Abstract
Background
Geogenic and anthropogenic activities such as Artisanal and illegal gold mining continue to have negative impacts on the environment and river basins in China. This work studied the hydrogeochemical characteristics of surface water from the Birim River basin and assess the quality of water for human consumption and agricultural activities. In addition, the ecological risk assessment for Cd, Zn, Pb and As in sediment was evaluated using pollution indices.
Results
The results show that the turbidity, temperature, colour and iron concentration in the water samples were above the World Health Organization guidelines. Multivariate analysis explained five components that accounted for 98.15% of the overall hydrogeochemistry and affected by anthropogenic and geogenic impacts. The surface water was observed to range from neutral to mildly acidic, with the dominance of HCO3−, Cl−, Ca2+, Mg2+, and Na+ in ionic strength. The Piper diagram reveals five major surface water types: Na–HCO3–Cl, Na–Cl–HCO3, Na–Ca–Mg–HCO3, Na–Ca–Mg–HCO3 and Ca–Na–Mg–HCO3. The Gibbs plot showed that the major ion chemistry of surface water was mostly influenced by atmospheric precipitation and the water quality index showed that the majority of the surface water from settlements within the Birim River basin were of poor quality for drinking and other domestic purposes. However, irrigation suitability calculations with reference to sodium adsorption ratio, residual sodium carbonate, and magnesium ratio values, together with Wilcox and USSL models indicated that the surface water within the area under study was suitable for agriculture. The potential ecological risk for single heavy metals pollution and potential toxicity response indices gave low to considerable ecological risks for the sediments, with greater contributions from Cd, Pb and As. Whilst geo-accumulation indices indicated that the sediments ranged from unpolluted to moderately polluted Modified degree of pollution and Nemerow pollution index calculations which incorporate multi-element effects, however, indicated no pollution.
Conclusion
There are some levels of both potential ecological risks and health hazards in the study area. Hence continuous monitoring should be undertaken by the relevant agencies and authorities so that various interventions could be put in place to prevent the situation from deteriorating further in order to protect the inhabitants of the settlements within the Birim River basin.
Similar content being viewed by others
Background
Surface water which is the most significant inland water resource for human consumption, agricultural activities, recreational and industrial purposes (Razmkhah et al. 2010) has always been the end point of wastewater disposal from the adjacent areas. Lithology of river basins, anthropogenic inputs, climatic and atmospheric conditions affects the quality of surface water at any point. A series of organic, inorganic and biological pollutants, such as highly toxic heavy metals (Demirak et al. 2006; Moore and Ramamoorthy 2012) or non-toxic, biodegradable materials, such as faeces, food waste and wastewater can affect the quality of surface water (Bain et al. 2014).
The hydrogeochemical characteristics of surface water rely on the chemical composition of rock-forming minerals, such as sulphide, carbonate and silicate, as well as the physical process of erosion, which generates favourable conditions for mineral dissolution. Consequently, water resources are being enriched with metals, metalloids or ions, which often tend to be toxic to mankind as well as the natural environment (Lang et al. 2006; Négrel et al. 2006; Robinson and Ayotte 2006). Other geochemical activities, such as sorption, redox reactions, ion exchange, and complexation may alter it’s hydrogeochemistry and subsequently affect the water quality. The hydrogeochemical characterization may be accomplished via several techniques, and among them are the typical hydrogeochemical ratios, which can evaluate the dominant and origin processes of water resources (Zhu et al. 2007), as well as multivariate statistical analysis (Saleem et al. 2015; Purushothaman et al. 2014). These two techniques may be valuable in identifying the factors that influence surface water chemistry, particularly hydrogeological and complex geological systems.
Information on the physicochemical parameters of water for the endurance of organisms, such as fauna and flora is essential for evaluating the quality and type of water (Liu et al. 2010). Temperature and pH are among essential features of the environment since they affect nutrition, growth, metabolic activity and human reproduction. Heavy metals have been revealed to pose a serious threat to human health due to their toxicity, persistence, bioaccumulation in the food chain and non-destructible nature in the environment (Asare-Donkor et al. 2015; Asefi and Zamani-Ahmadmahmoodi 2015; Boateng et al. 2015; Bortey-Sam et al. 2015; Singh et al. 2013; Zhang et al. 2014). Essential metals, such as Fe, Mn, Zn and Cu play a vital role in the biological processes but turn to be toxic above certain concentrations. Several studies have revealed that chlorine contents in water are related to senility, heart disease as well as cancer of the urinary tract, pancreas, liver, colon, and osteosarcomas (Kim et al. 2011; Comber et al. 2011).
There are diverse reports in literature which assess the geochemical characteristics of surface water systems, as well as anthropogenic contamination influenced by factors such as agricultural fertilizers, sewage effluents, evapotranspiration, water–rock interactions and ion-exchange in several parts of the world (Abdesselam et al. 2013; Alaya et al. 2014; Iranmanesh et al. 2014; Kim et al. 2015; Khashogji and El Maghraby 2013; Nandimandalam 2012; Singh et al. 2016). However, in Ghana, there are few such reports (Boateng et al. 2016; Helstrup et al. 2007; Fianko et al. 2010; Yidana et al. 2012). The determination of surface water composition is of extreme significance for the assessment of its suitability for drinking, irrigation and domestic purposes. The primary objective the study has been to evaluate the hydrochemical characteristics of surface water from the Birim River basin assess the ecological risk and the suitability for domestic and irrigation uses.
Methods
Study area
The Birim River basin is located between latitudes 5°45′N and 6°35′N; and longitudes 0°20′W and 1°15′W. The Birim River takes its source from the Atewa Hills, Eastern Region of Ghana and follows a course of 175 km2 southwards to join the Pra River. The river drains an area of approximately 3895 km2 with the major tributaries being Adim, Amaw, Kade and Si. It has an estimated area of 3875 km2 (Ansah-Asare and Asante 2000). The rainfall pattern in the drainage area varies seasonally with major peaks from June to September and dry spells from December to January. It has a temperature range of 25–30 °C and relative humidity of 70–80% throughout the year. The area is endowed with mineral deposits, such as gold, bauxite, diamond, and others. The main occupations the settlers are farming and small-scale mining activities known as “Galamsey”, mostly along the banks and inside of the Birim River.
Sampling
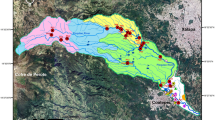
Forty-three samples of water and sediment were collected from 10 settlements (Fig. 1) within the Birim River basin. The sampling stations were Apapam (The source of the Birim River), Kibi, Adukrom, Bunso, Nsutam, Nsuapemso, Osino, Ankaase, Mampong, and Anyinam. Prior to sampling, water sample containers of 500 mL polyethylene bottles were rinsed with detergents and then washed thoroughly several times with distilled water. They were further soaked in 10% (v/v) HNO3 and left overnight. The surface water samples were collected at the mid-stream at 30 cm depths. Two sets of surface water samples (one for heavy metal and the other for physicochemical parameters) were taken from each sampling point. Electrical conductivity (EC), total dissolved solids (TDS) and pH were measured in situ. The samples for heavy metal analysis were filtered through pre-washed 0.45 m Millipore nitrocellulose filters to remove any suspensions treated with 2 mL of Analar HNO3 at pH < 2 to preserve them. The water samples were appropriately labelled and immediately transported in an ice chest to the laboratory and kept in the refrigerator at − 4 °C prior to analysis.
