Abstract
Background
Whitefly Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) is a plant-damaging insect in tropical and subtropical regions that causes agricultural damage worldwide, including in Viet Nam. The abuse of pesticides derived from chemicals has resulted in the evolution of insect-resistant strains, polluting the environment and threatening human health. Using entomopathogenic fungi (EPF) for biological control is an alternative strategy in integrated pest management. Hence, an attempt was conducted to isolate, characterize and evaluate the efficacy of EPF, Purpureocillium lilacinum against whitefly B. tabaci under laboratory and field conditions.
Results
Purpureocillium lilacinum PL1 (PL1) was isolated from the whitefly B. tabaci cadavers and subsequently identified using morphological study and internal transcribed spacer sequencing. Purpureocillium lilacinum PL1 had effectively grown and sporulated at temperatures ranging from 25 to 35 °C and throughout a broad pH range, which is particularly advantageous against the harsh tropical monsoon climate. Bioassay study indicated that 1 × 107 conidia/ml of P. lilacinum PL1 had a high lethality against the whitefly B. tabaci nymphs in vitro with efficiency was 88.24% after 7 days of treatment. The median lethal concentration (LC50) of P. lilacinum PL1 to B. tabaci after 7 days of treatment was 1.24 × 105 conidia/ml. In field conditions, 1 × 107 conidia/ml of P. lilacinum PL1 lowered the population of B. tabaci nymphs with efficacy was 78.86% after 2 batches, 7 days after treatments.
Conclusion
The findings indicated that P. lilacinum PL1 was effective in the biological control of B. tabaci nymphs, which could be a potential alternative to chemical pesticides for pest management.
Similar content being viewed by others
Background
Cassava (Manihot esculenta Crantz, Euphorbiaceae) is among the most important food crops in the world. It is cultivated on approximately 544,300 hectares in Viet Nam, with an annual production of 9.74 million tons (Kim et al. 2015). Cassava mosaic disease (CMD), which is caused by the begomovirus poses the greatest threat to global cassava productivity (Tokunaga et al. 2018). Cassava mosaic begomoviruses (CMB) are naturally transmitted by the whitefly Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae), the primary vector of begomoviruses, which has a relatively high reproductive capacity and rapid dispersal ability (Islam et al. 2018). CMD was originally found in Southeast Asia in 2015 in Ratanakiri, Cambodia, with the causative agent later identified as the Sri Lankan cassava mosaic virus (SLCMV) (Wang et al. 2016). SLCMV was recorded in Tay Ninh province, Viet Nam, in 2017 and represents a new danger to cassava farming in Viet Nam, reducing its tuber yield and starch content by 16–33%, respectively (Uke et al. 2018).
Bemisia tabaci is a polyphagous insect pest that has caused widespread damage to agricultural productivity around the world (Cruz-Estrada et al. 2013). Adults and nymphs alike feed on the sap, and their bile (sugar excreta) promotes the growth of “soot mold” on the leaves and fruits, reducing crop productivity (Gangwar et al. 2018). Bemisia tabaci might play an essential role in the transmission of over 350 plant virus species, which can severely damage crops’ productivity (Götz et al. 2016).
Insecticides derived from synthetic chemicals such as neonicotinoids and insect growth regulators have played a major role in eradicating B. tabaci over the past 2 decades (Khalid et al. 2021). Rising applications of chemical pesticides have raised a variety of concerns about human health and environmental damage (Pathak et al. 2022). In recent years, there has been a surge of interest in using biological control agents, such as entomopathogenic fungi (EPF), as an alternative strategy to chemical insecticides (Skinner et al. 2014). EPFs are biological control agents used to eliminate sap-sucking pests, and pests with chewing mouthparts. Using EPFs have many benefits beyond its effectiveness, such as reduced pesticide residues, maintenance of other natural enemies, and increase biodiversity in human-managed habitats (Lacey et al. 2001).
Purpureocillium spp. (Hypocreales: Ophiocordycipitaceae), which was previously classified as Paecilomyces, is well-known for its effectiveness as an EPF in the management of arthropods and nematodes (Goffré et al. 2015). Purpureocillium lilacinum is a well-known species that has been employed to control aphids, thrips, whiteflies, fruit flies, beetles, mosquitoes, and plant parasitic nematodes (Amala et al. 2013; Goffré et al. 2015). In the previous study, P. lilacinum was shown to be highly lethal to B. tabaci in vitro with efficacy up to 98% (Desoky et al. 2022). However, different EPFs have been shown to have varying effectiveness levels when administered in tropical and subtropical areas (Indriyanti et al. 2017). EPFs can be adapted to their environmental conditions depending on various geographical considerations (Indriyanti et al. 2017). Therefore, isolation of local EPFs, which have long been adapted to geographical and environmental conditions, is a useful strategy to exploit the potential for biological management of the whitefly B. tabaci. This study aimed to isolate an EPF strain and evaluate its efficacy in managing the whitefly, B. tabaci in cassava plantations in southern Vietnam, contributing to the development of an integrated pest management (IPM) approach for B. tabaci.
