Abstract
Rabies has been one of the most feared diseases throughout history. Human rabies remains an important public health problem in many developing countries. The WHO reports that more than 55,000 people die of this disease every year. Most of these cases occur in developing countries. In most Latin American countries, the major reservoirs of rabies are the dog and the hematophagous bat (Desmodus rotundus), which is present in the tropical and subtropical areas from Northern Mexico to Northern Argentina and Chile and transmits the disease to cattle. One of the better options for controlling rabies is vaccination. The expression of rabies virus G protein in different plant systems for developing an oral rabies vaccine could reduce costs of production and distribution and would be convenient for developing countries where the disease is endemic.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction to Rabies Virus
Rabies is derived from the Latin rabere, “to rage or to rave,” as is the corresponding adjective “rabid”; rabere possibly may have earlier origins in Sanskrit rhabas for “violence.” Since antiquity, rabies has been one of the most feared diseases. Human rabies remains an important public health problem in many developing countries (Wilkinson 2002; Woldehiwet 2002).
The World Health Organization (WHO) reports that more than 60,000 people die of this disease every year (WHO 2013). Most of these cases occur in the developing countries. In most countries of Latin America, the major reservoirs are the dog and, lately, the hematophagous bat (Desmodus rotundus), which is present in tropical and subtropical areas from Northern Mexico to Northern Argentina and Chile and transmits the disease mainly to cattle (Loza-Rubio et al. 2005; Delpietro et al. 2009). Vampire bat attacks on cattle are a major concern for cattle-raising areas. Blood loss and paralytic rabies due to bat bites can impose severe losses on the livestock industry (Arellano-Sota 1988).
Any tome which focuses upon some of the major rabies issues spanning the geographical extent from the US/Mexico border to Tierra del Fuego is long overdue, not least because Latin America is rich with historical, cultural, ecological, and viral diversity. One can only speculate about the primordial state of this disease, before canine rabies was imported during the sixteenth century with European colonization.
Clearly, the region has the greatest known diversity of rabies virus variants associated with the Chiroptera (the evolutionary well spring of the genus Lyssavirus), with representatives of major hosts among at least three bat families. Additionally, since the beginning of the twentieth century, a complex epizootiological relationship was identified between rabies viruses and hematophagous bats, leading to bovine paralytic rabies—unique in the entire globe. Similarly, only in the New World are non-human primates (e.g., marmosets in Brazil) believed to serve primary rabies virus reservoirs.
The Lyssavirus genus encompasses 15 viruses: rabies virus (RABV), Lagos bat virus (LBV), Mokola virus (MOKV), Duvenhage virus (DUVV), European bat lyssavirus 1 and 2 (EBLV 1 and 2), Australian bat lyssavirus, Aravan virus, Khujand virus, Irkut virus, West Caucasian virus (WCV), and Shimoni bat virus (SHIBV) (Loza-Rubio et al. 2012a, b; Kuzmin et al. 2010; Dietzgen et al. 2011). Another, two new Lyssavirus have been identified. One has been isolated from an insectivorous bat (Myotis nattereri) in Germany identified as Bokeloh (Freuling et al. 2011), and the other has been isolated from an African civet identified as Ikoma (IKOV) (Marston et al. 2012).
A new tentative Lyssavirus, Lleida bat lyssavirus, was found in a bent-winged bat (Miniopterus schreibersii) in Spain. It does not belong to phylogroup I or II, and it seems to be more closely related to the WCV bat virus and especially to the Ikoma lyssavirus (Aréchiga Ceballos et al. 2013). Classification of the genus is presented in Table 9.1.
Although several types of Lyssavirus are recognized worldwide, currently in the Americas only genotype 1 has been identified, even though there are several groups carrying out epidemiological surveillance in order to verify this situation or if at one point in time any other has been identified (Loza-Rubio et al. 2012a, b).
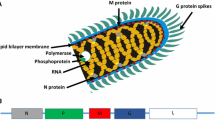
Rabies virus is the prototype species of the genus Lyssavirus in the family Rhabdoviridae. This RNA virus contains five genes which codified for the same number of proteins. The five structural proteins of the virion include a nucleocapsid (N), phosphoprotein (P, N, or NS), matrix protein (M), RNA polymerase (L), and a glycoprotein (G) (Fig. 9.1) (Schnell et al., 2010).
Structure of rabies virus. The five structural proteins of the virion include a nucleocapsid (N), phosphoprotein (P, N or NS), matrix protein (M), RNA polymerase (L), and glycoprotein (G) (Schnell et al. 2010). Source: http://viralzone.expasy.org/viralzone/all_by_species/22.html
2 Introduction to Glycoprotein (G)
2.1 Structure of G Protein
Rabies virus G protein is a transmembrane protein with 505 amino acids that weighs 65–67 kDa (kda) (Ross et al. 2008) and forms spicules that project outward from the infected cell forming trimers. This protein is used by the virus to join with the host cells and initiates the relationship between them when the cell receptors link. Amino acids 1 through 439 are responsible for the attachment of the virus to the cell receptors causing a fusion of the viral and cell membranes (Gaudin et al. 1993; Gaudin et al. 1999). The three protein-type membrane receptors for the rabies virus that have been identified are (1) the nicotinic receptor for acetylcholine, (2) the low-affinity neurotrophin receptor, and (3) the neural cell adhesion molecule (NCAM). These receptors are implicated in the adsorption of the rabies virus and the promotion of infection directly into the nerve ends and/or gangliosides located in neurons or at the point of axoplasmic transport of muscles. The virus moves along the dorsal ganglions and spinal cord; the brain is quickly infected causing apoptosis of the nerve cells and T cells (Lafon 2011). A total of five antigenic sites have been identified in the soluble region of the protein: I, II, III, IV, and “a,” which are located in residues 231, 330–338, 264, and 342–343, respectively. The antigenic site II, located in position 34–42 and 198–200, had only been recognized under denaturing conditions (Benmansour et al. 1991). Furthermore, the presence of a residue of the Glu amino acid in position 333 has been related with the invasion of neurons and pathogenicity (Wunner 2002); the lack of this amino acid causes nonrecognition of the virus and the strains become nonpathogenic.
It has been proposed that amino acid changes within the antigenic sites of the G protein could be the source of variants that are capable of escaping the host’s defenses and providing adaptation to new environments (Kobayashi et al. 2010; Khawplod et al. 2006).
2.2 Immunogenic Activity of G Protein
The G protein is a target of T lymphocytes and induces the formation of neutralizing antibodies against the virus (Loza-Rubio et al. 1998; Morales et al. 2006). This is the reason why this protein has been used for making vaccines, since it is the most exposed antigen of the virus.
The immune response that is triggered by the rabies virus is peculiar due to the immuno-privileged condition of the nervous system. This is based mainly on the restriction of T-cell migration and the deficiency in professional antigen-presenting cells (Lafon 2005). Protection against the rabies virus is mediated by the production of virus-neutralizing antibodies and/or T lymphocytes (helper CD4+ and cytotoxic CD8+). Although it is possible that the protection against an infection by the rabies virus is the result of various effector-host interactions, virus-neutralizing antibodies (VNA), which are mostly produced against the G protein, play an important role in the immunological protection against a rabies infection (Dietzschold et al. 1990; Desmeziéres et al. 1999). Furthermore, the G protein, besides inducing the formation of virus-neutralizing antibodies, also promotes the production of helper and cytotoxic T cells. It has been shown in intracerebral challenges that its structure is critical for both actions, the induction of neutralizing antibodies and protection (Drings et al. 1999; Hooper et al. 1994). The function of these T cells during rabies infection is to help in the induction of B cells and the production of antibodies, as well as to act as cell effectors in cytotoxic cell immunity response (Jackson 2003). After capture by macrophages and other antigen-presenting cells, antigens of the rabies virus are presented to CD4 or CD8 cells. This stimulation induces the production of cytokines such as IL-2, IL-4, and IFN-γ (Drings et al. 1999).