Sediment samples were taken from the top surface layer of sediment at a depth of 0–6 cm with a plastic trowel into open-mouth plastic containers. Sediment samples were obtained from the same location and at the same point where the water samples were taken. A sediment weight of about 30 g each was collected from each sampling station.
Sample analysis
Physicochemical parameters
Physical parameters, such as EC, pH and TDS were determined using cyberscan PC 650 multimeter series. Turbidity was measured using HANNA HI 93414 turbidity meter while Colour was determined using a spectrophotometer set at 465 nm. The temperature was determined using Mercury in Glass Thermometer and Total alkalinity was measured by the titrimetric method. The carbon dioxide content in the sample was determined by computations from the sample pH and total alkalinity. Total hardness was measured using EDTA titration and thus calculated as CaCO3 content (mg/L). Chloride concentration was determined using potentiometric titration with an AgNO3 solution with a glass and Ag–AgCl electrode system. The UV technique was used to determine the nitrogen concentration in the water samples as nitrates at a wavelength of 220 and 275 nm. Magnesium concentration was calculated as the difference between total hardness and calcium hardness multiplied by 0.243. The flame photometric method was used to analyse the potassium and sodium ion concentrations at a wavelength of 766.5 and 589 nm respectively.
Heavy metals
Water samples were digested by a method described by Asare-Donkor et al. (2015) and Arnold and Lenores (1989). Briefly, 100 mL of each water sample was transferred into a 250 mL beaker and 5 mL of conc. HNO3 was added. The mixture was gently heated on a hot plate after a few boiling chips had been added and evaporated to about 20 mL. Another 5 mL of conc. HNO3 was added and then heated for 10 min and allowed to cool. About 5 mL conc. HNO3 was used to rinse the sides of the beaker and the solution was quantitatively transferred into a 100 mL volumetric flask and made up to the mark with deionized distilled water.
The sediment samples were initially air-dried and further dried in an oven at 70 °C for 48 h to obtain a constant weight. The sediment was then crushed and ground into fine particles and further passed through a 2 mm sieve. The sediments were digested by a method described by Kouadia and Trefry (1987). About 1.0 g of the finely ground sediment sample was accurately weighed into a beaker and equal amounts of concentrated nitric acid, hydrofluoric acid and perchloric acid were added. The beaker was covered and set aside for several hours and evaporated to a few drops on a hot plate. Then 5 mL of HClO4 was added and evaporated to dryness. Conc HCl (10 mL) was added and the mixture was heated until the solution was clear and the fumes had ceased. Deionized distilled water was added and the digested material was filtered into a 100 mL volumetric flask, then the residue was washed several times with deionized distilled water and made to the mark. The heavy metals, such as Fe, Pb, Zn Cd and As were analysed with the VARIAN AA240FS atomic absorption spectrophotometer with an air-acetylene flame. Requisite lamps with appropriate operating absorption wavelength and other operating parameters for each element was employed for the determination. Each of the analysis was done in triplicate, in which the mean values were calculated.
Quality assurance
Replicate blanks and Standard Reference Material (SRM) of Fluka Analytical (Sigma-Aldrich Chemie GmbH, Product of Switzerland) were used for the quality control and method validation. Analytical results indicated a good agreement between those reported in this study and the certified value.
Statistical analysis
IBM SPSS-20 program was also used to analyse the Person correlation, principal component analysis (PCA) and cluster analysis (CA). XLSTAT ‘2016 statistical software and Origin 2016 Data Analysis and Graphing Software were used for the geochemical analysis.
Risk assessment methods
Enrichment factor (EF)
The enrichment factor (EF) is a convenient measure of geochemical trends and is used to characterize the degree of anthropogenic pollution through the establishment of enrichment ratios (Zakir et al. 2008). To evaluate the extent of contamination in the environment, the factors EF were computed relative to the abundance of species in the source material and to that found in the Earth’s crust (Sinex and Helz 1981).
where CM is the content of metal studied and CX is the content of the immobile element, immobile elements may be Fe (Zhang et al. 2007). In this study, the background geochemical compositions by Taylor and McLennan (1995) were used as the background values for the calculation. Five classes of enrichment factors have been identified EF < 2, depletion to mineral, 2 ≤ EF < 5: moderate, 5 ≤ EF < 20: significant, 20 ≤ EF < 40: very high and EF > 40: extremely high (Sutherland 2000).
Index of geo-accumulation (Igeo)
The \(I_{geo}\), which is the geochemical benchmark to assess heavy metals pollution in sediments was in was calculated based on Eq. (2):
where, \(B_{n}\) and \(C_{n}\) represent the geochemical background concentration and the measured concentration of the studied heavy metal n in the sediment, respectively. Because of the possible dissimilarities in background concentrations of any given heavy metal and anthropogenic influences, a factor value of 1.5 was used.
Modified degree of contamination
The modified degree of contamination (mCd) which has an advantage over single element indices since it takes account the synergistic effect of the contaminants at a study site (Brady et al. 2015) was calculated as follows:
where \(C_{f}^{i} = {\raise0.7ex\hbox{${C^{i} }$} \!\mathord{\left/ {\vphantom {{C^{i} } {C_{ref}^{i} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${C_{ref}^{i} }$}}\), \(C^{i}\) is the heavy metal concentration in sediment samples; \(C_{ref}^{i}\) the reference value of the element (Turekian and Wedepohl 1961; Hankason 1980) and \(C_{f}^{i}\) the contamination factor of each element.
Nemerow pollution index
The Nemerow pollution index takes into account the comprehensive effects of heavy metals (Yan et al. 2016) and can be used to interpret heavy metal pollution at particular sites (Duodu et al. 2017). The equation for the calculation is given by Eq. (4).
where \(P_{N}\) is the Nemerow pollution index, \(\overline{C}_{f}\), the arithmetic mean of contamination factors of all heavy metals, and \(C_{fmax}\) the maximum contamination factor among the heavy metals.
Potential ecological risk index
The total ecological risk index (RI) for heavy metals in sediments was calculated using Eq. (5)
where \(C_{f}^{i}\) is the contamination factor, \(T_{f}^{i}\) is the toxicity coefficient of metal i.
Table 1 shows the classification of heavy metal modified degree of contamination (mCd), Nemerow pollution index (PN), Index of geo-accumulation (Igeo) and potential ecological risk used for the sediment.
