Abstract
Rheumatoid arthritis affects millions of people worldwide and is considered a chronic multisystem disease whose causes are unknown. In general, the main objective of rheumatoid arthritis treatment is to improve the quality of life of patients by relieving pain, maintaining or improving functional capacity, preventing thus, disability. In recent years the role of adipokines in the pathogenesis of rheumatoid arthritis has been discussed but results are still conflicting. Although results from some studies have shown the implications of adipokines in the pathophysiology of autoimmune diseases, including rheumatoid arthritis, their role in the pathogenesis of disease progression is not clear. Thus, this review aimed to describe the association of key adipokines (leptin, resistin, visfatin and adiponectin) and rheumatoid arthritis, given the high prevalence of this disease and the important social impact caused by this chronic disabling disease.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic multisystem disease whose causes are unknown [1]. This disease presents a variety of systemic manifestations being the persistent inflammatory synovitis the most typical feature, compromising peripheral joints in a symmetric distribution. Mateen et al. (2016) [2] highlights RA as a disease, which is characterized in the majority of patients by the presence of rheumatoid factor (RF) and anti–citrullinated protein antibody (ACPA). The authors reinforce that cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-17 have an important role in the pathophysiology of RA since serum concentrations of these substances may indicate the severity of the disease.
The diagnosis of RA must be at earlier stages of disease and treatment should aim to relieve pain, maintain or improve functional capacity, preventing thus, disability, and improving patients quality of life [3].
Barbosa et al. (2012) [4] reported the important role of mediators synthesized in adipose tissue, named adipokines, in RA. Hutcheson (2015) [5] points out that knowledge about adiposity has changed and currently it appears as an important regulator of several key processes, including inflammation. Furthermore, adipokines have hormonal action supporting the regulation of appetite and glucose metabolism, and some of them such as leptin, resistin, adiponectin and visfatin have been associated to RA development. However the results are still conflicting [4, 5].
Thus, this review aimed to describe the association of key adipokines (leptin, resistin, visfatin and adiponectin) and RA, given the high prevalence of this disease and the important social impact caused by chronic disabling diseases of the articular system.
Methods
Study selection
This review is in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [6]. The search string was restricted to humans, including clinical studies, controlled clinical trials, meta-analysis, multicentric, observational studies, and randomized controlled trials. Relevant articles that were not retrieved in the main search but were cited in the publications were carefully reviewed and included if they met the criteria. As the main objective was to verify the association of adipokines with rheumatoid arthritis, most of the studies analyzed refer to observational studies such as cross-sectional, case-control and cohort studies presenting quantitative information regarding plasma or serum adipokines concentrations. The on line databases U.S. National Library of Medicine PUBMED, Periódicos Capes, Science Direct, and Scientific Electronic Library Online (SciELO) were searched for English, Spanish or Portuguese-language articles. The crossing of “rheumatoid arthritis” with the following descriptors separately was used to accomplish this review: “adipokines”, “leptin”, “resistin”, “visfatin”, and “adiponectin”. No exclusion criteria were established in view of the small number of articles regarding this current issue. The study selection process is described in Fig. 1.
Data extraction
The information sources were the results described in the articles selected. The following data were extracted from articles included in this review: authors, year of publication, study design, number of participants, disease characteristics, control groups and results regarding associations between each adipokine and markers of disease activity.
Results and discussion
Adipokines
Adipose tissue is a multifunctional organ responsible for lipid storage, thermogenesis, structural components and support of many organs such as joints, gastrointestinal tract and skin and nowadays also described as secretory and endocrine functions [7]. It is noteworthy in this context its role as an endocrine organ by synthesizing and secreting adipokines, which play an important role in the pathophysiology of insulin resistance, inflammation and atherogenesis [8, 9].
Leptin
Leptin is an adipokine produced in white adipose tissue. Discovered in 1994 [10], it is an Ob gene product [11], cloned and sequenced in mice and considered the adipokine responsible for the regulation of energy metabolism and homeostasis, as well as neuroendocrine functions [12]. It also assists the immunity and inflammation control through its receptor [13]. Thus, leptin is responsible for the regulation of various biological processes, being involved in the pathophysiology of many diseases. Leptin is considered a proinflammatory adipokine since it stimulates production of cytokines such as TNF-α, IL-6 and reactive oxygen species, and induces the production of CC chemokines by macrophages and alters the T helper (Th)1 / Th2 profile [13].