Regarding the immune response developed after vaccination, it involves the activation of specific differentiated B cells within plasma that produce antibodies and memory B cells. Produced antibodies are specific against the G protein (antibodies against other proteins are also generated with live or inactive virus vaccines) and are directed specifically against the antigenic components of the G protein and neutralize the virus (Lafon et al. 1990). Production of these virus-neutralizing antibodies involves a refined process of specificity adjustment which results in the selection of antigen avid cells. This specificity adjustment and antibody production depend on the correct folding of the protein; otherwise, the antigenic sites do not become exposed (Desmeziéres et al. 1999).
2.2.1 Heterologous G Protein Expression for Use in Immunizations
Because G protein induces antibodies against the rabies virus, this protein can be used as an immunogen when expressed in vectors such as Vaccinia, Canarypox, adenovirus, and yeast and in DNA vaccines, as well as in transgenic plants (Cadoz et al. 1992; Kieny et al. 1984; Xiang et al. 1996; Henderson et al. 2009; Sakamoto et al. 1999; Tacket 2009; Ventini et al. 2010).
The need for a safer and more effective vaccine has promoted the development of oral vaccines. In the case of wild rabies, specifically in foxes, the first massive oral vaccination was carried out in 1978 within the Rhône Valley in Switzerland and later it was extended to various other territories. This distribution was carried out manually using vaccine-laden bait (12–25 bait/km2) within endemic zones (Wandeler 2000, Bugnon et al. 2004). The first recombinant vaccine was the VR-G developed in 1984 in which the sequence of the G gene was inserted into a plasmid together with the Vaccinia promoter and flanked by the viral thymidine kinase gene. This plasmid was used to transfect cells that had been previously infected with a wild Vaccinia strain, and using homologous recombination, it was possible to obtain the recombinant plasmid (Kieny et al. 1984; Paolazzi et al. 1999. Oral immunization of foxes has allowed the large-scale elimination of the virus in areas of Europe where the baits have been placed. Oral vaccination with the vaccines Raboral V-RG (Vaccinia recombinant virus expressing G protein) (Kieny et al. 1984) and with Rabigen SAG2 (double mutant avirulent strain SAG2) (Artois et al. 1992) was effective for wild rabies in Europe which has helped to almost eradicate wild rabies in the western part of the continent (Desmettre et al. 1990) and has been successful for rabies control in Canada, the United States, and other countries (Lontai 1997; Mainguy et al. 2013). Nevertheless, until now, there is no effective oral vaccination for reservoir species. Notably, in the United States, there are no licensed vaccines for skunks.
Adenoviruses have been used as vectors for the expression of the G protein, promoting neutralizing antibodies and providing protection against intracerebral challenges in lactating mice that came from vaccinated mothers but that had no tolerance. It has been reported that this construct has higher efficacy when compared to conventional and VR-G vaccines (Xiang et al. 1996; Wang et al. 1997; Tims et al. 2000; Hu et al. 2006). In fact, its efficacy has been studied in dogs; in this species the efficacy can be compromised if there are antibodies against the same adenovirus, but only if the inoculation is intramuscular (Yuan et al. 2008). This prototype showed in cats the same efficacy to a challenge carried out at 12 months. Furthermore, a vaccine prototype using a type 2 adenovirus that expressed the rabies virus glycoprotein has been recently evaluated in sheep in which the said construct showed promising results when inoculated through either intramuscular or intradermal pathways (Bouet-Cararo et al. 2010).
Poxviruses have also been used as vectors for the expression of several antigens that induce both the cellular and the humoral responses. Avian poxvirus (Canarypox) has been preferred, since it does not infect humans, for the expression of the rabies G protein. It is known as the ALVAC-RG and has been shown to generate antibodies and promote protection immunity in cats and dogs. This vaccine is well tolerated in humans producing results that are at least similar to those of the diploid cell vaccine (Fries et al. 1996).
Some attenuated strains of Aujeszky’s disease virus have been used as vector for the G gene of the rabies virus. This prototype has shown its efficacy in dogs when administered through various pathways, including intramuscular, but most importantly the oral pathway (Yuan et al. 2008).
Furthermore, the baculovirus, which infects insects, has also been used since it allows a high expression of proteins. Tordo and colleagues reported in 1993 the cloning of the Mokola 3 genotype which was used as a vaccine (Tordo et al. 1993). This construct protected mice against a lethal challenge. In addition, the efficacy of a baculovirus that contained the G and N genes of the rabies virus was evaluated and its effectiveness was demonstrated. In another study the ectodomain of the rabies G gene was cloned into this system and compared with a DNA vaccine. The recombinant baculovirus induced antibody titers that were higher than the other vaccine.
The first demonstration that a plasmid could carry a protection antigen (G gene) of the rabies virus was published in 1994 (Xiang et al. 1996). This group showed that this immunogen was capable of promoting a protecting immune response when challenged in primates that were immunized with this vaccine and were capable of surviving a rabies virus challenge, while in others the DNA vaccine caused long-term protection levels through the production of neutralizing antibodies after a single immunization (Lodmell and Ewalt 2000). This group has also reported that 10 mg of G protein-codifying plasmid inoculated via intramuscular injection protects 100 % of mice, while the intradermal injection of 0.1 mg protects up to 83 %.
It also has been shown that DNA vaccines work in species such as dogs, cats, and horses (Osorio et al. 1999; Perrin et al. 2000; Fischer et al. 2003). Also, using normal syringes, various inoculation pathways have been evaluated such as intramuscular and intradermal (Perrin et al. 2000; Lodmell et al. 2006; Osinubi et al. 2009).
3 Description of the Systems Used to Produce the Protein
3.1 Theoretical Advantages of the Plant Process over Other Technologies
Literature indicates several potential advantages that are related to plant-derived vaccines, for example, heat-stable formulation for storage and transport (avoiding cold chain) which is important in tropical and subtropical areas and ease of delivery for better compliance leading to a reduced demand for skilled health-care professionals in developing and developed countries.
The use of recombinant gene technologies by the vaccine industry has revolutionized the way antigens are generated and has provided safer, more effective means of protecting host organisms against bacterial, viral, and parasitic pathogens (Lamphear et al. 2002; Loza-Rubio and Gomez-Lim, et al 2006) (Table 9.2).
In the case of viruses, no alternative to vaccines exists for animals since there are no antiviral drugs suitable for widespread application in the field. This underlines the need for controlling viral diseases of animals by vaccination. Advances in genetic engineering have made it possible to insert heterologous genes into several plant species, such as cereals and legumes. Plants are increasingly recognized as legitimate systems for the production of recombinant proteins and antigens. A wide range of proteins have been expressed and used for diagnostic purposes, industrial and pharmaceutical production of enzymes, food additives, therapeutic proteins, antibodies, and vaccine antigens (Streatfield 2006). However, despite nearly 20 years of development, there are only two plant-produced vaccine-related products that have gone all the way through all production and regulatory hurdles (Rybicki 2009).
3.2 Immunogenicity of Rabies Virus Antigen Expressed in Plants
The G protein, which is the main antigen of the rabies virus, has also been expressed in tomato, tobacco, and spinach plants. The first experience with the expression of the G protein of the rabies virus was in tomatoes (McGarvey et al. 1995). The full G gene of the virus was cloned into the BIN19 vector downstream of the 35S CaMV promoter. Later, tomato cells were transformed by infection with Agrobacterium tumefaciens. The expressed glycoprotein was purified by immunoprecipitation from leaves and fruits, and two bands, one of 60 kDa and another of 62 kDa, were detected with Western blot. This variation in protein weight could be due to differential glycosylation in the plant cell. The amount of recombinant glycoprotein in leaves was between approximately 1 and 10 ng/mg of soluble protein, while fruits had lower amounts.
