Abstract
Wound healing is a complex biological process with four main phases: hemostasis, inflammation, proliferation, and remodeling. Current treatments such as cotton and gauze may delay the wound healing process which gives a demand for more innovative treatments. Nanofibers are nanoparticles that resemble the extracellular matrix of the skin and have a large specific surface area, high porosity, good mechanical properties, controllable morphology, and size. Nanofibers are generated by electrospinning method that utilizes high electric force. Electrospinning device composed of high voltage power source, syringe that contains polymer solution, needle, and collector to collect nanofibers. Many polymers can be used in nanofiber that can be from natural or from synthetic origin. As such, electrospun nanofibers are potential scaffolds for wound healing applications. This review discusses the advanced electrospun nanofiber morphologies used in wound healing that is prepared by modified electrospinning techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skin is the largest organ in the body and accounts for 15% of the total body weight. It is the first line of defense that plays an important protective role against physical, chemical, and biological external [1]. The skin consists mainly of the epidermis, dermis, and hypodermis with the presence of other sublayers [2].
Skin wounds result from the disruption and damage of the skin layers [3]. Wounds can be acute or chronic. In acute wounds, the skin can self-heal and undergo normal healing stages. While in chronic wounds, self-healing property is insufficient and stages of healing is interrupted [4].
Wound dressings act as protective barriers to the applied surface and should be biocompatible, biodegradable, prevent microbial infection and resemble the extracellular matrix (ECM) of normal tissue, and provide an optimum environment for accelerated healing [5]. An ideal wound dressing should have an elastic mechanical structure but strong enough for easy handling and comfortable wear [6]. Too soft dressings are difficult to handle. On the other hand, high strength wound dressings often stick to wounds and may cause secondary injury [7].
Current treatments such as cotton, gauze films, foams, hydrogels, and hydrocolloid have low cost and high absorption capacity and play role in isolation of wound from contaminations [8]. Those treatments are often cause adhesion of the wound and delay on wound healing leading to decrease patient compliance [9]. Therefore, in order to improve the skin permeability of the drugs and to achieve better therapeutic effects, researchers have designed a variety of nanoparticle drug delivery agents for transdermal use, such as nanosheets, liposomes, hydrogels, wafers, nanospheres, dendrimers, nanosized colloids, and nanofibers [10]. Nanofibers resemble extracellular membrane (ECM) with large specific surface area, high porosity, good mechanical properties, and controllable morphology and size [11, 12].
Electrospun nanofiber properties used for wound healing including hydrophilicity, flexibility and strength, biocompatibility, and specific cell interactions are largely determined by the chemical composition of the polymers used [13]. Many different polymers used together with various bioactive ingredients could be introduced as electrospun nanofibers for wound healing purposes. Based on their origin, polymers can be classified as natural and synthetic polymers [14]. Natural polymeric dressings can be fabricated from protein polymers (e.g., gelatin [15], egg white [16], casein [17], whey protein [18] collagen [19, 20], silk fibroin [21], zein [22], keratin [23], marine [24], and soy protein [25]), plant polysaccharide (e.g., cellulose [26, 27], starch [28], and pectin [29, 30]), animal polysaccharide (e.g., chitosan [31,32,33] and hyaluronic acid [34,35,36]), fungal polysaccharide (e.g., pullulan [37, 38]), bacterial polysaccharides (e.g., dextran [39]), and alginates [40]. Synthetic polymeric dressings include polyvinyl alcohol (PVA) [41, 42], polyurethane (PU) [43, 44], polycaprolactone (PCL) [45], polylactic acid (PLA) [46], polyacrylic acid (PAA) [47], polyacrylonitrile (PAN) [48,49,50], poly-l-lactic acid (PLLA) [51], polyvinyl pyrrolidone (PVP) [52], polyethylene oxide (PEO) [53], polyethylene glycol (PEG) [54], polylactic-co-glycolic acid (PLGA) [55], polyglycolic acid (PGA) [56], polydopamine (PDA) [57], polyamide-6 (PA-6) [58], polyhydroxy butyrate (PHB) [59], polyvinylidene fluoride (PVDF) [60], poly-L-lactide-co-caprolactone (PLCL) [61], epsilon poly-lysine (ε-PL) [62], etc.
M. Wang et al. fabricated nanofibrous membrane of chitosan and PVA loaded with antibiotics at different ratios successfully, and they found that when low-molecular-weight chitosan to PVA ratio equaled 50/50, smooth and homogeneous fibers were obtained for potential wound healing applications [63]. H. Ezhilarasu et al. developed PCL/aloe vera (AV) nanofiber containing curcumin PCL/AV/CUR and tetracycline hydrochloride PCL/AV/TCH. The resulted fibers were nontoxic and have good mechanical properties within a range that resembles human skin properties that make them potential for wound healing applications [64]. F. Mwiiri , J. Brandner, and R. Daniels loaded birch bark dry extract (TE) on low-molecular-weight PVA fiber mats that showed significant increase in wound healing more than TE oleo gel with high drug permeation in a sustained release manner [65, 66]. Zaeri S, Karami F, and Assadi M prepared PVA solution containing 4% wt/vol propranolol and the result showed thin fibers that have good porosity and hydrophilicity with no toxic effects [67]. PU/PVA-gel nanofibers incorporated with cerium oxide (CeO2) nanoparticles and cinnamon essential oil (CEO) that showed good porosity, suitable fluid uptake capability with a slow degradation rate, and antibacterial effect on gram positive and gram negative bacteria [68].
In this context, nanofibrous scaffolds produced by electrospinning technique could potentially provide an excellent dressing for wound healing.
Wound Healing Phases
Wound healing process includes four subsequent phases: hemostasis, inflammation, proliferation, and remodeling with a timescale of seconds to hours, hours to days, days to weeks, and weeks to months, respectively [69, 70] (Fig. 1).
In hemostasis phase, the main goal is to prevent excessive blood loss and to protect vital function of the organ. Exposed extracellular matrix (ECM) components activate platelets, and three main steps occur: (a) platelet migration to the injured tissue; (b) secretion of alpha and dense granules containing adenosine diphosphate (ADP), thromboxane A2, and thrombin; and (c) aggregation. Chemical mediators released stimulate coagulation cascades. Thrombin catalyzes the conversion of fibrinogen to fibrin that promotes blood clot formation. This blood clot formed composed of platelets entrenched in a mesh of cross-linked fibrin fibers linked together by fibrinogen with smaller amounts of plasma fibronectin, vitronectin, and thrombospondin [71,72,73,74,75]. Activated platelets release multiple pro-inflammatory cytokines and chemokines, such as interleukin factors (IL-1α, IL-1β, IL-6, and IL-8) and tumor necrosis factor-α (TNF-α), and anti-inflammatory chemokines such as platelet factor-4 (PF-4), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and pro- and antiangiogenic factors such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) [76,77,78,79].
Inflammation phase divided into two stages, early stage and late stage, for the protection wounds against bacteria and removal of apoptotic tissues. The released mediators recruit neutrophils to the injury site first, followed by the accumulation of monocyte as well as mast cells [80]. At first, neutrophils kill and phagocytose bacteria and damaged matrix proteins within the wound bed [81]. Neutrophils also recruit TNF-1 and IL-1 which aid in the healing process [82]. Subsequently, neutrophils start to diminish, and monocytes differentiate into macrophages M1 and then migrate into the extravascular space to phagocytose bacteria as well as tissue debris [83]. Macrophages are divided into two categories based on their nature and function: inflammatory (M1) macrophages and anti-inflammatory (M2) macrophages. M1 macrophages are usually activated through various pro-inflammatory signals, such as tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ), and lipopolysaccharide (LPS); M1 phenotype secretes cytotoxic agents (nitric oxide), pro-inflammatory cytokines IL-1, IL-6, IL-12, IL-23, and TNF-α. M2 macrophages on the other hand are activated by IL-4 and IL-13, and they have various subtypes: M2a (alternatively activated macrophages), M2b (type 2 macrophages), and M2c (deactivated macrophages) [84]. The activation of M2a, M2b, and M2c macrophages occurs in response to IL-4 and IL-13, immune complexes and bacterial lipopolysaccharide (LPS), and glucocorticoids and TGF-b, respectively [85].
M2 macrophages contribute in cell proliferation phase by releasing of several growth factors such as PDGF, VEGF, TGF-β, insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), and fibroblast growth factor-2 (FGF-2) for the promotion of wound healing via angiogenesis and skin re-epithelialization [86]. M2 macrophages also act as regulatory cells by activating keratinocytes, fibroblasts, and endothelial cells that migrate into the clot and synthesize a new extracellular matrix components such as fibrin, collagen III, fibronectin, glycosaminoglycans, proteoglycans, and the matrix protein hyaluronan which contributes wound closure and initiate the formation of granulation tissue [87, 88].
In remodeling phase, replacement of collagen III by the stronger collagen I and rearrangement of collagen fibers leads the skin to reach its maximum elasticity and strength [89]. It also involves replacement of granulation tissue with the scar tissue by the fibroblasts and is completed to restore skin integrity [90].
Electrospinning Process
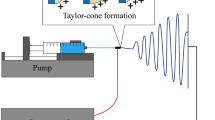
Many technologies have been developed to generate nanofibers including bioprinting, wet spinning, dry spinning, and electrospinning [91]. Electrospinning is a simple, cost-effective, and versatile setup process which depends on electrostatic concept in the presence of high electrical field [92]. Electrospinning device is mainly composed of high voltage power source, syringe that serves as reservoir for storing polymer solution, needle (spinneret) for dispensing of solution, and collector to collect nanofibers [93] (Fig. 2a).
Firstly, a high voltage is applied, and polymer solution is expelled from needle, and a strong electric field is formed between the needle and collector. Secondly, the continuous application of high voltage makes the polymer solution elongates to form a cone called Taylor cone. Thirdly, charged droplets of Taylor cone eject from the tip of the needle. Finally, the polymer solution is stretched and evaporated by the electric force and deposited on the collector to form nanofibers [94]. Insufficient entanglement of polymers due to instability of liquid jet and altered surface tension caused incomplete evaporation of solvent solution before reaching collector and led to the formation of beads instead of fibers [95,96,97] (Fig. 2b).
Electrospinning can be divided blend electrospinning, coaxial electrospinning, emulsion electrospinning, and side-by-side electrospinning [98, 99]. Blending electrospinning is the conventional and the most common drug incorporation method, especially for miscible polymer–drug solutions [100]. It involves dispersing or dissolving drugs in the polymer solution to form a homogenous solution [101]. Their drug release kinetics depends on the morphology and distribution of drug in the resulted fibers in addition to drug–polymer interaction which influence the release behavior as drugs blended electrospun nanofibers have frequently show burst release pattern [102, 103]. X. Chen et al. incorporated tannic acid TA by electrospinning into PCL nanofibers, and release study showed initial burst release of tannic acid that increased with increasing drug distribution in the polymer [104]. However, beside the burst release limitation, the direct contact of drug to solvent may lead to denature and decrease activity of sensitive bioactive agents (e.g., proteins and cytokines) that make a necessity for more recent electrospinning methods [105].
In contrast to conventional electrospinning, where polymer and drug are blended into one solution in which, coaxial electrospinning uses a core solution and a shell solution [106]. Coaxial electrospinning is an electrospinning technique in which the core solution and shell solution are placed into the core syringe and shell syringe, respectively [107] (Fig. 3a). The core–shell structure fabricated by coaxial electrospinning provides improved mechanical strength, declined the initial burst release, and protected drug from degradation by avoiding direct exposure to solvents by encapsulating the drug in the nanofiber [108,109,110]. Coaxial technique have many advantages such as the high encapsulation efficacy, the high variety in the selection of drugs and materials, and the simple procedure and cheapness [111]. Emulsion electrospinning is another method to encapsulate the drug inside a core of a core–shell fiber by electrospinning of oil-in-water (O/W) or water-in-oil (W/O) emulsions using the ordinary single needle instead of the two needles of coaxial electrospinning [112, 113] (Fig. 3b). In side-by-side electrospinning, two polymer solutions exposed to the electrical field in the presence of side-by-side needles for the different solutions, which makes it very easy for them to parting from each other allowing incorporation of solutions with different chemical properties (e.g., hydrophilic and hydrophobic) [114,115,116] (Fig. 3c).
Many techniques used to characterize and evaluate morphological, mechanical, chemical, and structural properties of the produced electrospun nanofiber [117]. Morphological properties include diameter, size distribution, and pore size distribution [118]. Diameter and size distribution of nanofibers can be measured by transmission electron microscopy (TEM) [119], scanning electron microscopy (SEM) [120], and atomic force microscopy (AFM) [121]. Pore size distribution and porosity can be measured by mercury intrusion porosimeter [122], liquid extrusion porosimeter [123], nuclear magnetic resonance [124], or capillary flow porometer [125]. Chemical properties of nanofibers can be characterized by Fourier transform infrared spectroscopy (FT-IR) [126], Raman spectroscopy [127], thermogravimetric analysis (TGA) [128], differential scanning calorimetry (DSC) [129], and differential thermal analysis (DTA). Structural evaluation can be characterized by X-day diffraction (XRD) technique [130]. Mechanical properties of the nanofibers can be characterized by tensile strength test according to the ISO 5270:1999 standard test methods using uniaxial tensile testing device [131, 132].