Methods
Isolation of entomopathogenic fungi from B. tabaci cadavers
The whitefly B. tabaci was obtained from a cassava field in Tan Chau, Tay Ninh province, Viet Nam. The whitefly population was maintained on cassava leaves in laboratory. EPFs were isolated following the protocol provided by (Sharma et al. 2018). Briefly, whitefly cadavers were obtained individually in sterile tubes and examined under a stereomicroscope (40×) to identify potential EPF based on the presence of hyphae surrounding the cuticle. The dead B. tabaci suspected of being infected with EPFs was surface sterilized using 70% ethanol for 1 min, followed by sterilized water twice for 1 min, dried on filter paper then transferred to potato dextrose agar (PDA) (Merck, Germany), supplemented with 0.1% chloramphenicol to prevent bacteria growth and incubated at 28 ± 1 °C, 50–60% RH for 14 days. The fungal colonies formed were then isolated by subculturing on PDA medium with the addition of 0.1% chloramphenicol and cultured at 28 °C (Sharma et al. 2018). After 3–7 days of cultivation, the microscopic characteristics of the isolated fungus strain were observed. The B. tabaci was then re-infected with fungal isolate to further investigation the causal relationship between the fungal isolate and the death of B. tabaci according to Koch’s postulate (Grimes 2006).
Morphological identification of fungal isolate
The 5 mm mycelia disk from the second isolation purification was inoculated on PDA monitored and recorded daily. After 7 days of cultivation, the color of colonies, and the presence of medium and incubated at 28 °C, 50–60% RH for 7 days. The radial expansion of the colonies and conidia were studied. Additionally, the microscopic of the fungal isolate was investigated. Briefly, the mycelia were collected by a sterile needle and placed in a drop of lactophenol cotton blue. The texture of hyphae, phialides, and conidial shape was observed at 1000×magnification using an Eclipse E100 microscope (Nikon Corp., Tokyo, Japan). The identification of morphological characteristics was based on the description provided by (Watanabe 2010) and (Humber 2012).
Molecular identification of fungal isolate
Molecular identification of fungal isolate used the internal transcribed spacer (ITS) region universal ITS primers: ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (Tangthirasunun et al. 2010). The PCR reaction was performed on a T100 thermal cycler (Bio-Rad, Irvine, CA, USA) at 95 °C for 5 min (pre-denaturation), 35 cycles of 95 °C for 1 min (denaturation), 55 °C for 1 min (annealing), and 72 °C for 1 min (extension), followed by 72 °C for 10 min (final extension). The PCR products were observed on a 0.8% agarose gel electrophoresis, using GelRed Loading Buffer (TBR, Ho Chi Minh City, Viet Nam), then purified and sequenced by Nam Khoa Biotek (Ho Chi Minh city, Viet Nam). The Sanger sequencing findings were edited with Bioedit and then aligned with published full-length sequences in the NCBI Basic Local Alignment Search Tool (BLAST) databases to collect fungal species references. Phylogenetic analysis based on ITS-5.8S rDNA sequence was performed with Clustal-W function and neighbor-joining method using MEGA 6.0.
Evaluation of chitinase activity of P. lilacinum PL1
Colloidal chitin was prepared using Hsu and Lockwood method (Hsu et al. 1975). About 5 g of chitin from shrimp shells (Sigma-Aldrich, Darmstadt, Germany) was mixed with 30 ml of HCl acid (35.5%) and incubated overnight at 4 °C. The colloidal chitin was precipitated by slowly adding 250 ml of chilled ethanol (50%), with constant stirring at 4 °C and left for overnight. The colloidal chitin was centrifuged at 10,000 g for 20 min, and sterile distilled water was used to wash the pellets until the pH value was neutral. The chitin medium contained 1% colloidal chitin, NaNO3 3.5 g/l, K2HPO4 1.5 g/l, MgSO4.7H2O, 0.5 g/l, KCl 0.5 g/l, and FeSO4.7H2O 0.01 g/l, and agar 20 g/l. The mycelia disk (5 mm) of P. lilacinum PL1 were cultured on 1% colloidal chitin agar and incubated at 28 °C, 50–60% RH. After 1–4 days of cultivation, chitin degradation ability was monitored by flooding P. lilacinum PL1 colonies in 1.5% Lugol solution and measuring the diameter of the halo around the colonies.
Evaluation of protease activity of P. lilacinum PL1
The casein medium contained casein 10 g/l, glucose 1 g/l, yeast extract 1 g/l, K2HPO4 1 g/l, KH2PO4 0.5 g/l, MgSO4.7H2O, and agar 20 g/l. The mycelia disk (5 mm) of P. lilacinum PL1 was cultured on casein medium agar and incubated at 28 °C, 50–60% RH for 1–4 days. The diameter of the bright halo surrounding the colonies was monitored as a characteristic of the biodegrade casein activity (Zhang et al. 2021).
Effect of temperature on the growth of P. lilacinum PL1
Suitability of temperature was investigated for the growth of P. lilacinum PL1 mycelia. The mycelia disk (5 mm) of P. lilacinum PL1 was cultured on PDA medium then incubated at different temperatures, viz. 25, 30, 35, and 40 °C, 50–60% RH for 14 days. The growth of P. lilacinum PL1 mycelia was investigated by measuring the diameter of the colony.
Effect of pH on the growth of P. lilacinum PL1
PDA culture medium was adjusted a pH 5, 6, 7 and 8 levels for this study, by using NaOH (0.1N) and HCl (0.1N) solution. The mycelia disk (5 mm) of P. lilacinum PL1 was cultured on PDA medium with a range of pH value from 5 to 8, then incubated at 28 °C, and subsequently monitored the colony diameter after 14 days of cultivation.
