Abstract
Food demand is directly associated with the human population. Due to various plant diseases, there has been a reduction in crop yield. There is an extreme necessity to low such losses in crop yield to meet the rising demand for food. Novel and eco-friendly control approaches should be developed for combating bacterial diseases of crops. Recent control strategies that involve the usage of antibiotics or chemicals are no more effective because of resistance developed by bacterial species. Furthermore, the usage of such agents has proven to be not environmentally friendly. To overcome these issues, bacteriophages are used as an alternative solution. Phages are viruses that attack specific bacterial species, and within current years much consideration is received by them in controlling different diseases caused by bacteria. Phages can be used for controlling different crop-related diseases. Several phage-based products are accessible in the market. Compared to chemical control methods, phage biocontrol offers several advantages. Mixtures of phages can be employed to target pathogenic bacteria. Unlike chemical control strategies, phage mixtures can be readily adjusted to counter any potential resistance. This review summarizes the use of phages as a biocontrol agent against phytopathogens.
Similar content being viewed by others
Background
The global population is expected to grow about 9.6 billion by the year 2050, which projects the emergence of scarcities of agricultural and food resources. To meet the demands of a growing food supply, crop production is likely to increase by about 70–80% by improving the efficiency of agricultural units (Raina et al. 2022). Normally, the crops are largely subjected to several factors like climate change, the gap in technology, pests, and plant diseases that lower the pace of production (Wang et al. 2022a, b). Specifically, plant diseases affect about 10% of global food in developing and emerging countries. The major plant pathogens include several parasitic plants, fungi, viruses, nematodes, and bacteria that are also known as phytopathogens (Daulagala 2021). Two hundred bacterial phytopathogens have been reported yet, and the most important genera of these pathogens are Xanthomonas, Ralstonia, Erwinia, Pseudomonas, and Pectobacterium. These pathogens are very high in virulence and can adapt to changing environments and are difficult to handle. Efficient disease control management is very crucial for a stable and effective food supply to consumers.
Antibiotics and copper compounds are considered the best antibacterial agents for the control of phytopathogenic bacteria. They are readily available and often used worldwide to control pests' attacks on several crops and affect productivity (Pereira et al. 2021). Antibiotics that are used widely include tetracycline, streptomycin, and kasugamycin, but they have some risks associated with them like emergence of resistant species, which cause hindrance in the management of plant diseases (Miller et al. 2022). The application of copper-based compounds as pesticide affects both the environment and the agricultural system. They cause phytotoxicity and bioaccumulation of these compounds on the surface of soil also occurs that leads to a reduction in microbial diversity (Tudi et al. 2021). Recently, several classes of control agents like pesticides and antibiotics have been completely banned in western countries due to their undesirable toxic characteristics (Alengebawy et al. 2021).
While taking into consideration all the damaging effects of pesticides on crop productivity, the alternative control agents of plant diseases having desirable characteristics are needed to be synthesized urgently (Elnahal et al. 2022). The best possible way to achieve this target is by using new tools and machinery based on biocontrol agents (BCAs) that synthesize a pest control agent with minimal negative impact on the environment. Biocontrol of plant diseases mainly includes the use of microorganisms (Izraeli et al. 2021). Biological control means utilizing the living entity for the control of any pathogens and parasites of plants. Biocontrol agents are capable to suppress the activities of pathogenic bacteria as well as their reproduction. Moreover, the basic idea of biocontrol agents majorly involves the strategy to reduce the incidence of disease by either direct or indirect manipulations of the microbial population (Bhardwaj et al. 2022).
The use of bacteriophage as a biocontrol agent (BCA) has become a major growing interest to deal with phytopathogens (Pandit et al. 2022). Phages are viruses that infect bacteria only with no harmful effect on plants and animals. Bacteriophages have been discovered in the twentieth century and are considered as most abundant and diverse in the natural environment and affect various ecological and biological processes having their role in bacterial mortality and genetic exchange (Chevallereau et al. 2022). They can be found in every domain or habitat, mainly in the soil and oceans. They infect the bacteria by using lysogenic or lytic cycles (Jamal et al. 2019). In the case of the lysogenic cycle, a bacteriophage integrates its genome directly into bacterial cell chromosomes after which they replicate and form daughter cells (Abedon 2022). They initiate the infection by attaching themselves to specific bacteria by adsorption and inserting their genome into the bacterial cytoplasm. In the case of the lytic cycle, phages are intended to utilize the ribosomes of host bacteria to make their proteins right after the injection of genetic material. At the end of this cycle, phage has produced some virion components that are lysin and holins. Lysins are also known as endolysin that cause lysis of bacterial cells (Murray et al. 2021). The virulent nature of lytic phages along with their exponential increase in numbers after each infection cycle makes them the best alternative of pesticides use (Boyer et al. 2022).
In the year 1920, phages were discovered, and right after a decade, they were subjected to be used as a potential therapeutic agent in the agriculture sector. Many plant diseases caused by bacterial pathogens like Xanthomonas campestris, Pseudomonas syringae, Dickeya solani, and Erwinia amylovora have been reported to be cured by bacteriophages (Holtappels et al. 2021). In this review, some major aspects regarding bacteriophages like history, life cycle, limitations, advantages as well as commercialization and control applications of bacteriophages as biocontrol agents have been summarized.
History of bacteriophages in controlling phytopathogens
Bacteriophages were discovered by Frederick Twort and Felix d’Herelle in the year 1917. Formerly, some other findings were also being reported about the bacteriophages, but these two were the first ones who suggested the existence of bacteriophage (Aswani and Shukla 2021). Moreover, the therapeutic potential of bacteriophage was being reported in the year 1919 by d’Herelle, while treating patients infected with diarrhea in Hôpital des Enfants-Malades in Paris (Leitner et al. 2021). After that, many other scientific studies were carried out to treat several bacterial infections like cholera and Staphylococcal infections by using bacteriophages in humans (Alomari et al. 2021). Furthermore, plants and crops were also subjected to phages for treating bacterial diseases. In the year 1924, scientists carried out studies regarding disease management in plants by using filtrate of cabbages after decomposition that successfully inhibited the growth of Xanthomonas campestris on cabbage crop (Nakayinga et al. 2021). Similarly, in 1925, a bioassay was carried out by a group of researchers in which soft rot diseases on carrots and potatoes were controlled effectively (Lee et al. 2021). Practically, the first-ever successful field trial of bacteriophage was carried out in 1935 in which Stewart’s wilt disease caused by Pantoea stewartia was treated (Jones et al. 2021). Bacteriophage was introduced to treat highly virulent strain of Agrobacterium tumefaciens and thus inhibited the activity of bacterial strain completely (Grace et al. 2021).