Electrospinning Parameters
Many parameters can affect electrospinning process which can be classified into process parameters (applied voltage, flow rate, diameter of needle to collector distance), solution parameters (concentration, molecular weight, viscosity, and conductivity), and environmental parameters (humidity and temperature) [133].
Process Parameters
The increase of applied voltage more than critical value will result in increase in diameter and formation of beaded fibers. This effect is attributed to that with the same flow rate, the size of Taylor cone decrease, and the velocity and length of the jet increase [134]. The application of high voltage to the polymer solution breaks the balance of its surface tension and creates a charge on the surface of the liquid. Reciprocated charge repulsion and the contraction of the surface charges to the counter electrode cause a force opposite to the surface tension. As the intensity of the electric field is increased, the hemispherical drop formed at tip of the needle tip gets converted into conical shape [135]. At voltage value lower than the critical value, the electrical force will not be enough to form the homogenous fibers [136].
L. Miranda Calderon et al. fabricated rifampicin-loaded methacrylic nanofiber and noticed that when decrease voltage from 20.3 to 14.2 KV lowered nanofiber diameter [137]. C. Kumar et al. developed of composite electrospun nanofibers based on PCL and collagen hydrolysate loaded with ferulic acid and increased applied voltage up to 18 kV that led to increased fiber diameter and further increase to 25 kV resulted in electrical bubbles due to the stagnant potential exceeding the confined air resistance [138]. M. Ignatova, N. Manolova, and I. Rashkov also noticed increased fiber diameter by increasing voltage [139]. N. Chinatangkula et al. designed shellac nanofiber loaded with monolaurin, and they noticed that moving capacity of polymer solution towards the tip was poor when the applied voltages was below 9 kV [140].
A minimum flow rate is required to produce fibers with lower defects and smaller diameter. At high flow rate, a larger drop is produced that results in a faster movement of solution to the collector lead to gradual increase in the volume of the Taylor cones, and the length of the straight fluid jets shortened as a result incomplete evaporation of the solvent occur and solvent-wet fibers produced that increase the probability of beads formation and increase fiber diameter [141, 142].
X. Wu et al. loaded magnesium l-ascorbic acid 2-phosphate and α-tocopherol acetate (MAAP/α-TAC) on PAN nanofibers to the form of core–shell structure and by increasing the flow rate of MAAP/α-TAC core solution and bead formation increased [143]. M. Almukainzi produced PEG/PVP nanofibers and noticed that nanofibers with increased diameter produced by increasing flow rate under the same conditions [144]. F. Davani et al. fabricated core–shell nanofiber by PEO, chitosan, and vancomycin in shell and PVP, gelatin, and imipenem/cilastatin in core compartments. They noticed that by increasing flow rate of both core and shell solutions, nanofibers with increased diameter produced [145]. M. Hajikhani, Z. Emam-Djomeh, and G. Askari encapsulated PLA\PEO\cefazolin inside within PVP shell, and when flow rate of core solution is less than 0.1 mL\h, extra thin fibers were formed that reduce cefazoline loading. On the other hand, when increase to more than 0.2 mL/h, it will cause jet break. In the case of shell solution, a flow rate of less than 0.6 mL/h resulted in the formation of numerous beads in the nanofibers due to insufficient solution to fully cover the surface of the core fibers. On the other hand, an excessive flow rate of about 1 mL/h resulted in the formation of large droplets on the tip of the needle that can fell on the collector and destroy the fabricated fibers [146].
Needle to collector distance determines the morphology of electrospun nanofibers. An optimized distance should be applied to allow all of the solvent to evaporate and to prevent bead formation [141]. The increase in the distance between needle and collector causes reduction in the electrostatic field strength and led to complete evaporation of the solvent occurs which make polymer solution fully stretched hence results in increased fiber diameter [147,148,149].
X. Zhang et al. fabricated silk fibroin nanofiber and showed that at 6 cm, continuous fibers with small number of beads were formed, while in distance above 9 cm, a smooth, bead-free, and fine fiber was formed. However, distance above 15 cm showed to be unsuitable for electrospinning [150].
Solution Parameters
Optimized polymer concentration range should be used. Below this range, only droplets are formed rather than fibers. This may occur because the polymer solution is not reaching the collector due to entangled polymer chains are broken under surface tension and electric field forces. On the other hand, when concentration is above this range, uncontrolled fibers morphologies may appear [151].
T. Baykara and G. Taylan developed core–shell nanofiber with PVA as shell solution and Nigella sativa seed oil as the core solution. Increasing concentration of PVA solution resulted in continuous and thicker fibers with low number of beads [152]. N. Chinatangkul et al. used shellac solution to develop nanofiber, and they noticed that by increasing shellac concentration, bead formation decreased [153]. B. Poornima and P. Korrapati incorporated ferulic acid and resveratrol into PCL\chitosan core–shell nanofiber. The found that chitosan optimum concentration was 2%, and when concentration was higher than 2% large, beaded fibers formed, while concentration less than 2% resulted in spray formation instead of fibers [154]. Z. Li et al. prepared core–shell fibers with small unilamellar vesicles (SUVs)\sodium hyaluronate in the core and PVP in the shell. Optimum sodium hyaluronate concentration was 2%, and when concentration was 1%, droplets were formed instead of fibers. On the other hand, increasing concentration to 3% caused blockage of needle during electrospinning process [155].
The molecular weight, controlled by the length of the polymer chain, therefore smooth and uniform fibers can be obtained if the molecular weight is appropriate. With extremely low molecular weight, beads are highly probable to be formed. Conversely, very high molecular weight increases fiber diameter and affects their morphology [156]. Viscosity is dependent on solution’s concentration and molecular weight. In extremely low viscosity, only droplets are formed, and fiber formation is interrupted. High viscosity prevents polymer solution flow through the needle [157, 158].
F. Zulkifli et al. fabricated hydroxyethyl cellulose (HEC)/PVA, and when the amount of HEC in the HEC/PVA increased from 30 to 50%, the viscosity of the solution was also increased [159]. W. Sarhan and H. Azzazy fabricated honey\chitosan\PVA nanofiber and found that increasing chitosan concentration from 1.5 to 3.5% resulted in highly viscous solution that is unable to spin [160]. L. Moradkhannejhada et al. prepared PLA nanofiber loaded with curcumin, and then PEG of different weights have been added. PEG addition led to a decrease in average weight of nanofibers because of the decreased viscosity of PLA solution; therefore, the jet solution with low viscosity can increase instability and consequently lead to fibers with small diameter. On the other hand, average size of nanofibers have been increased by increasing the PEG weight [161]. Z. Hadisi, J. Nourmohammadi, and S. Nassiri developed Lawsonia inermis–gelatin–starch nanofiber in which with an increase in gelatin content, the viscosity of the solution increased. They noticed that at low viscosities, the molecular entanglements between polymeric chains are not enough to form a uniform fiber, and thus, beads were formed [162]. M. Wang et al. PVP/PVDF core–shell shows the viscosity of neat PVDF and PVP is low, and once mixing the two solutions, the viscosity increased significantly. This change in viscosity resulted in good chain entanglement between the two substances, which lead to advanced electro-spinnability [163].
Polymer solution conductivity is dependent on intrinsic polymer properties, solvent, and ionizable salts. An increase in electric conductivity tends to decrease fiber diameter. Above the critical limit, polymer solutions become very unstable in the presence of strong electric fields, resulting in a broad diameter fibers and may prevent the formation of Taylor cone [164].
A. Abdel Gawad et al. fabricated PVA nanofibers from chitosan and iodoacetamide and complexes. Increasing the chitosan content increased conductivity due to progression of NH3+ groups from −NH2 in acidic medium which increases the charge density on the surface of the ejected jet formed during electrospinning, and therefore, it decreases diameter of the formed fibers [165]. Similar results are obtained by M. Ganesh [166].
Environmental Parameters
The humidity (RH%) influences the diffusive equilibrium between solvent and water vapor, affecting the fiber morphology. A decrease in the RH% humidity leads to fibers with decreased diameter. However, an excessive decrease in humidity tends to accelerate the evaporation rate of solvent and induce inadequate extension of the jet resulting in thicker nanofiber [167]. In contrast, humidity higher than certain limit inhibits solvent evaporation so that water vapor may penetrate into the jet leading to thinner fibers [168]. A highly humid environment may affect electrospinning and contribute to the formation of pores on the nanofibers surface. Additionally, high humidity in the environmental atmosphere can prevent blockage at the needle caused by quick evaporation of volatile solvent during electrospinning [169]. R. Augustine et al. loaded connective tissue growth factor (CTGF), and they found that humidity can lead to formation of secondary pores on fibers due to condensation of droplets from air and subsequent difference in the rate of evaporation of the solvent from the surface [170].
The temperature affects the rate of evaporation and the viscosity of polymer solution. The elevated temperature increases viscosity leading to increasing evaporation time and limiting further jet stretching. For low temperature, they decrease the viscosity and facilitate formation of thinner fibers [171].
Applications of Electrospun Nanofiber as Wound Healing Dressings
Morphology and structure of nanofibers play an important role in cell behavior by improvement of cell attachment and proliferation [172, 173]. Nanofibers are characterized by their similar structure to ECM, increasing cell viability, allowing gas exchange, and absorbing excess exudates from the wound as they have large surface area and high porosity [174]. They also have inert cell property which allow painless removal of wound dressing and protect newly formed skin layer combined with minimal scars [175].
Core–shell Nanofiber
Coaxial electrospinning and emulsion electrospinning are two new techniques for the fabrication of core–shell nanofibers, where the outer shell layer can encapsulate and prevent the release of the active components in the inner core layer [176].
Y. Li et al. suggested PLA\chitosan core–shell nanofiber as a potential scaffold for wound healing [177]. S. Afshar et al. prepared PLA\chitosan core–shell nanofiber by coaxial electrospinning loaded with curcumin. PLA-chitosan core–shell nanofiber showed better mechanical properties than that of neat chitosan nanofiber. Chitosan shell layer showed a burst release, and around 80% of drug released in less than 10 h. In contrast, curcumin inserted in the PLA exhibited a two-stage release behavior: an initial burst release of about 25% in the first 4 h followed by a sustained release in the second stage [178]. A. Joshi et al. used coaxial electrospinning to prepare PCL\gelatin core–shell nanofiber loaded with heparin. Compared to single-phase gelatin nanofiber, PCL\gelatin nanofiber showed improved mechanical and swelling properties. After that heparin\PCL\gel treated with bFGF that showed more accelerated healing than non-treated group [179]. C. Gao developed PCL/gelatin-ciprofloxacin/Fe3O4 multi-functional dressing that allowed controlled release of drug and improved re-epithelialization, granulation tissue formation, and collagen deposition at the wound site [180].
A. Basar et al. prepared ketoprofen-containing PCL and PCL/gelatin binary electrospun fibers by solution and emulsion electrospinning, respectively. PCL nanofiber exhibited a burst release profile that released approximately 90% of the drug after only 12 min. In contrast, PCL\gelatin binary structure extended release for about 4 days. Furthermore, electrospun PCL/gelatin binary nanofiber improved attachment and wettability more than PCL nanofiber [181]. M. Hussein et al. prepared core–shell and loaded phenytoin into PCL shell layer and silver-chitosan nanoparticles into PVA core layer that showed two-stage release behavior in addition to improved biocompatibility and mechanical properties [182]. X. Bai et al. prepared zeolite imidi framework (ZIF-8)-coated PCL/ε-PL core–shell nanofibers and found that ZIF-8 and ε-PL exhibited dual antibacterial properties and enhanced wound healing process [183].
C. Wang et al. fabricated core–shell nanofibers based on core layer involving gelatin, quaternary ammonium salt-grafted sulfonated chitosan and EGF/bFGF, shell layer of PCL, and polydopamine. This nanofiber improved mechanical properties and antimicrobial effect. Wounds treated with core–shell nanofiber exhibited the smallest wound area with superior angiogenesis effect and increased collagen deposition accompanied with decreased inflammatory mediators [184]. C. Cui et al. used coaxial electrospinning to encapsulate ciprofloxacin into PCL\chitosan core–shell nanofiber that showed ideal porosity and good mechanical properties. Nanofiber scaffolds showed three-stage release with an initial burst release of ciprofloxacin during the first 12 h, followed by a gradual release over more than 8 days. After 15 days, the release of ciprofloxacin reached a plateau at 56%. They also showed enhanced healing process with improved well-organized granulation tissue, better epithelialization, less lymphocyte, and neutrophil infiltration which were observed in the wounds [185]. N. Zandi et al. prepared core–shell nanofiber with gelatin with phenytoin as a shell layer and PVA\gelatin with lysozyme as a core layer, and release profiles can be considered in three stages including an initial burst release within 8 h followed by gradual release lasting to 33 h and then reached plateau [186].