Pollution load index
The pollution load index (PLI) was evaluated as the nth root of n multiplied contamination factor (Cf), as shown in Eq. (6):
PLI mainly allows a qualitative comparison between sites. PLI values < 1 signifies no pollution, PLI = 1 signifies baseline levels of pollutants and PLI > 1 signifies progressive site pollution. The pollution load index value of 0.08 indicated no deterioration of the site quality (PLI < 1).
Water quality index
Water quality index (WQI) was used in the evaluation of the status of water sources from the communities. In this process, each water quality parameter was assigned a specific weight (W) based on their relative significance on the water quality and the relative weight (W i ) was evaluated based on Eq. (7).
where w i represent the assigned weight of each parameter, W i is the sum of the assigned weights of all the parameters and the number of parameters (Table 2). A maximum weight of five was assigned to NO3− and Cl− owing to their significance to water quality, as well as human health.
Water quality index (WQI) allows easier illustrations of complex data to determine the status of water systems. The WQI calculations are depended on significant water quality parameters by providing a specific number to represent the overall water quality (Pius et al. 2012). According to WHO (2011), the quality rating scale (q i ) for each of the water parameter was evaluated following Eq. (8) and finally WQI was calculated using Eqs. (9) and (10):
where C i , q i and S i , respectively, represent the concentration of each chemical parameter in the water sample, the quality rating and the concentration of each parameter as per the WHO (2011) standard Table 2; SI i and W i represent the sub-index of the ith parameter and the sum of the assigned weights of all the parameters (Table 2), respectively.
Suitability for irrigation purposes
The suitability of surface water for irrigation purpose was evaluated by calculating the soluble sodium percentage (SSP), sodium adsorption ratio (SAR), Permeability index, Kelly’s ratio (KR), residual sodium carbonate (RSC) and magnesium ratio (MR). The SSP, SAR, KR, RSC and MR were calculated using Eqs. (11) to (15) and the values together with the PIs are given in Additional file 1: Table S3.
Results and discussion
Physicochemical parameters
Physicochemical parameter values of the surface water samples analysed in the Birim River basin are presented in Table 3. The water temperature ranged between 25.00 and 28.00 °C with a mean value of 26.26 °C. The colour of the water samples ranged from 364.62 to 34615.35 PtCo, with an average 14,583.67 PtCo. The levels of colour in all the surface water samples exceeded the WHO permissible limit of 15 PtCo. Highly collared water may be owned by the decaying vegetation in the water resources (Karikari and Ansa-Asare 2006). The pH values of all the surface water samples ranged between 6.36 and 7.02, with a mean pH of 6.68, indicating acidic to neutral to nature of Birim River basin water. The acidic pH was attributable to the naturally occurring silicate minerals. The pH values of all the surface water samples were below the WHO acceptable limits of 6.5–8.5 for drinking and other domestic purposes (WHO 2011). The TDS and EC concentrations in the surface water samples ranged from 29.03 to 64.33 mg/L and 58.03 to 128.97 μS/cm, with a mean of 42.60 mg/L and 25.21 μS/cm, respectively. The low TDS concentration in the water samples is due to the short residence time of the underground rocks as well as the slow weathering of granitic (Patel et al. 2016). The EC and TDS concentrations of all the river water samples are very much below the WHO acceptable limit of 500 μS/cm and < 600 mg/L, respectively (WHO 2011). Total alkalinity (TA) is a measure of HCO3− and CO32− ions. TA refers to the ability of water to neutralise strong acids. The alkalinity of the surface water samples ranged between 20.00 and 161.00 mg/L, with a mean of 79.20 mg/L. The alkalinity of all the surface water samples fell below the WHO permissible limits of 200.00 mg/L. Total hardness (TH) represent alkaline earth elements, such as magnesium and calcium within the water resources. The TH ranged from 51.76 to 168.57 mg/L, with a mean value of 92.31 mg/L. TH concentrations of all the river water samples were below the WHO guideline of 500 mg/L. Turbidity has an average value of 1652.86 NTU that ranges from 31.60 to 3000.00 NTU. All the surface water samples had turbidity concentration above the WHO acceptable limit of 5 NTU. The high turbidity can be ascribed to larger particles, such as dissolved solids and organic matter in the surface water samples (Schafer et al. 2010). The calcium hardness of the sampled surface water varied from 30.74 to 100.26 mg/L, with a mean of 55.15 mg/L, while magnesium hardness ranged between 21.02 and 68.31 mg/L, with a mean of 37.16 mg/L All the analysed surface water samples were below the WHO permissible limit of 200 mg/L for magnesium hardness. The free and total CO2 content in the surface water samples ranged from 17.57 to 44.63 and 35.17 to 172.11 mg/L with a mean of 30.02 and 99.24 mg/L, respectively.
Major ions and heavy metals
The concentrations of major ions, such as K+, Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, and CO32−) are summarised in Table 3. The sources of sodium ion (Na+) are from the weathering products of silicate rocks and its movement from the absorbed complex of soil and rocks by magnesium and calcium. The concentrations of Na+ varied between 2.90 and 5.10 mg/L, with a mean of 3.81 mg/L. The Na+ concentrations were below the WHO recommended limits of 200 mg/L. The levels of K+ in the surface water samples ranged between 0.40 and 3.90 mg/L, with a mean value of 1.00 mg/L and the levels were below the permissible limit of 12 mg/L by WHO (2011). The low concentration of K+ in the surface water samples was due to its affinity to be immobilised by clay minerals to partake in forming secondary minerals. The levels of Ca2+ ion in the Birim River basin ranged from 12.80 to 41.60 mg/L, with a mean 22.40 mg/L. The levels of Mg2+ varied between 5.13 and 16.66 mg/L, with a mean level of 8.90 mg/L. The concentration of Ca2+ and Mg2+ ions, respectively, in all the river water samples, fall below the WHO maximum acceptable limit of 75 and 150 mg/L. Calcium (Ca) and magnesium (Mg) are serious hydrochemical elements in water (Razowska-Jaworek 2014). Ca is a significant ion that affects the hardness of water and has been the most abundant elements found in water resources. The anion chemistry of Birim River basin was dominated by HCO3− followed by Cl−, SO42−, NO3− and CO32−. The Cl− content in the Birim water samples varied from 11.98 to 69.96 mg/L, with a mean of 27.76 mg/L and all the surface water samples were below the acceptable limits of 250 mg/L recommended by the WHO (2011). Sulphate (SO42−) ions are limited by Ca2+ ions in surface water. The major source of sulphate in water resources is sedimentary rock, such as anhydride and gypsum. The sulphate concentration in the Birim River basin ranged between bd and 1.00 mg/L, with a mean value of 11.98 mg/L. The SO42− contents in the Birim River samples were below the acceptable limits of 250 mg/L recommended by WHO. The HCO3− and CO32− concentrations ranged from 19.99 to 160.84 mg/L and 0.01 to 0.16 mg/L, with mean values of 78.63 and 0.05 mg/L, respectively. The levels of nitrate (NO3−) in surface water can be attributed to the oxidation of nitrogenous waste products in animal and human excreta, wastewater disposal and agricultural activity. The concentrations of NO3− in surface water can vary depending on surface runoff of fertiliser, denitrification by bacteria and uptake by phytoplankton. The level of NO3− in surface water was moderately low, ranged from bd to 1.00 mg/L, with a mean value of 0.38 mg/L. The levels of NO3− were below the acceptable limits of 45 mg/L. The level of iron in the surface water ranged between 0.63 and 6.44 mg/L, with a mean value of 3.41 mg/L. The level of zinc in the surface water ranged between bd and 0.02 mg/L, with a mean level of 0.01 mg/L. The levels of zinc were below the acceptable limit of 3 mg/L as per the WHO (2011) standard. The levels of As, Pb and Cd were however observed to be below detection in all the water samples analysed.