Paz-Filho et al. (2012) [14] described molecular mechanisms and pro-inflammatory systemic effects of leptin. It acts through its Ob receptor triggering inflammatory responses together with infectious and inflammatory stimuli of cytokines such as IL-1, lipopolysaccharide (LPS), and TNF-α, which may, in turn, increase levels of leptin. The interaction between leptin and inflammation are bidirectional, but all pro inflammatory since cytokines increases the synthesis and release of leptin, which in turn perpetuates the cycle of inflammation.
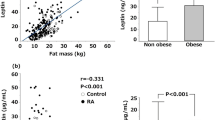
Leptin showed a significant effect on increasing the expression of Th1 cytokines. Experimental studies on mice demonstrated that these animals showed less severe stages of induced RA with lower levels of IL-1B and TNF-α in the synovial fluid and reduction in T-cell proliferative response induced by antigen [14]. However, clinical studies revealed paradoxical results about the effects of endogenous leptin in protecting joints in severe forms of erosive RA in humans [15].
In the last two decades, several studies have described the action of leptin in RA [16], leading researchers near to assume the hypothesis that this hormone has a key role in rheumatic diseases. [17] Thus, leptin levels may be a risk factor for the pathogenesis of RA [18].
Olama et al. (2012) [15] evaluated the ratio of synovial and serum leptin in patients with RA and found that the local utilization of leptin at the joint cavity has a protector role against the destructive course of RA. Rho et al. (2010) [19] also examined the hypothesis that the adipokines could influence insulin resistance and coronary atherosclerosis in patients with RA. Leptin was positively associated with insulin resistance assessed by Homeostasis Model Assessment - Insulin Resistance (HOMA-IR), even after adjusting for age, race, sex, body mass index (BMI), traditional cardiovascular risk factors and inflammation mediators. Targońska-Stepniak et al. (2010) [20] assessed leptin levels in patients with RA and demonstrated positive correlation between leptin levels and Disease Activity Score (DAS)-28. Yoshino et al. (2011) [21] found leptin levels significantly higher in RA patients compared to controls, and this adipokine correlated positively with C-Reactive Protein (CRP) levels, suggesting that leptin can act as a proinflammatory in this disease. On the other hand, Kontunen et al. (2011) [22] showed that leptin levels were increased only in patients with RA and concomitant diagnosis of metabolic syndrome (MetS).
Kang et al. (2013) [23] demonstrated that TNF-α was positively associated to leptin and the latter was associated with various metabolic risk factors, including insulin resistance. Bustos Rivera-Bahena et al. (2015) [24] evidenced that circulating levels of leptin correlate positively with clinical activity of RA, regardless of BMI. However, Xibille-Friedmann et al. (2015) [25] concluded that in a short term basal levels of leptin may predict disease activity independent of BMI. However, when submitted to treatment, this only occurred in patients with normal body weight.
Tian et al. (2014) [26] reported a review in which 23 studies were analyzed. The following results were obtained: 13 studies showed increased leptin levels; 8 studies did not demonstrate any significant difference and 2 had reduced leptin levels when compared to control subjects. Therefore, most of the studies have found higher levels of leptin in patients with RA, showing a possible role in the regulation of joint damage, and suggested that more studies are needed to understand the mechanisms of action of this adipokine. A meta-analysis conducted by Lee e Bae (2016) [27] confirmed these data showing that circulating levels of leptin were significantly higher in patients with RA with a positive correlation between this hormone and RA activity.
Despite the evidence demonstrated, some studies do not corroborate these associations. Allam e Radwan (2012) [28] and Abdalla et al. (2014) [29] found that although serum leptin level was significantly higher in RA patients than in control group, there was no correlation with clinical and laboratory markers of disease activity. Mirfeizi et al. (2014) [30] also stated that leptin has no effect on the process of joint damage in RA patients. Oner et al. (2015) [31] did not find any correlation between disease activity and serum leptin levels, indicating that this adipokine is not a good biomarker to monitor inflammation in RA.