M. Najafiasl et al. fabricated nanofibers using PVA/sodium alginate (SA) as core layer and chitosan as shell layer loaded with D-panthenol which exhibited enhanced mechanical properties and accelerated wound healing process [187]. A. Khan et al. incorporated ZnO nanoparticles and oregano essential oil into PLCL core–shell nanofiber, and results revealed good antibacterial activity and accelerated wound healing process. The untreated wound exhibited an elevated level of IL-6 which indicated inflammation in the wound area. Compared to untreated group, wounds treated by core–shell nanofiber showed complete epithelialization, and angiogenesis with highly organized collagen fibers in addition to that inflammatory activity decreased significantly, and scar was replaced by newly formed epithelium [188].
J. Wang et al. loaded nanohydroxyapatite (n-HAP) with tetracycline and subsequently encapsulated in chitosan\gelatin nanofiber. Compared to tetracycline\n-HAP and tetracycline\chitosan\gelatin which showed burst release of >90% of drug within 4 days, they showed sustained release where only 45% released within 4 days [189]. Z. Xie loaded chitosan and PEO nanofiber with VEGF- and PDGF-encapsulated PLGA nanoparticles embedded inside them. This scaffold has a biphasic release pattern: an initial burst release of VEGF followed by sustained release of PDGF. They facilitated easy detachment and promoted fast cell growth and proliferation in addition to complete wound closure within 2 weeks with less inflammatory cell presence and higher fibroblast cells. Furthermore, collagen deposition is shown to be more mature with more hair follicle formation [190].
Z. Dong et al. biological prepared ethyl cellulose-modified zein with tea carbon dots (TCDs) and calcium peroxide (CaO2) that shown to significantly accelerate the wound closure rate and the production of sebaceous glands and hair follicles. They also promoted the transformation of macrophages from M1 to M2 in diabetic rat wound models, shortened the duration of the inflammatory stage, and facilitated further wound healing [191]. C. Lee et al. prepared core–shell nanofiber loaded with insulin as core and PLGA\vildagliptin as shell. The core–shell nanofiber demonstrated improved wettability, porosity, surface area, and mechanical properties. It exhibited initial burst release in the first day followed by continuous release until day 30. The nanofiber promoted diabetic wound healing and reduced fibrotic effects [192].
S. Homaeigohar et al. developed PAN core–shell nanofiber together with bovine serum albumin (BSA) and calcium-deficient hydroxyapatite (HA) and showed to be nontoxic and resemble ECM of the skin with good mechanical properties [193]. M. Aljohani coated chitosan silver nanoparticles within poly-lactate calcium salt (PLCS) that revealed good water permeability and displayed high antimicrobial efficiency against gram positive and gram negative bacterial pathogens [194]. A. Aldalbahi et al. fabricated PVDF\cellulose acetate nanofiber that contains gold nanoparticles and displays enhanced cell spread and proliferation [195]. Z. Li et al. prepared core–shell nanofiber by cellulose acetate as shell and naproxen-loaded liposomes\sodium hyaluronate as core which showed biphasic release pattern with initial burst release of 47.1% naproxen in the first 8 h followed by sustained release of residual drug for about 12 days [196]. H. Zhang et al. synthesized 5-fluorouracil (5-Fu)-loaded dendritic mesoporous bioglass nanoparticles (dMBG) in PEO\poly (ether-ester-urethane) urea core–shell nanofiber that found to have good wettability and mechanical properties. The nanofiber effectively inhibits hypertrophic scars, accelerates wound healing process, and promotes angiogenesis and collagen deposition [197].
G. Jin et al. encapsulated multiple epidermal induction factors (EIF) such as the epidermal growth factor (EGF), insulin, hydrocortisone, and retinoic acid with gelatin and PLCL nanofiber that showed to promote cell proliferation. Compared to EIF blended nanofibers that showed burst release over a period of 15 days, there was no burst release which was detected from EIF core–shell nanofibers [198]. A. Li et al. loaded epigallocatechin-3-O-gallate (EGCG) in PLCL\gelatin core–shell nanofiber and results appropriate biocompatibility, antibacterial, and antioxidant ability, which could support cell viability and proliferation. Compared to gauze, wounds treated with core\shell nanofibers showed accelerated wound closure, angiogenesis, and re-epithelialization [199]. M. Movahedi et al. prepared PU\starch core–shell nanofibers and showed to have improved mechanical properties compared to starch nanofibers and higher cell proliferation compared to PU nanofibers. Wounds treated with the scaffold showed accelerated wound closure and presence of hair follicles and sebaceous glands [200].
Janus Nanofiber
Janus nanofiber involves two separate compartments which allow incorporation of solutions with different chemical properties [201]. F. Ao et al. designed Janus nanofiber contains hydrophilic and hydrophobic properties. They used ethyl cellulose as hydrophobic layer and ethyl cellulose\gelatin as hydrophilic layer [202].
K. Zhang et al. loaded curcumin in quaternized chitosan/PVA Janus nanofibrous aerogel, and the result showed uniform, homogenous, and biocompatible fibers with enhanced mechanical properties and liquid absorption capacity, while it retains the inherent soft texture and ECM architecture from nanofibers. It also showed noticeable antioxidant and anti-inflammatory effect. They were able to decrease TNF-α expression and increase IL-10 and VEGF expression [203]. Y. Shi et al. fabricated PVA\PLGA Janus nanofiber with copper sulfide nanoparticles (CuS), mupirocin (M), and valsartan (V) to form PLGAV-CuS/PVAM that showed good cytocompatibility and antibacterial activity. In the early stage of wound healing, the hydrophilic layer of the Janus fibrous membrane enables continuous and slow release of hydrophilic antimicrobial drugs (M), thereby avoiding infection [204]. X. Ji et al. incorporated Rana chensinensis skin peptides (RCSPs) and silver nanoparticles (Ag-NPs) into PCL\PVP Janus nanofibers that showed good wettability, mechanical properties like ECM, antibacterial activity, and effectively enhanced wound healing [205]. L. Li et al. prepared Janus nanofiber with PVA/hydroxylpropyl trimethyl ammonium chloride chitosan (HACC) on the hydrophilic side and thermoplastic polyurethane (TPU) on the hydrophobic side that showed to have good unidirectional wettability properties and high elasticity [206].
Multi-layer Electrospun Nanofibers
Multi-layer nanofibers are prepared by electrospinning of a second polymer solution on the same collector directly after the first electrospun nanofiber has been collected [207]. Each layer of produced multi-layer structures has its own biological, physical, and chemical properties to improve nanofiber characteristics [208]. A dense top layer can protect the wound site from mechanical stresses, dehydration, and microbial infections, while the sublayer is designed to resemble ECM in order to accelerate wound healing and improve cell proliferation [209,210,211]. Multi-layer nanofibers provide sustained release profile and prevent burst release when compared to single-layer structures [212]. Furthermore, multi-layer structures have the ability to load and release various drugs with different release profiles in each layer due to differences in morphological characteristics and degradability of different layers [213]. Layer-by-layer self-assembly nanofibers are formed by applying oppositely charged molecules to nanofibers followed by electrostatic interaction that results in molecules adsorption to the surface of nanofiber and formation of a multi-layer structure [214,215,216]. Table I shows the recent potential multi-layer nanofiber for wound healing.
Conclusion
Ideal wound dressing should have an elastic mechanical structure but strong enough for easy handling and comfortable wear. Nanofibers is characterized by its good mechanical strength and inert cell property allowing painless removal with minimal scars. They also resemble the ECM of the skin and provide a large surface area so they can absorb excess exudates from the wound. Many technologies have been developed for nanofibers preparation. Many parameters can affect electrospinning process that classified to process parameters (applied voltage, flow rate, diameter of needle to collector distance), solution parameters (concentration, molecular weight, viscosity, and conductivity), and environmental parameters (humidity and temperature). Electrospinning is a simple, cost-effective, and versatile setup process which depends on electrostatic concept in the presence of high electrical field. Conventional method involves dispersing or dissolving drugs in the polymer solution to form a homogenous solution. However, this method frequently shows burst release patterns and may lead to denature and decrease activity of sensitive bioactive agents. That gives the need for more advanced electrospinning techniques like coaxial electrospinning, emulsion electrospinning, and side-by-side electrospinning. Coaxial electrospinning and emulsion electrospinning are used for the fabrication of core–shell nanofibers, where the outer shell layer can encapsulate and prevent the release of the active components in the inner core layer. Janus nanofibers synthesized by side-by-side electrospinning incorporate two separate solutions with different chemical properties. Multi-layer nanofibers can be prepared either by electrospinning of a second polymer solution directly after the first nanofiber is collected or by self-assembly of oppositely charged molecules that adsorb on the surface of the nanofiber. In this context, we believe that electrospun nanofibers are promising dressings for wound healing applications.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Jiang T, Li Q, Qiu J, Chen J, Du S, Xu X, et al. Nanobiotechnology: applications in chronic wound healing. Int J Nanomedicine. 2022;17:3125–45. https://doi.org/10.2147/IJN.S372211.
Mssillou I, Bakour M, Slighoua M, Laaroussi H, Saghrouchni H, Ez-Zahra Amrati F, et al. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: a review. J Ethnopharmacol. 2022;298: 115663. https://doi.org/10.1016/j.jep.2022.115663.
Naderi N, Karponis D, Mosahebi A, Seifalian AM. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front Biosci (Landmark Ed. 2018;23:1038–59. https://doi.org/10.2741/4632.
Sideek SA, El-nassan HB, Fares AR, Elmeshad AN. Different curcumin-loaded delivery systems for wound healing applications : a comprehensive review. 2023;1–21.
Kharat Z, Amiri Goushki M, Sarvian N, Asad S, Dehghan MM, Kabiri M. Chitosan/PEO nanofibers containing Calendula officinalis extract: preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int J Pharm. 2021;609: 121132. https://doi.org/10.1016/j.ijpharm.2021.121132.
Vargas EAT, do Vale Baracho NC, de Brito J, de Queiroz AAA. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomater. 2010;6:1069–78. https://doi.org/10.1016/j.actbio.2009.09.018.
Lin CM, Chang YC, Cheng LC, Liu CH, Chang SC, Hsien TY, et al. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose. 2020;27:2651–67. https://doi.org/10.1007/s10570-019-02940-w.
Mouro C, Dunne CP, Gouveia IC. Designing new antibacterial wound dressings: development of a dual layer cotton material coated with poly(vinyl alcohol)_chitosan nanofibers incorporating Agrimonia eupatoria L. extract. Molecules. 2021;26. https://doi.org/10.3390/MOLECULES26010083.
Tang Y, Lan X, Liang C, Zhong Z, Xie R, Zhou Y, et al. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr Polym. 2019;219:113–20. https://doi.org/10.1016/j.carbpol.2019.05.004.
Zhang L, Du W, Li X, Ling G, Zhang P. Dissolving microneedles based on polysaccharide for dermatological diseases therapy. J Drug Deliv Sci Technol. 2022;78: 103913. https://doi.org/10.1016/j.jddst.2022.103913.
Cui C, Sun S, Wu S, Chen S, Ma J, Zhou F. Electrospun chitosan nanofibers for wound healing application. Eng Regen. 2021;2:82–90. https://doi.org/10.1016/j.engreg.2021.08.001.
Mirbagheri MS, Akhavan-Mahdavi S, Hasan A, Kharazmi MS, Jafari SM. Chitosan-based electrospun nanofibers for diabetic foot ulcer management; recent advances. Carbohydr Polym. 2023;120512. https://doi.org/10.1016/j.carbpol.2022.120512.
Haik J, Kornhaber R, Blal B, Harats M. The feasibility of a handheld electrospinning device for the application of nanofibrous wound dressings. Adv Wound Care. 2017;6:166–74. https://doi.org/10.1089/wound.2016.0722.
Pilehvar-Soltanahmadi Y, Nouri M, Martino MM, Fattahi A, Alizadeh E, Darabi M, et al. Cytoprotection, proliferation and epidermal differentiation of adipose tissue-derived stem cells on emu oil based electrospun nanofibrous mat. Exp Cell Res. 2017;357:192–201. https://doi.org/10.1016/j.yexcr.2017.05.015.
Liu X, Liu Y, Du J, Li X, Yu J, Ding B. Breathable, stretchable and adhesive nanofibrous hydrogels as wound dressing materials. Eng Regen. 2021;2:63–9. https://doi.org/10.1016/j.engreg.2021.05.001.
Lu T, Zou Q, Zhu K, Yuan D, Ma M, Ye C. Electrospun egg white/polyvinyl alcohol fiber dressing to accelerate wound healing. J Polym Res. 2021;28:1–15. https://doi.org/10.1007/s10965-021-02422-3.
Mishra B, Hossain S, Mohanty S, Gupta MK, Verma D. Fast acting hemostatic agent based on self-assembled hybrid nanofibers from chitosan and casein. Int J Biol Macromol. 2021;185:525–34. https://doi.org/10.1016/j.ijbiomac.2021.06.116.
Ahmed SM, Ahmed H, Tian C, Tu Q, Guo Y, Wang J. Whey protein concentrate doped electrospun poly(epsilon-caprolactone) fibers for antibiotic release improvement. Colloids Surfaces B Biointerfaces. 2016;143:371–81. https://doi.org/10.1016/j.colsurfb.2016.03.059.
Markov PA, Vinogradov II, Kostromina E, Eremin PS, Gilmutdinova IR, Kudryashova IS, et al. A wound dressing based on a track-etched membrane modified by a biopolymer nanoframe: physicochemical and biological characteristics. Eur Polym J. 2022;181: 111709. https://doi.org/10.1016/j.eurpolymj.2022.111709.