Correlation of water quality parameters
There was a strong correlation between Ca2+ and Mg2+ with TH indicates that Ca2+ and Mg2+ are the major contributors to water hardness. Ca2+ and Mg2+ has a significant positive correlation (r = 1.000, p < 0.01), indicating the contribution of dolomite to the levels of Ca2+ and Mg2+ ions in the surface water. The positive correlation of Cl− with Na+ and Ca2+ indicates the dominance of soluble salts. High positive correlation of EC with CO32− and HCO3− ions signify high mobility of CO32− and HCO3− ions in the surface water. In addition, the strong significant correlation between TDS and EC (r = 1.000, p < 0.01) might be ascribed to ions in TDS conducting electricity. The strong positive correlation between Mg2+ and SO42− ions (r = 0.703) indicates agricultural activities, such as chemical and organic fertilisers in the study area. The strong correlation between NO3− and SO42− signifies the influences of agricultural activities, evaporation, marine sources and poor drainage conditions on the surface water system. Surface water HCO3− was strongly correlated with pH, EC, alkalinity CO32− and TDS. Total CO2 was strongly correlated with HCO3−, CO32− and free CO2.
Hierarchical cluster analysis
The water quality differences between the sampling stations are given by dendrogram as shown in Fig. 2. Cluster 1, representing Adukrom, Nsutam, Osino and Anyinam are areas with high water pollution. Cluster 2 representing Kibi, Nsuapemso and Apapam are areas with moderate water pollution. Cluster 3 representing Ankaase, Mampong and Bunso are areas with lower water pollution.
Principal component analysis
The physicochemical parameters in the surface water samples were statistically evaluated with R-mode PCA to understand the correlation of the analysed parameters and identify the significant factors influencing the study area. Suitability of components for PCA was tested by performing Kaiser–Meyer–Olkin (KMO) tests. KMO test is an index used in relating the magnitude of the observed correlation and partial coefficients with a value of > 0.5. In this study, KMO was 0.83, which indicates the suitability of the physicochemical parameters results for component analysis. The component loadings are classified as strong, moderate and weak with absolute loading values of > 0.75, 0.75–0.50 and 0.50–0.30, respectively. The PCA on the composite data sets extracted five components with eigenvalue > 1 and these components explained 98.15% of the total variance as shown in Additional file 1: Table S1.
The first component (PC1) accounted for 49.14% of total variance and includes strong positive loadings of pH, turbidity, alkalinity, total hardness, colour, calcium hardness, magnesium hardness, Mg2+, Ca2+, SO42−, CO32−, HCO32− and total CO2; and moderate positive load of TDS, EC, free CO2 and Fe. This component signifies dissolution of carbonate minerals as well as geogenic attributes, such as surface runoff during the rainy season, soil erosion, and weathering of mineral bearing rocks. The second component (PC2) explaining 20.68% of the total variance, have a strong positive loading of temperature; moderate positive loadings of TDS and Fe and weak loadings of EC. The moderate positive load of Fe is influenced by geological activities (Adamu et al. 2015). The third component (PC3) explaining 13.71% of total variance, have a moderate loading of SO42−, colour, and turbidity, while NO3− and K+ has a weak positive load. The PC3 indicates agriculture practices, such as the use of chemical fertilisers, as well as anthropogenic contaminants induced by domestic wastes (Jalali 2007). The fourth component (PC4) which explained 9.05% of total variance has a moderate positive loading of Zn. The fifth component (PC5) explaining 5.56% of total variance, have a moderate and weak positive loading of Cl− and Na+, respectively.
Surface water evolution mechanisms
The geochemical evolution of surface water was analysed by plotting the levels of major cations and anions in a Piper trilinear diagram. The relative abundance of the anions and cations are shown in Fig. 3. The plot reveals five types of facies Ca–Na–Mg HCO3 (39%), Na–Cl–HCO3 (15%), Na–Ca–Mg–HCO3 (23%), Na–Ca–Mg–HCO3 (4%) and Na–HCO3–Cl (8%) of which Ca–Na–Mg HCO3 is the predominant facies type.
Gibbs plots were used to access the hydrochemical processes, such as evaporation, rock-water interaction and precipitation on surface water chemistry. Gibbs plots have been extensively utilised to evaluate the underlying mechanisms behind water evolution. All the studied water samples are in the lower part of the diagram (Fig. 4), signifying precipitation interactions as the leading factor controlling the surface water chemistry. Thus, there is a close connection between the surface water chemistry and atmospheric precipitation in the Birim River basin.
Hydrogeochemical facies
Molar ratios of major ions have been extensively utilised to ascertain the hydrogeochemical formation and process mechanisms of water resources (Murkute 2014; Singh et al. 2013; Marghade et al. 2011). Ca2+/Mg2+ ratio is normally used to evaluate the source of Ca2+ and Mg2+ in the water systems. The ratio of 1, signifies dissolution of dolomite, a ratio greater than 1 (> 1), indicates calcite contribution and a ratio greater than 2 (> 2), indicates dissolution of silicate minerals (Singh et al. 2013). All the water samples have Ca2+ + Mg2+ ratio greater than 2 (> 2) (Fig. 5a), indicating silicate minerals responsible for Ca2+/Mg2+ contribution. The bimodal process of Ca enhancement and weathering process (carbonate vs silicate) are shown in Fig. 5b, displaying the ratio of (Ca2+ + Mg2+)/(HCO3− + SO42−). If Ca2+, Mg2+, HCO3− and SO42− ions are from the dissolution of dolomite, gypsum, and calcite, a 1:1 stoichiometry of (Ca2+ + Mg2+) to (HCO3− + SO42−) might occur (Singh et al. 2014). Most of the water samples, however, deviate from 1:1, which indicates that Ca2+ and Mg2+ primarily occur from the dissolution of gypsum, dolomite, and calcite. The molar ratio also signifies that the sources of Ca2+ and Mg2+ were not only from carbonate since the ratio was not 1:2. The (Na+ + K+)/Cl− molar ratios in the analysed surface water from the Birim River basin were greater than 1 (> 1) (Fig. 5c), indicating that halite, as well as silicate weathering, such as potash plagioclase and sodium plagioclase, was the source Na+ and K+ ions (Lin et al. 2016a). If Ca2+ and HCO3− in the surface water originate mainly from dolomite and calcite, the molar ratio of Ca2+ and HCO3− ions within the surface water will be 1:2 and 1:4, respectively.