Mass production of P. lilacinum PL1 using solid state fermentation
Solid-state fermentation was applied for mass multiplication of P. lilacinum PL1, using broken rice as substrate (Dawar et al. 2003). Briefly, 1 kg of broken rice was spread on a tray with a size of 40 cm by 40 cm, supplement with 500 ml water, then sterilized by autoclaving. Purpureocillium lilacinum PL1 conidia was then harvested from PDA culture medium, resuspended at a density of 1 × 106 conidia/g with the sterilized substrate, then incubated at 28 °C for 14 days. After 14 days of cultivation, the P. lilacinum PL1 conidia density in broken rice substrate were determined using serial dilutions on PDA plates or counting by hemocytometer (Hirschmann, MO, USA).
Propagation of B. tabaci nymphs in the greenhouse
Greenhouse whitefly propagation was carried out according to the protocol described by (Mascarin et al. 2013). Briefly, a population of whitefly B. tabaci that had colonized cassava plantations was collected and reared in a greenhouse. Whitefly-free plants were planted in close proximity to cassava seedlings afflicted with adult whiteflies for 72 h to facilitate horizontal transmission. This method might produce approximately 30–50 eggs/leaf (Mascarin et al. 2013). The adult whiteflies were then removed and the plants carrying eggs were transported to a different glasshouse at 26–30 °C, 50–60% RH for 12–13 days, until the nymphs molted to the second instar. The second instar of B. tabaci nymphs were confirmed according to the pictorial guide (Naranjo et al. 2017).
Laboratory evaluation of the lethality of P. lilacinum PL1 on B. tabaci nymphs
Leaves contained second-instar B. tabaci nymphs were collected and placed with abaxial surface up in a 150-mm diameter Petri plate. The number of nymphs was adjusted to 50 individual/plate using a needle. Purpureocillium lilacinum PL1 conidia were harvested in broken rice substrate after 14 days of cultivation with a sterile spatula and suspended in sterile distilled water with Tween 80 (0.02% v/v), then and the conidia concentration was determined using a hemocytometer (Hirschmann, MO, USA). For the pathogenic assay, P. lilacinum PL1 conidia suspension was adjusted by a series of aqueous dilutions (1 × 104, 1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia/ml). Bioassays were conducted by treating B. tabaci nymphs with 5 ml of P. lilacinum PL1 conidia solutions. B. tabaci nymphs were treated with sterile distilled water with Tween 80 (0.02% v/v), were assigned as the control group. The leaves were then air-dried and transferred to another 150-mm diameter Petri plate containing a layer of 1.5% agarose gel, then incubate at 28 °C, 50–60% RH (Shah et al. 2020). Nymphs’ mortality rate was recorded daily for 9 days, starting on day 3rd after inoculation. Data on nymphs mortality were converted to the percentages of nymphs mortality at each time interval and dilution according to Abbott’s formula (Abbott 1925) as follows:
where m and n are for the percentages of dead nymphs in the treatment group and untreated group, respectively.
Evaluation of the lethality of P. lilacinum PL1 on B. tabaci nymphs under field conditions
Field trials were performed in 3 different cassava plantations in Tan Chau, Tay Ninh, Viet Nam. This location was chosen due to the abundance of the whitefly B. tabaci, and cassava plants showing symptoms of CMD. This study utilized the Randomized complete blocks design (RCBD) with 3 replicates for each treatment. The plot size was 10 m × 5 m × 1 m spacing, and each plot allocated 50 cassava plants. Before treatment, B. tabaci nymphs’ population was investigated on leaves from the top, middle, and bottom of each plant/plot. Insecticides were applied as foliar applications with 3 treatments: P. lilacinum PL1 at a concentration of 1 × 107 conidia/ml; fenobucarb (Nicotex Joint Stock, Binh Duong, Viet Nam) at a concentration of 2 mg/ml (as recommended by the manufacturer) as positive controls; and untreated plots were sprayed with water as negative control. Purpureocillium lilacinum PL1 or fenobucarb were applied as foliar sprays in the afternoon after 4 pm, with two sprays were performed at 14-day intervals. The spray volumes were subject to 300 l/hectares, according to the guideline of Food and Agriculture Organization of the United Nations (Lavers 2001). Bemisia tabaci nymphs were counted on leaves from the top, middle, and bottom of each plant/plot after 7 and 14 days after treatment. The efficacy of each treatment in the management of B. tabaci nymphs’ population was calculated using the Henderson–Tilton methodology (Henderson et al. 1955).
where n insect population; T treated; Co: control.
Results were calculated using the mean of triplicate readings.
Statistical analysis
The experiment was conducted using a completely randomized design (CRD) with 3 replicates for each treatment. Results were calculated using the mean of triplicate readings and presented as the mean ± standard error of the mean data were analyzed using SAS 9.4 software (SAS, Inc., Cary, NC, USA). Statistical significance was assessed between groups, using F-test and Duncan’s test. p < 0.05 was considered to indicate statistical significance. LC50 and LC90, as well as LT50 and LT90, were calculated with Probit analysis, using SAS 9.4 software (SAS, Inc., Cary, NC, USA).