Zulkifli FH, Jahir Hussain FS, Abdull Rasad MSB, Mohd Yusoff M. In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym Degrad Stab. 2014;110:473–81. https://doi.org/10.1016/j.polymdegradstab.2014.10.017.
Kang M, Chen P, Jin HJ. Preparation of multiwalled carbon nanotubes incorporated silk fibroin nanofibers by electrospinning. Curr Appl Phys. 2009;9:S95-7. https://doi.org/10.1016/j.cap.2008.08.014.
Fereydouni N, Astaneh ME. Fabrication of a zein membrane containing cerium oxide nanoparticles: physical, chemical and biological properties as a potential wound dressing. J Mol Struct. 2023;1291: 136006. https://doi.org/10.1016/j.molstruc.2023.136006.
Kim JW, Kim MJ, Ki CS, Kim HJ, Park YH. Fabrication of bi-layer scaffold of keratin nanofiber and gelatin-methacrylate hydrogel: implications for skin graft. Int J Biol Macromol. 2017;105:541–8. https://doi.org/10.1016/j.ijbiomac.2017.07.067.
Kikionis S, Koromvoki M, Tagka A, Polichronaki E, Stratigos A, Panagiotopoulos A, et al. Ulvan-based nanofibrous patches enhance wound healing of skin trauma resulting from cryosurgical treatment of keloids. Mar Drugs. 2022;20:1–15. https://doi.org/10.3390/md20090551.
Varshney N, Sahi AK, Poddar S, Mahto SK. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: fabrication and characterization. Int J Biol Macromol. 2020;160:112–27. https://doi.org/10.1016/j.ijbiomac.2020.05.090.
Wang Q, Li M, Zheng Z, Niu Y, Xue X, Ao C, et al. Polyethylenimine-functionalized nanofiber nonwovens electrospun from cotton cellulose for wound dressing with high drug loading and sustained release properties. Polymers (Basel). 2022;14. https://doi.org/10.3390/polym14091748.
López-Calderón HD, Avilés-Arnaut H, Galán-Wong LJ, Almaguer-Cantú V, Laguna-Camacho JR, Calderón-Ramón C, et al. Electrospun polyvinylpyrrolidone-gelatin and cellulose acetate bi-layer scaffold loaded with gentamicin as possible wound dressing. Polymers (Basel). 2020;12:2311. https://doi.org/10.3390/polym12102311.
Wadke P, Chhabra R, Jain R, Dandekar P. Silver-embedded starch-based nanofibrous mats for soft tissue engineering. Surfaces Interfaces. 2017;8:137–46. https://doi.org/10.1016/j.surfin.2017.05.008.
Chen S, Cui S, Hu J, Zhou Y, Liu Y. Pectinate nanofiber mat with high absorbency and antibacterial activity: a potential superior wound dressing to alginate and chitosan nanofiber mats. Carbohydr Polym. 2017;174:591–600. https://doi.org/10.1016/j.carbpol.2017.06.096.
Mouro C, Gomes AP, Ahonen M, Fangueiro R, Gouveia IC. Chelidonium majus l. Incorporated emulsion electrospun pcl/pva_pec nanofibrous meshes for antibacterial wound dressing applications. Nanomaterials. 2021;11. https://doi.org/10.3390/nano11071785.
Saatchi A, Arani AR, Moghanian A, Mozafari M. Cerium-doped bioactive glass-loaded chitosan/polyethylene oxide nanofiber with elevated antibacterial properties as a potential wound dressing. Ceram Int. 2021;47:9447–61. https://doi.org/10.1016/j.ceramint.2020.12.078.
Ali A, Shahid MA, Hossain MD, Islam MN. Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int J Biol Macromol. 2019;138:13–20. https://doi.org/10.1016/j.ijbiomac.2019.07.015.
Sannasimuthu A, Ramani M, Paray BA, Pasupuleti M, Al-Sadoon MK, Alagumuthu TS, et al. Arthrospira platensis transglutaminase derived antioxidant peptide-packed electrospun chitosan/ poly (vinyl alcohol) nanofibrous mat accelerates wound healing, in vitro, via inducing mouse embryonic fibroblast proliferation. Colloids Surfaces B Biointerfaces. 2020;193: 111124. https://doi.org/10.1016/j.colsurfb.2020.111124.
Gruppuso M, Iorio F, Turco G, Marsich E, Porrelli D. Hyaluronic acid/lactose-modified chitosan electrospun wound dressings – crosslinking and stability criticalities. Carbohydr Polym. 2022;288: 119375. https://doi.org/10.1016/j.carbpol.2022.119375.
Xue F, Zhang H, Hu J, Liu Y. Hyaluronic acid nanofibers crosslinked with a nontoxic reagent. Carbohydr Polym. 2021;259: 117757. https://doi.org/10.1016/j.carbpol.2021.117757.
Huerta-Angeles G, Brandejsová M, Knotková K, Hermannová M, Moravcová M, Šmejkalová D, et al. Synthesis of photo-crosslinkable hyaluronan with tailored degree of substitution suitable for production of water resistant nanofibers. Carbohydr Polym. 2016;137:255–63. https://doi.org/10.1016/j.carbpol.2015.10.077.
Ajalloueian F, Asgari S, Guerra PR, Chamorro CI, Ilchenco O, Piqueras S, et al. Amoxicillin-loaded multilayer pullulan-based nanofibers maintain long-term antibacterial properties with tunable release profile for topical skin delivery applications. Int J Biol Macromol. 2022;215:413–23. https://doi.org/10.1016/j.ijbiomac.2022.06.054.
Amjadi S, Gholizadeh S, Ebrahimi A, Almasi H, Hamishehkar H, Taheri RA. Development and characterization of the carvone-loaded zein/pullulan hybrid electrospun nanofibers for food and medical applications. Ind Crops Prod. 2022;183: 114964. https://doi.org/10.1016/j.indcrop.2022.114964.
Innocenti Malini R, Lesage J, Toncelli C, Fortunato G, Rossi RM, Spano F. Crosslinking dextran electrospun nanofibers via borate chemistry: proof of concept for wound patches. Eur Polym J. 2019;110:276–82. https://doi.org/10.1016/j.eurpolymj.2018.11.017.
Najafiasl M, Osfouri S, Azin R, Zaeri S. Alginate-based electrospun core/shell nanofibers containing dexpanthenol: a good candidate for wound dressing. J Drug Deliv Sci Technol. 2020;57. https://doi.org/10.1016/j.jddst.2020.101708.
Chuysinuan P, Thanyacharoen T, Techasakul S, Ummartyotin S. Electrospun characteristics of gallic acid-loaded poly vinyl alcohol fibers: release characteristics and antioxidant properties. J Sci Adv Mater Devices. 2018;3:175–80. https://doi.org/10.1016/j.jsamd.2018.04.005.
Teixeira MA, Antunes JC, Seabra CL, Fertuzinhos A, Tohidi SD, Reis S, et al. Antibacterial and hemostatic capacities of cellulose nanocrystalline-reinforced poly(vinyl alcohol) electrospun mats doped with Tiger 17 and pexiganan peptides for prospective wound healing applications. Biomater Adv. 2022;137: 212830. https://doi.org/10.1016/j.bioadv.2022.212830.
Shababdoust A, Zandi M, Ehsani M, Shokrollahi P, Foudazi R. Controlled curcumin release from nanofibers based on amphiphilic-block segmented polyurethanes. Int J Pharm. 2020;575: 118947. https://doi.org/10.1016/j.ijpharm.2019.118947.
Liu C, Shi H, Yang H, Yan S, Luan S, Li Y, et al. Fabrication of antibacterial electrospun nanofibers with vancomycin-carbon nanotube via ultrasonication assistance. Mater Des. 2017;120:128–34. https://doi.org/10.1016/j.matdes.2017.02.008.
Rashtchian M, Hivechi A, Bahrami SH, Milan PB, Simorgh S. Fabricating alginate/poly(caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr Polym. 2020;233: 115873. https://doi.org/10.1016/j.carbpol.2020.115873.
Sun X, Li K, Chen S, Yao B, Zhou Y, Cui S, et al. Rationally designed particle preloading method to improve protein delivery performance of electrospun polyester nanofibers. Int J Pharm. 2016;512:204–12. https://doi.org/10.1016/j.ijpharm.2016.08.053.
Doğan D, Karaduman FR, Horzum N, Metin AÜ. Boron nitride decorated poly(vinyl alcohol)/poly(acrylic acid) composite nanofibers: a promising material for biomedical applications. J Mech Behav Biomed Mater. 2023;141: 105773. https://doi.org/10.1016/j.jmbbm.2023.105773.
Chen P, Chai M, Mai Z, Liao M, Xie X, Lu Z, et al. Electrospinning polyacrylonitrile (PAN) based nanofiberous membranes synergic with plant antibacterial agent and silver nanoparticles (AgNPs) for potential wound dressing. Mater Today Commun. 2022;31: 103336. https://doi.org/10.1016/j.mtcomm.2022.103336.
kalwar K, Xi J, Dandan L, Gao L. Fabrication of PAN/FeNPs electrospun nanofibers: nanozyme and an efficient antimicrobial agent. Mater Today Commun. 2021;26:102168. https://doi.org/10.1016/j.mtcomm.2021.102168.
Yang J, Liu CL, Ding YN, Sun TC, Bai XH, Cao ZK, et al. Synergistic antibacterial polyacrylonitrile/gelatin nanofibers coated with metal-organic frameworks for accelerating wound repair. Int J Biol Macromol. 2021;189:698–704. https://doi.org/10.1016/j.ijbiomac.2021.08.175.
Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. Composite poly-l-lactic acid/poly-(α, β)-dl-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater Sci Eng C. 2012;32:1443–51. https://doi.org/10.1016/j.msec.2012.04.024.
Sadeghi-Avalshahr AR, Nokhasteh S, Molavi AM, Mohammad-Pour N, Sadeghi M. Tailored PCL scaffolds as skin substitutes using sacrificial PVP fibers and collagen/chitosan blends. Int J Mol Sci. 2020;21:2311. https://doi.org/10.3390/ijms21072311.
El-Naggar ME, Abdelgawad AM, Salas C, Rojas OJ. Curdlan in fibers as carriers of tetracycline hydrochloride: controlled release and antibacterial activity. Carbohydr Polym. 2016;154:194–203. https://doi.org/10.1016/j.carbpol.2016.08.042.
Bui HT, Chung OH, Dela Cruz J, Park JS. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res. 2014;22:1288–96. https://doi.org/10.1007/s13233-014-2179-6.
Gao S, Chen T, Wang Z, Ji P, Xu L, Cui W, et al. Immuno-activated mesenchymal stem cell living electrospun nanofibers for promoting diabetic wound repair. J Nanobiotechnology. 2022;20:1–20. https://doi.org/10.1186/s12951-022-01503-9.
Fang Y, Zhu X, Wang N, Zhang X, Yang D, Nie J, et al. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur Polym J. 2019;116:30–7. https://doi.org/10.1016/j.eurpolymj.2019.03.050.
Zhao J, Han F, Zhang W, Yang Y, You D, Li L. Toward improved wound dressings: effects of polydopamine-decorated poly(lactic-co-glycolic acid) electrospinning incorporating basic fibroblast growth factor and ponericin G1. RSC Adv. 2019;9:33038–51. https://doi.org/10.1039/c9ra05030b.
Khataei S, H.Al-Musawi M, Asadi K, Ramezani S, Abbasian M, Ghorbani M. Effect of molecular weight and content of polyvinylpyrrolidone on cell proliferation, loading capacity and properties of electrospun green tea essential oil-incorporated polyamide-6/polyvinylpyrrolidone nanofibers. J Drug Deliv Sci Technol. 2023;82:104310. https://doi.org/10.1016/j.jddst.2023.104310.
Vimala Devi M, Liji Sobhana SS, Shiny PJ, Ramanathan G, Grace Felciya SJ, Poornima V, et al. Durable nanofibrous matrices augmented with hydrotalcite-like compounds for cutaneous regeneration of burn wounds. Appl Clay Sci. 2020;187: 105476. https://doi.org/10.1016/j.clay.2020.105476.
Venault A, Lin KH, Tang SH, Dizon GV, Hsu CH, Maggay IVB, et al. Zwitterionic electrospun PVDF fibrous membranes with a well-controlled hydration for diabetic wound recovery. J Memb Sci. 2020;598: 117648. https://doi.org/10.1016/j.memsci.2019.117648.
Wang G, Ju S, Li X, Cai Y, Li Y, Li W, et al. Preclinical animal study of electrospun poly (L-lactide-co-caprolactone) and formulated porcine fibrinogen for full-thickness diabetic wound regeneration. Biomed Pharmacother. 2023;162: 114734. https://doi.org/10.1016/j.biopha.2023.114734.
Yang Q, Xie Z, Hu J, Liu Y. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater Sci Eng C. 2021;128: 112319. https://doi.org/10.1016/j.msec.2021.112319.
Wang M, Roy AK, Webster TJ. Development of chitosan/poly(vinyl alcohol) electrospun nanofibers for infection related wound healing. Front Physiol. 2017;7:2016–8. https://doi.org/10.3389/fphys.2016.00683.