In the Ca2+/HCO3− plot (Fig. 5d), the Ca2+ and HCO3− molar ratio for some of the surface water was between 1:1 and 1:2, signifying that calcite was the only source of Ca2+ and HCO3− ions in the surface water. Nonetheless, most of the surface water samples had a molar ratio less than 1:2 (< 1:2), suggesting dolomite as the dominant sources of Ca2+ and HCO3− ions. A high ratio of (Ca2+ + Mg2+)/(Na+ + K+) and (Na+ + K+)/total cation demonstrate that the chemical composition of surface water in the Birim River basin was mainly influenced by carbonate weathering with a small contribution of silicate weathering as shown in Fig. 5e, f. The plot of Ca2+ + Mg2+/Cl− obviously showed that salinity decrease with increase in Ca2+ + Mg2+, and this phenomenon can be ascribed to ion exchange (Fig. 5g). Ca2+ + Mg2+/HCO3− plot (Fig. 5h) revealed a horizontal trend line, signifying that Ca2+ + Mg2+/HCO3− ratio does not alter during the increase of HCO3−. Thus, the contribution of Ca2+ + Mg2+ and HCO3− are from different sources. Enrichment of HCO3− and depletion of Ca2+ + Mg2+ may be ascribed to cation exchange. The scatter diagram of Ca2+ + Mg2+ versus total cations revealed that the data falls below the 1:1 trend line, which demonstrates an increased contribution of Na+ and K+ as TDS increases (Fig. 5i).
Water quality index
According to Sahu and Sikdar (2008), WQI values are grouped into five classifications: excellent (< 50), Good (50.1–100), poor (100.1–200), very poor (200.1–300) and unfit for drinking (< 300.1).
The observed WQI in analysed surface water samples ranged between 32.80 and 167.71 (Table 4). Based on the surface water quality index, 50% fall under poor type, 20% are the moderate type and 30% shows excellent water type. The WQI results indicate that the majority of the surface water from the Birim River are of poor quality for drinking and other domestic uses and may pose health problems to communities in the area. Generally high values of water quality indices indicate that most of the study area have excessive levels of one or more water quality parameters. From the sensitivity analysis it was observed that the WQI did not vary much due the removal of an individual parameter with the exception of iron where the variation was very significant. The high WQI values were particularly observed in Nsutam, Osino, Mampong, Anyinam and Adukrom where iron concentrations were observed to be very high and some mining activities have been taking place.
Heavy metals concentrations in sediments and component analysis
Results of heavy metals concentration in sediments samples at the different sampling sites are presented in Table 6.
From Table 5, heavy metals levels in the sediment samples follow the order: Fe > Zn > Pb > Cd > As. The concentrations of As and Zn in all the sediment samples were below the corresponding values of effect range median (ERM), effect range low (ERL), Interim sediment quality guideline (ISQG) and probable effect level (PEL). Lead contents at all sites, was higher than ERL, ERM, PEL and ISQG guidelines, suggesting that Pb in sediments from the Birim River might be periodically expected to induce adverse biological effects on the biota. Cadmium levels in almost all the communities were higher than the ERM and ISQG guidelines except at Anyinam. Pearson correlation coefficients among the heavy metals in sediments showed significant positive correlations among Zn/As (r =0.734, p <0.05), Zn/Fe (r =0.812, p <0.01) and As/Fe (r =0.774, p <0.01), signifying that these heavy metals were related to each other and could have a common natural or anthropogenic source (Hu et al. 2013). The data were normalised to minimise the grain size effect on the heavy metals. The rotated component matrices are presented in Additional file 1: Table S2. The first two components with eigenvalues > 2 account for 77.77% of the total variability. PC1 has very high positive loadings of Zn, Fe, and As. Therefore, this component is supposed to reflect the contribution of natural geological and anthropogenic sources, such as industrial, agricultural and transportation and can originate from similar pollution sources. PC1 results concur with the correlation analysis results. PC2 has very high positive loadings of Cd, which suggested that persistent application of phosphate fertilisers in the study area may have led to an increase in Cd accumulation in the sediments (Guo and He 2013). PCA has been employed to ascertain the hypothetical source of heavy metals (Sun et al. 2010; Yi et al. 2011).
Heavy metal contamination and its ecological risks in sediments
Enrichment factor
On the basis of the magnitude of the enrichment factor, five contamination categories have been recognized generally (Sutherland 2000). Additionally, if the EF value of an element is greater than unity it indicates that the metal is more abundant in the sample relative to that found in the Earth’s crust. EF values less than 5, though of less significance, are indicative of metal accumulation since such small enrichments may arise from differences in the composition of sample material with respect to the reference Earth’s crust ratio values used in the EF calculations. If the EF values are greater than 5, samples are considered contaminated (Atgin et al. 2000). In accordance with the above suppositions, sediments from the Birim River basin (Table 6) are not contaminated with the metals studied (EF < 5), but show moderate enrichment (2 ≤ EF < 5) for only Pb and As. The mean values of EF in the sediments were 2.11, 0.11, 0.71 and 3.77 for Pb, Cd, Zn and As, respectively. These signify the anthropogenic source of Pb and As, which is in agreement with supposition by Zhang and Liu (2002) who stated that EF values greater than 1.5 suggest that the sources are more likely to be anthropogenic. It is generally presumed that high EF values are indicative of the anthropogenic source of heavy metals.
Geo-accumulation and pollution indices
The \(I_{geo}\), which is the geochemical benchmark to assess heavy metals pollution in sediments Because of the possible dissimilarities in background concentrations of any given heavy metal and anthropogenic influences, a factor value of 1.5 was used. The Igeo values of the surface sediments in the Birim river varied from − 2.51 to − 1.78 (average − 2.18) for Pb, 1.45 to 2.17 (average 1.92) for Cd, − 4.71 to − 3.36 (average − 3.99) for Zn, and − 5.48 to − 4.13 (average − 4.81) for As (Table 7). The Igeo index values for Pb, Zn and As were negative and could be included in the unpolluted status (class 0), but pollution from Cd was moderately contaminated in the study area (Igeo: 1–2). PLI mainly allows a qualitative comparison between sites. PLI values < 1 signifies no pollution, PLI = 1 signifies baseline levels of pollutants and PLI > 1 signifies progressive site pollution. The pollution load index value of 0.08 indicated no deterioration of the site quality (PLI < 1).