Thus, leptin seems to have a role in the pathophysiology of RA and comorbidities associated, such as obesity and metabolic syndrome. Leptin is considered a pro inflammatory adipokine by the great majority of authors who suggest a predominantly deleterious action on the joint. Only one survey showed that increased levels of leptin can act as a protective factor against the destructive course of RA.
Table 1 summarizes the main findings of leptin in RA patients.
Resistin
Isolated in rodents, resistin was first described in 2001. It is a protein rich in cysteine, compounded by 108 amino acids, called RELMs (resistin-like molecules) also known as FIZZ 332 [32]. In humans, it is originated mainly from circulating monocytes and macrophages [33].
It was initially correlated to the pathogenesis of insulin resistance in obesity and some cardiovascular diseases (CVD) but now is also considered an important link between obesity and inflammation [34]. Resistin has been found in areas of inflammation and seems to be mediated by IL-6 and TNF α [35].
Due to its implication in inflammation processes, the involvement of resistin in the pathogenesis of RA has been investigated. Kassem et al. (2010) [36] studied if there is a role of resistin in the pathogenesis of RA by investigating possible correlations between resistin concentration in serum and synovial fluid with disease activity and radiographic joint damage. The authors’ results supported the hypothesis that resistin is involved in the pathogenesis of RA and suggested serum resistin as a good marker of prognosis of the disease in RA patients. Yoshino et al. (2011) [21] compared serum resistin levels from RA patients and healthy control subjects. The authors found that the level of resistin in serum did not differ between patients and controls, but observed that serum resistin were positively associated with CRP levels in RA patients, suggesting a pro inflammatory action of this cytokine.
Kontunen et al. (2011) [22] reported that high levels of resistin are associated with RA, regardless of the presence of MetS. Fadda et al. (2013) [37] compared resistin levels in serum and synovial fluid of patients with RA and osteoarthritis and found higher levels in patients with RA. This result indicates a possible role of resistin in the pathogenesis of inflammatory rheumatic diseases. The high levels of this adipokine in the synovial fluid could suggest a bad prognosis for progression of RA, but the authors point out that more studies are needed to confirm if resistin is a good marker to evaluate the progression of this disease. Kang et al. (2013) [23] reinforced this hypothesis. The authors found an association between resistin levels and inflammatory markers in patients with RA. Recently, Bustos Rivera-Bahena et al. (2015) [24] demonstrated that resistin levels correlated positively with clinical manifestations of disease activity in patients with RA, albeit of patient body mass index. Huang et al. (2015) [38] in a meta-analysis concluded that serum resistin levels were significantly higher in RA patients compared to control group.
However, some authors did not show significant associations between serum resistin and HOMA-IR, nor differences between serum and synovial fluid resistin levels between RA patients and controls [19]. Al-Kady et al. (2010) [39] after studying the levels of resistin in of RA patients found no significant differences in resistin levels between RA patients and controls. Hammad et al. (2014) [40] also found no correlation between serum levels of resistin with clinical or laboratory markers in RA patients.
Thus, there is an important role of adipokines in the pathogenesis of obesity, CVD and inflammatory processes. The pro inflammatory action of resistin was observed in most studies of patients with RA, which suggest that this adipokines is a good marker to assess the progression of this disease.
Table 2 summarizes the main findings of resistin in RA patients.
Visfatin
Also known as PBEF (pre-B-cell colony-enhancing factor) or Nicotinamide Phosphoribosyltransferase (Nampt) [41], visfatin is a protein with molecular weight of 52 kDa, first described by Samal et al. (1994) [42]. It is primarily found in liver, bone marrow and muscle tissue, but also produced by adipose tissue and secreted by macrophage [43]. Its production is influenced by TNF-α, IL-6, Toll-like receptor (TLR) and chemokines [44]. Stofkova (2010) [45] reports that visfatin may contribute to inflammation processes, triggering production of cytokines and activation of nuclear factor kappa beta (NF-κβ). Thus, some studies have suggested some relation between this adipokine and the pathogenesis of type 2 diabetes and obesity [46] and increased cardiovascular risk [47].