Ezhilarasu H, Ramalingam R, Dhand C, Lakshminarayanan R, Sadiq A, Gandhimathi C, et al. Biocompatible aloe vera and tetracycline hydrochloride loaded hybrid nanofibrous scaffolds for skin tissue engineering. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20205174.
Mwiiri FK, Brandner JM, Daniels R. Electrospun bioactive wound dressing containing colloidal dispersions of birch bark dry extract. Pharmaceutics. 2020;12:991. https://doi.org/10.3390/pharmaceutics12100991.
Mwiiri FK, Daniels R. Influence of PVA molecular weight and concentration on electrospinnability of birch bark extract-loaded nanofibrous scaffolds intended for enhanced wound healing. Molecules. 2020;25:4799. https://doi.org/10.3390/molecules25204799.
Zaeri S, Karami F, Assadi M. Propranolol-loaded electrospun nanofibrous wound dressing: from fabrication and characterization to preliminary wound healing evaluation. Iran J Basic Med Sci. 2021;24:1279–91. https://doi.org/10.22038/ijbms.2021.57770.12857.
Hussein MAM, Gunduz O, Sahin A, Grinholc M, El-Sherbiny IM, Megahed M. Dual spinneret electrospun polyurethane/PVA-gelatin nanofibrous scaffolds containing cinnamon essential oil and Nanoceria for chronic diabetic wound healing: preparation, physicochemical characterization and in-vitro evaluation. Molecules. 2022;27:2146. https://doi.org/10.3390/molecules27072146.
He X, Xue J, Shi L, Kong Y, Zhan Q, Sun Y, et al. Recent antioxidative nanomaterials toward wound dressing and disease treatment via ROS scavenging. Mater Today Nano. 2022;17. https://doi.org/10.1016/j.mtnano.2021.100149.
Wen S, Hu Y, Zhang Y, Huang S, Zuo Y, Min Y. Dual-functional core-shell electrospun mats with precisely controlled release of anti-inflammatory and anti-bacterial agents. Mater Sci Eng C. 2019;100:514–22. https://doi.org/10.1016/j.msec.2019.02.076.
Si Y, Shi S, Hu J. Applications of electrospinning in human health: from detection, protection, regulation to reconstruction. Nano Today. 2023;48: 101723. https://doi.org/10.1016/j.nantod.2022.101723.
Kumar M, Hilles AR, Ge Y, Bhatia A, Mahmood S. A review on polysaccharides mediated electrospun nanofibers for diabetic wound healing: their current status with regulatory perspective. Int J Biol Macromol. 2023;234. https://doi.org/10.1016/j.ijbiomac.2023.123696.
Trinh XT, Long N Van, Van Anh LT, Nga PT, Giang NN, Chien PN, et al. A comprehensive review of natural compounds for wound healing: targeting bioactivity perspective. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23179573.
Singh S, Young A, McNaught C-E. The physiology of wound healing. Surg. 2017;35:473–7. https://doi.org/10.1016/j.mpsur.2017.06.004.
Dushenkov A, Jungsuwadee P. Coagulation and platelet disorders. In: Chisholm-Burns MA, Schwinghammer TL, Malone PM, Kolesar JM, Bookstaver PB, Lee KC, editors. Pharmacother Princ Pract 6th Ed. New York, NY: McGraw-Hill Education; 2022.
Liao HT, Lai YT, Kuo CY, Chen JP. A bioactive multi-functional heparin-grafted aligned poly(lactide-co-glycolide)/curcumin nanofiber membrane to accelerate diabetic wound healing. Mater Sci Eng C. 2021;120: 111689. https://doi.org/10.1016/j.msec.2020.111689.
Gowda BHJ, Mohanto S, Singh A, Bhunia A, Abdelgawad MA, Ghosh S, et al. Nanoparticle-based therapeutic approaches for wound healing: a review of the state-of-the-art. Mater Today Chem. 2023;27: 101319. https://doi.org/10.1016/j.mtchem.2022.101319.
Opneja A, Kapoor S, Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. 2019;179:56–63. https://doi.org/10.1016/j.thromres.2019.05.001.
Pattnaik S, Mohanty S, Sahoo SK, Mohanty C. A mechanistic perspective on the role of phytoconstituents-based pharmacotherapeutics and their topical formulations in chronic wound management. J Drug Deliv Sci Technol. 2023;84: 104546. https://doi.org/10.1016/j.jddst.2023.104546.
Bian D, Wu Y, Song G. Basic fibroblast growth factor combined with extracellular matrix-inspired mimetic systems for effective skin regeneration and wound healing. Mater Today Commun. 2023;35: 105876. https://doi.org/10.1016/j.mtcomm.2023.105876.
Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. https://doi.org/10.1016/j.clindermatol.2006.09.007.
Chandrasekaran R, Krishnan M, Bupesh G, Chacko S, Gawade O, Hasan S, et al. Prospective features of functional 2D nanomaterial graphene oxide in the wound healing process. J Drug Deliv Sci Technol. 2023;82: 104352. https://doi.org/10.1016/j.jddst.2023.104352.
Liang Y, Liang Y, Zhang H, Guo B. Antibacterial biomaterials for skin wound dressing. Asian J Pharm Sci. 2022;17:353–84. https://doi.org/10.1016/j.ajps.2022.01.001.
Dutta SD, Ganguly K, Patil TV, Randhawa A, Lim KT. Unraveling the potential of 3D bioprinted immunomodulatory materials for regulating macrophage polarization: state-of-the-art in bone and associated tissue regeneration. Bioact Mater. 2023;28:284–310. https://doi.org/10.1016/j.bioactmat.2023.05.014.
Azari Z, Gorgani S, Hosseini SA, Wang AZ, Kim H, Kargozar S. The role of immune cells in therapeutic angiogenesis: concepts in tissue engineering. Curr Opin Biomed Eng. 2023;28: 100470. https://doi.org/10.1016/j.cobme.2023.100470.
Juncos Bombin AD, Dunne NJ, McCarthy HO. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C. 2020;114: 110994. https://doi.org/10.1016/j.msec.2020.110994.
Wolf SJ, Melvin WJ, Gallagher K. Macrophage-mediated inflammation in diabetic wound repair. Semin Cell Dev Biol. 2021;119:111–8. https://doi.org/10.1016/j.semcdb.2021.06.013.
Scull G, Brown AC. Development of novel microenvironments for promoting enhanced wound healing. Curr Tissue Microenviron Reports. 2020;1:73–87. https://doi.org/10.1007/s43152-020-00009-6.
Maaz Arif M, Khan SM, Gull N, Tabish TA, Zia S, Ullah Khan R, et al. Polymer-based biomaterials for chronic wound management: promises and challenges. Int J Pharm. 2021;598:120270. https://doi.org/10.1016/j.ijpharm.2021.120270.
Yaşayan G, Nejati O, Ceylan AF, Karasu Ç, Kelicen Ugur P, Bal-Öztürk A, et al. Tackling chronic wound healing using nanomaterials: advancements, challenges, and future perspectives. Appl Mater Today. 2023;32. https://doi.org/10.1016/j.apmt.2023.101829.
Kong B, Liu R, Guo J, Lu L, Zhou Q, Zhao Y. Tailoring micro/nano-fibers for biomedical applications. Bioact Mater. 2023;19:328–47. https://doi.org/10.1016/j.bioactmat.2022.04.016.
Yan B, Zhang Y, Li Z, Zhou P, Mao Y. Electrospun nanofibrous membrane for biomedical application. SN Appl Sci. 2022;4. https://doi.org/10.1007/s42452-022-05056-2.
Mao Y, Shen W, Wu S, Ge X, Ao F, Ning Y, et al. Electrospun polymers: using devices to enhance their potential for biomedical applications. React Funct Polym. 2023;186: 105568. https://doi.org/10.1016/j.reactfunctpolym.2023.105568.
Chen S, Tian H, Mao J, Ma F, Zhang M, Chen F, et al. Preparation and application of chitosan-based medical electrospun nanofibers. Int J Biol Macromol. 2023;226:410–22. https://doi.org/10.1016/j.ijbiomac.2022.12.056.
Yang G, Li X, He Y, Ma J, Ni G, Zhou S. From nano to micro to macro: electrospun hierarchically structured polymeric fibers for biomedical applications. Prog Polym Sci. 2018;81:80–113. https://doi.org/10.1016/j.progpolymsci.2017.12.003.
Barakat HS, Freag MS, Gaber SM, Al Oufy A, Abdallah OY. Development of verapamil hydrochloride-loaded biopolymer-based composite electrospun nanofibrous mats: in vivo evaluation of enhanced burn wound healing without scar formation. Drug Des Devel Ther. 2023;17:1211–31. https://doi.org/10.2147/DDDT.S389329.
Sedghi R, Shaabani A. Electrospun biocompatible core/shell polymer-free core structure nanofibers with superior antimicrobial potency against multi drug resistance organisms. Polymer (Guildf). 2016;101:151–7. https://doi.org/10.1016/j.polymer.2016.08.060.
Zhu Y, Wang Z, Bai L, Deng J, Zhou Q. Biomaterial-based encapsulated probiotics for biomedical applications: current status and future perspectives. Mater Des. 2021;210:110018. https://doi.org/10.1016/j.matdes.2021.110018.
Xu H, Zhang F, Wang M, Lv H, Yu DG, Liu X, et al. Electrospun hierarchical structural films for effective wound healing. Biomater Adv. 2022;136. https://doi.org/10.1016/j.bioadv.2022.212795.
Habibi S, Mohammadi T, HMTShirazi R, Atyabi F, Kiani M, Asadi AA. A bilayer mupirocin/bupivacaine-loaded wound dressing based on chitosan/poly (vinyl alcohol) nanofibrous mat: preparation, characterization, and controlled drug release. Int J Biol Macromol. 2023;240:124399. https://doi.org/10.1016/j.ijbiomac.2023.124399.
Altinkok C, Sagdic G, Daglar O, Ercan Ayra M, Yuksel Durmaz Y, Durmaz H, et al. A new strategy for direct solution electrospinning of phosphorylated poly(vinyl chloride)/polyethyleneimine blend in alcohol media. Eur Polym J. 2023;183. https://doi.org/10.1016/j.eurpolymj.2022.111750.
Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011;32:4243–54. https://doi.org/10.1016/j.biomaterials.2011.02.042.
Fahimirad S, Abtahi H, Satei P, Ghaznavi-Rad E, Moslehi M, Ganji A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr Polym. 2021;259:117640. https://doi.org/10.1016/j.carbpol.2021.117640.
Chen X, Zhang Q, Wang Y, Meng J, Wu M, Xu H, et al. Fabrication and characterization of electrospun poly(caprolactone)/tannic acid scaffold as an antibacterial wound dressing. J Biomed Mater Res B Appl Biomater. 2021;109:1478–87. https://doi.org/10.1002/jbm.b.34807.
Jiang H, Wang L, Zhu K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J Control Release. 2014;193:296–303. https://doi.org/10.1016/j.jconrel.2014.04.025.
Martin A, Cai J, Schaedel AL, van der Plas M, Malmsten M, Rades T, et al. Zein-polycaprolactone core–shell nanofibers for wound healing. Int J Pharm. 2022;621:121809. https://doi.org/10.1016/j.ijpharm.2022.121809.
Gao Z, Liu S, Li S, Shao X, Zhang P, Yao Q. Fabrication and properties of the multifunctional rapid wound healing panax notoginseng@Ag electrospun fiber membrane. Molecules. 2023;28. https://doi.org/10.3390/molecules28072972.
Zare MR, Khorram M, Barzegar S, Asadian F, Zareshahrabadi Z, Saharkhiz MJ, et al. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int J Pharm. 2021;603. https://doi.org/10.1016/j.ijpharm.2021.120698.
Adeli-Sardou M, Yaghoobi MM, Torkzadeh-Mahani M, Dodel M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int J Biol Macromol. 2019;124:478–91. https://doi.org/10.1016/j.ijbiomac.2018.11.237.
Shitole AA, Raut P, Giram P, Rade P, Khandwekar A, Garnaik B, et al. Poly (vinylpyrrolidone)-iodine engineered poly (ε-caprolactone) nanofibers as potential wound dressing materials. Mater Sci Eng C. 2020;110:110731. https://doi.org/10.1016/j.msec.2020.110731.
Sena S, Sumeyra KN, Ulkugul G, Sema A, Betul K, Muge SB, et al. Controlled release of metformin hydrochloride from core-shell nanofibers with fish sarcoplasmic protein. Med. 2019;55:1–13. https://doi.org/10.3390/medicina55100682.
Fahimirad S, Ajalloueian F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int J Pharm. 2019;566:307–28. https://doi.org/10.1016/j.ijpharm.2019.05.053.
Mu X, Liu Y, Fang D, Wang Z, Nie J, Ma G. Electric field induced phase separation on electrospinning polyelectrolyte based core-shell nanofibers. Carbohydr Polym. 2012;90:1582–6. https://doi.org/10.1016/j.carbpol.2012.07.034.
Badmus M, Liu J, Wang N, Radacsi N, Zhao Y. Hierarchically electrospun nanofibers and their applications: a review. Nano Mater Sci. 2021;3:213–32. https://doi.org/10.1016/j.nanoms.2020.11.003.