Results
Isolation and characterization of isolate fungi from infected-whitefly B. tabaci
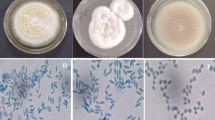
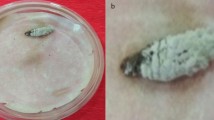
The cadavers of naturally infected whitefly B. tabaci with mycelia on the cuticle were collected for isolation of the EPF (Fig. 1A, B). The macroscopic portion of the isolated fungus strain had a purple appearance (Fig. 1C). Under a microscope at 1000×magnification, the conidia were unicellular, ovoid, and formed chains (Fig. 1D). Phialides are distinguished by a long, tapering neck and a bulbous base (Fig. 1D). Koch’s postulates were applied to indicate the causal relationship between the fungal isolates and the death of B. tabaci. The fungus isolated was responsible for the death of healthy whitefly B. tabaci and exhibited macroscopic and microscopic similarities with the isolated fungus strain (Fig. 1E, F). These data indicated that the fungal isolate was the cause of death in whiteflies B. tabaci, potentially serving as an EPF.
Characterization of Purpureocillium lilacinum PL1 isolated from Bemisia tabaci. A Dead B. tabaci with the presence of mycelia on the cuticle. B Bemisia tabaci cadavers under stereomicroscope at 40×magnification. C Macroscopic morphology of P. lilacinum PL1 isolates in PDA culture medium. D Microscopic morphology of P. lilacinum PL1 isolates under microscope at 1000×magnification. E Macroscopic morphology of P. lilacinum PL1 re-isolated from B. tabaci using Koch postulates F Microscopic morphology of P. lilacinum PL1 re-isolated from B. tabaci using Koch postulates. G Phylogenetic tree based on the ITS gene sequences of P. lilacinum PL1 in this study and other known P. lilacinum strains in the GenBank database. The P. lilacinum PL1 in this study are marked in bold
Molecular identification of isolated fungi
The rDNA region PCR product was further amplified and then purified to evaluate the nucleotide sequence of the fungus isolate. A phylogenetic analysis was performed using Purpureocillium reference fungal species from GenBank to determine the taxonomic identity of isolates. BLAST analysis revealed that the isolated fungus strain was 100% homologous to P. lilacinum. The phylogenetic tree in (Fig. 1G) was developed using MEGA 4.1. The phylogenetic tree based on ITS rDNA indicated that the isolated fungus strain and P. lilacinum belong to the same branch, while other Paecilomyces species indicated a greater gap among them (Fig. 1G). Thus, the fungus isolate strain was identified as P. lilacinum and referred as P. lilacinum PL1.
Chitinase and protease activity
The biosynthesis of extracellular chitinase or protease in P. lilacinum PL1 was also evaluated using an agar plate assay for the degradation of chitin or casein. The results in (Fig. 2) demonstrated that P. lilacinum PL1 might synthesize chitinase and protease. The chitin degrading halo zone diameter was 13.3 mm on day 1st and increased to 28.70 mm after 4 days of cultivation (Fig. 2A, B). Furthermore, the protein degrading ability, as indicated by the casein degrading halo zone, gradually increased from 13.30 to 30.00 mm after 4 days of cultivation (Fig. 2A, C). Thus, P. lilacinum PL1 might synthesize extracellular protease and chitinase, satisfying the candidate criteria of an EPF.
Chitinase and protease activity of Purpureocillium lilacinum PL1 on agar plate. A Halo diameter of chitin (black) and casein (gray) degradation of P. lilacinum PL1 at different time intervals. B Representative images of the chitin degradation ability of P. lilacinum PL1 on agar plate. C Representative images of the casein degradation ability of P. lilacinum PL1 on agar plate. * or # Indicates statistically significant differences relative to the day 1 group
Effect of pH and temperature on the growth of P. lilacinum PL1
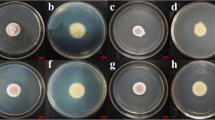
The effect of pH and temperature on the growth of P. lilacinum PL1 was further investigated. Purpureocillium lilacinum PL1 was cultivated in PDA culture medium with a pH range of 5.0, 6.0, 7.0, and 8.0. The mean of colony diameters was measured with the whole culture after 14 days of cultivation. The results presented in (Fig. 3A, B) indicated that there was non-significant difference between the pH values on the growth of P. lilacinum PL1 mycelia. Furthermore, the effect of temperature on the growth of P. lilacinum PL1 mycelia was also screened. P. lilacinum PL1 mycelia had effectively grown, and there was non-significant difference in the diameter of colonies between 25 and 35 °C (Fig. 3C, D). However, the growth of P. lilacinum PL1 mycelia was attenuated by 25% at 40 °C (Fig. 3C, D).
Effect of pH (A) and temperature (B) on the growth of Purpureocillium lilacinum PL1 mycelial indicated by radial growth after 4 days of cultivation. C Representative images of P. lilacinum PL1 mycelial on PDA culture medium at different pH value after 14 days of cultivation. D Representative images of P. lilacinum PL1 mycelial on PDA culture medium at different temperature after 14 days of cultivation. Different superscript lowercase letters (a–c) within a line and capital letters (A–D) within a column indicate statistically significant differences between groups (p < 0.05)
Laboratory evaluation of the lethality of P. lilacinum PL1 on B. tabaci nymphs
The effect of P. lilacinum PL1 on the mortality of B. tabaci nymphs was investigated in vitro with a range of concentrations from 1 × 104 to 1 × 109 conidia/ml for 9 days. The results in (Table 1) demonstrated that P. lilacinum PL1 at a concentration of 1 × 107 conidia/ml decreased the B. tabaci nymphs’ population in vitro after 7 days of treatment (Table 1). The elimination efficiency of B. tabaci nymphs was 88.24% after 7 days of treatment with P. lilacinum PL1 at the concentration of 1 × 107 conidia/ml and there was non-statistically significant difference between the lethality caused by the concentration of 1 × 108 conidia/ml and 1 × 109 conidia/ml as well as efficiency after 9 days of treatment (Table 1). Therefore, P. lilacinum PL1 at the concentration of 1 × 107 conidia/ml was further applied to the management of B. tabaci nymphs under field conditions.