Furthermore, other studies have reported that the oral administration of the rabies virus ribonucleoprotein induces the production of neutralizing antibodies when afterwards an inactive virus vaccine booster is applied in mice (Dietzschold et al. 1987). Also, as a way to improve the expression of proteins in plants, other viruses have been used as vectors to infect plant tissue such as the alfalfa mosaic virus (AIMV) using the coat protein (Cp) which serves a carrier for the peptides to be expressed. In this manner, Yusibov et al. (1997) expressed using this system the G and N proteins of the rabies virus and the human immunodeficiency virus type 1 (HIV-1). These constructs were inoculated into tobacco plants (Nicotiana benthamiana) in order to later isolate the virus from the leaves and semi-purify the viral particles for their inoculation of mice. Animals received seven doses (10 μg per dose) via intraperitoneal injection and assessing the response in the presence or absence of adjuvant. Using antibodies against the Cp protein, the presence of a 28.9 kDa band was found in Western blot which corresponds to the fusion protein formed by the Cp protein of AIMV and the viral peptides of the rabies virus. The identification of each peptide was carried out using monoclonal antibodies against each of them. Finally, it was demonstrated that the viral particles that were inoculated promoted an immune response in mice against the rabies virus antigens, as well as those of HIV-1, regardless of the adjuvant was present or not.
In another study, using the constructs reported by Yusibov’s group, infection of tobacco and spinach plants was carried out (Modelska et al. 1998). In this study, mice were immunized with protein purified from transformed leaves by oral and intraperitoneal route. Inoculation was carried out using 50 μg of purified recombinant virus in three doses. These same particles were administered orally through gastric intubation in four doses (250 μg per dose). Another group was fed for 7 days with the transformed spinach leaves (1 g per dose containing 15 μg of antigen). In all groups, serum samples and fecal pellets were collected 2 days before each immunization and the neutralizing activity of rabies virus-specific serum antibodies was determined. In animals immunized via intraperitoneal route, the presence of antibodies was observed after the second immunization. The mice immunized by oral route showed the presence of IgG and IgA. The higher levels of immune response generated by the leaf-feeding approach as compared with gastric intubation raise the possibility that the plant cells enhanced the delivery of virus particles to the sites of immune responses. A total of 40 % of the animals survived the challenge.
Using this same transient expression system in these two previous studies, Yusibov and collaborators in 2002 assessed these plants not only in mice but also in people (Yusivov et al. 2002). In the oral immunity study, three lots of spinach (3,000 plants) were inoculated with the recombinant virus that expresses the peptides of the rabies virus. Mice were immunized via intraperitoneal route with the purified recombinant protein (250 mg = 35 μg of peptide per dose) together with Freund’s adjuvant and later challenged. Two groups were formed in the experiment with people. The first group (five individuals) were previously immunized against rabies and then were fed using 20 g of fresh transformed spinach containing 0.6 mg of recombinant virus (84 μg of protein). The second group was composed of nine volunteers without previous immunization who received 150 g of fresh spinach tissue per dose (700 μg of protein).
Afterwards they received a dose of commercial vaccine intramuscularly and the presence of IgG and IgA was determined in serum. The leaves of spinach were found to contain 0.4 ±0.007 mg of recombinant virus in fresh tissue that contained 84 mg of the chimeric peptide. A 19.3 kDa band, corresponding to the fusion peptide, was detected using Western blot. The whole (100 %) of the mice immunized with the extract survived the challenge, 43 % of those immunized with the synthetic peptides, and 20 % of those immunized with the alfalfa mosaic virus. Furthermore, three of the five volunteers mounted an effective response against the antigen after ingesting the transformed spinach. In six of the nine volunteers, the antibody titers increased against the recombinant virus. Four of these individuals showed IgG and 2/7 showed IgA. In 5/9 of the volunteers there was an increase in IgG in serum after receiving three doses of the spinach leaves. In none of these experiments was tolerance observed.
Using tobacco also, Ashraf et al. (2005) expressed the glycoprotein fused to an endoplasmic reticulum retention sequence (SEKDEL) in order to improve its expression. The gene was cloned downstream of a double CaMV35S promoter. Transformation was measured using Agrobacterium tumefaciens. The protein was purified and 25 μg of this extract was used to immunize five mice via intraperitoneal receiving boosters at days 7, 14, and 28. These were later challenged using a standard laboratory strain (CVS). The protein purified from plant leaves showed a single band of ~66 kDa corresponding at G protein. Transformed plants contained chimeric G protein at 0.38 % of the total soluble protein. The rabies glycoprotein expressed in tobacco is glycosylated and is not degraded during the purification. These proteins show immunoreactivity to antirabies virus antibodies and elicit a high level of immune response in mice. The plant-derived GP gave 100 % protection similar to the commercial vaccine. In comparison with other studies, the protein that accumulates in tobacco is of higher molecular mass, comparable to the native protein.
In Mexico, edible vaccines have been developed using corn and carrots, proving their efficiency in mice. In some cases these provided 100 % protection in animals when challenged with a lethal virus originating from vampire bats (Lerma 2005; Rojas et al. 2009; Loza-Rubio et al. 2008).
In the first report carried out by our study group, we reported the expression of the gene that codes for the glycoprotein in corn. Corn is a cereal rich in protein which is used for both human and animal consumption; this plant has been an adequate experimental model because of the high levels of expression of transgenes obtained. It was perceived as a species with great potential for producing an edible vaccine. The vector used for the transformation of the plant was pGHCNS. G gene rabies virus was cloned downstream of the promoter and the maize ubiquitin promoter 35ScaMV. The expression cassette was flanked by matrix attachment region (MARs). Maize embryogenic calluses were transformed with the above construction by biolistics. Regenerated maize plants were recovered and grown in greenhouse. The presence of the G gene and its products was detected in vegetal tissue by PCR and Western blot. A fine powder was prepared from transformed grains and administered as pellet (50 μg of recombinant G protein). Other groups of mice were immunized intramuscularly with 50 μg of G protein using a commercial vaccine. All mice were challenged intracerebrally at day 90 post-vaccination using a vampire bat rabies virus which is used to evaluate commercial vaccine in Mexico. Embryogenic calluses were transformed by biolistics and herbicide-resistant plants were obtained. Twenty-five plants were recovered and 92 % contained the G gene as detected by PCR. The rabies G protein was identified by Western blot and presented a size of about 69 kDa. This increase in molecular weight may be due to posttranslational modifications, and this modification does not seem to have any adverse effect on the antigenic properties of the protein. Similar modifications were showed by McGarvey et al. (1995). Protein was expressed at 1 % of total soluble protein, which is equivalent to about 50 μg per gram of fresh weight in mass. This study obtained a higher level of expression than ever reported. The level of expression obtained in this study is comparable to results obtained by others in maize when expressing the spike protein of the transmissible gastroenteritis coronavirus and the fusion protein of Newcastle disease virus (Lamphear et al. 2002; Guerrero-Andrade et al. 2006). In sera, mice were seronegative at the start of the experiment, but by day 90 post-vaccination, titers varied by more than 0.5 IU. The animals were protected at 100 %, similar at the commercial vaccine. This work has demonstrated that the systems for transformation, selection, and regeneration of mice developed in this study are efficient. Likewise, the plant-based G protein was able to induce viral neutralizing antibodies and protect mice after challenge.
In another assay, the glycoprotein was expressed using carrots to be used in the immunization of mice (Rojas et al. 2009). This plant model was used since carrot is a vegetable that is widely distributed and easy to produce and can be consumed raw.