Modified degree of contamination and Nemerow pollution index
In the environment, heavy metals co-exist together with other organic compounds and their negative effect results from their combined effects. Since the single element indices do not adequately take into account this synergistic effect this study employed the multi-element indices such as the combined degree of contamination and Nemerow pollution indices. The results of both mCd and PN as shown in Table 7 indicate that they fall within the zero class < 1.5 and < 1 respectively (Table 1). Hence the sediments in the settlements under study are unpolluted.
Ecological risk assessment
The potential ecological risk assessment properly combines the ecological effects and toxicology. This approach was employed to analyse the level of pollution of heavy metals in the surface sediments of Birim River basin. The overall potential ecological index and potential ecological risk from the single metals (\(E_{f}^{i}\)) results are shown in Table 8.
The \(E_{f}^{i}\) values range from 1.93 to 3.20 for Pb, 97.26–160.42 for Cd, 0.07–0.18 for Zn, and 0.55–1.40 for As, with mean values of 2.45, 135.93, 0.12 and 0.92, respectively. Based on the Eri results, the ecological risks associated with As, Zn and Pb are generally low whiles that associated with Cd are a considerable risk. The RI values are 101.09–163.61, with an average of 139.36, indicative of a moderate risk. Cd has high (average 97%) contributions to the RI values in the sediments of Birim River basin, and these results are similar to those reported in China (Li et al. 2016; Lin et al. 2016b).
Suitability for irrigation purposes
The evaluation of the suitability of surface water for irrigation purpose relies on TDS, EC, and relative concentration of Ca2+, Mg2+, Na+ and HCO3− ions. The high content of salts, particularly Na+ ion in the irrigation water influences the soil structure, reduces aeration and permeability, as well as resulting in alkaline soil, which can affect plant growth. Sodium adsorption ratio and percent sodium (% Na) is the sodium hazard induced by an excessive Na+ ion in the irrigation water (Alam 2014). High Na+ ion concentrations in irrigation water can negatively affect soil physical properties, causing soil particle dispersion when large amounts of Na+ ion are adsorbed onto the soil particles (Bob et al. 2016; Al-Omran et al. 2016; Arveti et al. 2011). SAR is a measure of the extent to which Na+ ion in the water systems might be absorbed by the soil. Generally, the higher the SAR value, the larger the risk of sodium hazard on plant growth. SAR values > 2.0, signify unsuitability of water for irrigation purpose (Ayuba et al. 2013; Vasanthavigar et al. 2010). The SAR value in the study area ranged between 0.73 and 1.52, with a mean value of 1.01.
The % Na content is also another parameter for evaluating the suitability of water for agriculture purposes. Since the combination of sodium with CO32− or Cl− results in the formation of alkaline or saline soils. The % Na value ranged between 29.61 and 70.10, with a mean value of 48.91. Hence, virtually all the water samples are suitable for irrigation except at Nsutam and Nsuapemso with % Na values slightly higher than the permitted limit of 60% for irrigation purposes.
KR is the amount of Na+ ions measured against Ca2+ and Mg2+. A KR greater than unity (KR > 1) is indicative of an excess amount of Na+ ions in the water and is considered as of alkali hazard to the soil thereby making the water unsuitable for irrigation. The KR value for all the water samples was above unity, indicating unsuitability of this water for irrigation purpose.
RSC occurs when the excess CO32− combine with Na+ ion to form NaHCO3. RSC shows the potential to eliminate Ca2+ and Mg2+ ions from the soil solution. High RSC value in irrigation water can lead to solidification and salinization of agricultural soils (Zaidi et al. 2015). However, as the soil solution becomes more concentrated, there is a high affinity for Ca2+ and Mg2+ ions to precipitate out as CO32− which increases the relative proportion of sodium (Ravikumar et al. 2013). According to Li et al. (2016), water for irrigation purposes are classified as suitable, not suitable and marginally suitable for irrigation when RSC value are < 1.25, > 2.5 and 1.25–2.5 meq/L, respectively. In this study, RSC values ranged between—2.42 and 125.15 meq/L, signifying that not almost all the water samples are suitable for irrigation except at Bunso sampling sites.
Magnesium adsorption ratio expresses the relationship between calcium and magnesium concentration in surface water (Ayuba et al. 2013). Moreover, the excess amount of Mg2+ ion can affect the quality of soil, since high levels of Mg2+ ions in the soil can cause infiltration problems. MAR value > 50, represent unsuitable of water for irrigation purposes (Ayuba et al. 2013). The MR value in the surface water samples ranged from 28.53 to 28.77. All the MR in the surface water samples were below the acceptable limit of 50, indicating their suitability for irrigation purpose.
Permeability is greatly affected by Na+, Ca2+, Mg2+, HCO3− and Cl− contents in the soil. On the basis of PI, water for irrigation purposes can be classified as suitable, marginally suitable and unsuitable for irrigation when the PI values are 100% maximum permeability (class I), 75% maximum permeability (class II) and 25% maximum permeability (class III), respectively. The calculated PI value ranged between 22.88 and 61.65 (Additional file 1: Table S3). According to the classification of PI, almost all the water samples fall under the class II, indicating that they are marginally suitable for irrigation purposes with the exception of those from Adukrom and Anyinam.
This study utilised diagrams established by the United States Salinity Laboratory (USSL) as well as Wilcox to evaluate water quality for irrigation purposes (Fig. 6).
The USSL diagram as shown in Fig. 6a signifies that the alkalinity and salinity values of all the surface water samples were very low (C1–S1), and thus no alkali hazard to crop growth. The Wilcox diagram (Fig. 6b) signifies that all the surface water samples fell under the ‘‘Excellent to good’’ class. Thus both Wilcox and USSL diagrams demonstrated the suitability of all the surface water samples from the Birim River basin for irrigation.
Conclusion
The results of this study provided valuable information about the hydrochemistry and water quality of surface water as well as ecological risks of some heavy metal contents of sediment from different settlements within the Birim River basin. The hydrogeochemical analysis of the surface water samples revealed that the water was neutral to mildly acidic and the hydrochemical facies of the area was dominated by the Ca–Na–Mg–HCO3 (39%) and Na–Ca–Mg–HCO3 (23%) water type. According to the geo-accumulation and Pollution indexes for the studied metals, settlements in the Birim River basin have moderately contaminated sediment. However multi-element indices such as the modified degree of contamination and nemerow pollution indices indicate no pollution in the study area so far as the heavy metals studied are concerned. Based on the Eri results, the ecological risks associated with As, Zn and Pb are generally low whiles that associated with Cd are a considerable whilst the RI values are indicative of a moderate risk. All these observations notwithstanding a systematic investigation are recommended to monitor the hydrochemistry, metal loading and change in the surface water, as well as sediment quality as both legal and illegal mining activities, is intensifying in the area.