Other studies have demonstrated a correlation between serum and synovial fluid levels of visfatin and the pathogenesis of RA [13, 41, 48, 49]. This adipokine can act as a regulator of inflammation and the destruction process of joints [35] and induce stimulation of great quantities of chemokines [50], thus possibly contributing to the inflammatory state of RA. However, its association to disease activity is not yet fully known [51].
Alkady et al. (2011) [52] showed that visfatin levels correlated with disease activity and may be involved in the progression of RA. Khalifa et al. (2013) [53] suggested that visfatin has a role in the pathogenesis of RA, and it may be considered as a marker of the disease and the radiographic bone lesion score. Therefore, it can be a potential therapeutic target for RA. El-Hini et al. (2013) [54] demonstrated positive and significant correlation between visfatin and insulin resistance and also with serum cholesterol, low density lipoprotein cholesterol (LDL-c) and triglycerides. Additionally, the disease activity score was positively correlated with visfatin.
Sglunda et al. (2014) [55] observed that visfatin levels in serum were significantly higher in RA patients compared to healthy individuals and suggested that reduction in visfatin concentrations could reduce disease activity in patients at early stage of RA. They also found positive association between this adipokine and elevated levels of total cholesterol, but not with the atherogenic index. Mirfeizi et al. (2014) [30] found that serum levels of visfatin in RA patients with radiographic joint damage were significantly higher than in patients without joint damage.
Nonetheless, Rho et al. (2010) [19] did not evidence relationship between visfatin and insulin resistance nor coronary atherosclerosis in patients with RA and Meyer et al. (2013) [56] did not show any correlation between serum levels of visfatin and radiographic progression of the disease.
Table 3 summarizes the main findings of visfatin in RA patients.
Adponectin
Adiponectin is an anti-inflammatory adipokine compounded by 244 amino acids and is produced and secreted mainly by adipocytes. [57, 58] Studies suggest that monomeric form of adiponectin appears to occur only in adipocytes, but there are three forms of adiponectin circulating in the body: trimmers (low molecular weight, LMW), hexamers (middle molecular weight, MMW) and multimers (high molecular weight, HMW) are the plasma circulating forms of adiponectin [58]. The receptors are AdipoR1and AdipoR2, respectively present at skeletal muscles and liver [59].
Several studies have demonstrated the role of this important anti-inflammatory cytokine in obesity, diabetes mellitus type 2, atherosclerosis and metabolic syndrome, being the highest levels a protective factor for these diseases [35, 60,61,62].
Paradoxically, in the pathogenesis of rheumatoid arthritis adiponectin seems to have proinflammatory effects in the joints, because its ability to stimulate the secretion of inflammatory mediators [63] and may also be associated to disease activity [52]. Scotece et al. (2012) [64] described the major effects of increased synovial and circulating levels of adiponectin in RA. They concluded that adiponectin in synovial fibroblasts induced prostaglandin (PG)E2, IL-6, IL-8, matrix metalloproteinase (MMP)-1 and MMP-13; in human chondrocytes induced nitric oxide (NO), IL-6, MMP-3, MMP-9, monocyte chemoattractant protein (MCP)-1 and IL-8 and promoted inflammation by increasing TNF-α, IL-6 and IL-8.
Krysiak et al. (2012) [65] suggested that these different actions can be explained by different mechanisms: LMW adiponectin has anti-inflammatory activities, while the HMW adiponectin has proinflammatory activities. However, Frommer et al. (2012) [66] showed a proinflammatory and destructive role of all isoforms of adiponectin in patients with RA, suggesting a much more harmful than beneficial action of adiponectin in chronic inflammatory diseases. Several studies evidenced association of adiponectin in radiographic progression of RA [67, 68]. Thus, serum adiponectin levels could be a good biomarker to evaluate the early stages of disease progression [56]. However, this association was not mediated by the selective effect of HMW adiponectin. [69] Recently, Skalska and Kontny (2016) [18] observed that HMW and MMW adiponectins potentially stimulated the secretion of rheumatoid ASC (adipose-derived stem cells) in patients with RA, but did not exert a strong impact on ASC towards RA-FLS (fibroblast-like synoviocytes) and peripheral blood mononuclear cells.