Yang J, Wang K, Yu DG, Yang Y, Bligh SWA, Williams GR. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater Sci Eng C. 2020;111:110805. https://doi.org/10.1016/j.msec.2020.110805.
Zhang H, Sun L, Guo J, Zhao Y. Hierarchical spinning of janus textiles with anisotropic wettability for wound healing. Research. 2023;6:1–9. https://doi.org/10.34133/research.0129.
Aruchamy K, Mahto A, Nataraj SK. Electrospun nanofibers, nanocomposites and characterization of art: insight on establishing fibers as product. Nano-Structures and Nano-Objects. 2018;16:45–58. https://doi.org/10.1016/j.nanoso.2018.03.013.
Kulkarni D, Musale S, Panzade P, Paiva-Santos AC, Sonwane P, Madibone M, et al. Surface functionalization of nanofibers: the multifaceted approach for advanced biomedical applications. Nanomaterials. 2022;12:1–35. https://doi.org/10.3390/nano12213899.
Yoosefi S, Rakhshani A, Montazeri V, Tavakoli M, Aliabadi A, Fatahi Y, et al. Dual drug delivery system based on layered double hydroxides/carboxymethyl cellulose-poly ethylene oxide bionanocomposite electrospun fibrous mats: Fabrication, characterization, in-vitro and in-vivo studies. Int J Biol Macromol. 2022;222:3142–54. https://doi.org/10.1016/j.ijbiomac.2022.10.087.
Rath G, Hussain T, Chauhan G, Garg T, Goyal AK. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater Sci Eng C. 2016;58:242–53. https://doi.org/10.1016/j.msec.2015.08.050.
Yang S, Han X, Jia Y, Zhang H, Tang T. Hydroxypropyltrimethyl ammonium chloride chitosan functionalized-PLGA electrospun fibrous membranes as antibacterialwound dressing: in vitro and in vivo evaluation. Polymers (Basel). 2017;9:1–19. https://doi.org/10.3390/polym9120697.
Lee SY, Jang DH, Kang YO, Kim OB, Jeong L, Kang HK, et al. Cellular response to poly(vinyl alcohol) nanofibers coated with biocompatible proteins and polysaccharides. Appl Surf Sci. 2012;258:6914–22. https://doi.org/10.1016/j.apsusc.2012.03.135.
Ilomuanya MO, Adebona AC, Wang W, Sowemimo A, Eziegbo CL, Silva BO, et al. Development and characterization of collagen-based electrospun scaffolds containing silver sulphadiazine and Aspalathus linearis extract for potential wound healing applications. SN Appl Sci. 2020;2:1–13. https://doi.org/10.1007/s42452-020-2701-8.
Chen X, Zhu Q, Wen Y, Li Z, Cao S, Yan H, et al. Chemical modification of alginate via the oxidation-reductive amination reaction for the development of alginate derivative electrospun composite nanofibers. J Drug Deliv Sci Technol. 2022;68:103113. https://doi.org/10.1016/j.jddst.2022.103113.
Chen H, Huang J, Yu J, Liu S, Gu P. Electrospun chitosan-graft-poly (ɛ-caprolactone)/poly (ɛ-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int J Biol Macromol. 2011;48:13–9. https://doi.org/10.1016/j.ijbiomac.2010.09.019.
Yang S, Zhang X, Zhang D. Electrospun chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane with ciprofloxacin antibiotic drug for potential wound dressing application. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20184395.
Sharikova A, Foraida ZI, Sfakis L, Peerzada L, Larsen M, Castracane J, et al. Characterization of nanofibers for tissue engineering: chemical mapping by confocal Raman microscopy. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2020;227:117670. https://doi.org/10.1016/j.saa.2019.117670.
Zhao R, Li X, Sun B, Zhang Y, Zhang D, Tang Z, et al. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int J Biol Macromol. 2014;68:92–7. https://doi.org/10.1016/j.ijbiomac.2014.04.029.
Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm. 2013;452:333–43. https://doi.org/10.1016/j.ijpharm.2013.05.012.
Scheidt DT, Pellá MCG, Breitenbach GL, Simões MR, Caetano J, Martins CVB, et al. Blend composition effect on the diameter of electrospun chitosan (CHT)/poly(ethylene oxide) (PEO) nanofibers. Colloids Surfaces A Physicochem Eng Asp. 2023;670. https://doi.org/10.1016/j.colsurfa.2023.131516.
Salami MS, Bahrami G, Arkan E, Izadi Z, Miraghaee S, Samadian H. Co-electrospun nanofibrous mats loaded with bitter gourd (Momordica charantia) extract as the wound dressing materials: in vitro and in vivo study. BMC Complement Med Ther. 2021;21:1–12. https://doi.org/10.1186/s12906-021-03284-4.
Samadian H, Zamiri S, Ehterami A, Farzamfar S, Vaez A, Khastar H, et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: in vitro and in vivo studies. Sci Rep. 2020;10:1–12. https://doi.org/10.1038/s41598-020-65268-7.
Ibrahim HM, Klingner A. A review on electrospun polymeric nanofibers: production parameters and potential applications. Polym Test. 2020;90: 106647. https://doi.org/10.1016/j.polymertesting.2020.106647.
Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2018;11:1165–88. https://doi.org/10.1016/j.arabjc.2015.11.015.
Bubakir MM, Li H, Barhoum A, Yang W. Advances in melt electrospinning technique. Handb Nanofibers. 2019. https://doi.org/10.1007/978-3-319-53655-2_8.
Zarghami A, Irani M, Mostafazadeh A, Golpour M, Heidarinasab A, Haririan I. Fabrication of PEO/chitosan/PCL/olive oil nanofibrous scaffolds for wound dressing applications. Fibers Polym. 2015;16:1201–12. https://doi.org/10.1007/s12221-015-1201-8.
Miranda-Calderon L, Yus C, Landa G, Mendoza G, Arruebo M, Irusta S. Pharmacokinetic control on the release of antimicrobial drugs from pH-responsive electrospun wound dressings. Int J Pharm. 2022;624: 122003. https://doi.org/10.1016/j.ijpharm.2022.122003.
Kumar CS, Soloman AM, Thangam R, Perumal RK, Gopinath A, Madhan B. Ferulic acid-loaded collagen hydrolysate and polycaprolactone nanofibres for tissue engineering applications. IET Nanobiotechnology. 2020;14:202–9. https://doi.org/10.1049/iet-nbt.2019.0281.
Ignatova M, Manolova N, Rashkov I. Electrospinning of poly(vinyl pyrrolidone)–iodine complex and poly(ethylene oxide)/poly(vinyl pyrrolidone)–iodine complex – a prospective route to antimicrobial wound dressing materials. Eur Polym J. 2007;43:1609–23. https://doi.org/10.1016/j.eurpolymj.2007.02.020.
Chinatangkul N, Limmatvapirat C, Nunthanid J, Luangtana-Anan M, Sriamornsak P, Limmatvapirat S. Design and characterization of monolaurin loaded electrospun shellac nanofibers with antimicrobial activity. Asian J Pharm Sci. 2018;13:459–71. https://doi.org/10.1016/j.ajps.2017.12.006.
Medeiros GB, Lima F de A, de Almeida DS, Guerra VG, Aguiar ML. Modification and functionalization of fibers formed by electrospinning: a review. Membranes (Basel). 2022;12. https://doi.org/10.3390/membranes12090861.
Yang Y, Zhu T, Liu ZP, Luo M, Yu DG, Annie Bligh SW. The key role of straight fluid jet in predicting the drug dissolution from electrospun nanofibers. Int J Pharm. 2019;569. https://doi.org/10.1016/j.ijpharm.2019.118634.
Wu XM, Branford-White CJ, Yu DG, Chatterton NP, Zhu LM. Preparation of core-shell PAN nanofibers encapsulated α-tocopherol acetate and ascorbic acid 2-phosphate for photoprotection. Colloids Surfaces B Biointerfaces. 2011;82:247–52. https://doi.org/10.1016/j.colsurfb.2010.08.049.
Almukainzi M, El-Masry TA, Negm WA, Elekhnawy E, Saleh A, Sayed AE, et al. Co-delivery of gentiopicroside and thymoquinone using electrospun m-PEG/PVP nanofibers: in-vitro and in vivo studies for antibacterial wound dressing in diabetic rats. Int J Pharm. 2022;625:122106. https://doi.org/10.1016/j.ijpharm.2022.122106.
Davani F, Alishahi M, Sabzi M, Khorram M, Arastehfar A, Zomorodian K. Dual drug delivery of vancomycin and imipenem/cilastatin by coaxial nanofibers for treatment of diabetic foot ulcer infections. Mater Sci Eng C. 2021;123:111975. https://doi.org/10.1016/j.msec.2021.111975.
Hajikhani M, Emam-Djomeh Z, Askari G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int J Biol Macromol. 2021;172:143–53. https://doi.org/10.1016/j.ijbiomac.2021.01.051.
Shadman-Manesh V, Gholipour-Kanani A, Najmoddin N, Rabbani S. Preclinical evaluation of the polycaprolactone-polyethylene glycol electrospun nanofibers containing egg-yolk oil for acceleration of full thickness burns healing. Sci Rep. 2023;13:919. https://doi.org/10.1038/s41598-023-28065-6.
Rather HA, Thakore R, Singh R, Jhala D, Singh S, Vasita R. Antioxidative study of cerium oxide nanoparticle functionalised PCL-gelatin electrospun fibers for wound healing application. Bioact Mater. 2018;3:201–11. https://doi.org/10.1016/j.bioactmat.2017.09.006.
Shahrousvand M, Haddadi-Asl V, Shahrousvand M. Step-by-step design of poly (ε-caprolactone)/chitosan/Melilotus officinalis extract electrospun nanofibers for wound dressing applications. Int J Biol Macromol. 2021;180:36–50. https://doi.org/10.1016/j.ijbiomac.2021.03.046.
Zhang X, Khan MMR, Yamamoto T, Tsukada M, Morikawa H. Fabrication of silk sericin nanofibers from a silk sericin-hope cocoon with electrospinning method. Int J Biol Macromol. 2012;50:337–47. https://doi.org/10.1016/j.ijbiomac.2011.12.006.
Liu Z, Gu Y, Bi L. Applications of electrospun nanofibers in solid oxide fuel cells – a review. J Alloys Compd. 2023;937:168288. https://doi.org/10.1016/j.jallcom.2022.168288.
Baykara T, Taylan G. Coaxial electrospinning of PVA/Nigella seed oil nanofibers: processing and morphological characterization. Mater Sci Eng B. 2021;265:115012. https://doi.org/10.1016/j.mseb.2020.115012.
Chinatangkul N, Pengon S, Krongrawa W, Chansatidkosol S, Limmatvapirat C, Limmatvapirat S. Designing electrospun shellac nanofibers with mupirocin using the Box-Behnken approach for topical wound care. J Drug Deliv Sci Technol. 2022;76:103720. https://doi.org/10.1016/j.jddst.2022.103720.
Poornima B, Korrapati PS. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr Polym. 2017;157:1741–9. https://doi.org/10.1016/j.carbpol.2016.11.056.
Li Z, Kang H, Li Q, Che N, Liu Z, Li P, et al. Ultrathin core-sheath fibers for liposome stabilization. Colloids Surfaces B Biointerfaces. 2014;122:630–7. https://doi.org/10.1016/j.colsurfb.2014.07.042.
Zhang L, Yin M, Wei X, Sun J, Xu D. Recent advances in morphology, aperture control, functional control and electrochemical sensors applications of carbon nanofibers. Anal Biochem. 2022;656: 114882. https://doi.org/10.1016/j.ab.2022.114882.
Li X, Chen Y, Huang H, Mai YW, Zhou L. Electrospun carbon-based nanostructured electrodes for advanced energy storage - a review. Energy Storage Mater. 2016;5:58–92. https://doi.org/10.1016/j.ensm.2016.06.002.
Abid M Bin, Wahab RA, Salam MA, Gzara L, Moujdin IA. Desalination technologies, membrane distillation, and electrospinning, an overview. Heliyon. 2023;9:e12810. https://doi.org/10.1016/j.heliyon.2023.e12810.
Zulkifli FH, Hussain FSJ, Rasad MSBA, Mohd Yusoff M. Nanostructured materials from hydroxyethyl cellulose for skin tissue engineering. Carbohydr Polym. 2014;114:238–45. https://doi.org/10.1016/j.carbpol.2014.08.019.
Sarhan WA, Azzazy HME. High concentration honey chitosan electrospun nanofibers: biocompatibility and antibacterial effects. Carbohydr Polym. 2015;122:135–43. https://doi.org/10.1016/j.carbpol.2014.12.051.
Moradkhannejhad L, Abdouss M, Nikfarjam N, Shahriari MH, Heidary V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J Drug Deliv Sci Technol. 2020;56:101554. https://doi.org/10.1016/j.jddst.2020.101554.
Hadisi Z, Nourmohammadi J, Nassiri SM. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int J Biol Macromol. 2018;107:2008–19. https://doi.org/10.1016/j.ijbiomac.2017.10.061.