The G gene of the rabies virus arctic fox strain was subcloned between the double enhancer cauliflower mosaic virus 35S promoter and 35S CaMV terminator in the vector pUCpSS; this construct was named pUCpSSrabG. For transformation, we decided to use the minimal cassette expression approach (promoter-gene-terminator). We employed carrot seeds for induction of carrot callus. We were able to regenerate 300 adult plants from 100 calli selected in liquid medium, and 93.3 % of the analyzed plants showed integration of the transgene with levels of expression varying from 0.2 to 1.4 % TSP. In our project, the plant-produced band migrated slightly above the native G protein (~70 vs 65 kDa); one likely explanation for this was glycosylation of the protein by the plant. For selection, we employed the gene coding for phosphinothricin acetyltransferase (bar), which confers resistance to herbicide Basta. Embryogenic calluses were transformed by biolistics and herbicide-resistant plants were obtained in liquid medium. The presence of the G gene in leaves was determined by PCR and the protein was detected by Western blot using rabbit polyclonal serum against rabies G protein. In order to evaluate the carrot as vaccine, 24 mice were divided into four groups: G1, fed standard mouse chow (negative control); G2, received an intramuscular dose of inactivated rabies vaccine; G3, mice fed 50 μg of rabies virus G protein in 2 g of raw carrot; and G4, mice received 50 μg rabies virus G protein contained in 2 g of raw carrot plus 50 μg of N protein rabies virus (N protein was orally administered) since this molecule has been reported as adjuvant in some rabies vaccines. Mice vaccinated were challenged intracerebrally 60 days post-vaccination. We showed that the ingestion of antigen expressed in carrot resulted in protective rabies antibodies (66 %). These results are consistent with previous studies where the glycoprotein of rabies virus was expressed either in tobacco or in spinach. In this study, we did not observe a 100 % protection of the mice; this is possibly because a greater concentration of G protein is necessary. To improve the protective dose, 100 μg (4 g of carrots) could be administered instead of the 50 μg used in this study.
Recently transgenic corn was used supplied under controlled conditions at various dosages in sheep via oral administration of a single dose. The results showed that 2 g of the G protein of the rabies virus protected more than 80 % of the animals challenged with a lethal vampire bat origin virus (Loza-Rubio et al. 2012a, b). This assay used the same conditions for obtaining transformed corn reported previously by Loza-Rubio et al. (2008). Similarly the Basta herbicide was used for selecting transformed plants.
When the plants reached adulthood, kernels expressing the glycoprotein were identified by PCR and Western blot and were subsequently pooled and quantified before immunization. The animals were divided into six groups containing six animals per group as follows: Group 1, sheep fed 0.5 mg of rabies virus G protein in 20 g of ground maize kernels; Group 2, sheep fed 1.0 mg of rabies virus G protein in 40 g of ground maize kernels; Group 3, sheep fed 1.5 mg of rabies virus G protein in 60 g of ground maize kernels; Group 4, sheep fed 2.0 mg of rabies virus G protein in 80 g of ground maize kernels; Group 5, sheep vaccinated with an inactivated rabies vaccine administered intramuscularly; and Group 6, animals fed 40 g of non-transformed ground maize kernels. Once all groups had been immunized, they were deprived of feed and water for 4 h. The animals were bled to evaluate immune response in serum. Sheep were challenged by the injection at 120 days post-vaccination. The G protein was detected slightly above the native G protein (~70 kDa). The same increase in molecular weight was observed in polyacrylamide gel electrophoresis; the differentially expressed band seems to be heavier than the native G protein (positive control). The expression level obtained from this analysis was an average of 25 μg of G recombinant protein/g of fresh tissue in the three different lines.
At day 30 post-vaccination, rabies virus antibodies were detected in all vaccinated groups. Animals that received one or two doses of antigen (0.5 and 1.0 mg, respectively) showed a survival rate of 50 % (three deaths in six vaccinated animals). In Group 3, which was immunized with 1.5 mg of protein G, only two sheep died of rabies (2/6), with a survival rate of 66 %. The lowest mortality was found among sheep immunized with the commercial vaccine and those receiving 2.0 mg of protein, which protected 83 % of the animals (1/6). In this study, a large amount of protein was needed to elicit an immune response because a significant portion of the recombinant protein was likely degraded in the rumen. Although we did not observe any signs of tolerance, this could be because the sheep were fed the edible vaccine only once. These results demonstrated the effectiveness of the oral immunization of sheep with corn that expresses the rabies virus G protein. This is the first study in which an orally administered edible vaccine has shown efficacy in a polygastric species.
3.3 Benchmarks of What Is Needed to Commercialize the Product in This System
This new technology could contribute to global vaccination programs and have a dramatic impact on public and veterinary health, not only in our country but also in others with similar problems. Nevertheless, the fact that transformed corn must not be grown on open fields must be taken into account since it is an open pollination plant. It should be grown in greenhouses with the highest biosecurity. There are also issues that still need to be resolved, such as the antigen dose that each plant produces since it could produce tolerance (Loza-Rubio and Rojas-Anaya 2010). One possible alternative for the production of antigenic proteins using plants as an expression system is the use of suspension plant cell in pellets that express the antigens of interest. This would avoid the need for growing the plants in greenhouses. The administration would be oral, which is a significant advantage that could be of interest to pharmaceutical companies.
4 Technical Progress
4.1 Improvements to the Production System for a Rabies Virus Vaccine in Plants
The plant systems evaluated by our group were carrots and corn with the best results, in terms of protein expression and antibody production, obtained with corn.
Genetic modification of cereals has been carried out by direct DNA transfer (i.e., the introduction, integration, and expression of foreign genes) into protoplasts or intact cells grown in vitro using polyethylene glycol treatment and electroporation. These methods have their disadvantages, such as low transformation efficiency of monocotyledon plants and expression levels, which make them impractical. Thus, biolistics was a good option for improving the expression of recombinant proteins (Klein and Fitzpatrick-McElligott 1993; Christou 1995). Other factors for obtaining high levels of expression are described below.
This technique allows the introduction of naked DNA (biologically active) into intact plant cells by the acceleration of DNA-covered microparticles (tungsten or gold) through an explosion mechanism (pressure gun) or by gas bursts (carbon dioxide, nitrogen, or helium) (Klein et al. 1988). Biolistics revolutionized the genetic engineering of monocotyledon species, such as corn.
4.2 Challenges for the Optimization of Protein Expression
An important aspect for obtaining an edible vaccine is to develop efficient expression levels in terms of total soluble protein (≥1 %) since low percentages (0.01 %) require purification of the protein. There are several strategies for increasing the expression levels such as the optimization of the gene, use of strong promoters (tissue specific), non-translated leading sequences in the 3′ region, subcellular target signals, crossing of transgenic strains with high expression, germplasm crossing, plastid transformation, and the use of vegetable virus expression systems (Mett et al. 2008; Streatfield 2006; Potenza et al. 2004; Walmsley and Arntzen 2000; Sala et al. 2003; Gleba et al. 2005).
4.2.1 Use of Specific Promoters
Currently there is a wide range of promoters that have been used for regulating the expression of the transgene in transformed plants. Regulation can occur at any step of the expression process, and the promoters that manage it ensure the said control during transcription. The election of a promoter for plant transformation depends on the objective and purpose for which the plant is being transformed, as well as its species. Promoters that are specific to the species or tissue can be used, as well as those for seeds or grains, flowers, pollen, roots, leaves, or even aerial tissue (Buchanan et al. 2000; Potenza et al. 2004). Within the promoter are regulating regions, sequence motifs, or cis elements; these regulating sequences are known as increasers. These can be located upstream or downstream of the coding region. These regions are required to carry out the maximum transcription of a gene, and it is due to this reason that they are used within plant transformation vectors (Alberts et al. 2002).
The most common of the constitutive promoters used in plant transformation is the 35S promoter from the cauliflower mosaic virus (35S CaMV) (Odell et al. 1985), which is highly valued since it has high expression levels at practically all regions of a transgenic plant. This promoter can achieve high expression levels of the transgene both in monocotyledons and in dicotyledonous plants, although in the former it is somewhat recalcitrant (Battraw and Hall 1990; Benfey et al. 1990).
