int J Environ Res Public Health. 2014;11(4):4049–65.
Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, et al. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur J Med Res. 2010;15(Suppl 2):120–2.
Su SC, Pei D, Hsieh CH, Hsiao FC, Wu CZ, Hung YJ. Circulating pro-inflammatory cytokines and adiponectin in young men with type 2 diabetes. Acta Diabetol. 2011;48(2):113–9.
Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–95.
West NA, Hamman RF, Mayer-Davis EJ, D’Agostino RB, Marcovina SM, Liese AD, et al. Cardiovascular risk factors among youth with and without type 2 diabetes: differences and possible mechanisms. Diabetes Care. 2009;32(1):175–80.
Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146(8):3417–27.
Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab. 2016;101(6):2423–31.
De Filippo G, Rendina D, Moccia F, Rocco V, Campanozzi A. Interleukin-6, soluble interleukin-6 receptor/interleukin-6 complex and insulin resistance in obese children and adolescents. J Endocrinol Investig. 2015;38(3):339–43.
Shalitin S, Deutsch V, Tauman R. Hepcidin, soluble transferrin receptor and IL-6 levels in obese children and adolescents with and without type 2 diabetes mellitus/impaired glucose tolerance and their association with obstructive sleep apnea. J Endocrinol Investig. 2018;41(8):969–75.
Sopasakis VR, Nagaev I, Smith U. Cytokine release from adipose tissue of nonobese individuals. Int J Obes. 2005;29(9):1144–7.
Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A. 2009;106(33):13998–4003.
Hansson GK. Inflammation and immune response in atherosclerosis. Curr Atheroscler Rep. 1999;1(2):150–5.
Pacifico L, Di RL, Anania C, Osborn JF, Ippoliti F, Schiavo E, et al. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154(5):691–7.
Wei Z, Huang Y, Xie N, Ma Q. Elevated expression of secreted autocrine growth factor progranulin increases cervical cancer growth. Cell Biochem Biophys. 2015;71(1):189–93.
Luo L, Lu L, Lu Y, Zhang L, Li B, Guo K, et al. Effects of hypoxia on progranulin expression in HT22 mouse hippocampal cells. Mol Med Rep. 2014;9(5):1675–80.
Matsubara T, Mita A, Minami K, Hosooka T, Kitazawa S, Takahashi K, et al. PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab. 2012;15(1):38–50.
Youn BS, Bang SI, Kloting N, Park JW, Lee N, Oh JE, et al. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes. 2009;58(3):627–36.
Eriksen JL, Mackenzie IR. Progranulin: normal function and role in neurodegeneration. J Neurochem. 2008;104(2):287–97.
Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, et al. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 2008;17(4):711–24.
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332(6028):478–84.
Qu H, Deng H, Hu Z. Plasma progranulin concentrations are increased in patients with type 2 diabetes and obesity and correlated with insulin resistance. Mediat Inflamm. 2013;2013:360190.
Li H, Zhou B, Xu L, Liu J, Zang W, Wu S, et al. Circulating PGRN is significantly associated with systemic insulin sensitivity and autophagic activity in metabolic syndrome. Endocrinology. 2014;155(9):3493–507.
Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis. 2004;14(5):228–32.
Alissa EM, Sutaih RH, Kamfar HZ, Alagha AE, Marzouki ZM. Serum progranulin levels in relation to insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2017;30(12):1251–6.
Daxer J, Herttrich T, Zhao YY, Vogel M, Hiemisch A, Scheuermann K, et al. Nocturnal levels of chemerin and progranulin in adolescents: influence of sex, body mass index, glucose metabolism and sleep. J Pediatr Endocrinol Metab. 2017;30(1):57–61.
Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin--the classical, resistin--the controversical, adiponectin--the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19(4):525–46.
Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the first law of thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2(8):447–58.
Iikuni N, Lam QL, Lu L, Matarese G, La CA. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70–9.
Berglund ED, Vianna CR, Donato J Jr, Kim MH, Chuang JC, Lee CE, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122(3):1000–9.
Morales A, Wasserfall C, Brusko T, Carter C, Schatz D, Silverstein J, et al. Adiponectin and leptin concentrations may aid in discriminating disease forms in children and adolescents with type 1 and type 2 diabetes. Diabetes Care. 2004;27(8):2010–4.
Abdelgadir M, Elbagir M, Eltom M, Berne C, Ahren B. Reduced leptin concentrations in subjects with type 2 diabetes mellitus in Sudan. Metabolism. 2002;51(3):304–6.
Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in First Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes. 2009;10(4):269–77.
Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60(6):1647–56.
Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60(5):1474–7.
Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–31.
Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–10.
Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39(8):715–28.
Navarro P, de DO, Gavela-Perez T, Jois A, Garces C, Soriano-Guillen L. High-sensitivity C-reactive protein and leptin levels related to body mass index changes throughout childhood. J Pediatr. 2016;178:178–82.
Reinehr T, Woelfle J, Wiegand S, et al. Leptin but not adiponectin is related to type 2 diabetes mellitus in obese adolescents. Pediatr Diabetes. 2016 Jun;17(4):281–8. https://doi.org/10.1111/pedi.12276.
Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278(41):40352–63.
Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031–41.
Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26.
Statnick MA, Beavers LS, Conner LJ, Corominola H, Johnson D, Hammond CD, et al. Decreased expression of apM1 in omental and subcutaneous adipose tissue of humans with type 2 diabetes. Int J Exp Diabetes Res. 2000;1(2):81–8.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–5.
Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, et al. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab. 2007;92(12):4569–74.
Reinehr T, Roth C, Menke T, Andler W. Adiponectin before and after weight loss in obese children. J Clin Endocrinol Metab. 2004;89(8):3790–4.
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290(3):1084–9.
Tabak AG, Brunner EJ, Miller MA, Karanam S, McTernan PG, Cappuccio FP, et al. Low serum adiponectin predicts 10-year risk of type 2 diabetes and HbA1c independently of obesity, lipids, and inflammation: Whitehall II study. Horm Metab Res. 2009;41(8):626–9.
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–88.
Coimbra S, Brandao PJ, Santos-Silva A, Neuparth MJ. Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: a close linkage with obesity and length of the disease. Biomed Res Int. 2014;2014:701915.
Yaghootkar H, Lamina C, Scott RA, Dastani Z, Hivert MF, Warren LL, et al. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes. 2013;62(10):3589–98.
Boord JB, Fazio S, Linton MF. Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr Opin Lipidol. 2002;13(2):141–7.
Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1(2):107–19.
Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134(9):2464S–8S.
Reinehr T, Stoffel-Wagner B, Roth CL. Adipocyte fatty acid-binding protein in obese children before and after weight loss. Metabolism. 2007;56(12):1735–41.
Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab. 2002;13(1):18–23.
Norata GD, Ongari M, Garlaschelli K, Raselli S, Grigore L, Catapano AL. Plasma resistin levels correlate with determinants of the metabolic syndrome. Eur J Endocrinol. 2007;156(2):279–84.
Perseghin G, Burska A, Lattuada G, Alberti G, Costantino F, Ragogna F, et al. Increased serum resistin in elite endurance athletes with high insulin sensitivity. Diabetologia. 2006;49(8):1893–900.
Reinehr T, Roth CL, Menke T, Andler W. Resistin concentrations before and after weight loss in obese children. Int J Obes. 2006;30(2):297–301.
Castan-Laurell I, Boucher J, Dray C, Daviaud D, Guigne C, Valet P. Apelin, a novel adipokine over-produced in obesity: friend or foe? Mol Cell Endocrinol. 2005;245(1–2):7–9.
Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146(4):1764–71.
Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res. 2008;79(2):279–86.
Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132(1–3):27–32.
Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(1):E59–67.
Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, Garcia-Almeida JM, Garcia-Arnes J, Tinahones FJ, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19(11):1574–80.
Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114(10):544–8.
Heinonen MV, Laaksonen DE, Karhu T, Karhunen L, Laitinen T, Kainulainen S, et al. Effect of diet-induced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19(9):626–33.
Castan-Laurell I, Vitkova M, Daviaud D, Dray C, Kováciková M, Kovacova Z, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158(6):905–10.
Tapan S, Tascilar E, Abaci A, Sonmez A, Kilic S, Erbil MK, et al. Decreased plasma apelin levels in pubertal obese children. J Pediatr Endocrinol Metab. 2010;23(10):1039–46.
Reinehr T, Woelfle J, Roth CL. Lack of association between apelin, insulin resistance, cardiovascular risk factors, and obesity in children: a longitudinal analysis. Metabolism. 2011;60:1349–54.
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–30.
Revollo JR, Korner A, Mills KF, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6(5):363–75.
Kiess W, Petzold S, Topfer M, et al. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab. 2008;22(1):135–53.
Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54(10):2911–6.
Retnakaran R, Youn BS, Liu Y, Hanley AJG, Lee NS, Park JW, et al. Correlation of circulating full-length visfatin (PBEF/NAMPT) with metabolic parameters in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol. 2008;69(6):885–93.
Friebe D, Neef M, Kratzsch J, Erbs S, Dittrich K, Garten A, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54:1200–11.
Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–61.
de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655–61.