The highest median lethal concentration (LC50) and LC90 of P. lilacinum PL1 on B. tabaci nymphs after 7 days of treatment was recorded as 1.24 × 105 conidia/ml and 1.08 × 108 conidia/ml, respectively (Table 2). Furthermore, the highest median lethal time (LT50) of 1 × 107 conidia/ml of P. lilacinum PL1 on B. tabaci nymphs was recorded as 4.36 days (Table 2).
Evaluation of the efficacy of P. lilacinum PL1 for the management of B. tabaci nymphs in cassava field in Tay Ninh, Viet Nam
The effectiveness of P. lilacinum PL1 in the management of B. tabaci nymphs was evaluated in a cassava farm in Tan Chau district, Tay Ninh, Viet Nam in April 2022 with temperature range between 26 and 35 °C, air humidity of approximately 50–65% RH, and an absence of raining. The densities of whitefly B. tabaci nymphs on cassava leaves were also investigated, the results in (Table 3) indicated that B. tabaci nymphs appeared at densities ranging from 5.94 to 6.12 nymphs/leaf, and an urgent strategy was required to restrict the spread of B. tabaci.
In the above assays, P. lilacinum PL1 was identified, having a high mortality rate against B. tabaci nymphs in vitro. Therefore, P. lilacinum PL1 was the leading candidate for the management of whitefly in cassava fields. The following experimental conditions were included for the field conditions: P. lilacinum PL1 was obtained at a concentration of 1 × 107 conidia/ml. The chemical insecticide fenobucarb at the recommended concentration of 2 mg/ml according to the manufacturer, was used as a positive control. Bemisia tabaci nymphs’ densities varied significantly after 7 days of the first treatments. The results in (Table 3) indicated that both P. lilacinum PL1 and fenobucarb significantly reduced the density of B. tabaci nymphs relative to the untreated group. Under field conditions, B. tabaci nymphs’ population showed variability in both the control and treatment groups, which differed than the controlled laboratory conditions. In order to evaluate the efficacy of P. lilacinum PL1 in the percentage of nymph B. tabaci reduction under field conditions, the Henderson–Tilton formula, which is a modified version of Abbott's formula (Abbott 1925), was employed to compare the untreated (control) and treated groups before and after the treatment was applied (Henderson et al. 1955). The results in (Table 3) indicated that after 7 days of the first treatment, P. lilacinum PL1 was more effective than fenobucarb in the management of B. tabaci nymphs, with efficacy rates of 77.46 and 56.08%, respectively. The second treatment was performed 7 days following the first treatment in order to improve the efficacy of B. tabaci nymphs’ management under field conditions. Results shown in (Table 3) indicated that 7 days after the second treatment, the B. tabaci nymphs’ density in the control groups increased to 13.2 nymphs/leaf. However, there were only 6.57 B. tabaci nymphs/leaf in the fenobucarb treatment group and 2.27 nymphs/leaf in the P. lilacinum PL1 treatment group (Table 3), for respective efficiencies of 50.27 and 78.86% (Table 3).
Discussion
Numerous studies have indicated that Purpureocillium spp. isolated from infected insect cadavers might be used as a biological agent for pest management (Johny et al. 2012). The macroscopic morphology of P. lilacinum is characterized by the purple-colored and suede-like floccose on the colony surface. Conidiophores are erect, bearing branches with densely clustered phialides. Phialides are swollen at their bases, gradually tapering into a slender neck. Conidia are ellipsoidal to fusiform and are produced in divergent chains (Sun et al. 2021). In this study, P. lilacinum PL1 isolated from infected B. tabaci developed colonies with a similar morphological appearance on PDA culture medium. Pairwise comparisons of ITS sequence data showed that P. lilacinum PL1 isolated was 100% homologous with the previously described P. lilacinum strains (GenBank MT732891.1:1–634). Thus, based on colony characteristics, microscopic morphology, spore morphology, and molecular characteristics, the fungal propagules were identified as Purpureocillium lilacinum.
Southern Viet Nam has a tropical monsoon climate with high temperatures, humidity, and abundant rainfall throughout the year (Kurbatova et al. 2015). These environmental conditions are suitable for the growth of EPFs (Akıner et al. 2020). Our findings indicated that P. lilacinum PL1 mycelia and sporulation were optimally grown at 25–35 °C. Colony radial growth was roughly 25% lower at 40 than 30 °C. Furthermore, the sporulation of P. lilacinum PL1 characterized by purple-colored colonies was still maintained at 40 °C. This contrasts with the previous study, which indicated that Purpureocillium spp. isolated in Germany could not be grown and sporulated above 36 °C (Kiewnick 2006). Native EPFs have been shown to be superior for pest management since they are biologically compatible with their location and habitat type (Bidochka et al. 2002). Thus, the P. lilacinum PL1 isolate in this study was appropriate for the tropical monsoon climate in Viet Nam, characterized by high temperatures (Kosanic et al. 2019). Furthermore, pH is one of the abiotic variables impacting EPFs activity in both laboratory survival and efficacy in field conditions. As an agricultural application, P. lilacinum PL1 had effectively grown and sporulated across a large pH range (from 5 to 8), which was highly beneficial as it would be amenable to application in various habitats with different pH ranges.