Regarding the expression of the G protein of the rabies virus in corn, the use of matrix attachment regions (MARs) that flanked the expression cassette became relevant, as they allowed the expression of the proteins to become constitutive. In carrots it was decided that a double 35S promoter of the cauliflower mosaic virus within vector pUCpSS was to be used as promoter, while in corn it was decided to use the corn ubiquitin promoter (Ubi). The use of this promoter has demonstrated that it increases the expression levels of heterologous genes in several cereal species (Cornejo et al. 1993; Gallo-Meagher and Irving 1993; Taylor et al. 1993; Vasil 1994). In contrast with other studies which have purified the recombinant proteins, our study did not require such process and remarkably the expression of the G protein ranged from 0.01 % up to 1.4 % of the total soluble protein.
Finally, although the edible vaccine system is attractive and novel, in practice its application can become complicated due to the following reasons:
-
1.
Dosing of the antigen will depend on its expression level within the plant tissue, even though good expression levels have been demonstrated generally. In order to determine this parameter, each plant line produced would need to be analyzed (individual plants).
-
2.
The expression levels that are obtained are not always inheritable to the next generations of the transgenic line. Furthermore, the seeds that develop from these lines can have genotypic and phenotypic characteristics that are undesirable, such as loss of fertility and others.
5 Nontechnical Hurdles
5.1 Production
One of the most important publications of the Mexican regulations in this issue is the “special protection regime for corn.” In the context of plant-derived vaccines, it is important to mention that the regime establishes: “The experimentation or release into the environment of genetically modified corn that has characteristics that prevent or limit its use or consumption by humans or animals shall not be allowed, as well as their use in the processing of food for human consumption.” The aforementioned blocks the experimentation with corn in Mexico for the development of antigens and other biopharmaceuticals. This is because Mexico is the origin center and diversity of this cereal. An alternative might be the development of these edible vaccines in plants different to the maize. In other regions, such as the United States and Europe, the development of transgenic corn is not allowed because the regulation in these countries is more flexible. The regulatory framework of other countries has been discussed by Loza-Rubio and coworkers previously (Loza-Rubio and Rojas-Anaya 2010).
5.2 Regulatory
Once the regulatory item be resolved, there are other important points that must be covered regarding the design and elaboration of a vaccine derived from plants, such as assuring transgene stability and antigen expression in the following generations, guaranteeing the consistency and reproducibility of the methods used to obtain the vaccine, evaluation and monitoring of the environment to avoid contamination of endemic species, making sure that efficient methods are available for quantification of the antigen to determine correct dosing and bioavailability of the vaccine, and assessment of the handling and transporting techniques with regard to wastes produced from the transformed plants. Because of all this, legislation is necessary, both at global and local levels, on the research and development of biological products using transgenic plants as a platform. In this regard, Hungary became the first Central European country to adopt legislation on the regulation of genetic engineering activity. Its Gene Technology Law entered into force in January 1999 with the concomitant establishment of an advisory body (The Gene Technology Committee). Subsequently, several countries of Central and Eastern Europe adopted regulations about genetically modified organisms (GMOs).
Before 2000, the differences between the US and the European community in their approach to the regulation of biotechnology had arisen because of the different initial cognitive frameworks, a different level of trust in the government, and the dissimilar agro-political situation. The dissimilar cognitive frameworks arise primarily because many Europeans appear to view the environment as a fragile ecosystem that may be easily unbalanced by transgenic plants. In contrast, the dominant view in the United States is of a resilient environment that can easily adapt. In September 2003, the Cartagena Protocol, an international treaty governing the movements of living modified organisms (LMOs) resulting from modern biotechnology from one country to another, was adopted. One of the principal objectives of the protocol is to provide information to importing countries to assist their decision making when accepting imports of LMOs. Now, boasting almost 190 member governments all around the world (known as “Parties”), the Convention has three goals: the conservation of biodiversity, the sustainable use of the components of biodiversity, and the fair and equitable sharing of the benefits arising from the use of genetic resources (Loza-Rubio and Rojas-Anaya 2010).
During the FAO Global Biotechnology Forum in 2005 (Ruane and Sonino, 2008), countries discussed about the kinds of GMO regulatory systems that might be appropriate for developing countries; it is important to consider that GMOs for food and agriculture are a very heterogeneous group, for example, the potential environmental risks from GM forest trees and the release of a GM yeast to make bread are different. In addition, within each of these sectors, GMOs may vary considerably, requiring different kinds of regulations, for example:
-
Some species are not grown for food (e.g., cotton), so food safety regulations are not strictly an issue although it should be kept in mind that some material, e.g., pollen/honey derived from GM, may still enter the food chain.
-
The same species may be modified for very different traits, e.g., an agricultural crop or animal may be modified to produce human pharmaceuticals as tomatoes producing vaccines against virus or animals producing hormones. “Pharmed” products under development include vaccines, antibodies, and industrial proteins and, in the crop sector, involve banana, maize, potato, and tomato plants. Special regulations covering potential gene flow to their conventional counterparts may be necessary;
-
Regulations may vary depending on whether the GM species is produced for export or domestic use. For this reason, GMO commercialization is subject to a strict marketability requirement. Otherwise, GMO varieties are not approved for commercialization. When exports are not a significant factor (e.g., in the case of cotton), commercial release can be approved irrespective of the regulatory status elsewhere, since there are no “sensitive” markets for the product.
On the other hand, there are twelve countries that are noteworthy due to their high levels of biodiversity: Brazil, Indonesia, Colombia, Australia, Mexico, Madagascar, Peru, China, the Philippines, India, Ecuador, and Venezuela, known as mega-diverse; therefore, it is very important to preserve their germplasm (Tovar 2008).
The identity of Mexico in terms of its biodiversity is important therefore its global role must be recognized. Mexico is a country with domestic and wild plant species of which it is their center of origin, so their protection has been requested. In this context, Mexico is one of the countries signatory to the Cartagena Protocol that was adopted on 29 January 2000 (Cartagena Protocol, 2000). The majority of the problems related to gene flow corresponding to GMOs and the issues regarding the responsibility/compensation were examined in the legislation framework of the Cartagena Protocol on Biosafety to the Convention on Biological Diversity. Article 1 mentions: “In accordance with the precautionary approach contained in Principle 15 of the Rio Declaration on Environment and Development, the objective of this Protocol is to contribute to ensuring an adequate level of protection in the field of the safe transfer, handling and use of living modified organisms resulting from modern biotechnology that may have adverse effects on the conservation and sustainable use of biological diversity, taking also into account risks to human health, and specifically focusing on transboundary movements.”
Before 2005, the Ecological Equilibrium and Environmental Protection General Act (Tovar 2008) was the national judicial instrument that provided the basis for regulation regarding GMOs. It has the objective of “regulating the activities of confined use, experimental release, pilot program release, commercial release, marketing, import and export of genetically modified organisms” in order to prevent, avoid, or reduce the possible risks that these activities could cause to human health, as well as to the health of animals, plants, and water animals, the environment, and the biological diversity of the country. It establishes as competent authorities for issuing permits and sanctions the Ministry of the Environment and Natural Resources; the Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food; and the Ministry of Health. It also establishes the basis for the operation of the Inter-ministry Commission on Biosafety for Genetically Modified Organisms (CIBIOGEM) through which the various aforementioned ministries must collaborate regarding the biosafety of GMO.
The law established that a permit will be required for carrying out the following activities (Ley de bioseguridad de Organismos Geneticamente Modificados, 2005):
-
(a)
Experimental release into the environment, including imports for this activity, of one or more GMOs
-
(b)
Release into the environment in a pilot program, including imports for this activity
-
(c)
The commercial release into the environment, including imports for this activity, of GMOs
In order to establish a risk assessment of the aforementioned points, each case is to be analyzed individually through scientific and technical studies carried out by the interested parties, evaluating the possible risks of the experimental release into the environment and to the biological diversity, as well as to the health of animals, plants, and fisheries. The studies must include possible risks to human health. Up to the development of this document, no reference has been made regarding transformed plants that express an antigen (plant-derived antigens).