Yamawaki H, Tsubaki N, Mukohda M, Okada M, Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393(4):668–72.
Herder C, Ouwens DM, Carstensen M, Kowall B, Huth C, Meisinger C, et al. Adiponectin may mediate the association between omentin, circulating lipids and insulin sensitivity: results from the KORA F4 study. Eur J Endocrinol. 2015;172(4):423–32.
Oswiecimska J, Suwala A, Swietochowska E, et al. Serum omentin levels in adolescent girls with anorexia nervosa and obesity. Physiol Res. 2015;64(5):701–9.
Buyukinan M, Atar M, Can U, Pirgon O, Guzelant A, Deniz I. The association between serum vaspin and omentin-1 levels in obese children with metabolic syndrome. Metab Syndr Relat Disord. 2018;16(2):76–81.
Zhang M, Tan X, Yin C, Wang L, Tie Y, Xiao Y. Serum levels of omentin-1 are increased after weight loss and are particularly associated with increases in obese children with metabolic syndrome. Acta Paediatr. 2017;106(11):1851–6.
Chavan SS, Hudson LK, Li JH, Ochani M, Harris Y, Patel NB, et al. Identification of pigment epithelium-derived factor as an adipocyte-derived inflammatory factor. Mol Med. 2012;18:1161–8.
Galhardo J, Hunt LP, Shield JP. Serum levels of pigment epithelium-derived factor (PEDF) are positively associated with acanthosis nigricans in obese adolescents. Diabet Med. 2012;29(7):e117–20.
Crowe S, Wu LE, Economou C, Turpin SM, Matzaris M, Hoehn KL, et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10(1):40–7.
Gattu AK, Birkenfeld AL, Jornayvaz F, et al. Insulin resistance is associated with elevated serum pigment epithelium-derived factor (PEDF) levels in morbidly obese patients. Acta Diabetol. 2012;49(Suppl 1):S161–9.
Sunderland KL, Tryggestad JB, Wang JJ, Teague AM, Pratt LV, Zhang SX, et al. Pigment epithelium-derived factor (PEDF) varies with body composition and insulin resistance in healthy young people. J Clin Endocrinol Metab. 2012;97(11):E2114–8.
Tryggestad JB, Wang JJ, Zhang SX, Thompson DM, Short KR. Elevated plasma pigment epithelium-derived factor in children with type 2 diabetes mellitus is attributable to obesity. Pediatr Diabetes. 2015;16(8):600–5.
Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282(38):28175–88.
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148(10):4687–94.
Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, et al. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept. 2009;154(1–3):102–6.
Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582(5):573–8.
Hatziagelaki E, Herder C, Tsiavou A, Teichert T, Chounta A, Nowotny P, et al. Serum chemerin concentrations associate with beta-cell function, but not with insulin resistance in individuals with non-alcoholic fatty liver disease (NAFLD). PLoS One. 2015;10(5):e0124935.
Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-alpha stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis. 2013;9(2):306–14.
Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151(5):1998–2007.
Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab. 2009;94(8):3085–8.
Ye Z, Wang S, Yang Z, He M, Zhang S, Zhang W, et al. Serum lipocalin-2, cathepsin S and chemerin levels and nonalcoholic fatty liver disease. Mol Biol Rep. 2014;41(3):1317–23.
Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol. 2010;72(3):342–8.
Landgraf K, Friebe D, Ullrich T, Kratzsch J, Dittrich K, Herberth G, et al. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab. 2012;97(4):E556–64.
Redondo MJ, Rodriguez LM, Haymond MW, Hampe CS, Smith EO, Balasubramanyam A, et al. Serum adiposity-induced biomarkers in obese and lean children with recently diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2014;15(8):543–9.
Sledzinska M, Szlagatys-Sidorkiewicz A, Brzezinski M, Kazmierska K, Sledzinski T, Kaminska B. Serum chemerin in children with excess body weight may be associated with ongoing metabolic complications - a pilot study. Adv Med Sci. 2017;62(2):383–6.
Maghsoudi Z, Kelishadi R, Hosseinzadeh-Attar MJ. The comparison of chemerin, adiponectin and lipid profile indices in obese and non-obese adolescents. Diabetes Metab Syndr. 2016;10(2 Suppl 1):S43–6.
Niklowitz P, Rothermel J, Lass N, Barth A, Reinehr T. Link between chemerin, central obesity, and parameters of the metabolic syndrome: findings from a longitudinal study in obese children participating in a lifestyle intervention. Int J Obes. 2018.
Dimova R, Tankova T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed Res Int. 2015;2015:823481.