Entomopathogenic fungi are well-known for parasitizing arthropods by adhering to and interacting with the insect’s epicuticular layer through the formation of physical or enzymatic activity after penetrating the insect cuticle (Ortiz-Urquiza et al. 2013). EPFs establish a pathogenic connection with the insect by producing an infective structure known as the appressorium (Sandhu et al. 2012) then further penetrate the insect cuticle via mechanical pressure and cuticle-degrading enzymes such as proteases and chitinases (Ibrahim et al. 2016; Sunitha et al. 2013). The present findings suggested that P. lilacinum PL1 may produce the cuticle-degrading enzymes protease and chitinase, which are required for insect parasitism.
In the present study, the effect of P. lilacinum PL1 isolated against the whitefly B. tabaci under laboratory conditions, followed by field experiments was examined. From the data presented, it is apparent that a dilution of P. lilacinum PL1 at 1 × 107 conidia/ml considerably reduced the B. tabaci nymphs’ populations in vitro after 7 days of treatment, with an efficiency of 88.24%. This result corroborated a previous study showing that P. lilacinum isolate XI-1 caused the highest adult mortality rate of whitefly B. tabaci (86.81%) within 7 days of treatment at a concentration of 1 × 107 conidia/ml (Sun et al. 2021), lending further credence to our proposal of using EPF as a suitable agent for pest management. The LC50 of P. lilacinum PL1 on whitefly B. tabaci nymphs deduced from the bioassay studies was 1.24 × 105 conidia/ml at day 7th after treatment. Thus, P. lilacinum PL1 expressed the same pattern in the management of whitefly B. tabaci in the comparison to P. lilacinum isolate XI-1 in the previous study (Sun et al. 2021).
The field evaluation study in assessing the lethality of P. lilacinum PL1 at concentration of 1 × 107 conidia/ml, in the present study revealed the percentage of B. tabaci nymphs’ reduction as 77.46% at day 7th after the first spraying. B. tabaci nymphs’ mortality was considerably higher in the fungal treatment plots after the second spraying (78.86%) than the untreated group, demonstrating that the B. tabaci nymphs’ population was suppressed, following 2 batches of 7-day after treatment of P. lilacinum PL1. The concentration of 1 × 107 conidia/ml used in this study was comparable to previous studies for insect management (Cabanillas et al. 2009). In previous study, P. fumosoroseus and P. lilacinus lowered the glasshouse whitefly T. vaporariorum over 70% (Gökçe et al. 2005). P. fumosoroseus Apopka-97, on the other hand, reduced the T. vaporariorum population by 48% (Alma et al. 2007). Thus, the efficacy of P. lilacinum PL1 is considered to be equivalent to or higher than several previous studies. Furthermore, the P. lilacinum PL1 application was more effective than the fenobucarb treatment plots at managing the B. tabaci nymphs’ population.
Pesticides are currently the mainstay of whitefly management. Considering the threats associated with pesticide abuse, B. bassiana, M. anisopliae, and I. fumosorosea are effective EPFs that play a role in the management of whitefly nymphs as part of an integrated pest management strategy (Gul et al. 2014; Mora et al. 2016). Thus, P. lilacinum PL1 identified in this investigation was found to be very efficient and possesses exceptional potential as a biopesticide. Despite the fact that EPFs have significant advantages over conventional pesticides, there are several limitations to their application for pest management. The insecticidal activity of EPF in open fields in the tropics can be negatively affected by environmental conditions such as temperature, humidity and UV exposure (Loong et al. 2013). Therefore, further studies on the effects of humidity, temperature, and UV protection agents are required to enhance the efficacy of P. lilacinum PL1 in the management of sucking insects.