Recent applications of bacteriophages for controlling phytopathogens
Almost every phage-based research and publication is confined to the isolation and characterization of bacteriophages, while some of them gave insight into the potential of phage therapy (Hatfull et al. 2022). Bacteriophages possess a wide range of biocontrol applications that are widely used in the field of agriculture to protect plants from various bacterial diseases (Stefani et al. 2021). Several transgenic plants express the specific bacteriophage enzymes for their survival and protection from bacterial pathogens (Harshitha et al. 2022). In the last decade, several bacterial plant pathogens were described by numerous scientists and pathologists. Out of them, one of the most important plant pathogens is the P. syringae responsible to cause several plant diseases in monocots as well as in dicot plants from all over the world (Sakata et al. 2021). To check the efficacy of phage in this case, two different field trials were conducted on a phage cocktail of about six isolated bacteriophages. This trial was carried out by using a phage as a biocontrol against P. syringae that caused the bacterial blight disease in leek. So, it was evident from the experiment that the phage cocktail has the potential therapeutic cure for the bacterial blight disease and successfully eradicates the plant infection caused by the P. syringe (Liu et al. 2021a, b).
Bacteriophages that are used for biocontrol in agriculture must be stable enough in the environment to tolerate (UV) radiations, temperature fluctuations, and chemical agents and must be lytic (Wang et al. 2022a, b). Agriphage is a phage-based product, produced by the approved US company Omnilytics, which can control the bacterial spot diseases of peppers and tomatoes. Several bacteriophage enzymes such as ΦXo411 and Lys411 have been isolated showing lytic activity against the Xanthomonas (Grabowski et al. 2021). Phage should be applied directly while treating bacterial infections at early stages for better and more efficient results. Referring to experimental trials, Xanthomonas campestris pv. pruni is a bacterial pathogen that causes leaf spots. It was subjected to two different treatment approaches. In the initial phase, the treatment was administered one hour prior to the bacterial inoculations, while in the subsequent phase, it was applied 24 h in advance of the bacterial inoculation (Clavijo-Coppens et al. 2021). The results were quite astonishing during the first trial. Lately, the sensitivity was checked to monitor the phage survival in different climatic conditions in both controlled and uncontrolled conditions. Fruits were also treated with phage suspension, and 92% of tested fruits did not develop the disease. The 10 times decrease in population of phage was noticed compared to phage applied in controlled climatic chamber. The only reason for the decrease in the phage population is adverse environmental conditions like dehydration, UV radiations, and high temperature (AI Raish et al. 2021). Moreover, scientists have also demonstrated that the activity of phage concerning their time for a specific application like dawn and dusk is the most convenient time for the better activity of phage. At those specific times, the reduction of UV radiation is observed, which increases the efficiency of phage activity (Wintachai et al. 2022).
The selection of suitable bacteriophages in growing conditions is also an important element. RSL1 is a phage against bacterium Ralstonic solanaceum, which showed a significant resistance pattern when exposed to high temperatures, i.e., 37–50 °C. Tomato plants, infected with bacterium R. solanaceum, were wilted after 4 days of infection. However, when these plants were exposed to phage RSL1, no wilting pattern was observed. Phage RS1 can prevent the wilting pattern in these plants by limiting the growth of R. solanaceum cells (Sasaki et al. 2021). In recent studies, potatoes and tomatoes are the two most important crops that are highly benefited from phage biocontrol applications. In European countries, a potential phage has also been formulated for the control of D. solani. In the last decade, a bioassay was conducted in a field trial in which phage (LIMEstone1) was used. In this bioassay, seed tubers (cultivar Bintje) were inoculated with a specific host bacterium and phage (with an MOI of 100) (Bartnik et al. 2022). Tubers inoculated with phage and bacterium showed only 10% maceration of tissues as compared to the control, which exhibited 40% tissue maceration. Streptomyces scabies is a Gram-positive bacterium responsible for causing infections on potatoes leading to the formation of corky lesions generally known as common scabs and results in reduced growth of seedlings. The pathogen can be effectively treated by using phage so the chances of infection in potato crop can be successfully eliminated. In summary, the above studies demonstrate phage potential in treating various plant diseases (Abdelrhim et al. 2021).
Bacteriophages have also been used in field conditions and greenhouses. To control R. solanacearum, phage was applied directly into the rhizosphere through soil drenching that was effective in the suppression of the development of wilting in the tomato plant (Kizheva et al. 2021). In the case of other soil-borne pathogens like Xanthomonas euvesicatoria, X. campestris pv. campestris, and P. carotovorum subsp. carotovorum, foliar spraying method was used to eradicate the disease incidence in plants caused by these pathogens. Furthermore, another application regarding the potential of filamentous phage has also been reported. The filamentous ΦRSM-type phage enhances the eradication of R. solanacearum in tomato plants (Umrao et al. 2021).
Besides biocontrol applications, some significant factors affect the phage effectiveness used in the field of agriculture. In a recent evident study, it was demonstrated that the pH of the fruit has a vital impact on the phage stability as well as its activity (Bastas and Baysal 2022). In the case of fresh-cut melon, application of the potential phage cocktail that is specific to Salmonella was effective, as the bacterial population was significantly reduced by 3.5 logs at 10 °C and 2.5 logs at 20 °C. Conversely, in the case of apple, the same phage cocktail was applied and no reduction in bacterial population was observed. It was predicted that it may be possible due to the low pH of apples (Śliwka et al. 2022).
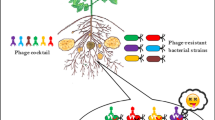
To ensure the formulations of phage in such a way that it can possess a wide host range, multiple phages were combined into a mixture known as a bacteriophage cocktail. It is an effective way to recompense the host range limitation of a single bacteriophage. This technique can also eradicate the chances of the development of phage-resistant bacteria (Rahimzadeh et al. 2021). Studies regarding the effectiveness of phage cocktail on Xanthomonas are also quite important to invective the impact of different environmental factors on phage therapy (Nga et al. 2021). Recently, a study reported regarding the biocontrol activity of phages against P. syringae. Aerosol of single phage containing 5% sucrose and 3% corn flour sprayed on bean leaves before inoculation of P. syringae. The disease severity was reduced about 60% when a single phage was used and about 70% reduction was observed by using a phage cocktail (Rasool et al. 2021). Moreover, further research is needed in the field of agriculture to implement the role of bacteriophages on a global scale. In crops propagation, the stability of phage cocktails mainly depends upon phage resistance to adverse environmental factors (Farooq et al. 2022).