Chang HM, Lee HJ, Park HS, Kang JH, Kim KS, Song YS, et al. Effects of weight reduction on serum vaspin concentrations in obese subjects: modification by insulin resistance. Obesity (Silver Spring). 2010;18(11):2105–10.
Handisurya A, Riedl M, Vila G, Maier C, Clodi M, Prikoszovich T, et al. Serum vaspin concentrations in relation to insulin sensitivity following RYGB-induced weight loss. Obes Surg. 2010;20(2):198–203.
Korner A, Neef M, Friebe D, et al. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int J Obes. 2011;35(4):578–86.
Youn BS, Kloting N, Kratzsch J, Lee N, Park JW, Song ES, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57(2):372–7.
Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, et al. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287(13):10301–15.
Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9(3):144–52.
Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–9.
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35.
Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–53.
So WY, Cheng Q, Chen L, Evans-Molina C, Xu A, Lam KSL, et al. High glucose represses beta-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: involvement of peroxisome proliferator-activated receptor gamma signaling. Diabetes. 2013;62(11):3751–9.
Song A, Xu M, Bi Y, Xu Y, Huang Y, Li M, et al. Serum fetuin-A associates with type 2 diabetes and insulin resistance in Chinese adults. PLoS One. 2011;6(4):e19228.
Iyidir OT, Degertekin CK, Yilmaz BA, Altinova AE, Toruner FB, Bozkurt N, et al. Serum levels of fetuin A are increased in women with gestational diabetes mellitus. Arch Gynecol Obstet. 2014;291(4):933–7.
Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Häring HU, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008;3(3):e1765.
Diaz-Delfin J, Hondares E, Iglesias R, Giralt M, Caelles C, Villarroya F. TNF-alpha represses beta-Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology. 2012;153(9):4238–45.
Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555(7698):673–7.
Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–53.
Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–81.
Chen C, Cheung BM, Tso AW, et al. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care. 2011;34(9):2113–5.
Xiao Y, Xu A, Law LS, et al. Distinct changes in serum fibroblast growth factor 21 levels in different subtypes of diabetes. J Clin Endocrinol Metab. 2012;97(1):E54–8.
Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215(1):1–7.
Reinehr T, Karges B, Meissner T, Wiegand S, Fritsch M, Holl RW, et al. Fibroblast growth factor 21 and fetuin-A in obese adolescents with and without type 2 diabetes. J Clin Endocrinol Metab. 2015;100(8):3004 jc20152192.
Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57(10):2762–7.
Kalabay L, Chavin K, Lebreton JP, Robinson KA, Buse MG, Arnaud P. Human recombinant alpha 2-HS glycoprotein is produced in insect cells as a full length inhibitor of the insulin receptor tyrosine kinase. Horm Metab Res. 1998;30:1):1–6.
Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51(8):2450–8.
Ix JH, Biggs ML, Mukamal KJ, Kizer JR, Zieman SJ, Siscovick DS, et al. Association of fetuin-a with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation. 2012;125(19):2316–22.
Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93(11):4479–85.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69.
Bouillon R, Bex M, Van HE, et al. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80(4):1194–202.
Pietschmann P, Schernthaner G, Woloszczuk W. Serum osteocalcin levels in diabetes mellitus: analysis of the type of diabetes and microvascular complications. Diabetologia. 1988;31(12):892–5.
Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes. 2010;34(5):852–8.
Park KH, Zaichenko L, Brinkoetter M, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(12):4899–907.
Sesti G, Andreozzi F, Fiorentino TV, Mannino GC, Sciacqua A, Marini MA, et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014;51(5):705–13.
Staiger H, Bohm A, Scheler M, et al. Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived ‘browning’ factor irisin, determines insulin sensitivity. PLoS One. 2013;8(4):e61903.
Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8.
Anastasilakis AD, Polyzos SA, Saridakis ZG, Kynigopoulos G, Skouvaklidou EC, Molyvas D, et al. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J Clin Endocrinol Metab. 2014;99(9):3247–55.
Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769–78.
Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99(11):E2154–61.
Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity--correlation with body mass index. Peptides. 2013;39:125–30.
Reinehr T, Elfers C, Lass N, Roth CL. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J Clin Endocrinol Metab. 2015;100(5):2123–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Thomas Reinehr and Christian Ludwig Roth declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Type 2 and Monogenic Diabetes
Rights and permissions
About this article
Cite this article
Reinehr, T., Roth, C.L. Inflammation Markers in Type 2 Diabetes and the Metabolic Syndrome in the Pediatric Population. Curr Diab Rep 18, 131 (2018). https://doi.org/10.1007/s11892-018-1110-5
Published:
DOI: https://doi.org/10.1007/s11892-018-1110-5