Conclusions
A highly pathogenic strain of P. lilacinum PL1 was isolated from B. tabaci cadavers infected with EPFs. The lethality of P. lilacinum PL1 against B. tabaci nymphs was assessed in the laboratory and in the field conditions. According to a biological assay, P. lilacinum PL1 was highly lethal to B. tabaci nymphs under laboratory conditions. In addition, P. lilacinum PL1 was superior to the chemical agent fenobucarb for the management of B. tabaci nymphs in cassava fields. Our findings authenticate the potential of P. lilacinum PL1 in the management of the whitefly B. tabaci; consequently, P. lilacinum PL1 might be utilized as a biopesticide as part of an integrated pest management (IPM) strategy to manage whiteflies in a sustainable manner.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Abbreviations
- CMB:
-
Cassava mosaic begomoviruses
- CMD:
-
Cassava mosaic disease
- EPF:
-
Entomopathogenic fungi
- g :
-
Gravity force or relative centrifugal force
- ITS:
-
Internal transcribed spacer
- LC:
-
Lethal concentration
- LT:
-
Lethal time
- PDA:
-
Potato dextrose agar
- RCBD:
-
Randomized complete blocks design
- SLCMV:
-
Sri Lankan cassava mosaic virus
- PCR:
-
Polymerase chain reaction
- RH:
-
Relative humidity
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Akıner MM, Öztürk M, Güney İ, Usta A (2020) Natural infection potential and efficacy of the entomopathogenic fungus Beauveria bassiana against Orosanga japonica (Melichar). Egyp J Biol Pest Control 30:1–9
Alma CR, Goettel MS, Roitberg BD, Gillespie DR (2007) Combined effects of the entomopathogenic fungus, Paecilomyces fumosoroseus Apopka-97, and the generalist predator, Dicyphus hesperus, on whitefly populations. Biocontrol 52:669–681
Amala U, Jiji T, Naseema A (2013) Laboratory evaluation of local isolate of entomopathogenic fungus, Paecilomyces lilacinus Thom Samson (ITCC 6064) against adults of melon fruit fly, Bactrocera cucurbitae Coquillett. J Trop Agric 51(1):132–134
Bidochka MJ, Menzies FV, Kamp AM (2002) Genetic groups of the insect-pathogenic fungus Beauveria bassiana are associated with habitat and thermal growth preferences. Arch Microbiol 178:531–537
Cabanillas HE, Jones WA (2009) Pathogenicity of Isaria sp.(Hypocreales: Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Protect 28(4):333–337
Cruz-Estrada A, Gamboa-Angulo M, Borges-Argáez R, Ruiz-Sánchez E (2013) Insecticidal effects of plant extracts on immature whitefly Bemisia tabaci Genn. (Hemiptera: Aleyroideae). Electron J Biotechnol 16(1):6–6
Dawar S, Ghaffar A (2003) Screening of substrates for mass production of biocontrol agents. Pakistan J Bot (Pakistan)
Desoky SMA, Abdelall MF, Ahmed Y (2022) Isolation, Identification, evaluation of Purpureocillium Lilacinum Egyptian isolate toxicity test in vitro and analysis its bioactive products. J Adv Agric Res 27(4):602–617
Gangwar R, Gangwar C (2018) Lifecycle, distribution, nature of damage and economic importance of whitefly, Bemisia tabaci (Gennadius). Acta Sci Agric 2(4):36–39
Goffré D, Folgarait PJ (2015) Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. J Invertebr Pathol 130:107–115
Gökçe A, Er MK (2005) Pathogenicity of Paecilomyces spp to the glasshouse whitefly, Trialeurodes vaporariorum, with some observations on the fungal infection process. Turk J Agric For 29(5):331–340
Götz M, Winter S (2016) Diversity of Bemisia tabaci in Thailand and Vietnam and indications of species replacement. J Asia-Pac Entomol 19(2):537–543
Grimes DJ (2006) Koch’s postulates-then and now. Microb-Am Soc Microbiol 1(5):223
Gul HT, Saeed S, Khan FA (2014) Entomopathogenic fungi as effective insect pest management tactic: a review. Appl Sci Bus Econ 1(1):10–18
Henderson CF, Tilton EW (1955) Tests with acaricides against the brown wheat mite. J Econ Entomol 48(2):157–161
Hsu S, Lockwood J (1975) Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol 29(3):422–426
Humber RA (2012) Identification of entomopathogenic fungi, Manual Tech Invertebr Pathol: 151–187
Ibrahim A, Mohamed H, El-Naggar S, Swelim M, Elkhawaga O (2016) Isolation and selection of entomopathogenic fungi as biocontrol agent against the greater wax moth, Galleria mellonella L.(Lepidoptera: Pyralidae). Egypt J Biol Pest Control 26(2):249
Indriyanti DR, Widiyaningrum P, Slamet M, Maretta YA (2017) Effectiveness of Metarhizium anisopliae and Entomopathogenic nematodes to control Oryctes rhinoceros larvae in the rainy season. Pak J Biol Sci: PJBS 20(7):320–327
Islam W, Akutse KS, Qasim M, Khan KA, Ghramh HA, Idrees A, Latif S (2018) Bemisia tabaci-mediated facilitation in diversity of begomoviruses: evidence from recent molecular studies. Microb Pathog 123:162–168
Johny S, Kyei-Poku G, Gauthier D, van Frankenhuyzen K (2012) Isolation and characterisation of Isaria farinosa and Purpureocillium lilacinum associated with emerald ash borer, Agrilus planipennis in Canada. Biocontrol Sci Tech 22(6):723–732
Khalid MZ, Ahmed S, Al-Ashkar I, El Sabagh A, Liu L, Zhong G (2021) Evaluation of resistance development in Bemisia tabaci Genn.(Homoptera: Aleyrodidae) in cotton against different insecticides. InSects 12(11):996
Kiewnick S (2006) Effect of temperature on growth, germination, germ-tube extension and survival of Paecilomyces lilacinus strain 251. Biocontrol Sci Tech 16(5):535–546
