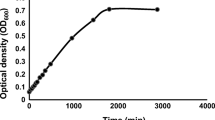
Abstract
Halophilic phage are a type of virus that exist in salty environments within halophilic archaeal or bacterial hosts. However, relatively few reports on halophilic bacteriophages exist, and our overall understanding of halophilic bacteriophages is quite limited. We used SYBR Green I fluorescent staining to detect the abundance of viruses in Yuncheng Saline Lake, China. Using the double-layer plate method, a lytic phage that could infect halophilic bacterium Salinivibrio sp. YM-43 was isolated and named YXM43. We studied host range, optimal host, morphological characteristics, nucleic acid type, protein composition, and other biological characteristics of the virus. Results reveal a high abundance of this halophilic virus in Yuncheng Saline Lake. The newly isolated bacteriophage YXM43 has a narrow host range, with the most suitable host being Virgibacillus sp. SK39. After purification and enrichment, YXM43 is observed as a spherical particle with a diameter of approximately 30 nm, with no tail. No lipid envelope can be seen in YXM43. The capsid protein of the virus can be separated into seven proteins with molecular weights ranging from 62.0 to 13.0 kDa. YXM43 is a DNA virus with a genome approximately 23 kb. The virus is tolerant of low salinity, and its activity is highest at a temperature of 60 °C and a pH of 10. YXM43 is temperature and pH tolerant, and can adapt to environmental change, even withstanding chloroform treatment. The results indicate that bacteriophage YXM43 is a novel halophilic bacteriophage with broad tolerance to environmental change.






Similar content being viewed by others
References
Forest R, Rebecca VT (2009) Viruses manipulate the marine environment. Nature 459:207–212. https://doi.org/10.1038/nature08060
Priya N, Sheila P, Juan AU et al (2012) De novo metagenomic assembly reveals abundant novel major lineage of Archaea in hypersaline microbial communities. ISME J 6:81–93. https://doi.org/10.1038/ismej.2011.78
Francisco RV, Ana B, Martin C et al (2009) Explaining microbial population genomics through phage predation. Nat Rev Microbiol 7:828–836. https://doi.org/10.1038/nrmicro2235
Atanasova NS, Oksanen HM, Bamford DH (2015) Haloviruses of archaea, bacteria, and eukaryotes. Curr Opin Microbiol 25:40–48. https://doi.org/10.1016/j.mib.2015.04.001
Alison L, Timothy W, Susanne E et al (2014) Viruses of Haloarchaea. Life 4:681–715. https://doi.org/10.3390/life4040681
Pietilä MK, Atanasova NS, Manole V et al (2012) Virion architecture unifies globally distributed pleolipoviruses infecting halophilic archaea. J Virol 86:5067–5079. https://doi.org/10.1128/JVI.06915-11
Pietilä MK, Laurinmäki P, Russell DA et al (2013) Insights into head-tailed viruses infecting extremely halophilic archaea. J Virol 87:3248–3260. https://doi.org/10.1128/JVI.03397-12
Ana S, Elina R (2014) A Glimpse of the genomic diversity of haloarchaeal tailed viruses. Front Microbiol 5:84–89. https://doi.org/10.3389/fmicb.2014.00084
Ackermann HW, Prangishvili D (2012) Prokaryote viruses studied by electron microscopy. Arch Virol 157:1843–1849. https://doi.org/10.1007/s00705-012-1383-y
Ana S, Deborah JS, Daniel AR et al (2013) Snapshot of haloarchaeal tailed virus genomes. RNA Biol 10:803–816. https://doi.org/10.4161/rna.24045
Aharon O, Mikal H, Svein N et al (2002) Intracellular ion and organic solute concentrations of the extremely halophilic bacterium Salinibacter ruber. Extremophiles 6:491–498. https://doi.org/10.1007/s00792-002-0286-3
Oren A, Ventosa A (2014) International committee on systematics of prokaryotes; subcommittee on the taxonomy of Halobacteriaceae. Int J Syst Evol Microbiol 64:3914. https://doi.org/10.1099/ijs.0.069922-0
Francisco RV, Ana-Belen MC, Beltran RB et al (2009) Explaining microbial population genomics through phage predation. Nat Rev Microbiol 7:828–836. https://doi.org/10.1038/nrmicro2235
Curtis AS (2007) Marine viruses-major players in the global ecosystem. Nat Rev Microbiol 5:801–812. https://doi.org/10.1038/nrmicro1750