Wang M, Fang D, Wang N, Jiang S, Nie J, Yu Q, et al. Preparation of PVDF/PVP core-shell nanofibers mats via homogeneous electrospinning. Polymer (Guildf). 2014;55:2188–96. https://doi.org/10.1016/j.polymer.2014.02.035.
Soares PIP, Borges JP. Recent advances in magnetic electrospun nanofibers for cancer theranostics application. Prog Nat Sci Mater Int. 2021;31:835–44. https://doi.org/10.1016/j.pnsc.2021.11.003.
Abdelgawad AM, El-Naggar ME, Hudson SM, Rojas OJ. Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. Int J Biol Macromol. 2017;94:96–105. https://doi.org/10.1016/j.ijbiomac.2016.07.061.
Ganesh M, Aziz AS, Ubaidulla U, Hemalatha P, Saravanakumar A, Ravikumar R, et al. Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: synthesis and evaluation on synergism in wound healing. J Ind Eng Chem. 2016;39:127–35. https://doi.org/10.1016/j.jiec.2016.05.021.
Raksa A, Numpaisal P, Ruksakulpiwat Y. The effect of humidity during electrospinning on morphology and mechanical properties of SF/PVA nanofibers. Mater Today Proc. 2021;47:3458–61. https://doi.org/10.1016/j.matpr.2021.03.459.
Ziliang Li, Yukai Hou, Yufei Ma, Fuqiang Zhai Joshi MK. Recent advances in one-dimensional electrospun semiconductor nanostructures for UV photodetector applications: a review. J Alloys Compd. 2023;130784. https://doi.org/10.1016/j.jallcom.2023.169718.
Nguyen TTT, Ghosh C, Hwang SG, Chanunpanich N, Park JS. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int J Pharm. 2012;439:296–306. https://doi.org/10.1016/j.ijpharm.2012.09.019.
Augustine R, Zahid AA, Hasan A, Wang M, Webster TJ. Ctgf loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int J Nanomedicine. 2019;14:8573–88. https://doi.org/10.2147/IJN.S224047.
Cui J, Li F, Wang Y, Zhang Q, Ma W, Huang C. Electrospun nanofiber membranes for wastewater treatment applications. Sep Purif Technol. 2020;250:117116. https://doi.org/10.1016/j.seppur.2020.117116.
Zhou F, Cui C, Sun S, Wu S, Chen S, Ma J, et al. Electrospun ZnO-loaded chitosan/PCL bilayer membranes with spatially designed structure for accelerated wound healing. Carbohydr Polym. 2022;282: 119131. https://doi.org/10.1016/j.carbpol.2022.119131.
Huang R, Li W, Lv X, Lei Z, Bian Y, Deng H, et al. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials. 2015;53:58–75. https://doi.org/10.1016/j.biomaterials.2015.02.076.
Dodero A, Scarfi S, Mirata S, Sionkowska A, Vicini S, Alloisio M, et al. Effect of crosslinking type on the physical-chemical properties and biocompatibility of chitosan-based electrospun membranes. Polymers (Basel). 2021;13. https://doi.org/10.3390/polym13050831.
Unnithan AR, Ghavami Nejad A, Sasikala ARK, Thomas RG, Jeong YY, Murugesan P, et al. Electrospun zwitterionic nanofibers with in situ decelerated epithelialization property for non-adherent and easy removable wound dressing application. Chem Eng J. 2016;287:640–8. https://doi.org/10.1016/j.cej.2015.11.086.
Li C, Luo X, Li L, Cai Y, Kang X, Li P. Carboxymethyl chitosan-based electrospun nanofibers with high citral-loading for potential anti-infection wound dressings. Int J Biol Macromol. 2022;209:344–55. https://doi.org/10.1016/j.ijbiomac.2022.04.025.
Li Y, Chen F, Nie J, Yang D. Electrospun poly(lactic acid)/chitosan core–shell structure nanofibers from homogeneous solution. Carbohydr Polym. 2012;90:1445–51. https://doi.org/10.1016/j.carbpol.2012.07.013.
Afshar S, Rashedi S, Nazockdast H, Ghazalian M. Preparation and characterization of electrospun poly(lactic acid)-chitosan core-shell nanofibers with a new solvent system. Int J Biol Macromol. 2019;138:1130–7. https://doi.org/10.1016/j.ijbiomac.2019.07.053.
Joshi A, Xu Z, Ikegami Y, Yoshida K, Sakai Y, Joshi A, et al. Exploiting synergistic effect of externally loaded bFGF and endogenous growth factors for accelerated wound healing using heparin functionalized PCL/gelatin co-spun nanofibrous patches. Chem Eng J. 2021;404:126518. https://doi.org/10.1016/j.cej.2020.126518.
Gao C, Zhang L, Wang J, Cheng Y, Chen Z, Yang R, et al. Coaxial structured drug loaded dressing combined with induced stem cell differentiation for enhanced wound healing. Mater Sci Eng C. 2021;134:112542. https://doi.org/10.1016/j.msec.2021.112542.
Basar AO, Castro S, Torres-Giner S, Lagaron JM, Turkoglu Sasmazel H. Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater Sci Eng C. 2017;81:459–68. https://doi.org/10.1016/j.msec.2017.08.025.
Mohamady Hussein MA, Guler E, Rayaman E, Cam ME, Sahin A, Grinholc M, et al. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr Polym. 2021;270:118373. https://doi.org/10.1016/j.carbpol.2021.118373.
Bai XH, Zhang J, Cheng GT, Liu XF, Sun TC, Yang J, et al. Dual antibacterial polypeptide-coated PCL@ZIF-8 nanofiber reduces infection and inflammation in burn wounds. J Mater Sci. 2022;57:3678–87. https://doi.org/10.1007/s10853-021-06832-y.
Wang C, Zhang Q, Hou G, Wang C, Yan H. Sustained release of EGF/bFGF growth factors achieved by mussel-inspired core–shell nanofibers with hemostatic and anti-inflammatory effects for promoting wound healing. Eur Polym J. 2023;190:112003. https://doi.org/10.1016/j.eurpolymj.2023.112003.
Cui C, Sun S, Li X, Chen S, Wu S, Zhou F, et al. Optimizing the chitosan-PCL based membranes with random/aligned fiber structure for controlled ciprofloxacin delivery and wound healing. Int J Biol Macromol. 2022;205:500–10. https://doi.org/10.1016/j.ijbiomac.2022.02.118.
Zandi N, Lotfi R, Tamjid E, Shokrgozar MA, Simchi A. Core-sheath gelatin based electrospun nanofibers for dual delivery release of biomolecules and therapeutics. Mater Sci Eng C. 2020;108:110432. https://doi.org/10.1016/j.msec.2019.110432.
Najafiasl M, Osfouri S, Azin R, Zaeri S. Fabrication, characterization and in vivo evaluation of dexpanthenol sustained-release nanofibers for wound healing. Polym Test. 2020;91:106827. https://doi.org/10.1016/j.polymertesting.2020.106827.
Khan A ur R, Huang K, Khalaji MS, Yu F, Xie X, Zhu T, et al. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact Mater. 2021;6:2783-800. https://doi.org/10.1016/j.bioactmat.2021.01.040.
Wang J, Cai N, Chan V, Zeng H, Shi H, Xue Y, et al. Antimicrobial hydroxyapatite reinforced-polyelectrolyte complex nanofibers with long-term controlled release activity for potential wound dressing application. Colloids Surfaces A Physicochem Eng Asp. 2021;624:126722. https://doi.org/10.1016/j.colsurfa.2021.126722.
Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013;9:9351–9. https://doi.org/10.1016/j.actbio.2013.07.030.
Dong Z, Yin J, Zhou X, Li S, Fu Z, Liu P, et al. Natural and biocompatible dressing unit based on tea carbon dots modified core-shell electrospun fiber for diabetic wound disinfection and healing. Colloids Surfaces B Biointerfaces. 2023;226:113325. https://doi.org/10.1016/j.colsurfb.2023.113325.
Lee CH, Hung KC, Hsieh MJ, Chang SH, Juang JH, Hsieh IC, et al. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomedicine Nanotechnology, Biol Med. 2020;24:102123. https://doi.org/10.1016/j.nano.2019.102123.
Homaeigohar S, Tsai TY, Zarie ES, Elbahri M, Young TH, Boccaccini AR. Bovine serum albumin (BSA)/polyacrylonitrile (PAN) biohybrid nanofibers coated with a biomineralized calcium deficient hydroxyapatite (HA) shell for wound dressing. Mater Sci Eng C. 2020;116:111248. https://doi.org/10.1016/j.msec.2020.111248.
Aljohani M, Alkabli J, Abualnaja MM, Alrefaei AF, Almehmadi SJ, Mahmoud MHH, et al. Electrospun AgNPs-polylactate nanofibers and their antimicrobial applications. React Funct Polym. 2021;167:104999. https://doi.org/10.1016/j.reactfunctpolym.2021.104999.
Aldalbahi A, El-Naggar ME, Ahmed MK, Periyasami G, Rahaman M, Menazea AA. Coreeshell Au@Se nanoparticles embedded in cellulose acetate/polyvinylidene fluoride scaffold for wound healing. J Mater Res Technol. 2020;9:15045–56. https://doi.org/10.1016/j.jmrt.2020.10.079.
Li Z, Kang H, Che N, Liu Z, Li P, Li W, et al. Controlled release of liposome-encapsulated Naproxen from core-sheath electrospun nanofibers. Carbohydr Polym. 2014;111:18–24. https://doi.org/10.1016/j.carbpol.2014.04.017.
Zhang H, Guo M, Zhu T, Xiong H, Zhu LM. A careob-like nanofibers with a sustained drug release profile for promoting skin wound repair and inhibiting hypertrophic scar. Compos Part B Eng. 2022;236:109790. https://doi.org/10.1016/j.compositesb.2022.109790.
Jin G, Prabhakaran MP, Kai D, Ramakrishna S. Controlled release of multiple epidermal induction factors through core-shell nanofibers for skin regeneration. Eur J Pharm Biopharm. 2013;85:689–98. https://doi.org/10.1016/j.ejpb.2013.06.002.
Li A, Li L, Zhao B, Li X, Liang W, Lang M, et al. Antibacterial, antioxidant and anti-inflammatory PLCL/gelatin nanofiber membranes to promote wound healing. Int J Biol Macromol. 2022;194:914–23. https://doi.org/10.1016/j.ijbiomac.2021.11.146.
Movahedi M, Asefnejad A, Rafienia M, Khorasani MT. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: in vitro and in vivo evaluation. Int J Biol Macromol. 2020;146:627–37. https://doi.org/10.1016/j.ijbiomac.2019.11.233.
Osali S, Ghiyasi Y, Esfahani H, Jose R, Ramakrishna S. Electrospun nanomembranes at the liquid–liquid and solid–liquid interface - a review. Mater Today. 2023. https://doi.org/10.1016/j.mattod.2023.05.005.
Ao F, Luo X, Shen W, Ge X, Li P, Zheng Y, et al. Multifunctional electrospun membranes with hydrophilic and hydrophobic gradients property for wound dressing. Colloids Surfaces B Biointerfaces. 2023;225:113276. https://doi.org/10.1016/j.colsurfb.2023.113276.
Zhang K, Jiao X, Zhou L, Wang J, Wang C, Qin Y, et al. Nanofibrous composite aerogel with multi-bioactive and fluid gating characteristics for promoting diabetic wound healing. Biomaterials. 2021;276:121040. https://doi.org/10.1016/j.biomaterials.2021.121040.
Shi Y, Zhou M, Zhao S, Li H, Wang W, Cheng J, et al. Janus amphiphilic nanofiber membranes synergistically drive antibacterial and anti-inflammatory strategies for skin wound healing. Mater Des. 2023;227:111778. https://doi.org/10.1016/j.matdes.2023.111778.
Ji X, Li R, Liu G, Jia W, Sun M, Liu Y, et al. Phase separation-based electrospun Janus nanofibers loaded with Rana chensinensis skin peptides/silver nanoparticles for wound healing. Mater Des. 2021;207:109864. https://doi.org/10.1016/j.matdes.2021.109864.
Li L, Mai Y, Wang Y, Chen S. Stretchable unidirectional liquid-transporting membrane with antibacterial and biocompatible features based on chitosan derivative and composite nanofibers. Carbohydr Polym. 2022;276:118703. https://doi.org/10.1016/j.carbpol.2021.118703.
Lee H, Xu G, Kharaghani D, Nishino M, Song KH, Lee JS, et al. Electrospun tri-layered zein/PVP-GO/zein nanofiber mats for providing biphasic drug release profiles. Int J Pharm. 2017;531:101–7. https://doi.org/10.1016/j.ijpharm.2017.08.081.
Jafari A, Amirsadeghi A, Hassanajili S, Azarpira N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int J Pharm. 2020;583:119413. https://doi.org/10.1016/j.ijpharm.2020.119413.
Eskandarinia A, Kefayat A, Agheb M, Rafienia M, Amini Baghbadorani M, Navid S, et al. A novel bilayer wound dressing composed of a dense polyurethane/propolis membrane and a biodegradable polycaprolactone/gelatin nanofibrous scaffold. Sci Rep. 2020;10:1–15. https://doi.org/10.1038/s41598-020-59931-2.