The latest amendment to the Regulations of the Genetically Modified Organisms Biosafety Act was published after 2009. These regulations establish, among other things:
-
(a)
The characteristics that must be contained within the request for permission to carry out activities using GMOs
-
(b)
The requirements for permits for release into the environment
-
(c)
Considerations on the import and export of GMOs that are destined for their release into the environment
-
(d)
Characteristics of the Internal Biosafety Commissions of public and private institutions
-
(e)
Determination of the centers of origin and genetic diversity
-
(f)
Establishment of the National Biosafety Information System
-
(g)
Determination of the list of GMOs that are to be issued by the competent ministries
On the other hand, Brazil, another mega-diverse country with great advances and biotechnology development, promulgated Decree 6.041 of the Policy on the Development of Biotechnology in which the objective is “To promote and carry out actions in order to establish the adequate environment for developing biotechnology products and innovative processes, promote the greatest efficiency of the national productive structure, the innovative capacity of Brazilian companies, the adsorption of technologies, the generation of business and the expansion of exports (Ley No. 1.105, 2005; Biotechnology Development Policy 2007).” In comparison with the Mexican regulation, it also established the competences of the ministries regarding activities with GMOs, the integration of Biosafety Committees, etc. It is noteworthy that in the said document, it is established as a strategic objective to stimulate the production of recombinant proteins using plants, animals, and microorganisms as bioreactors and the plants resistant to biotic and abiotic stress. The aforementioned emphasizing on the coexistence of transgenic and conventional varieties promoting the development of mechanisms and technologies for preserving the genetic identity of cultivars, as well as the development of geographical information systems for monitoring and zoning of the activities related to distance biotechnology safety.
Recent developments in genetic modification and the use of LMOs in agriculture have ignited a debate over the potential effects of these organisms on biological diversity. The regime does allow states to enact national protective measures to preserve human and animal health as well as natural resources, based on scientific evidence. However, it is necessary to ensure that this is not only on paper but is carried out in order to avoid ecological imbalances that could affect all species including humans.
5.3 Public Perception
One of the main challenges that modern biotechnology currently has is the acceptance by consumers of the products developed by it, especially of products derived from transgenic plants for their use as food. This is known as “biotechnophobia,” which is the rejection of anything that has been derived from biotechnology due to its denomination as something “not natural” and/or “potentially dangerous.” Unfortunately, this has been promoted by ecologist associations that have a strong penetration in mass media. It is important that scientific institutions and community promote the use of everyday language to describe the benefit of new technological discoveries. For example, the use of the word “transgenic” is perceived as something that is against nature, and in some cases, it has been used instead of “transgender” in mass media causing further confusion. Another such word that should be avoided is “mutation” since it is associated with an organism that causes harm and that it is against nature, association that comes about due to its use in science fiction.
6 Conclusions
The use of biolistics as a method for plant genetic transformation and the use of plant expression vectors with constitutive promoters helped to achieve the development of carrot and corn plants that express the G protein of the rabies virus. These plants were successful antigen production systems. According to the results obtained in this study, it was found that the G protein expressed in the plant systems under evaluation was functional, even though it had suffered some posttranslational modifications. When immunity assays were carried out, it was found that corn tissue was the most effective in providing greater protection when challenged with rabies virus from hematophagous bats, which are the main transmitter of this virus in Mexico. Results show that both carrot and corn are convenient systems for the expression of the G protein since good expression levels were achieved and such levels would allow the production of a subunit vaccine. Nevertheless, the expression of the N protein in tomato plants was not satisfactory. It is noteworthy that the use of biolistics allows permanent expression since the transgene is integrated into the plant genome and from there it segregates into future generations thus obtaining a good candidate for an edible vaccine against the rabies virus in animals and humans.
This study allows us to visualize a rabies vaccine derived from plants since it was demonstrated that plant cells from both species under evaluation are capable of expressing the G protein of the virus in sufficient quantities. It is recommended that further studies be carried out on wild animals, which are transmitters of the disease.
Commercial production of vaccine of this type depends on overcoming regulatory frameworks on the use of plant cells as protection antigen producers without the need for using a whole plant as the biological medium.
References
Alberts B, Jonson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Aréchiga Ceballos N, Vázquez Morón S, Berciano JM, Nicolás O, Aznar López C, Juste J, Rodríguez Nevado C, Aguilar Setién A, Echevarría JE (2013) Novel lyssavirus in bat, Spain. Emerg Infect Dis 19:793–795
Arellano-Sota C (1988) Vampire-bat transmitted rabies in cattle. Rev Infect Dis 10:707–709
Artois M, Guittré C, Thomas I, Leblois H, Brochier B, Barrat J (1992) Potential pathogenicity for rodents of vaccines intended for oral vaccination against rabies: a comparison. Vaccine 10:524–528
Ashraf S, Singh PK, Yadav DK, Shahnawaz M, Mishra S, Sawant SV, Tuli R (2005) High level expression of surface glycoprotein of rabies virus in tobacco leaves and its immunoprotective activity in mice. J Biotechnol 119:1–14
Battraw MJ, Hall TC (1990) Histochemical analysis of CaMV 35S promoter-B- glucuronidase gene expression in transgenic rice plants. Plant Mol Biol 15:527–538
Benfey PN, Ren L, Chua N-H (1990) Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J 9:1677–1684
Benmansour A, Leblois H, Coulon P, Tuffereau C, Gaudin Y, Flamand A, Lafay F (1991) Antigenicity of rabies virus glycoprotein. J Virol 65:4198–4203
Biotechnology Development Policy (2007) National Biotechnology Committee. Brazil
Boehm R (2007) Bioproduction of therapeutic proteins in the 21st century and the role of plants and plant cell as production platforms. Ann NY Acad Sci 102:121–131
Bouet-Cararo C, Contreras V, Fournier A, Jallet C, Guilbert JM, Dubois E, Thiery R, Bréard E, Tordo N, Richardson J, Scwartz-Cornil I, Ziéntara S, Klonjkowoki B (2010) Canine adenoviruses elicit both humoral and cell-mediated immune responses against rabies following immunization of sheep. Vaccine 29:1304–1310
Bower DM, Kristala L, Prather J (2009) Engineering of bacterial strains and vectors for the production of plasmid DNA. Appl Microbiol Biotechnol 82:805–813
Buchanan BB, Gruissem W, Jones RL (eds) (2000) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, MD, pp 340–342