Concepción C, Ana GP, Victoria B et al (1988) Isolation and characterization of phage F9–11 from a lysogenic Deleya halophila strain. Curr Microbiol 17:49–53. https://doi.org/10.1007/BF01568819
Conceptión C, Ana GP, Fernando MC et al (1994) Behaviour of two D. halophila bacteriophages with respect to salt concentrations and other environmental factors. Toxicol Environ Chem 43:85–93. https://doi.org/10.1080/02772249409358020
Fu C, Zhao Q, Li Z et al (2017) Complete genome sequence of Halomonas ventosae virulent halovirus QHHSV-1. Arch Virol 162:3215–3219. https://doi.org/10.1007/s00705-017-3415-0
Rodela ML, Sabet S, Peterson A et al (2019) Broad environmental tolerance for a salicola host-phage pair isolated from the cargill solar saltworks, Newark, CA, USA. Microorganisms 7:106–122. https://doi.org/10.3390/microorganisms7040106
Yoon SH, Ha SM, Kwon S et al (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Tiiu K, Hans-W A, Usha G et al (1991) A bacteriophage of a moderately halophilic bacterium. Arch Microbiol 156:435–438. https://doi.org/10.1007/BF00245388
Usha G, Tiiu K, Donn JK et al (1996) A moderately halophilic Vibrio from a Spanish saltern and its lytic bacteriophage. Can J Microbiol 42:1015–1023. https://doi.org/10.1139/m96-130
Khayat R, Tang L, Larson ET et al (2005) Structure of an archaeal virus capsid protein reveals a common ancestry to eukaryotic and bacterial viruses. PNAS 102:18944–18949. https://doi.org/10.1073/pnas.0506383102
Fu CQ, Zhao Q, Li ZY et al (2016) A novel Halomonas ventosae-specific virulent halovirus isolated from the Qiaohou salt mine in Yunnan, Southwest China. Extremophiles 20:101–110. https://doi.org/10.1007/s00792-015-0802-x
Nuttall SD, Dyall-Smith ML (1993) HF1 and HF2: novel bacteriophages of halophilic archaea. Virology 197:678–684. https://doi.org/10.1006/viro.1993.1643
Hanna MK, Elina R, Petra K et al (2006) Quantitative dissociation of archaeal virus SH1 reveals distinct capsid proteins and a lipid core. Virology 356:4–11. https://doi.org/10.1016/j.virol.2006.07.027
Wang CX, Li X (2018) JMT-1: a novel, spherical lytic halotolerant phage isolated from Yuncheng saline lake. Braz J Microbiol 49:262–268. https://doi.org/10.1016/j.bjm.2018.03.004
Suttle CA, Fuhrman JA (2010) Enumeration of virus particles in aquatic or sediment samples by epifluorescence microscopy. MAVE 15:145–153. https://doi.org/10.4319/mave.2010.978-0-9845591-0-7.145
Porter K, Tang SL, Chen CP et al (2013) PH1: an archaeovirus of Haloarcula hispanica related to SH1 and HHIV-2. Archaea 2013:456318. https://doi.org/10.1155/2013/456318
Kukkaro P, Bamford DH (2009) Virus-host interactions in environments with a wide range of ionic strengths. Environ Microbiol Rep 1:71–77. https://doi.org/10.1111/j.1758-2229.2008.00007.x
Yu HY, Cao JB, Li X (2015) Isolation and characterization of a bacteriophage CJL-1 infecting halophilic bacteria. Biotechnology 25:56–60. https://doi.org/10.16519/j.cnki.1004-311x.2015.01.011
Nikita Z, Elina C, Andris D et al (2020) Isolation and characterization of the novel Virgibacillus-infecting bacteriophage Mimir87. Arch Virol 165:737–741. https://doi.org/10.1007/s00705-019-04516-2
Acknowledgements
This work was supported by Science and Technology Innovation Project of Higher Education Institutions in Shanxi Province (2019L0859); Discipline project of “Characteristic Agricultural Products Development” (XK-2019012); Key discipline construction of Yuncheng University; Shanxi Basic Research Program (Free Exploration) (20210302123080). We also thank Steven M. Thompson from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human or Animals Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, CX., Zhao, AH., Yu, HY. et al. Isolation and Characterization of a Novel Lytic Halotolerant Phage from Yuncheng Saline Lake. Indian J Microbiol 62, 249–256 (2022). https://doi.org/10.1007/s12088-022-01005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-022-01005-0