Zhu P, Yin H, Wei J, Wu J, Ping D, Zhang X. A bilayer biocompatible polycaprolactone/zinc oxide/Capparis spinosa L ethyl acetate extract/polylactic acid nanofibrous composite scaffold for novel wound dressing applications. Int J Biol Macromol. 2023;242:125093. https://doi.org/10.1016/j.ijbiomac.2023.125093.
Shie Karizmeh M, Poursamar SA, Kefayat A, Farahbakhsh Z, Rafienia M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Biomater Adv. 2022;135. https://doi.org/10.1016/j.msec.2022.112667.
Chogan F, Mirmajidi T, Rezayan AH, Sharifi AM, Ghahary A, Nourmohammadi J, et al. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta Biomater. 2020;113:144–63. https://doi.org/10.1016/j.actbio.2020.06.031.
Mirhaj M, Tavakoli M, Varshosaz J, Labbaf S, Salehi S, Talebi A, et al. Preparation of a biomimetic bi-layer chitosan wound dressing composed of A-PRF/sponge layer and L-arginine/nanofiber. Carbohydr Polym. 2022;292:119648. https://doi.org/10.1016/j.carbpol.2022.119648.
Ma X, Wu G, Dai F, Li D, Li H, Zhang L, et al. Chitosan/polydopamine layer by layer self-assembled silk fibroin nanofibers for biomedical applications. Carbohydr Polym. 2021;251:117058. https://doi.org/10.1016/j.carbpol.2020.117058.
Song R, Yan J, Xu S, Wang Y, Ye T, Chang J, et al. Silver ions/ovalbumin films layer-by-layer self-assembled polyacrylonitrile nanofibrous mats and their antibacterial activity. Colloids Surfaces B Biointerfaces. 2013;108:322–8. https://doi.org/10.1016/j.colsurfb.2013.03.008.
Zhao Y, Tian C, Liu Y, Liu Z, Li J, Wang Z, et al. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials. 2023;295:122029. https://doi.org/10.1016/j.biomaterials.2023.122029.
Gao Z, Su C, Wang C, Zhang Y, Wang C, Yan H, et al. Antibacterial and hemostatic bilayered electrospun nanofibrous wound dressings based on quaternized silicone and quaternized chitosan for wound healing. Eur Polym J. 2021;159:110733. https://doi.org/10.1016/j.eurpolymj.2021.110733.
Lemraski EG, Jahangirian H, Dashti M, Khajehali E, Sharafinia S, Moghaddam RR, et al. Antimicrobial double-layer wound dressing based on chitosan/polyvinyl alcohol/copper: in vitro and in vivo assessment. Int J Nanomedicine. 2021;16:223–35. https://doi.org/10.2147/IJN.S266692.
Yao CH, Lee CY, Huang CH, Chen YS, Chen KY. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater Sci Eng C. 2017;79:533–40. https://doi.org/10.1016/j.msec.2017.05.076.
Figueira DR, Miguel SP, de Sá KD, Correia IJ. Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int J Biol Macromol. 2016;93:1100–10. https://doi.org/10.1016/j.ijbiomac.2016.09.080.
Chagas PAM, Schneider R, dos Santos DM, Otuka AJG, Mendonça CR, Correa DS. Bilayered electrospun membranes composed of poly(lactic-acid)/natural rubber: a strategy against curcumin photodegradation for wound dressing application. React Funct Polym. 2021;163:104889. https://doi.org/10.1016/j.reactfunctpolym.2021.104889.
Tehrani FK, Sheikhi M, Rafiemanzelat F, Esmaeili F, Ghodsi S, Koohmareh GA, et al. Protein and polysaccharide-based asymmetric mat with tuned bilayer configuration for enhanced wound healing efficiency. Carbohydr Polym. 2022;292:119666. https://doi.org/10.1016/j.carbpol.2022.119666.
Wu S, Zhao W, Sun M, He P, Lv H, Wang Q, et al. Novel bi-layered dressing patches constructed with radially-oriented nanofibrous pattern and herbal compound-loaded hydrogel for accelerated diabetic wound healing. Appl Mater Today. 2022;28:101542. https://doi.org/10.1016/j.apmt.2022.101542.
Zahid S, Khalid H, Ikram F, Iqbal H, Samie M, Shahzadi L, et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater Sci Eng C. 2019;101:438–47. https://doi.org/10.1016/j.msec.2019.03.080.
Gao Z, Qi Q, Li R, Li C, Xie X, Hou G. A nanofiber/sponge double-layered composite membrane capable of inhibiting infection and promoting blood coagulation during wound healing. Colloids Surfaces B Biointerfaces. 2023;224:113209. https://doi.org/10.1016/j.colsurfb.2023.113209.
Pandey G, Pandey P, Arya DK, Kanaujiya S, Deepak Kapoor D, Gupta RK, et al. Multilayered nanofibrous scaffold of polyvinyl alcohol/gelatin/poly (lactic-co-glycolic acid) enriched with hemostatic/antibacterial agents for rapid acute hemostatic wound healing. Int J Pharm. 2023;638:122918. https://doi.org/10.1016/j.ijpharm.2023.122918.
Kang YO, Im JN, Park WH. Morphological and permeable properties of antibacterial double-layered composite nonwovens consisting of microfibers and nanofibers. Compos Part B Eng. 2015;75:256–63. https://doi.org/10.1016/j.compositesb.2015.01.029.
Yang BY, Hu CH, Huang WC, Ho CY, Yao CH, Huang CH. Effects of bilayer nanofibrous scaffolds containing curcumin/lithospermi radix extract on wound healing in streptozotocin-induced diabetic rats. Polymers (Basel). 2019;11. https://doi.org/10.3390/polym11111745.
Jeckson TA, Neo YP, Sisinthy SP, Foo JB, Choudhury H, Gorain B. Formulation and characterisation of deferoxamine nanofiber as potential wound dressing for the treatment of diabetic foot ulcer. J Drug Deliv Sci Technol. 2021;66:102751. https://doi.org/10.1016/j.jddst.2021.102751.
Palo M, Rönkönharju S, Tiirik K, Viidik L, Sandler N, Kogermann K. Bi-layered polymer carriers with surface modification by electrospinning for potential wound care applications. Pharmaceutics. 2019;11. https://doi.org/10.3390/pharmaceutics11120678.
Nikfarjam S, Aldubaisi Y, Swami V, Swami V, Xu G, Vaughan MB, et al. Polycaprolactone electrospun nanofiber membrane with skin graft containing collagen and bandage containing MgO nanoparticles for wound healing applications. Polymers (Basel). 2023;15. https://doi.org/10.3390/polym15092014.
Arasteh S, Khanjani S, Golshahi H, Mobini S, Jahed MT, Heidari-Vala H, et al. Efficient wound healing using a synthetic nanofibrous bilayer skin substitute in murine model. J Surg Res. 2020;245:31–44. https://doi.org/10.1016/j.jss.2019.07.017.
Chandika P, Oh GW, Heo SY, Kim SC, Kim TH, Kim MS, et al. Electrospun porous bilayer nano-fibrous fish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Mater Sci Eng C. 2021;121:111871. https://doi.org/10.1016/j.msec.2021.111871.
Chen DWC, Liao JY, Liu SJ, Chan EC. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: an in vitro and in vivo study. Int J Nanomedicine. 2012;7:763–71. https://doi.org/10.2147/IJN.S29119.
Chen DW, Hsu YH, Liao JY, Liu SJ, Chen JK, Ueng SWN. Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured PLGA/collagen nanofibrous membranes. Int J Pharm. 2012;430:335–41. https://doi.org/10.1016/j.ijpharm.2012.04.010.
Shahghasempour L, Hosseinzadeh S, Haddadi A, Kabiri M. Evaluation of Lactobacillus plantarum and PRGF as a new bioactive multi-layered scaffold PU/PRGF/gelatin/PU for wound healing. Tissue Cell. 2023;82:102091. https://doi.org/10.1016/j.tice.2023.102091.
Yang M, Yu S, Zhao P, Shi G, Guo Y, Xie L, et al. Fabrication of biologically inspired electrospun collagen/silk fibroin/bioactive glass composited nanofibrous to accelerate the treatment efficiency of wound repair. Int Wound J. 2023;20:687–98. https://doi.org/10.1111/iwj.13910.
Sadeghi A, Fatemi MJ, Zandi M, Bagheri T, Ghadimi T, Tamimi M, et al. Multilayered 3-D nanofibrous scaffold with chondroitin sulfate sustained release as dermal substitute. Int J Biol Macromol. 2022;206:718–29. https://doi.org/10.1016/j.ijbiomac.2022.03.061.
Nada AA, Ali EA, Soliman AAF, Shen J, Abou-Zeid NY, Hudson SM. Multi-layer dressing made of laminated electrospun nanowebs and cellulose-based adhesive for comprehensive wound care. Int J Biol Macromol. 2020;162:629–44. https://doi.org/10.1016/j.ijbiomac.2020.06.184.
Abdel Khalek MA, Abdel Gaber SA, El-Domany RA, El-Kemary MA. Photoactive electrospun cellulose acetate/polyethylene oxide/methylene blue and trilayered cellulose acetate/polyethylene oxide/silk fibroin/ciprofloxacin nanofibers for chronic wound healing. Int J Biol Macromol. 2021;193:1752–66. https://doi.org/10.1016/j.ijbiomac.2021.11.012.
He C, Liu X, Zhou Z, Liu N, Ning X, Miao Y, et al. Harnessing biocompatible nanofibers and silver nanoparticles for wound healing: sandwich wound dressing versus commercial silver sulfadiazine dressing. Mater Sci Eng C. 2021;128:112342. https://doi.org/10.1016/j.msec.2021.112342.
Tavakoli M, Mirhaj M, Varshosaz J, Salehi S, Mohanna SM, Salehi S, et al. Asymmetric tri-layer sponge-nanofiber wound dressing containing insulin-like growth factor-1 and multi-walled carbon nanotubes for acceleration of full-thickness wound healing. Biomater Adv. 2023;151:213468. https://doi.org/10.1016/j.bioadv.2023.213468.
Derakhshan MA, Nazeri N, Khoshnevisan K, Heshmat R, Omidfar K. Three-layered PCL-collagen nanofibers containing Melilotus officinalis extract for diabetic ulcer healing in a rat model. J Diabetes Metab Disord. 2022;21:313–21. https://doi.org/10.1007/s40200-022-00976-7.
Shokrollahi M, Bahrami SH, Nazarpak MH, Solouk A. Multilayer nanofibrous patch comprising chamomile loaded carboxyethyl chitosan/poly(vinyl alcohol) and polycaprolactone as a potential wound dressing. Int J Biol Macromol. 2020;147:547–59. https://doi.org/10.1016/j.ijbiomac.2020.01.067.
Hussein MAM, Su S, Ulag S, Woźniak A, Grinholc M, Erdemir G, et al. Development and in vitro evaluation of biocompatible PLA-based trilayer nanofibrous membranes for the delivery of Nanoceria: a novel approach for diabetic wound healing. Polymers (Basel). 2021;13. https://doi.org/10.3390/polym13213630.
Talukder ME, Hasan KMF, Wang J, Yao J, Li C, Song H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J Mater Sci. 2021;56:12814–34. https://doi.org/10.1007/s10853-021-06123-6.
Ma B, Xie J, Jiang J, Wu J. Sandwich-type fiber scaffolds with square arrayed microwells and nanostructured cues as microskin grafts for skin regeneration. Biomaterials. 2014;35:630–41. https://doi.org/10.1016/j.biomaterials.2013.09.111.
Mahjour SB, Fu X, Yang X, Fong J, Sefat F, Wang H. Rapid creation of skin substitutes from human skin cells and biomimetic nanofibers for acute full-thickness wound repair. Burns. 2015;41:1764–74. https://doi.org/10.1016/j.burns.2015.06.011.
Albright V, Xu M, Palanisamy A, Cheng J, Stack M, Zhang B, et al. Micelle-coated, hierarchically structured nanofibers with dual-release capability for accelerated wound healing and infection control. Adv Healthc Mater. 2018;7:1800132. https://doi.org/10.1002/adhm.201800132.
Yingjie Z, Shuying Z, Zhimin T, Yan L, Lu W. Layer-by-layer assembly of peptides-decorated coaxial nanofibrous membranes with antibiofilm and visual pH sensing capability. Colloids Surfaces B Biointerfaces. 2022;220:112860. https://doi.org/10.1016/j.colsurfb.2022.112860.
Zhou B, Li Y, Deng H, Hu Y, Li B. Antibacterial multilayer films fabricated by layer-by-layer immobilizing lysozyme and gold nanoparticles on nanofibers. Colloids Surfaces B Biointerfaces. 2014;116:432–8. https://doi.org/10.1016/j.colsurfb.2014.01.016.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.M.A.: writing, original draft. M.A.E.-N.: supervision and writing, review and editing. M.H.T.: supervision and writing, review and editing. E.A.: supervision and writing, review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelhakeem, E., Monir, S., Teaima, M.H.M. et al. State-of-the-Art Review of Advanced Electrospun Nanofiber Composites for Enhanced Wound Healing. AAPS PharmSciTech 24, 246 (2023). https://doi.org/10.1208/s12249-023-02702-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02702-9