Bugnon P, Breitenmoser U, Peterhans E, Zanoni R (2004) Efficacy of oral vaccination in the final stage of fox rabies elimination in Switzerland. J Vet Med 51:433–437
Cadoz M, Strady A, Meigner B, Taylor J, Tartaglia J, Paoletti E et al (1992) Immunisations with canarypox virus expressing rabies glycoprotein. Lancet 339:1429–1432
Cartagena protocol on biosafety to the convention on biological diversity. United Nations. Montreal, 2000
Christou P (1995) Particle bombardment. In: Galbraith DW, Bourque DP, Bohnert HJ (eds) Methods in cell biology, vol 50. Academic, St. Louis, MO, pp 375–382
Cornejo M, Luth D, Blankenship KM, Anderson OD, Blenchl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:558–567
Delpietro HA, Lord RD, Russo RG, Gury-Dhomen F (2009) Observations of sylvatic rabies in Northern Argentina during outbreaks of paralytic cattle rabies transmitted by vampire bats (Desmodus rotundus). J Wildl Dis 45:1169–1173
Desmettre P, Languet B, Chappuis G, Brochier B, Thomas I, Lecocq L-P, Kieny M- P, Blancou J, Aubert M, Artois M, Pastoret P-P (1990) Use of vaccinia recombinant for oral vaccination of wildlife. Vet Microbiol 23:227–236
Desmeziéres E, Jacob Y, Saron MF, Delpeyroux F, Tordo N, Perrin P (1999) Lyssavirus glycoproteins expressing immunologically potent foreign B cell and cytotoxic T lymphocyte epitopes as prototypes for multivalent vaccines. J Gen Virol 80:2343–2351
Dietzgen RG, Calisher CH, Kurath G, Kusmin IV, Rodríguez LL, Stone DM (2011) Family Rhabdoviridae. In: King AM, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus taxonomy: classification and nomenclature of viruses. Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier, San Diego
Dietzschold B, Wang H, Rupprecht C, Celis E, Tollis M, Ertl H, Heber-Katz E, Koprowski H (1987) Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci U S A 84:9165–9169
Dietzschold B, Gore M, Casali P, Ueki Y, Rupprecht CE, Notkins AL, Koprowski H (1990) Biological characterization of human monoclonal antibodies to rabies virus. J Virol 64:3087–3090
Dioario Oficial de la Federación (2009) Reglamento de la Ley de Bioseguridad de Organismos Geneticamente Modificados
Drings A, Jallet C, Chambert B, Tordo N, Perrin P (1999) Is there an advantage to including the nucleoprotein in a rabies glycoprotein subunit vaccine? Vaccine 17:1549–1557
Fischer L, Minke J, Dufay N, Baudu P, Audonnet JC (2003) Rabies DNA vaccine in the horse: strategies to improve serological responses. Vaccine 21:4593–4596
Food and Agriculture Organisation of the United Nations (2011) Investigating the role of bats in emerging zoonoses: balancing ecology, conservation and public health interests. In: Newman SH, Field HE, de Jong CE, Epstein JH (eds). FAO Animal Production and Health
Freuling CM, Beer M, Conraths FJ, Finke S, Hoffmann KB, Kiemt J, Mettenleiter TC, Mühlbach E, Teifke J, Wohlsein P, Müller T (2011) Novel lyssavirus in Nattere’s bat Germany. Emerg Infect Dis 17:1519–1522
Fries LF, Tartaglia J, Taylor J, Kauffman EK, Meignier B, Paoletti E, Plotkin S (1996) Human safety and immunogenicity of canarypox-rabies glycoprotein recombinant vaccine and alternative poxvirus vector system. Vaccine 14:428–434
Gallo-Meagher M, Irving JE (1993) Effects to tissues type and promoter strength on transient GUS expression in sugar cane following particle bombardment. Plant Cell Rep 12:666–670
Gaudin Y, Ruigrok RW, Knossow M, Flamand A (1993) Low-pH conformational changes of rabies virus glycoprotein and their role in membrane fusion. J Virol 67:1365–1372
Gaudin Y, Moreira S, Benejean J, Blondel D, Flamand A, Tuffereau C (1999) Soluble ectodomain of rabies virus glycoprotein expressed in monomeric conformation that is antigenically distinct from the native state of the complete, membrane-anchored glycoprotein. J Gen Virol 80:1647–1656
Gleba Y, Klimyuk S, Marillonet S (2005) Magnifection: a new platform for expressing recombinant vaccines in plants. Vaccine 23:2042–2048
Guerrero-Andrade O, Loza-Rubio E, Olivera-Flores MT, Feérvari-Bone T, Gómez-Lim MA (2006) Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res 15:455–463
Henderson H, Jackson F, Bean K, Panasuk B, Niezgoda M, Slate D et al (2009) Oral immunization of raccoons and skunks with a canine adenovirus recombinant rabies vaccine. Vaccine 27:194–197
Hooper DC, Pierrard L, Modelska A, Otvos L, Fu Z, Koprowski H, Dietzschold B (1994) Rabies nucleocapsid as an oral immunogen immunological enhancer. Proc Natl Acad Sci U S A 91:10908–10911
Hu R, Zhang S, Fooks AR, Yuan H, Liu Y, Li H, Tu C, Xia X, Xiao Y (2006) Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes Infect 8:1090–1097
Jackson AC (2003) Rabies virus infection: an update. J Neurovirol 9:253–258
Johnson N, Cunningham AF, Fooks AR (2010) The immune response to rabies virus infection and vaccination. Vaccine 28:3896–3901
Khawplod P, Shoji Y, Ubol S, Mitmoonpitak C, Wilde H, Nishizono A, Kurane I, Morimoto K (2006) Genetic analysis of dog rabies viruses circulating in Bangkok. Infect Genet Evol 6:235–240
Kieny MP, Lathe R, Drillien R, Spenhner D, Skory S, Schmitt D et al (1984) Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature 312:163–166
Klein TM, Fitzpatrick-McElligott S (1993) Particle bombardment: a universal approach for gene transfer to cells and tissues. Curr Opin Biotechnol 4:583–590
Klein TM, Fromm M, Weissinger A, Dwight T, Schaaf S, Sletten M, Sanford JC (1988) Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc Natl Acad Sci U S A 85:4305–4309
Kobayashi Y, Suzuki Y, Itou T, Carvalho AA, Cunha EM, Ito FH, Gojobori T, Sakai T (2010) Low genetic diversities of rabies virus population within different host in Brazil. Infect Genet Evol 10:278–283
Kuzmin IV, Mayer AE, Niezgoda M, Markotter W, Agwanda B, Breiman RF, Rupprecht CE (2010) Shimoni bat virus, a new representative of the Lyssavirus genus. Virus Res 149:197–210
Lafon M (2005) Modulation of the immune response in the nervous system by rabies virus. CTMI 289:239–258
Lafon M (2011) Evasive strategies in rabies virus infection. Adv Virus Res 79:33–53
Lafon M, Edelman I, Bouvet JP, Lafange M, Martchatre E (1990) Human monoclonal antibodies specific for the rabies virus glycoprotein and N protein. J Gen Virol 71:1689–1696
Lamphear BJ, Streatfield SJ, Jilka JM, Brooks CA, Barker DK, Turner DD, Delaney DE, Garcia M, Wiggins B, Woodard SL, Hood EE, Tizard IR, Lawhorn B, Howard JA (2002) Delivery of subunit vaccines in maize seed. J Control Release 85:169–180
Lerma BSL (2005) Caracterización en plantas de maíz obtenidas a partir de callos embriogénicos transformados con el gen de la proteína G del virus de la rabia. Master Thesis. CINVESTAV-Irapuato. Marzo
Ley de Bioseguridad de Organismos Geneticamente Modificados (2005) Diario Oficial de la Federación. México
Ley No. 1.105 decreto No. 5.591 Normas de seguridad y mecanismos de fiscalización de actividades relacionadas con OGM. Brasil. 2005
Lodmell DL, Ewalt CL (2000) Rabies vaccination: comparison of neutralizing antibody responses after priming and boosting with different combinations of DNA, inactivated o recombinant vaccinia virus vaccines. Vaccine 18:2394–2398
Lodmell DL, Ewalt LC, Parnell MJ, Rupprecht CE, Hanlon C (2006) One-time intradermal DNA vaccination in ear pinnae one year prior to infection protects dogs against rabies virus. Vaccine 24:412–416
Lontai I (1997) The current state of rabies prevention in Europe. Vaccine 15S:S16–S19
Loza-Rubio E, Gomez-Lim MA (2006) De Pasteur a nuestros días, batalla contra la rabia. Ciencia y Desarrollo 176:26–33
Loza-Rubio E, Rojas-Anaya E (2010) Vaccine production in plant systems–an aid to the control of viral diseases in domestic animals: a review. Acta Vet Hung 58:511–522