Abbreviations
- WQI:
-
water quality index
- SSP:
-
soluble sodium percentage
- SAR:
-
sodium adsorption ratio
- KR:
-
Kelly’s ratio
- RSC:
-
residual sodium carbonate
- MR:
-
magnesium ratio
- ERM:
-
effect range median
- ERL:
-
effect range low
- ISQG:
-
interim sediment quality guideline
- PEL:
-
probable effect level
- PLI:
-
pollution load index
- CF:
-
contamination factor
- CD:
-
contamination degree
- Igeo :
-
Geo-accumulation Index
- PERI:
-
potential ecological risk index
- ER:
-
ecological risk
- Ti :
-
toxicity coefficient
- RI :
-
total ecological risk index
- USSL:
-
United States Salinity Laboratory
- KMO:
-
Kaiser–Meyer–Olkin tests
- PCA:
-
principal component analysis
References
Abdesselam S, Halitim A, Jan A, Trolard F, Bourrié G (2013) Anthropogenic contamination of groundwater with nitrate in arid region: case study of southern Hodna (Algeria). Environ Earth Sci 70:2129–2141
Adamu CI, Nganje TN, Edet A (2015) Heavy metals contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ Nanotechnol Monit Manag 3:10–21
Alam F (2014) Evaluation of hydrogeochemical parameters of groundwater for suitability of domestic and irrigational purposes: a case study from central Ganga Plain, India. Arab J Geosci 7(10):4121–4131
Alaya MB, Saidi S, Zemni T, Zargouni F (2014) Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ Earth Sci 71:3387–3421
Al-Omran AM, Aly AA, Al-Wabel MI, Sallam AS, Al-Shayaa MS (2016) Hydrochemical characterization of groundwater under agricultural land in arid environment: a case study of Al-Kharj, Saudi Arabia. Arab J Geosci 9:1–17
Ansah-Asare O, Asante K (2000) The water quality of Birim river in South-East Ghana. West Afr J Appl Ecol 1:23–34
Arnold G, Lenores C (1989) Standard methods for examination of water and waste water, 17th edn. American Public Health Association, New York, pp 3–8
Arveti N, Sarma MRS, Aitkenhead-Peterson JA, Sunil K (2011) Fluoride incidence in groundwater: a case study from Talupula, Andhra Pradesh, India. Environ Monit Assess 172:427–443
Asare-Donkor NK, Kwaansa-Ansah EE, Opoku F, Adimado AA (2015) Concentrations, hydrochemistry and risk evaluation of selected heavy metals along the Jimi River and its tributaries at Obuasi a mining enclave in Ghana. Environ Syst Res 4:1
Asefi M, Zamani-Ahmadmahmoodi R (2015) Mercury concentrations and health risk assessment for two fish species, Barbus grypus and Barbus luteus, from the Maroon River, Khuzestan Province, Iran. Environ Monit Assess 187:1–10
Atgin RS, El-Agha O, Zararsiz A, Kocatas A, Parlak H, Tuncel G (2000) Investigation of the sediment pollution in Izmir Bay: heavy elements, Spectrochim. Acta B 55(7):1151–1164
Ayuba R, Omonona O, Onwuka O (2013) Assessment of groundwater quality of Lokoja basement area, North-Central Nigeria. J Geol Soc India 82:413–420
Bain R et al (2014) Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Trop Med Int Health 19:917–927
Boateng TK, Opoku F, Acquaah SO, Akoto O (2015) Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu–Juaben Municipality, Ghana. Environ Syst Res 4:1
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu–Juaben Municipality, Ghana. Environ Earth Sci 75:1–14
Bob M, Rahman NA, Elamin A, Taher S (2016) Assessment of groundwater suitability for irrigation in Madinah City, Saudi Arabia. Arab J Geosci 9:1–11
Bortey-Sam N, Nakayama SMM, Ikenaka Y, Akoto O, Baidoo E, Mizukawa H, Ishizuka M (2015) Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environ Monit Assess 187:1–12
Brady JP, Ayoko GA, Martens WN, Goonetilleke A (2015) Development of a hybrid pollution index for heavy metals in marine and estuarine sediments. Environ Monit Assess 187(5):306
Comber H, Deady S, Montgomery E, Gavin A (2011) Drinking water fluoridation and osteosarcoma incidence on the island of Ireland. Cancer Causes Control 22:919–924
Duodu GO, Ogogo KN, Mummullage S, Harden F, Goonetilleke A, Ayoko GA (2017) Source apportionment and risk assessment of PAH in Brisbane River sediment, Australia. Ecol Indicators 73:784–799
Demirak A, Yilmaz F, Tuna AL, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63(9):1451–1458
Fianko JR, Nartey VK, Donkor A (2010) The hydrochemistry of groundwater in rural communities within the Tema District, Ghana. Environ Monit Assess 168:441–449
Guo R, He X (2013) Spatial variations and ecological risk assessment of heavy metals in surface sediments on the upper reaches of Hun River, Northeast China. Environ Earth Sci 70:1083–1090
Hankason L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. Water Res. 14(8):975–1001
Helstrup T, Jørgensen NO, Banoeng-Yakubo B (2007) Investigation of hydrochemical characteristics of groundwater from the Cretaceous–Eocene limestone aquifer in southern Ghana and southern Togo using hierarchical cluster analysis. Hydrogeol J 15:977–989
Hu B, Li G, Li J, Bi J, Zhao J, Bu R (2013) Spatial distribution and ecotoxicological risk assessment of heavy metals in surface sediments of the southern Bohai Bay, China. Environ Sci Pollut Res 20:4099–4110
Iranmanesh A, Ii RAL, Wimmer BT (2014) Multivariate statistical evaluation of groundwater compliance data from the Illinois Basin–Decatur Project. Energy Procedia 63:3182–3194
Jalali M (2007) Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah basin in western Iran. Environ Monit Assess 130:347–364. https://doi.org/10.1007/s10661-006-9402-7
Karikari AY, Ansa-Asare OD (2006) Physico-chemical and microbial water quality assessment of Densu River of Ghana. West Afr J Appl Ecol 10:87–100
Khashogji MS, El Maghraby MMS (2013) Evaluation of groundwater resources for drinking and agricultural purposes, Abar Al Mashi area, south Al Madinah Al Munawarah City, Saudi Arabia. Arab J Geosci 6:3929–3942
Kim FM et al (2011) An assessment of bone fluoride and osteosarcoma. J Dent Res 90:1171–1176
Kim K-H, Yun S-T, Mayer B, Lee J-H, Kim T-S, Kim H-K (2015) Quantification of nitrate sources in groundwater using hydrochemical and dual isotopic data combined with a Bayesian mixing model. Agric Ecosyst Environ 199:369–381
Kouadia L, Trefry JH (1987) Sediment heavy metal contamination in the Ivory Coast West Africa. Water Air Soil Pollut 32:145–154
Lang Y-C, Liu C-Q, Zhao Z-Q, Li S-L, Han G-L (2006) Geochemistry of surface and ground water in Guiyang, China: water/rock interaction and pollution in a karst hydrological system. Appl Geochem 21:887–903
Li P, Wu J, Qian H (2016) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9:1–17
Lin M-L, Peng W-H, Gui H-R (2016a) Hydrochemical characteristics and quality assessment of deep groundwater from the coal-bearing aquifer of the Linhuan coal-mining district, Northern Anhui Province, China. Environ Monit Assess 188:1–13