Lytic proteins of bacteriophages for controlling phytopathogens
Bacteriophages have evolved numerous strategies to release hundreds of new progenies from the host to the external environment (Łoś et al. 2021). Most filamentous phages constantly release new virions from the host cell through extracellular vesicles without lysing the bacterium. While in some cases, bacteriophages release their progenies through lytic enzymes which are a quite beneficial in controlling the pathogenic bacteria as the phages possess a wide range of hosts. That’s why they can easily eliminate the risk of the development of resistant strains by reproducing and infecting a wide variety of bacterial strains.
Bacteriophages have evolved group of lytic enzymes that are responsible for the degradation of the peptidoglycan layer of the bacterial host. These enzymes are known as endolysin (Abdelrhim et al. 2021). During the late stages of replication, these hydrolases are activated with the help of holin proteins and destroy the peptidoglycan (PG) layer of the bacterial host which results in the release of progeny virions. Endolysins are classified into five different groups based on the bonds in peptidoglycan they target. Phage infecting Gram-negative bacteria produces endolysin with a single globular domain known as enzymatically active domain EAD that is responsible for the digestion of the PG layer (Deka et al. 2022).
Endolysins CN77 and CMP1 have also been reported for lysing Clavibacter nebraskensis and C. michiganensis respectively. The endolysin can lyse C. michiganensis subspecies specifically without affecting the soil bacteria (Zhang et al. 2022). Moreover, endolysins have extended activity and they are capable to infect other opportunistic pathogens like Stenotrophomonas maltophilla, Pseudomonas aeruginosa as well as Xanthomonas species by simply degrading their peptidoglycan (Diallo et al. 2021).
Bacterial leaf blight is a rice crop disease caused by Xanthmonas oryzae (Liu et al. 2021a, b). Several endolysins such as ΦXo411and Lys411 have been isolated to show wide lytic against Xanthomonas spp (Rahman et al. 2021). Recently, a new endolysin is discovered from bacteriophages effective against Agrobacterium tumefaciens. It is a soil-borne pathogen that causes severe diseases in orchard crops (Valencia-Hernandez et al. 2023).
Expression of genes of endolysin in several plants is a way to reduce resistance in pathogenic bacteria. Clavibacter michiganensis is a species that causes bacterial infections such as wilt and canker in the tomato plant (Sato 2022). This infection can be prevented by using a bacteriophage endolysin, namely CMP1, normally expressed by transgenic tomato plants. The application of endolysin was not only limited to bacteria as its effect has also been noticed in various cases of fungi. A remarkable resistance pattern to infection has been observed when T4 lysozymes bacteriophage was introduced into those plants that have been infected by several species of fungi like Magnaporthe oryzae and Rhizoctonia solana.
The endolysin can disrupt the cell wall of Gram-negative and Gram-positive bacteria when applied exogenously and have the potential to hydrolyze the peptidoglycan layer of Gram-positive bacteria more easily due to the absence of their outer membrane (Lee et al. 2022). This characteristic makes them an alternative source of antimicrobials especially in controlling bacterial drug resistance. Their modular structure with different binding and catalytic domains are a tool for the development of bioengineered lysin products with higher activity and desired properties. The engineering of endolysins allows swapping among different domains to enhance their efficiency by increasing their lytic activity. The engineering of endolysin also results in the production of chimeric enzymes with improved solubility and binding affinity (Sabri et al. 2021).
Use of bacteriophages with other strategies for controlling plant diseases
Researchers have started examining outcomes caused by a combination of phages with other disease control measures. The hypersensitive response of different phages in several infected plant models has been studied against Xanthomonas spp (Abdelsattar et al. 2021). The combined technique of phage with other control agents and chemicals like copper hydroxide with mancozeb resulted in synergistic benefits in the control of pathogens of various plants. Copper and mancozeb can help in the control of plant disease as they can enhance the penetration of the plasma membrane (Sulley et al. 2021).
Other evidence regarding the combination of phage with other control strategies in plants has also been reported. Pantoea agglomerans has been considered as an excellent biocontrol agent as they can suppress fire blight in some plants caused by Erwinia amylovora (Biosca et al. 2021). Besides, the enhanced biocontrol activity would be achieved if it is used with the combination of phage. A similar enhancement has also been observed in the case of treating tobacco bacterial wilt. When Ralstonia solanacearum, a bacteriocin-producing strain, was used with the combination of phage, remarkable results were obtained (Nakao et al. 2021). Besides, there is an organic compound, namely Acibenzolar-S-methyl (ASM), that is commonly used as a fungicide has shown increased control activity when it was combined with bacteriophage against bacterial disease in the tomato plant (Gao et al. 2021).
The combination of phage with antimicrobial agents has contributed well to the reduction of several plant diseases. Systemic acquired resistance (SAR) is a kind of resistance in plants acquired due to exposure to several virulent and pathogenic microbes (Das 2021). SAR inducers combined with other antibacterial agents have been evaluated. Bacteriophage shows an effective control phenomenon when used in the combination of acibenzolar-S-methyl (ASM). The ASM is a synthetic compound related to the plant defence hormone salicylic acid and helps in inducing systemic acquired resistance (SAR) against several pathogens in plants.
Employing carrier bacteria with bacteriophages for controlling phytopathogens
Besides several environmental factors, the abundance of host bacteria can also cause variations in phage count. To improve the activity and efficiency of phage, the idea of the combination of phage with non-pathogenic bacteria has been raised (Düzgüneş et al. 2021). Non-pathogenic carrier bacterium should be used with the combination of phage, as it will not cause any harm to the plant nor phage properties. In this regard, several strategies have been investigated (Gayder 2021). Recently, a group of researchers have isolated Erwinia amylovora phages to characterize them and to use them with carrier bacteria. Initially, a temperate phage was used that was capable to infect E. amylovora species as well as a saprophyte (Doukkali et al. 2022). Currently, a carrier system of P. agglomerans has been combined with the phages that had reduced the risk of blossom blight diseases. This combination also reduced the risk of fire blight in the plant as well. In a nutshell, co-application of phage with carrier bacteria has reduced the risk of infection to a greater rate (Meile et al. 2022). This process is considered as economical as they do not need any purification which is an advantage too.