Loza-Rubio E, Pedroza-Requénes R, Montano-Hirose JA, Aguilar Setién A (1998) Caracterización con anticuerpos monoclonales de virus de la rabia aislados de fauna doméstica y silvestre de México. Vet Mex 29:345–349
Loza-Rubio E, Rojas AE, Banda RVM, Nadin-Davis SA, Cortez GB (2005) Detection of multiple strains of rabies virus RNA using primers designed to target Mexican vampire bat variants. Epidemiol Infect 133:927–934
Loza-Rubio E, Rojas AE, Gómez NL, Olivera FMTJ, Gómez-Lim M (2008) Development of an edible rabies vaccine in Maize using the Vnukovo strain. Dev Biol (Karger) 131:477–482
Loza-Rubio E, Nadin-Davis SA, Morales SE (2012a) Molecular and biological properties of rabies viruses circulating in Mexican Skunks: focus on P gene variation. Rev MexCiencPecu 3:155–170
Loza-Rubio E, Rojas AE, Gómez NL, Flores OMT, Gómez-Lim MA (2012b) Protein G from rabies virus expressed in maize protects mice against a challenge with an heterologous rabies virus. Vaccine 30:5551–5556
Mainguy J, Fehlner-Gardiner C, Slate D, Rudd RJ (2013) Oral rabies vaccination in raccoons: comparison of ONRAB and RABORAL V-RG vaccine-bait field performance in Québec, Canada and Vermont, USA. J Wildl Dis 49:190–193
Marston DA, Horton DL, Ngeleja C, Hampson K, McElhinney LM, Banyard AC, Haydon D, Cleaveland S, Rupprecht CE, Bigambo M, Fooks AR, Lembo T (2012) Ikoma lyssavirus, highly divergent novel Lyssavirus in an African Civet. Emerg Infect Dis 18:664–667
McGarvey PB, Hammond J, Dienelt MM, Hooper CD, Fu FZ, Dietzschold B et al (1995) Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology 13:1484–1487
Mett V, Farrance EC, Green BJ, Yusibov V (2008) Plants as biofactories. Biologicals 36:354–358
Modelska A, Dietzschold B, Fleysh N, Fu ZF, Steplewski K, Hooper C et al (1998) Immunization against rabies with plant-derived antigen. Proc Natl Acad Sci USA 95:2481–2485
Morales MM, Rico RG, Gómez OJ, Aguilar SA (2006) Importancia inmunológica de la proteína N en la infección por virus de la rabia. Rev Vet Méx 37:352–364
Odell J, Nagy F, Chua N-H (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313:810–812
Osinubi MOV, Wu X, Franka R, Niezgoda M, Nok AJ, Ogunkoya AB, Rupprecht CE (2009) Enhancing comparative rabies DNA vaccine effectiveness through glycoprotein gene modifications. Vaccine 27:7214–7218
Osorio JE, Tomlinson RS, Frank EJ, Haanes K, Rushlow JR, Haynes JR, Stinchcomb DT (1999) Immunization of dogs and cats with a DNA vaccine against rabies virus. Vaccine 17:1109–1116
Paolazzi CC, Pérez O, De Filipo J (1999) Rabies vaccine. Mol Biol 11:137–147
Peeters K, Wilde CHD, Jeager G, Angenon G, Depicker A (2001) Production of antibodies and antibody fragments in plants. Vaccine 19:2756–2761
Perrin P, Jacob Y, Aguilar SA, Loza-Rubio E, Jallet C, Aubert M et al (2000) Immunization with DNA vaccine induces protection against rabies virus. Vaccine 18:479–486
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol-Plant 40:1–22
Rojas AE, Loza-Rubio E, Flores OMT, Gómez-Lim MA (2009) Expression of rabies virus G protein in carrots (Daucus carota). Transgenic Res 18:911–919
Ross BA, Favi MC, Vásquez V (2008) Glicoproteína del virus rábico: Estructura, inmunogenicidad y rol en la patogenia. Rev Cil Infect 25:S14–S18
Ruane J, Sonino A (2008) Results from the FAO biotechnology forum. FAO, Rome
Rybicki EP (2009) Third International conference on plant-based vaccines and antibodies. Expert Rev Vaccines 8:1151–1155
Sakamoto S, Ide T, Tokiyoshi S, Nakao J, Hamada F, Yamamoto M et al (1999) Studies on the structures and antigenic properties of rabies virus glycoprotein analogues produced in yeast cells. Vaccine 17:205–218
Sala F, Rigano M, Barbante A, Basso B, Walsley AM, Castiglione S (2003) Vaccine antigen production in transgenic plants: strategies, gene constructs and Perspectives. Vaccine 21:803–808
Schnell MJ, McGettigan JP, Wirblich C, Papaneri A (2010) The cell biology of rabies virus: using stealth to reach the brain. Nat Rev Microbiol 8:51–61
Streatfield SJ, Lane JR, Brooks CA, Barker DK, Poage ML, Mayor JM, Lamphear BJ et al (2003) Corn as production systems for human and animal vaccines. Vaccine 21:812–815
Streatfield SJ (2006) Engineered chloroplasts as vaccine factories to combat bioterrorism. Trends Biotechnol 24:339–342
Tacket C (2009) Plant-based oral vaccines: results of human trials. Curr Top Microbiol Immunol 332:103–117
Taylor MG, Vasil I, Vasil IK (1993) Enhanced GUS expression in cereal/grass cell suspension and immature embryos using the maize ubiquitin-based plasmid pAHC25. Plant Cell Rep 12:491–495
Tillman U (2004) Advances in the production of human therapeutic proteins in yeast and filamentous fungi. Nat Biotechnol 22:1409–1414
Tims T, Briggs DJ, Davis RD, Moore SM, Xiang Z, Ertl HCJ, Fu ZF (2000) Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titer of neutralizing antibodies. Vaccine 18:2804–2807
Tordo N, Bourhy H, Sather S, Ollo R (1993) Structure and expression in baculovirus of the Mokola virus glycoprotein: an efficient recombinant vaccine. Virology 194:59–69
Tovar MP (2008) Ley General del Equilibrio ecologico y la Protección al Ambiente. In: Bioseguridad en la amplicación de la Biotecnología y el uso de los organismos geneticamente modificados. CIBIOGEM. pp 287
Vasil IK (1994) Molecular improvement of cereals. Plant Mol Biol 25:925–937
Ventini DC, Astray RM, Jemos MA, Jorge SA, Riquelme CC, Suazo CA et al (2010) Recombinant rabies virus glycoprotein synthesis in bioreactor by transfected Drosophila melanogaster S2 cells carrying a constitutive or an inducible promoter. J Biotechnol 146:169–172
Walmsley AM, Arntzen CJ (2000) Plants for delivery of edible vaccines. Curr Opin Biotechnol 11:126–129
Wandeler AI (2000) Oral immunization against rabies: afterthoughts and foresight. Scheweiz Ach Tierheilk 142:455–462
Wang Y, Xiang Z, Pasquini S, Erlt HC (1997) The use an E1-deleted, replication defective adenovirus recombinant expressing the rabies virus glicoproteína for early vaccination of mice against rabies virus. J Vilol 71:3677–3683
Wilkinson L (2002) History. In: Jackson CA, Wunner HW (eds) Rabies. Elsevier, San Diego, CA, pp 1–21
Woldehiwet Z (2002) Rabies: recent developments. Res Vet Sci 73:17–25
World Health Organization (2013) WHO expert consultation on rabies. No. 982. Geneva
Wunner WH (2002) Rabies virus. In: Jackson AC, Wunner WH (eds) Rabies, 2nd edn. Academic, San Diego, pp 23–68
Xiang ZQ, Yang Y, Wilson JM, Ertl HCJ (1996) A replication-defective human adenovirus recombinant serves as highly efficacious vaccine carrier. Virology 219:220–227
Yuan Z, Zhang S, Liu Y, Zhang F, Fooks RA, Li Q, Rongliang H (2008) A recombinant pseudorabies virus expressing rabies virus glycoprotein: safety and immunogenicity in dogs. Vaccine 26:1314–1321
Yusibov V, Modelska A, Steplewski K, Agadjanyan M, Weiner D, Hooper C, Koprowski H (1997) Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc Natl Acad Sci U S A 94:5784–5788
Yusivov V, Hooper DC, Spitsin SV, Fleysh N, Kean RB, Mikheeva T, Deka D, Karasev A, Cox S, Randall J, Koprowsky H (2002) Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 20:3155–3164
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Loza-Rubio, E., Rojas-Anaya, E. (2014). Edible Rabies Vaccines. In: Howard, J., Hood, E. (eds) Commercial Plant-Produced Recombinant Protein Products. Biotechnology in Agriculture and Forestry, vol 68. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43836-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-43836-7_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43835-0
Online ISBN: 978-3-662-43836-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)