Furthermore, Rho et al. (2010) [19] did not find any association between adiponectin levels and insulin resistance or coronary artery calcium score. Yoshino et al. (2011) [21] also observed higher levels of adiponectin in serum of RA patients, but it was negatively associated with CRP levels. Bustos Rivera-Bahena et al. (2015) [24] did not evidenced association between adiponectin and disease activity and Chennareddy et al. (2016) [70] reported that despite serum levels of adiponectin are higher in RA patients than in controls there was no correlation with disease activity, duration, BMI and waist-to-hip ratio.
Despite the protective effect of adiponectin in the pathogenesis of obesity, diabetes mellitus, atherosclerosis, and metabolic syndrome, it is unclear whether this effect is reproduced in RA. Several studies emphasize that adiponectin appears to play a pro inflammatory role in the pathogenesis of RA, particularly in the joints, by stimulating the secretion of inflammatory mediators. In this scenario, it highlights the importance of developing new research elucidating the real role of adipokines in the pathogenesis of RA.
Table 4 summarizes the main findings of adipnectin in RA patients.
Conclusion
In recent years, it has been studied the importance of adipokines in the pathogenesis of RA, however the results are still conflicting and the exactly role of adipose tissue in RA is not yet fully understood. Despite studies have been demonstrating the implications of adipokines in the pathophysiology of autoimmune diseases, including RA, it is not yet clear their role in the progression of disease. It is noteworthy the complex pathophysiology of this disease, thus requiring better knowledge about the mechanisms of action of these adipokines in RA as well as the changes that drugs can promote in the circulating levels of these adipokines in these patients.
Abbreviations
- ACPA:
-
Anti–citrullinated protein antibody
- ASC:
-
Adipose-derived stem cells
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular diseases
- DAS:
-
Disease Activity Score
- FLS:
-
Fibroblast-like synoviocytes
- HMW:
-
High molecular weight
- HOMA-IR:
-
Homeostasis Model Assessment - Insulin Resistance
- IL:
-
Interleukin
- LDL-c:
-
Low density lipoprotein cholesterol
- LMW:
-
Low molecular weight
- LPS:
-
Lipopolysaccharide
- MCP:
-
Monocyte chemoattractant protein
- MetS:
-
Metabolic syndrome
- MMP:
-
Matrix metalloproteinase
- MMW:
-
Middle molecular weight
- Nampt:
-
Nicotinamide phosphoribosyltransferase
- NF-κβ:
-
Nuclear factor kappa beta
- NO:
-
Nitric oxide
- PBEF:
-
Pre-B-cell colony-enhancing factor
- PG:
-
Prostaglandina
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RA:
-
Rheumatoid arthritis
- RELM:
-
Resistin-like molecules
- RF:
-
Rheumatoid factor
- SciELO:
-
Scientific Electronic Library Online
- Th:
-
T helper
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
References
Lipsky PE. Artrite Reumatoide. In: Medicina interna de Harrison. 14th ed. Rio de Janeiro: Amgh Editora; 1998. p. 1996–7.
Mateen S, Zafar A, Moin S, Khan AQ, Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta. 2016;455:161–71.
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46(2):328–46. http://www.ncbi.nlm.nih.gov/pubmed/11840435
Barbosa VDS, Rêgo J, Antônio N. Possível papel das adipocinas no lúpus eritematoso sitêmico e na artrite reumatoide. Rev Bras Reumatol. 2012;52(2):278–87.
Hutcheson J. Adipokines influence the inflammatory balance in autoimmunity. Cytokine. 2015;75(2):272–9.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349 http://www.bmj.com/content/349/bmj.g7647
Neumann E, Frommer KW, Vasile M, Müller-Ladner U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 2011;63(5):1159–69.
Freitas Lima LC, Braga VA, do Socorro de França Silva M, Cruz JC, Sousa Santos SH, de Oliveira Monteiro MM, et al. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol. 2015;6:1–15.
Dichi I, Simão ANC. Metabolic syndrome: new targets for an old problem. Expert Opin Ther Targets. 2012;16(2):147–50. http://www.tandfonline.com/doi/full/10.1517/14728222.2012.648924
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. http://www.ncbi.nlm.nih.gov/pubmed/7984236
Guimarães DED, Sardinha FL DC, Mizurini D DM, Das GT Do CM. Adipocitocinas: uma nova visão do tecido adiposo. Rev Nutr. 2007;20(5):549–59. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-52732007000500010&lng=pt&nrm=iso&tlng=pt
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim S, et al. Leptin in human physiology and pathophysiology. AJP Endocrinol Metab. 2011;301:567–84.