Lin Q, Liu E, Zhang E, Li K, Shen J (2016b) Spatial distribution, contamination and ecological risk assessment of heavy metals in surface sediments of Erhai Lake, a large eutrophic plateau lake in southwest China. CATENA 145:193–203
Liu Y-Q, Moy B, Kong Y-H, Tay J-H (2010) Formation, physical characteristics and microbial community structure of aerobic granules in a pilot-scale sequencing batch reactor for real wastewater treatment. Enzyme Microb Technol 46:520–525
Marghade D, Malpe DB, Zade AB (2011) Geochemical characterization of groundwater from northeastern part of Nagpur urban, Central India. Environ Earth Sci 62:1419–1430. https://doi.org/10.1007/s12665-010-0627-y
Moore JW, Ramamoorthy S (2012) Heavy metals in natural waters: applied monitoring and impact assessment. Springer, Berlin
Murkute YA (2014) Hydrogeochemical characterization and quality assessment of groundwater around Umrer coal mine area Nagpur District, Maharashtra, India. Environ Earth Sci 72:4059–4073. https://doi.org/10.1007/s12665-014-3295-5
Nandimandalam JR (2012) Evaluation of hydrogeochemical processes in the Pleistocene aquifers of middle Ganga plain, Uttar Pradesh, India. Environ Earth Sci 65:1291–1308
Négrel P, Casanova J, Brulhet J (2006) REE and Nd isotope stratigraphy of a late jurassic carbonate platform, Eastern paris basin, France. J Sediment Res 76(3):605–617
Patel P, Raju NJ, Reddy BSR, Suresh U, Gossel W, Wycisk P (2016) Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin, Andhra Pradesh, India. Environ Earth Sci 75:1–24
Pius A, Jerome C, Sharma N (2012) Evaluation of groundwater quality in and around Peenya industrial area of Bangalore, South India using GIS techniques. Environ Monit Assess 184:4067–4077. https://doi.org/10.1007/s10661-011-2244-y
Purushothaman P, Rao MS, Rawat YS, Kumar CP, Krishan G, Parveen T (2014) Evaluation of hydrogeochemistry and water quality in Bist-Doab region, Punjab, India. Environ Earth Sci 72:693–706
Ravikumar P, Mehmood MA, Somashekar R (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3:247–261
Razmkhah H, Abrishamchi A, Torkian A (2010) Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: a case study on Jajrood River (Tehran, Iran). J Environ Manage 91:852–860
Razowska-Jaworek L (ed) (2014) Calcium and magnesium in groundwater: occurrence and significance for human health. CRC Press, New York. https://doi.org/10.1201/b17085
Robinson GR, Ayotte JD (2006) The influence of geology and land use on arsenic in stream sediments and ground waters in New England, USA. Appl Geochem 21:1482–1497
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ Geol 55:823–835
Saleem M, Jeelani G, Shah RA (2015) Hydrogeochemistry of Dal Lake and the potential for present, future management by using facies, ionic ratios, and statistical analysis. Environ Earth Sci 74:3301–3313
Schafer A, Rossiter H, Owusu P, Richard B, Awuah E (2010) Developing country water supplies: physico-chemical water quality in Ghana. Desalination 251:193–203
Sinex SA, Helz GR (1981) Regional geochemistry of heavy elements in Chesapeake Bay sediment. Environ Geol 3:315–323
Singh NKS, Devi CB, Sudarshan M, Meetei NS, Singh TB, Singh NR (2013) Influence of Nambul River on the quality of fresh water in Loktak Lake. Int J Water Resourc Environ Eng 5:321–327
Singh CK, Rina K, Singh RP, Mukherjee S (2014) Geochemical characterization and heavy metals contamination of groundwater in Satluj River basin. Environ Earth Sci 71:201–216
Singh S, Raju NJ, Gossel W, Wycisk P (2016) Assessment of pollution potential of leachate from the municipal solid waste disposal site and its impact on groundwater quality, Varanasi environs, India. Arab J Geosci 9:1–12
Sun Y, Zhou Q, Xie X, Liu R (2010) Spatial, sources and risk assessment of heavy metals contamination of urban soils in typical regions of Shenyang, China. J Hazard Mater 174:455–462
Sutherland RA (2000) Bed sediment-associated heavy metals in an urban stream, Oahu, Hawaii. Environ Geol 39:611–627
Taylor SR, McLennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth's crust. Geol Soc Am Bull 72(2):175–192
Vasanthavigar M et al (2010) Application of water quality index for groundwater quality assessment: thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Yan N, Liu WB, Xie HT, Gao LR, Han Y, Wang MT, Li HF (2016) Distribution and assessment of heavy metals in the surface sediments of Yellow River, China. J Environ Sci China 39:45–51
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Yidana SM, Banoeng-Yakubo B, Sakyi PA (2012) Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. J Earth Syst Sci 121:491–507
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess 187:1–18
Zakir HM, Shikazono N, Otomo K (2008) Geochemical distribution of heavy metals and assessment of anthropogenic pollution in sediments of Old Nakagawa River. Tokyo Jpn Am J Environ Sci 4(6):661–672
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54(6):1051–1070
Zhang LP, Ye X, Feng H (2007) Heavy metal contamination in Western Xiamen Bay sediments and its vicinity, China Mar. Pollut Bull 54:974–982
Zhang R, Jiang D, Zhang L, Cui Y, Li M, Xiao L (2014) Distribution of nutrients, heavy metals, and PAHs affected by sediment dredging in the Wujin’gang River basin flowing into Meiliang Bay of Lake Taihu. Environ Sci Pollut Res 21:2141–2153
Zhu GF, Li ZZ, Su YH, Ma JZ, Zhang YY (2007) Hydrogeochemical and isotope evidence of groundwater evolution and recharge in Minqin basin, Northwest China. J Hydrol 333:239–251
Authors’ contributions
JOO carried out the sampling, laboratory analyses and participated in the drafting of the manuscript. NKA and AAA conceived the study, participated in its design and coordination, performed the statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are very grateful to the National Council for Tertiary Education (NTCE), Ghana for a research Grant under the Teaching and Learning Innovation Fund (TALIFKNUSTR/3/005/2005).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The National Council for Tertiary Education (NTCE) under the Teaching and Learning Innovation Fund funded the collection of samples, cost of analysis and interpretation of data with Grant Number TALIFKNUSTR/3/005/2005.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
Additional tables.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Asare-Donkor, N.K., Ofosu, J.O. & Adimado, A.A. Hydrochemical characteristics of surface water and ecological risk assessment of sediments from settlements within the Birim River basin in Ghana. Environ Syst Res 7, 9 (2018). https://doi.org/10.1186/s40068-018-0113-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-018-0113-1