Challenges and issues associated with bacteriophages for controlling phytopathogens
Though the use of bacteriophages to control bacterial plant pathogens was successful at early stages, phage therapy did not end up to its potential and applied antibacterial stratagem for the control of pathogenic plant-bacteria because of issues with reliability and efficacy. In 1963, Okabe, a forerunner in the field, concluded, in general, that phages appeared to be inefficient as a controlling agent. Furthermore, their narrow range of action against a particular bacterial strain puts them in a difficult spot against various antibacterial agents, i.e., antibiotics, which have had an extensive range of action (Majkowska‐Skrobek et al. 2021).
Emergence of bacterial-resistant strains against bacteriophages
While considering phages to be used as biological control agents, the real concern is the possibility of bacterial mutation that makes them resistant to specific phages. As various studies have shown, when identifying an appropriate phage as a biocontrol agent, the major factor that could be drastic is genetic variability shown by bacterial strains related to specific plants that might not have existed previously (Larrahondo-Rodríguez et al. 2022). For example, Xanthomonas campestris pv vesicatoria, a bacteria linked with the bacterial spot disease of pepper and tomato, now has about four different species (Balogh et al. 2008). Different phages are required to control the disease for each of the four strains because phages are mostly specific to a particular strain of a species of bacteria. Besides, there could be a significant change in the specificity of phage to different strains in a bacterial species.
Selection of right phage
Different criteria for selection have been used by researchers to identify phages to be used as part of the biological control of phytopathogens. For biological control experiments, from a collection of eight phages, a lytic phage with the widest host range was chosen against X. campestris pruni, a pathogen linked with a bacterial spot of peach and plum. Balogh (2006) observed in a study on X. citri that causes bacterial citrus canker, that on the surface of leaves of grapefruit, phages differ in their ability to multiply and interact with host bacterium. It was also tracked down in vitro traits, for example, plaque size, the ability to reproduce in liquid media, or the efficacy to decrease bacterial counts in liquid media against bacterial tomato spot (Balogh, un-published results). Evidently, before the implementation of phages as efficient a biocontrol agent their pre-screening is recommended rather than randomly choosing them based on their lytic activity only (Villarroel et al. 2016).
Persistence of bacteriophages in rhizosphere and phyllosphere
Because the efficiency of biocontrol is affected by the density of targeted pathogen and biocontrol agents, maintaining the high populaces of biocontrol agents in a close environment to the targeted bacterium is quite important. To target the bacterium, phage should be present above the particular threshold level; lower than the threshold level it will have a lesser effect on the targeted bacterium and consequently control of the disease (Abedon 2015). The physical accessibility of targeted bacterium to phage is one factor that must be considered related to the phyllosphere other than target bacterium and phage densities. In 2015, Abedon mentioned the probability of targeted bacterium living in spatial escapes, which are unreachable to the biocontrol agent (Dewald-Wang et al. 2022a, b).
The administered phage populations could decrease over time because of the extremely deleterious environment of the phyllosphere. In the phyllosphere, the major limiting factor for phage therapy is the short-lived persistence on surfaces of the plant leaves. The viruses were inactivated by low and high pH; sunlight and increased temperature were easily removed by rain. The most destructive of all the ecological factors are the spectra of sunlight (wavelength range 280–400 nm) UV-A and UV-B for the survival of the virus. Though the application of phage in the early evening or late afternoon helped in the persistence of the phage, letting the phage population increases to react with bacterial strains on the surfaces of the leaf.
Advantages of using bacteriophages over other techniques
Without any harm, humans are exposed to phages daily as phages exist in the environment naturally, as compared to chemical biocides. If the target bacterial host is reachable to the phage, their numbers increase after application (Majdinasab et al. 2021). However, phages manage to multiply if the target host is available to them. Unlike metal-based pesticides, phage does not efficiently accumulate in the soil (López-Martín et al. 2021). Generally, phages only target a specific strain of bacterium within a species. Basit et al. (1992) isolated a phage that could kill the bacterium that did not help in nitrogen fixation, but was not able to infect the strain Bradyrhizobium japonicum that help in soyabean growth because of its nitrogen-fixing properties, ultimately leading to better crop growth (Azeredo et al. 2021).
A significant factor in the virulence of plant pathogens is biofilm formation. It is involved in the resistance of bacterial plant pathogens to metal bactericides (Fessia et al. 2022). But phages have adapted to cope this problem of biofilm, by the release of depolymerase enzymes that destroy the biofilm material, making the receptors on the surface of the target bacterium accessible to the phage (Born et al. 2015). The increasing consumers demand for chemical-free preservatives, and biocide-free food products have led to limited use of chemicals on crops. Phages could be registered as biopesticides as they naturally exist in the environment, which makes them appropriate for organic consumer-friendly farming.
Methods for applications of bacteriophages for getting maximum activity
Poor persistence on the phyllosphere is one of the limiting factors for efficient biocontrol on crops by phage, though various methods have been studied to minimize this issue. With the availability of living hosts in the rhizosphere and phyllosphere, phage persistence could be improved (Balogh et al. 2003). Phage-based control could be improved by applying the phage during dark hours. Certainly, longer phage persistence was observed in the phyllosphere when phage was applied in the evening, allowing phage to infect and kill their targeted bacteria (Buttimer et al. 2017).
A study conducted by Born et al. (2015) with various substances to examine whether they protect phage against UV showed that pure aromatic amino acids, astaxanthin, tween 80, and casein as well as natural extracts from beetroot, red pepper, and carrot are protective against UV radiations. None had any negative effect on the stability and infectivity of phage. Hence, it shows that a vast range of substances, which absorb UV resulting in limiting its exposure to phage, can boost the performance of phage in the phyllosphere. These protective effects are also observed with the use of biodegradable polymers (Nga et al. 2021). Also, Balogh et al. (2003) showed improved activity of phage by applying the following combinations with phage: (1) 0.5% sucrose and 0.75% skim milk, (2) 0.5% sucrose and 0.5% (PCF) pregelatinized corn, and (3) 0.25% (PCF) pregelatinized corn, 0.5% sucrose and 0.5% Casecrete NH400. These experiments were performed with phages against Xanthomonas campestris pv. vesicatoria on tomato plants in field and greenhouse trials. All formulations mostly showed improved disease control (Collinge et al. 2022).