Del Prete A, Salvi V, Sozzani S. Adipokines as potential biomarkers in rheumatoid arthritis. Mediat Inflamm. 2014;2014:1–12.
Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong M-L, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol. 2012;56(9):597–607. http://www.ncbi.nlm.nih.gov/pubmed/23329181
Olama SM, Senna MK, Elarman M. Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int. 2012;32(3):683–90. http://www.ncbi.nlm.nih.gov/pubmed/21140264
Toussirot É, Michel F, Binda D, Dumoulin G. The role of leptin in the pathophysiology of rheumatoid arthritis. Life Sci. 2015;140:29–36. http://linkinghub.elsevier.com/retrieve/pii/S002432051500257X
Scotece M, Conde J, López V, Lago F, Pino J, Gómez-Reino JJ, et al. Adiponectin and leptin: new targets in inflammation. Basic Clin Pharmacol Toxicol. 2014;114(1):97–102.
Skalska U, Kontny E. Adiponectin isoforms and Leptin impact on rheumatoid adipose Mesenchymal stem cells function. Stem Cells Int. 2016;2016:1–7.
Rho YH, Chung CP, Solus JF, Raggi P, Oeser A, Gebretsadik T, et al. Adipocytokines, insulin resistance, and coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2010;62(5):1259–64.
Targońska-Stepniak B, Dryglewska M, Majdan M. Adiponectin and leptin serum concentrations in patients with rheumatoid arthritis. Rheumatol Int. 2010;30:731–7.
Yoshino T, Kusunoki N, Tanaka N, Kaneko K, Kusunoki Y, Endo H, et al. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern Med. 2011;50(4):269–75. https://www.ncbi.nlm.nih.gov/pubmed/21325757
Kontunen P, Vuolteenaho K, Nieminen R, Lehtimäki L, Kautiainen H, Kesäniemi Y, et al. Resistin is linked to inflammation, and leptin to metabolic syndrome, in women with inflammatory arthritis. Scand J Rheumatol. 2011;40(4):256–62. http://www.ncbi.nlm.nih.gov/pubmed/21453187
Kang Y, Park H-J, Kang M-I, Lee H-S, Lee S-W, Lee S-K, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):1–7. http://www.ncbi.nlm.nih.gov/pubmed/24245495
Bustos Rivera-Bahena C, Xibillé-Friedmann DX, González-Christen J, Carrillo-Vázquez SM, Montiel-Hernández JL. Peripheral blood Leptin and Resistin levels as clinical activity biomarkers in Mexican rheumatoid arthritis patients. Reumatol Clin. 2016;12(6):323–6.
Xibille-Friedmann DX, Ortiz-Panozo E, Bustos Rivera-Bahena C, Sandoval-Rios M, Hernandez-Gongora SE, Dominguez-Hernandez L, et al. Leptin and adiponectin as predictors of disease activity in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(4):471–7.
Tian G, Liang J-N, Wang Z-Y, Zhou D. Emerging role of leptin in rheumatoid arthritis. Clin Exp Immunol. 2014;177(3):557–70. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4137840&tool=pmcentrez&rendertype=abstract
Lee YH, Bae S-C. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: a meta-analysis. Z Rheumatol. 2016;75(10):1021–7.
Allam A, Radwan A. The relationship of serum leptin levels with disease activity in Egyptian patients with rheumatoid arthritis. Egypt Rheumatol. 2012;34(4):185–90.
Abdalla M, Effat D, Sheta M, Hamed WE. Serum Leptin levels in rheumatoid arthritis and relationship with disease activity. Egypt Rheumatol. 2014;36(1):1–5.