Kim H, Mai NTT, Mai NB, Howeler R (2015) Cassava conservation and sustainable development in Vietnam. In: Paper presented at the sustainable cassava production in asia for multiple uses and for multiple markets. Proceedings of the 9th regional cassava workshop, Nanning, Guangxi, China
Kosanic A, Kavcic I, van Kleunen M, Harrison S (2019) Climate change and climate change velocity analysis across Germany. Sci Rep 9(1):1–8
Kurbatova YA, Kuricheva O, Avilov V, Dinh BD, Kuznetsov A (2015) Fluxes of energy, H2O, and CO2 between the atmosphere and the monsoon tropical forest in Southern Vietnam. Paper presented at the Doklady Biological Sciences
Lacey LA, Frutos R, Kaya H, Vail P (2001) Insect pathogens as biological control agents: do they have a future? Biol Control 21(3):230–248
Lavers A (2001) Guidelines on good practice for ground application of pesticides. Food and Agricultural Organization (FAO) of the United Nations, Rome
Loong C, Ahmad SS, Hafidzi MN, Dzolkifli O, Faizah A (2013) Effects of UV-B and solar radiation on the efficacy of Isaria fumosorosea and Metarhizium anisopliae (Deuteromycetes: Hyphomycetes) for controlling bagworm, Pteroma pendula (Lepidoptera: Psychidae). J Entomol 10(2):53–65
Mascarin GM, Kobori NN, Quintela ED, Delalibera I Jr (2013) The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and their conidial production using solid substrate fermentation. Biol Control 66(3):209–218
Mora MAE, Rouws JRC, Fraga ME (2016) Occurrence of entomopathogenic fungi in Atlantic forest soils. Microbiol Discov 4(1)
Naranjo SE, Ellsworth PC (2017) Methodology for developing life tables for sessile insects in the field using the whitefly, Bemisia tabaci, in cotton as a model system. JoVE (J Vis Exp) 129:e56150
Ortiz-Urquiza A, Keyhani NO (2013) Action on the surface: entomopathogenic fungi versus the insect cuticle. InSects 4(3):357–374
Pathak VM, Verma VK, Rawat BS, Kaur B, Babu N, Sharma A, Dewali S, Yadav M, Kumari R, Singh S (2022) Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: a comprehensive review. Front Microbiol. https://doi.org/10.3389/fmicb.2022.962619
Sandhu SS, Sharma AK, Beniwal V, Goel G, Batra P, Kumar A, Jaglan S, Sharma A, Malhotra S (2012) Myco-biocontrol of insect pests: factors involved, mechanism, and regulation. J Pathog. https://doi.org/10.1155/2012/12689
Shah R, Al-Sadi AM, Nasser Al-Sabahi J, Al-Raeesi AA, Khamis Said Al-Rawahi K, Saud Al-Rashdi A, Fadhil Madad Al-Hinai S, Velazhahan R (2020) Efficacy of an Omani strain of Cordyceps javanica and its culture filtrate against whitefly (Bemisia tabaci) under laboratory conditions. All Life 13(1):615–622
Sharma L, Oliveira I, Torres L, Marques G (2018) Entomopathogenic fungi in Portuguese vineyards soils: Suggesting a ‘Galleria-Tenebrio-bait method’as bait-insects Galleria and Tenebrio significantly underestimate the respective recoveries of Metarhizium (robertsii) and Beauveria (bassiana). MycoKeys 38:1
Skinner M, Parker BL, Kim JS (2014) Role of entomopathogenic fungi in integrated pest management. Integr Pest Manag: 169–191
Sun T, Wu J, Ali S (2021) Morphological and molecular identification of four Purpureocillium isolates and evaluating their efficacy against the sweet potato whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt J Biol Pest Control 31(1):1–9
Sunitha V, Nirmala Devi D, Srinivas C (2013) Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J Agric Sci 9(1):1–9
Tangthirasunun N, Poeaim S, Soytong K, Sommartya P, Popoonsak S (2010) Variation in morphology and ribosomal DNA among isolates of Metarhizium anisopliae from Thailand. J Agric Technol 6(2):317–329
Tokunaga H, Baba T, Ishitani M, Ito K, Kim O-K, Ham LH, Le HK, Maejima K, Natsuaki KT, Van Dong N (2018) Sustainable management of invasive cassava pests in Vietnam, Cambodia, and Thailand. Crop Prod under Stressful Cond. https://doi.org/10.1007/978-981-10-7308-3_8
Uke A, Hoat TX, Quan M, Liem N, Ugaki M, Natsuaki KT (2018) First report of Sri Lankan cassava mosaic virus infecting cassava in Vietnam. Plant Dis 102(12):2669
Wang H-L, Cui X-Y, Wang X-W, Liu S, Zhang Z, Zhou X (2016) First report of Sri Lankan cassava mosaic virus infecting cassava in Cambodia. Plant Dis 100(5):1029–1029
Watanabe T (2010) Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC Press
Zhang X, Shuai Y, Tao H, Li C, He L (2021) Novel method for the quantitative analysis of protease activity: the casein plate method and its applications. ACS Omega 6(5):3675–3680
Acknowledgements
The authors are especially grateful to Nguyen Tat Thanh University and HUTECH University for providing all the resources needed for this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HNT and ADD designed this study; KYPL, NDTT, and TDN performed experiments; ADD wrote the paper. All authors approved this final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent for publication was taken from the co-authors.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen Thi, H., Phung Le, K.Y., Thai Thien, N.D. et al. Insecticidal activity of isolated Purpureocillium lilacinum PL1 against whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) on cassava plantations in southern Viet Nam. Egypt J Biol Pest Control 33, 44 (2023). https://doi.org/10.1186/s41938-023-00691-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00691-2