Commercialization of bacteriophages as a biocontrol agent
In the last decade, various biocontrol agents have been introduced into the market commercially. Omnilytics is a first-ever USA-based company that received registration regarding phage-based biocontrol products. Agriphage is a phage-based product that can control the bacterial spots of peppers and tomatoes (Nawaz et al. 2016). Another Hungarian-based company Enviroinvest received registration regarding their product, namely Erwiphage, designed to control apple tree disease (Epstein and Bassein 2003). Besides, a Scottish company owns a product, namely APS biocontrol, specialized for the control of soft rot diseases caused by Enterobacteriace (Crane and Giddings 2004). However, in some countries, there are some gaps and delays in legislation to allow the biocontrol agent in controlling bacterial plant diseases. This problem was arisen due to the constant changing of newly emerging strains of target bacterium. The problem could be overcome by the adaptations of new emerging strains as well as constant updating. Moreover, the legislation related to phage biocontrol should be pliable for the best applications and implementation of biocontrol agents (Murray et al. 2008).
Legal status of bacteriophages as biopesticides
Notably, microbial biocontrol agents (MBAs) are highly effective biopesticides made of living microorganisms. Though viruses ought not to be categorized this way, the international regulatory framework categorizes viruses along with other true microorganisms, i.e., fungi and bacteria (Chatzopoulou et al. 2020). Concerning various pesticides, before marketing and registration, MBAs ought to go through very cautious hazard assessment to environmental fate and food safety. The evaluation system of the EU was first established in regulation 91/414/EEC, followed by directive 1107/2009 which annulled and succeeded the regulation. Along with its execution, just 26% of registered Plant Protection Products (PPP) and active substances qualified the audit, compared to those under regulation 91/414/EEC. Hence, because of increased awareness about environmental and consumers safety, only 1/4th of the active substances have not been annulled in agriculture. In the USA, the regulatory framework is less complicated, because only two administrations are concerned, the Food and Drug Administration (FDA) and Environmental Protection Agency (EPA) (Hazards et al. 2017). On the other hand, in European Union EU has at least four main administrations involved: the committee on Plants, Animals, Food and Feed (PAFF Committee), the Directorate-General for Health and Food Safety (DG SANTE), the European Food Safety Agency (EFSA), that provides risk communication and risk assessment and the Rapporteur Member State, where the candidate presents a report that contains all related data on the MBA (Spök et al. 2008). The participant states are categorized into three assessment zones. The MBA could be incorporated in the list of permitted active substances, at the end of the procedure that requires 26–36 months. On patenting bacteriophages, a comprehensive insight is provided by Holtappels et al. (2021), where they found a great association between the patent documents and published papers. Approved patent calls are quite comprehensive covering application methodology. The increased patenting activity and publishing show there is great interest in bacteriophages. On the contrary, very few bacteriophages based legal biopesticides are available commercially. In a reported opinion, EFSA removed bacteriophages from the Qualified Presumption of Safety (QPS). The QPS list enlists microorganisms deliberately introduced into the feed and food production chain. In 2007, it was first established that in the framework of commercial approval, it is updated and revised every year by EFSA. List as: (1) the last level of taxonomy (Caudovirales order) is too big, (2) shortage of detailed assessment at the genomic level to differentiate between non-transducing and transducing bacteriophages or if they contain virulence factors (Svircev et al. 2018). Therefore, raised safety concerns in the EU end up leading to comparatively rigid registration procedures and regulations before the products could be commercialized in the market. Ultimately, Svircev et al. (2018), presenting the potential of bacteriophages in agricultural sciences, precisely detected potential complications of bacteriophages used as biological control agents. Other than the fact that it could lead to phage resistance in host bacteria, real hurdles for its registration as a Microbial biocontrol agent are the formation of pseudo lysogens or lysogens and that bacteriophages can act as a carrier for MGEs (mobile genetic elements), comprising AMR (antimicrobial resistance genes), also stated by various scientists (Svircev et al. 2018).
Conclusions
To control plant disease effectively, a proper disease management strategy involving various integrated techniques is usually required. At present, phage biocontrol is a new, but very uncommon approach. Phages, on the other hand, have many qualities that can help to expand the arsenal of crop disease management. Furthermore, phages have the natural ability to evolve to overcome resistance to new bacterial strains or phage resistance. They can be used in conjunction with other biocontrol or chemical agents. Their sensitivity to certain soil conditions and UV light could be a drawback to their utilization. However, some of these limitations have been overcome by changing the time of phage application, and the use of UV protectant formulas to the crops to prevent interactions with chemical pesticides.
Pesticide companies are focusing more on biopesticides and shifting their investments away from chemical pesticides. The pesticide industry is about $56 billion worth, with biopesticides accounting for only $2–3 billion. On the other hand, biopesticides in the future are likely to overtake chemical pesticides. This shift is thought to be the result of the rising demand of customers for chemical-free foods and the increased legalization of synthetic pesticides in some parts of the world. Furthermore, many biopesticides are potentially less expensive to develop and commercialize. With the current economic environment, increasing activity in the development of phage as biocontrol agents as a feasible crop disease control strategy is expected in the future. Being all-natural makes bacteriophages ideal for organic farming.
Availability of data and materials
Not applicable.
Abbreviations
- AMR:
-
Antimicrobial resistance
- ASM:
-
Acibenzolar-S-methyl
- BCA:
-
Biocontrol assay
- DG SANTE:
-
Directorate-General for Health and Food Safety
- EAD:
-
Enzymatically active domain
- EFSA:
-
European Food safety Agency
- EPA:
-
Environmental Protection Agency
- FDA:
-
Food and Drug Administration
- MBA:
-
Microbial Biocontrol Agents
- MGE:
-
Mobile genetic element
- MOI:
-
Multiplicity of infection
- PAFF:
-
Plants, Animals, Food and Feed (Committee)
- PCF:
-
Pregelatinized corn flour
- PG:
-
Peptidoglycan
- PPP:
-
Plant protection product
- QPS:
-
Qualified presumption safety
- SAR:
-
Systemic acquired resistance
- USA:
-
United States of America
- UV:
-
Ultraviolet
References
Abdelrhim AS, Ahmad AA, Omar MO, Hammad AM, Huang Q (2021) A new Streptomyces scabies-infecting bacteriophage from Egypt with promising biocontrol traits. Arch Microbiol 203:4233–4242
Abdelsattar AS, Nofal R, Makky S, Safwat A, Taha A, El-Shibiny A (2021) The synergistic effect of biosynthesized silver nanoparticles and phage zcse2 as a novel approach to combat multidrug-resistant salmonella enterica. Antibiotics 10(6):678
Abedon ST (2015) Ecology of anti-biofilm agents II: bacteriophage exploitation and biocontrol of biofilm bacteria. Pharmaceuticals 8(3):559–589
Abedon ST (2022) Bacteriophages as drivers of evolution: an evolutionary perspective. Springer
Al Raish SM, Saeed EE, Alyafei DM, El-Tarabily KA, AbuQamar SF (2021) Evaluation of streptomycete actinobacterial isolates as biocontrol agents against royal poinciana stem canker disease caused by the fungal pathogen Neoscytalidium dimidiatum. Biol Control 164:104783
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9(3):42
Alomari MMM (2021) Bacteriophages as an alternative method for control of zoonotic and foodborne pathogens. Viruses 13(12):2348
Aswani VH, Shukla SK (2021) An early history of phage therapy in the united states: is it time to reconsider? Clin Med Res 19(2):82–89
Azeredo J, García P, Drulis-Kawa Z (2021) Targeting biofilms using phages and their enzymes. Curr Opin Biotechnol 68:251–261
Balogh B (2006) Characterization and use of bacteriophages associated with citrus bacterial pathogens for disease control. PhD Dissertation, Gainesville, FL: University of Florida.