Mirfeizi Z, Noubakht Z, Rezaie AE, Jokar MH, Sarabi ZS. Plasma levels of leptin and visfatin in rheumatoid arthritis patients; is there any relationship with joint damage? Iran J Basic Med Sci. 2014;17(9):662–6. http://www.ncbi.nlm.nih.gov/pubmed/25691942
Oner SY, Volkan O, Oner C, Mengi A, Direskeneli H, Tasan DA. Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatol Port. 2015;40(1):50–4.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. http://www.ncbi.nlm.nih.gov/pubmed/11201732
Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88(10):4848–56. http://www.ncbi.nlm.nih.gov/pubmed/14557464
Codoñer-Franch P, Alonso-Iglesias E. Resistin: insulin resistance to malignancy. Clin Chim Acta. 2015;438:46–54.
Abella V, Scotece M, Conde J, López V, Lazzaro V, Pino J, et al. Review article Adipokines. Metabolic Syndrome and Rheumatic Diseases J Immunol Researc. 2014;2014:1–15.
Kassem E, Mahmoud L, Salah W. Study of Resistin and YKL-40 in rheumatoid arthritis. J Am Sci. 2010;6(10):1004–12.
Fadda SMH, Gamal SM, Elsaid NY, Mohy AM. Resistin in inflammatory and degenerative rheumatologic diseases: relationship between resistin and rheumatoid arthritis disease progression. Z Rheumatol. 2013;72(6):594–600.
Huang Q, Tao S-S, Zhang Y-J, Zhang C, Li L-J, Zhao W, et al. Serum resistin levels in patients with rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Clin Rheumatol. 2015:1713–20. http://link.springer.com/10.1007/s10067-015-2955-5
Al-kady EA, Ahmed HM, Tag L, Adel M, Al-Kady EA. Adipocytokines: Adiponectin, Resistin and Visfatin in serum and synovial fluid of rheumatoid arthritis patients and their relation to disease activity. Med J Cairo Univ. 2010;78(2):723–9.
Hammad MH, Nasef S, Musalam D, Ahmed MM, Osman I, Hammad MH. Resistin, an adipokine , its relation to inflammation in Systemic Lupus Erythematosus and Rheumatoid Arthritis. Middle East J Intern Med. 2014;7(3):3–9.
Bao JP, Chen WP, Wu LD. Visfatin: a potential therapeutic target for rheumatoid arthritis. J Int Med Res. 2009;37(6):1655–61. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20146863
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell Colony-enhancing. Mol Cell Biol. 1994;14(2):1431–7.
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–30. http://www.ncbi.nlm.nih.gov/pubmed/15604363
Kerekes G, Nurmohamed MT, González-Gay MA, Seres I, Paragh G, Kardos Z, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol. 2014;10(11):691–6. http://www.ncbi.nlm.nih.gov/pubmed/25090948
Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul. 2010;44(1):25–36. http://www.ncbi.nlm.nih.gov/pubmed/20151765
Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91(4):1578–81. http://www.ncbi.nlm.nih.gov/pubmed/16449335
Romacho T, Sánchez-ferrer CF, Peiró C. Review article Visfatin / Nampt: an Adipokine with cardiovascular impact. Mediat Inflamm. 2013;2013:1–16.
Naguib A, Elsawy N, Aboul-enein F, Hossam N. The relation between serum visfatin levels and cardiovascular involvement in rheumatoid arthritis. Alexandria J Med. 2011;47(2):117–24. https://doi.org/10.1016/j.ajme.2011.07.005%5Cnhttp://linkinghub.elsevier.com/retrieve/pii/S2090506811000479
Gómez R, Suarez A, Villalvilla A, Herrero-Beaumont G, Largo R, Young DA. Visfatin: a new player in rheumatic diseases. Immunometabolism. 2013;1:10–5. http://www.degruyter.com/view/j/immun.2013.1.issue/immun-2013-0002/immun-2013-0002.xml
Meier FMP, Frommer KW, Peters MA, Brentano F, Lefèvre S, Schröder D, et al. Visfatin/pre-B-cell colony-enhancing factor (PBEF), a proinflammatory and cell motility-changing factor in rheumatoid arthritis. J Biol Chem. 2012;287(34):28378–85.
Kim KS, Choi HM, Ji HI, Song R, Yang HI, Lee SK, et al. Serum adipokine levels in rheumatoid arthritis patients and their contributions to the resistance to treatment. Mol Med Rep. 2014;9(1):255–60.