Balogh B, Jones JB, Momol MT, Olson SM, Obradovic A, King P, Jackson LE (2003) Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Dis 87(8):949–954
Balogh B, Canteros BI, Stall RE, Jones JB (2008) Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis 92(7):1048–1052
Bartnik P, Lewtak K, Fiołka M, Czaplewska P, Narajczyk M, Czajkowski R (2022) Resistance of Dickeya solani strain IPO 2222 to lytic bacteriophage ΦD5 results in fitness tradeoffs for the bacterium during infection. Sci Rep 12(1):10725
Basit HA, Angle JS, Salem S, Gewaily EM (1992) Phage coating of soybean seed reduces nodulation by indigenous soil bradyrhizobia. Canadian J Microbiol 38(12):1264–1269
Bastas KK, Baysal Ö (2022) Microbial battling of fire blight disease on pome fruits. Microbial biocontrol: food security and post harvest management, vol 2. Springer, pp 211–226
Bhardwaj K, Adunphatcharaphon S, Banerjee K, Elliott C, Petchkongkaew A, Kolawole O (2022) A review of the fundamental factors and processes leading to the accumulation of aflatoxins in cereal crops. Preprints.org, 2022010400
Biosca EG, Català-Senent JF, Figàs-Segura À, Bertolini E, López MM, Álvarez B (2021) Genomic analysis of the first European bacteriophages with depolymerase activity and biocontrol efficacy against the phytopathogen Ralstonia solanacearum. Viruses 13(12):2539
Born Y, Bosshard L, Duffy B, Loessner MJ, Fieseler L (2015) Protection of Erwinia amylovora bacteriophage Y2 from UV-induced damage by natural compounds. Bacteriophage 5(4):e1074330
Boyer M, Wisniewski-Dyé F, Combrisson J, Bally R, Duponnois R, Costechareyre D (2022) Nettle manure: an unsuspected source of bacteriophages active against various phytopathogenic bacteria. Adv Virol 167(4):1099–1110
Buttimer C, McAuliffe O, Ross RP, Hill C, O’Mahony J, Coffey A (2017) Bacteriophages and bacterial plant diseases. Front Microbiol 8:34
Chatzopoulou S, Eriksson NL, Eriksson D (2020) Improving risk assessment in the European food safety authority: lessons from the European Medicines Agency. Front Plant Sci 11:349
Chevallereau A, Pons BJ, van Houte S, Westra ER (2022) Interactions between bacterial and phage communities in natural environments. Nat Rev Microbiol 20(1):49–62
Clavijo-Coppens F, Ginet N, Cesbron S, Briand M, Jacques MA, Ansaldi M (2021) Novel virulent bacteriophages infecting mediterranean isolates of the plant pest Xylella fastidiosa and Xanthomonas albilineans. Viruses 13(5):725
Collinge DB, Jensen DF, Rabiey M, Sarrocco S, Shaw MW, Shaw RH (2022) Biological control of plant diseases—what has been achieved and what is the direction? Plant Pathol 71(5):1024–1047
Crane M, Giddings JM (2004) “Ecologically acceptable concentrations” when assessing the environmental risks of pesticides under European directive 91/414/EEC. Hum Ecol Risk Assess 10(4):733–747
Das AK (2021) Bacteriophage mediated horizontal gene transfer. Brac University
Daulagala PWHKP (2021) Chitinolytic endophytic bacteria as biocontrol agents for phytopathogenic fungi and nematode pests: a review. Asian J Res Bot 5(3):14–24
Deka D, Annapure US, Shirkole SS, Thorat BN (2022) Bacteriophages: an organic approach to food decontamination. J Food Process Preserv 46(10):e16101
Dewald-Wang EA, Parr N, Tiley K, Lee A, Koskella B (2022) Multiyear time-shift study of bacteria and phage dynamics in the phyllosphere. Am Nat 199(1):126–140
Diallo A, Zougrana S, Sawadogo M, Kone D, Silué D, Szurek B, Wonni I, Hutin M (2021) First report of Bacterial Leaf Streak disease of rice caused by Xanthomonas oryzae pv. oryzicola in Ivory Coast. Plant Dis 105(12):4147
Doukkali L, Radouane N, Ezrari S, Tahiri A, Tazi B, Guenoun F, Amiri S, Lahlali R (2022) Lessons learnt from the fire blight epidemics: a mini review. Indian Phytopathol 75(3):611–625
Düzgüneş N, Sessevmez M, Yildirim M (2021) Bacteriophage therapy of bacterial infections: the rediscovered frontier. Pharmaceuticals 14(1):34
Elnahal AS, El-Saadony MT, Saad AM, Desoky ESM, El-Tahan AM, Rady MM, AbuQamar SF, El-Tarabily KA (2022) The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol 162(4):759–792
Epstein L, Bassein S (2003) Patterns of pesticide use in California and the implications for strategies for reduction of pesticides. Annu Rev Phytopathol 41(1):351–375
Farooq T, Hussain MD, Shakeel MT, Tariqjaveed M, Aslam MN, Naqvi SAH, Amjad R, Tang Y, She X, He Z (2022) Deploying viruses against phytobacteria: potential use of phage cocktails as a multifaceted approach to combat resistant bacterial plant pathogens. Viruses 14(2):171
Fessia A, Barra P, Barros G, Nesci A (2022) Could Bacillus biofilms enhance the effectivity of biocontrol strategies in the phyllosphere? J Appl Microbiol 133(4):2148–2166
Gao H, Guo M, Song J, Ma Y, Xu Z (2021) Signals in systemic acquired resistance of plants against microbial pathogens. Mol Biol Rep 48(4):3747–3759
Gayder SC (2021) Interactions and population dynamics between Erwinia amylovora, Pantoea agglomerans, and their bacteriophages for effective phage therapy
Grabowski Ł, Łepek K, Stasiłojć M, Kosznik-Kwaśnicka K, Zdrojewska K, Maciąg-Dorszyńska M, Węgrzyn G, Węgrzyn A (2021) Bacteriophage-encoded enzymes destroying bacterial cell membranes and walls, and their potential use as antimicrobial agents. Microbiol Res 248:126746
Grace ER, Rabiey M, Friman VP, Jackson RW (2021) Seeing the forest for the trees: use of phages to treat bacterial tree diseases. Plant Pathol 70(9):1987–2004
Harshitha N, Rajasekhar A, Saurabh S, Sonalkar R, Tejashwini M, Mitra SD (2022) Bacteriophages: potential biocontrol agents and treatment options for bacterial pathogens. Clin Microbiol Newsl 44(5):41–50
Hatfull GF, Dedrick RM, Schooley RT (2022) Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med 73:197–211
Hazards EPOB (2017) Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J 15(3):e04664
Holtappels D, Fortuna K, Lavigne R, Wagemans J (2021) The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr Opin Biotechnol 68:60–71
Izraeli Y, Lalzar M, Mozes-Daube N, Steinberg S, Chiel E, Zchori-Fein E (2021) Wolbachia influence on the fitness of Anagyrus vladimiri (Hymenoptera: Encyrtidae), a bio-control agent of mealybugs. Pest Manag Sci 77(2):1023–1034
Jamal M, Bukhari SM, Andleeb S, Ali M, Raza S, Nawaz MA, Shah SS (2019) Bacteriophages: an overview of the control strategies against multiple bacterial infections in different fields. J Basic Microbiol 59(2):123–133
Jones JB, Svircev AM, Obradović AŽ (2021) Crop use of bacteriophages. Bacteriophages Biol Technol Ther 5:839–856
Kizheva Y, Eftimova M, Rangelov R, Micheva N, Urshev Z, Rasheva I, Hristova P (2021) Broad host range bacteriophages found in rhizosphere soil of a healthy tomato plant in Bulgaria. Heliyon 7:5
Larrahondo-Rodríguez E, Liao YY, Huerta AI (2022) Diagnostic guide for bacterial spot of tomato and pepper. Plant Health Progress 23(3):355–361
Lee S, Vu NT, Oh EJ, Rahimi-Midani A, Thi TN, Song YR, Oh CS (2021) Biocontrol of soft rot caused by Pectobacterium odoriferum with bacteriophage phiPccP-1 in Kimchi cabbage. Microorganisms 9(4):779
Lee C, Kim H, Ryu S (2022) Bacteriophage and endolysin engineering for biocontrol of food pathogens/pathogens in the food: recent advances and future trends. Crit Rev Food Sci Nutr 54:1–20
Leitner L, Ujmajuridze A, Chanishvili N, Goderdzishvili M, Chkonia I, Rigvava S, Chkhotua A, Changashvili G, McCallin S, Schneider MP, Liechti MD (2021) Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect Dis 21(3):427–436
Liu J, Chia SL, Tan GH (2021a) Isolation and characterization of novel phages targeting Xanthomonas oryzae: culprit of bacterial leaf blight disease in rice. Ther Appl Res 2(3):142–151
Liu Y, Liu M, Hu R, Bai J, He X, Jin Y (2021b) Isolation of the novel phage PHB09 and its potential use against the plant pathogen pseudomonas syringae pv. actinidiae. Viruses 13(11):2275
López-Martín M, Dubern JF, Alexander MR, Williams P (2021) Abam regulates quorum sensing, biofilm formation, and virulence in Acinetobacter baumannii. J Bacteriol 203(8):10–1128
Łoś J, Zielińska S, Krajewska A, Michalina Z, Małachowska A, Kwaśnicka K, Łoś M (2021) Temperate phages, prophages, and lysogeny. Bacteriophages Biol Technol Ther 8:119–150
Majdinasab M, Daneshi M, Marty JL (2021) Recent developments in non-enzymatic (bio) sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 232:122397
Majkowska-Skrobek G, Markwitz P, Sosnowska E, Lood C, Lavigne R, Drulis-Kawa Z (2021) The evolutionary trade-offs in phage-resistant Klebsiella pneumoniae entail cross-phage sensitization and loss of multidrug resistance. Environ Microbiol 23(12):7723–7740
Meile S, Du J, Dunne M, Kilcher S, Loessner MJ (2022) Engineering therapeutic phages for enhanced antibacterial efficacy. Curr Opin Virol 52:182–191
Miller SA, Ferreira JP, LeJeune JT (2022) Antimicrobial use and resistance in plant agriculture: a one health perspective. Agriculture 12(2):289
Murray CW, Egan SK, Kim H, Beru N, Bolger PM (2008) US food and drug administration’s total diet study: dietary intake of perchlorate and iodine. J Eposure Sci Environ Epidemiol 18(6):571–580
Murray E, Draper LA, Ross RP, Hill C (2021) The advantages and challenges of using endolysins in a clinical setting. Viruses 13(4):680
Nakao S, Watanabe H, Yano T, Yamaoka Y, Ishii H (2021) Control efficacy of the systemic acquired resistance (SAR) inducer acibenzolar-S-methyl against Venturia nashicola in Japanese pear orchards. J Gen Plant Pathol 87(5):307–315
Nakayinga R, Makumi A, Tumuhaise V, Tinzaara W (2021) Xanthomonas bacteriophages: a review of their biology and biocontrol applications in agriculture. BMC Microbiol 21(1):1–20
Nawaz M, Mabubu JI, Hua H (2016) Current status and advancement of biopesticides: microbial and botanical pesticides. J Entomol Zool Stud 4(2):241–246
Nga NTT, Tran TN, Holtappels D, Kim Ngan NL, Hao NP, Vallino M, Tien DTK, Khanh-Pham NH, Lavigne R, Kamei K, Wagemans J (2021) phage biocontrol of bacterial leaf blight disease on welsh onion caused by Xanthomonas axonopodis pv. allii. Antibiotics 10(5):517
Pandit MA, Kumar J, Gulati S, Bhandari N, Mehta P, Katyal R, Rawat CD, Mishra V, Kaur J (2022) Major biological control strategies for plant pathogens. Pathogens 11(2):273
Pereira C, Costa P, Pinheiro L, Balcão VM, Almeida A (2021) Kiwifruit bacterial canker: an integrative view focused on biocontrol strategies. Planta 253:1–20
Poveda J, Roeschlin RA, Marano MR, Favaro MA (2021) Microorganisms as biocontrol agents against bacterial citrus diseases. Biol Control 158:104602
Rahimzadeh G, Saeedi M, Moosazadeh M, Hashemi SMH, Babaei A, Rezai MS, Kamel K, Asare-Addo K, Nokhodchi A (2021) Encapsulation of bacteriophage cocktail into chitosan for the treatment of bacterial diarrhea. Sci Rep 11(1):15603