Alkady EAM, Ahmed HM, Tag L, Abdou MA. Adiponectin, Resistin und Visfatin in Serum und Gelenkflüssigkeit bei Patienten mit rheumatoider Arthritis. Z Rheumatol. 2011;70(7):602–8. http://link.springer.com/10.1007/s00393-011-0834-2
Khalifa IA, Abdelfattah A. Relation between serum visfatin and clinical severity in different stages of rheumatoid arthritis. Egypt Rheumatol Rehabil. 2013;40(1):1–8.
El-Hini SH, Mohamed FI, Hassan AA, Ali F, Mahmoud A, Ibraheem HM. Visfatin and adiponectin as novel markers for evaluation of metabolic disturbance in recently diagnosed rheumatoid arthritis patients. Rheumatol Int. 2013;33(9):2283–9.
Sglunda O, Mann H, Hulejová H, Kuklová M, Pecha O, Pleštilová L, et al. Decreased circulating visfatin is associated with improved disease activity in early rheumatoid arthritis: data from the PERAC cohort. PLoS One. 2014;9(7):1–5.
Meyer M, Sellam J, Fellahi S, Kotti S, Bastard J-P, Meyer O, et al. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: results from the ESPOIR cohort. Arthritis Res Ther. 2013;15(6):1–13.
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–9. http://www.ncbi.nlm.nih.gov/pubmed/7592907
Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ. Adiponectin, the controversial hormone. Public Health Nutr. 2007;10(10A):1145–50. http://www.ncbi.nlm.nih.gov/pubmed/17903323
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. http://www.ncbi.nlm.nih.gov/pubmed/17268472
Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94(10):2137–42.
Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64(1):1–10. http://www.ncbi.nlm.nih.gov/pubmed/23850004
Simão TNC, Lozovoy MAB, Simão ANC, Oliveira SR, Venturini D, Morimoto HK, et al. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br J Nutr. 2013;110(10):1885–94. http://www.journals.cambridge.org/abstract_S0007114513001207
Chen X, Lu J, Bao J, Guo J, Shi J, Wang Y. Adiponectin: a biomarker for rheumatoid arthritis? Cytokine Growth Factor Rev. 2013;24(1):83–9.
Scotece M, Conde J, Gómez R, López V, Pino J, González A, et al. Role of adipokines in atherosclerosis: interferences with cardiovascular complications in rheumatic diseases. Mediat Inflamm. 2012;2012:1–14.
Krysiak R, Handzlik-Orlik G, Okopien B. The role of adipokines in connective tissue diseases. Eur J Nutr. 2012;51(5):513–28.
Frommer KW, Schäffler A, Büchler C, Steinmeyer J, Rickert M, Rehart S, et al. Adiponectin isoforms: a potential therapeutic target in rheumatoid arthritis? Ann Rheum Dis. 2012;71(10):1724–32. http://www.ncbi.nlm.nih.gov/pubmed/22532632
Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis [Internet]. 2011;70(9):1562–8. http://www.ncbi.nlm.nih.gov/pubmed/21571734
Klein-Wieringa IR, Van Der Linden MPM, Knevel R, Kwekkeboom JC, Van Beelen E, Huizinga TWJ, et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2011;63(9):2567–74.
Klein-Wieringa IR, Andersen SN, Herb-Van Toorn L, Kwekkeboom JC, Van Der Helm-Van Mil AHM, Meulenbelt I, et al. Are baseline high molecular weight adiponectin levels associated with radiographic progression in rheumatoid arthritis and osteoarthritis? J Rheumatol. 2014;41(5):853–7.
Chennareddy S, Kishore Babu KV, Kommireddy S, Varaprasad R, Rajasekhar L. Serum adiponectin and its impact on disease activity and radiographic joint damage in early rheumatoid arthritis – a cross-sectional study. Indian J Rheumatol. 2016;11(2):82–5.
Funding
The authors declare that they had no funding for this study.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Authors and Affiliations
Contributions
ECSF and FTR made substantial contributions to acquisition and interpretation of data, and writing the manuscript. ANCS and ID contributed to conception of the study, and revising it critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fatel, E.C.d., Rosa, F.T., Simão, A.N.C. et al. Adipokines in rheumatoid arthritis. Adv Rheumatol 58, 25 (2018). https://doi.org/10.1186/s42358-018-0026-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-018-0026-8