Rahman MU, Wang W, Sun Q, Shah JA, Li C, Sun Y, Li Y, Zhang B, Chen W, Wang S (2021) Endolysin, a promising solution against antimicrobial resistance. Antibiotics 10(11):1277
Raina A, Laskar RA, Wani MR, Khan S (2022) Plant breeding strategies for abiotic stress tolerance in cereals. Omics approach to manage abiotic stress in cereals. Springer, Singapore, pp 151–177
Rasool M, Akhter A, Soja G, Haider MS (2021) Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci Rep 11(1):6092
Sabri M, Benkirane R, Habbadi K, Sadik S, Ou-Zine M, Diouri M, Achbani EH (2021) Phages as a potential biocontrol of phytobacteria. Arch Phytopathol Plant Protect 54(17–18):1277–1291
Sakata N, Ishiga T, Masuo S, Hashimoto Y, Ishiga Y (2021) Coronatine contributes to Pseudomonas cannabina pv. alisalensis virulence by overcoming both stomatal and apoplastic defenses in dicot and monocot plants. Mol Plant Microbe Interact 34(7):746–757
Sasaki R, Miyashita S, Ando S, Ito K, Fukuhara T, Takahashi H (2021) Isolation and characterization of a novel jumbo phage from leaf litter compost and its suppressive effect on rice seedling rot diseases. Viruses 13(4):591
Sato H (2022) Development and future application of transgenic tall fescue (Festuca arundinacea Schreb.) with improved important forage and turf traits. Japan Agric Res Quart 56(1):1–6
Śliwka P, Ochocka M, Skaradzińska A (2022) Applications of bacteriophages against intracellular bacteria. Crit Rev Microbiol 48(2):222–239
Spök A, Twyman RM, Fischer R, Ma JK, Sparrow PA (2008) Evolution of a regulatory framework for pharmaceuticals derived from genetically modified plants. Trends Biotechnol 26(9):506–517
Stefani E, Obradović A, Gašić K, Altin I, Nagy IK, Kovács T (2021) Bacteriophage-mediated control of phytopathogenic xanthomonads: a promising green solution for the future. Microorganisms 9(5):1056
Sulley S, Babadoost M, Hind SR (2021) Biocontrol agents from cucurbit plants infected with Xanthomonas cucurbitae for managing bacterial spot of pumpkin. Biol Control 163:104757
Svircev A, Roach D, Castle A (2018) Framing the future with bacteriophages in agriculture. Viruses 10(5):218
Tudi M, Daniel Ruan H, Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung DT (2021) Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 18(3):1112
Umrao PD, Kumar V, Kaistha SD (2021) Biocontrol potential of bacteriophage ɸsp1 against bacterial wilt-causing Ralstonia solanacearum in Solanaceae crops. Egypt J Biol Pest Control 31(1):1–12
Valencia-Hernandez JA, Solano-Alvarez N, Rico-Rodriguez MA, Rodriguez-Ontiveros A, Torres-Pacheco I, Rico-Garcia E, Guevara-Gonzalez RG (2023) Eustressic dose of cadmium in soil induces defense mechanisms and protection against Clavibacter michiganensis in tomato (Solanum lycopersicum L.). J Plant Growth Regul 42(1):407–414
Villarroel J, Kleinheinz KA, Jurtz VI, Zschach H, Lund O, Nielsen M, Larsen MV (2016) HostPhinder: a phage host prediction tool. Viruses 8(5):116
Wang CX, Zhao AH, Yu HY, Wang LL, Li X (2022a) Isolation and characterization of a novel lytic halotolerant phage from Yuncheng Saline lake. Indian J Microbiol 62(2):249–256
Wang C, Wang X, Jin Z, Müller C, Pugh TA, Chen A, Wang T, Huang L, Zhang Y, Li LX, Piao S (2022b) Occurrence of crop pests and diseases has largely increased in China since 1970. Nat Food 3(1):57–65
Wintachai P, Surachat K, Singkhamanan K (2022) Isolation and characterization of a novel autographiviridae phage and its combined effect with tigecycline in controlling multidrug-resistant acinetobacter baumannii-associated skin and soft tissue infections. Viruses 14(2):194
Zhang Y, Huang HH, Duc HM, Masuda Y, Honjoh KI, Miyamoto T (2022) Application of endolysin LysSTG2 as a potential biocontrol agent against planktonic and biofilm cells of Pseudomonas on various food and food contact surfaces. Food Control 131:108460
Acknowledgements
The authors thank Higher Education Commission of Pakistan for providing financial assistance to carry out this work (No. 20-16428/NRPU/R&D/HEC/2021). The authors thank International Foundation for Science for providing financial support for this project (No. I-1-F-6676-1).
Funding
Higher Education Commission of Pakistan provided financial assistance to carry out this work (No. 20-16428/NRPU/R&D/HEC/2021). International Foundation for Science provided financial support for this project (No. I-1-F-6676-1).
Author information
Authors and Affiliations
Contributions
SK, AN and AAS did conceptualization; SK and AAS helped in resources, project administration and supervision; AN and SZ were involved in data curation; AN, MS and NK done writing—original draft preparation; SM SMAUSB, SZ, AAS, MB and SK contributed to writing—review and editing ; . All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approve the submission of the article.
Competing interests
The authors declare that no financial or any other conflicts of interest associated with the manuscript exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawaz, A., Zafar, S., Shahzadi, M. et al. Bacteriophages: an overview of the control strategies against phytopathogens. Egypt J Biol Pest Control 33, 108 (2023). https://doi.org/10.1186/s41938-023-00751-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00751-7