Abstract
The multilayered Djeffara aquifer system, south-eastern Tunisia, has been intensively used as a primary source to meet the growing needs of the various sectors (drinking, agricultural and industrial purposes). The analysis of groundwater chemical characteristics provides much important information useful in water resources management. Detailed knowledge of the geochemical evolution of groundwater and assessing the water quality status for special use are the main objective of any water monitoring study. An attempt has been made for the first time in this region to characterize aquifer behavior and appreciate the quality and/or the suitability of groundwater for drinking and irrigation purposes. In order to attend this objective, a total of 54 groundwater samples were collected and analyzed during January 2008 for the major cations (sodium, calcium, magnesium and potassium), anions (chloride, sulfate, bicarbonate), trace elements (boron, strontium and fluoride), and physicochemical parameters (temperature, pH, total dissolved salts and electrical conductivity). The evolution of chemical composition of groundwater from recharge areas to discharge areas is characterized by increasing sodium, chloride and sulfate contents as a result of leaching of evaporite rock. In this study, three distinct chemical trends in groundwater were identified. The major reactions responsible for the chemical evolution of groundwater in the investigated area fall into three categories: (1) calcite precipitation, (2) gypsum and halite dissolution, and (3) ion exchange. Based on the physicochemical analyses, irrigation quality parameters such as sodium absorption ratio (SAR), percentage of sodium, residual sodium carbonate, residual sodium bicarbonate, and permeability index (PI) were calculated. In addition, groundwater quality maps were elabortaed using the geographic information system to delineate spatial variation in physico-chemical characteristics of the groundwater samples. The integration of various dataset indicates that the groundwater of the Djeffara aquifers of the northern Gabes is generally very hard, brackish and high to very high saline and alkaline in nature. The water suitability for drinking and irrigation purposes was evaluated by comparing the values of different water quality parameters with World Health Organization (WHO) guideline values for drinking water. Piper trilinear diagram was constructed to identify groundwater groups where the relative major anionic and cationic concentrations are expressed in percentage of the milliequivalent per liter (meq/l), and it was demonstrated that the majority of the samples belongs to SO4–Cl–Ca–Na, Cl–SO4–Na–Ca and Na–Cl hydrochemical facies. As a whole, all the analyzed waters from this groundwater have revealed that this water is unsuitable for drinking purposes when comparing to the drinking water standards. Salinity, high electric conductivity, sodium adsorption ratio and sodium percentages indicate that most of the groundwater samples are inappropriate for irrigation. The SAR vary from medium (S2) to very high (S4) sodicity. Therefore, the water of the Djeffara aquifers of the northern Gabes is dominantly of the C4–S2 class representing 61.23 % of the total wells followed by C4–S3 and C4–S4 classes at 27.27 and 11.5 % of the wells, respectively. Based on the US Salinity Classification, most of the groundwater is unsuitable for irrigation due to its high salt content, unless certain measures for salinity control are undertaken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, fresh water resources are becoming more and more limited with increasing demand in the world. Thus, understanding the hydrochemical characteristics and water quality is becoming critical for groundwater planning and management, to ensure sustainable safe use of the resources for drinking, agricultural and industrial purposes. Groundwater resources of the Mediterranean coastal plains in the southern bank of the basin (Middle East and North Africa) show a qualitative and quantitative deterioration through time (Custodio and Bruggeman 1987; Edmunds et al. 2003) resulting from climatic constraints (semi-arid climate and low rainfall amount) and anthropological activities (limited resources, pumping higher rates than the present recharge, largely increased for urban, domestic supply and multiplication of the diffuse sources of pollution). Therefore, it is necessary to subject the water to routine quality analyses to assess its suitability for human consumption. The groundwater chemistry could have important information on the suitability of the groundwater for domestic, industrial and agricultural purposes, and its contamination has been recognized as one of the most serious problems in the world (Adams et al. 2001; Jalali 2007, 2009, Djabri et al. 2007; Wen et al. 2008; Giridharan et al. 2008; Khazaei et al. 2006; Tayfur et al. 2008; Anku et al. 2009; Trabelsi et al. 2009, 2011; Rouabhia et al. 2009; Fehdi et al. 2009). Groundwater chemistry depends on a number of factors such as general geology, degree of chemical weathering of the various rock types, quality of recharge water, and inputs from sources other than water–rock interaction. Such factors and their interactions result in a complex groundwater quality (Domenico and Schwartz 1990; Edmunds et al. 2003; Risacher et al. 2003; Guler and Thyne 2004; Vengosh et al. 2005; Boughriba et al. 2006; Ayenew et al. 2008; Giridharan et al. 2008; Han et al. 2009; Trabelsi et al. 2009, 2011). In Tunisia and other similar parts worldwide, common studies have been carried out to assess the geochemical characteristics of groundwater (Subba Rao et al. 1998; Graniel et al. 1999; Umar and Sami Ahmad 2000; Guler et al. 2002; Guler and Thyne 2004; Elango et al. 2003; Krishnakumar 2004; Jeevanandam et al. 2006; Ravikumar et al. 2009, 2010; Aghazadeh and Mogaddam 2011; Hamzaoui-Azaza et al. 2011, 2012; Ketata et al. 2011). The development of groundwater resources in these arid and semi-arid regions is a sensitive issue, and careful management is required to avoid water-quality degradation (Al-Bassam and Al-Rumikhani 2003; Al-Shaibani 2008; Dassi et al. 2005; Dassi 2010; Trabelsi et al. 2005, 2007).
In Tunisia, the gradient of average annual rainfall decreases from north to south from approximately 1,300 to <200 mm. In fact, in the northern Tunisia, freshwater of dams are used for agricultural and domestic needs, while in southern Tunisia, surface waters are limited and water resources are based on groundwater of variable quality. As part of Southern Tunisia, the Gabes-north region has a semi-arid climate with very irregular rainfall, which makes the groundwater resources quite fragile.
The Djeffara aquifers of the northern Gabes are well known by their groundwater potential and their artesian conditions. Recently, confining pressure was dramatically reduced due to overexploitation in agricultural and industrial purposes. This region has major difficulties in managing its water resources, which are in decline, especially since, for the last decades, the scarcity of groundwater.
This work represents a first attempt for the establishment of a comprehensive study in the area. It also intends to characterize the main factors and mechanisms controlling the chemistry and to assess the quality of groundwater with references to its suitability to drinking and irrigation. Further, water quality parameters were compared with the international standards (WHO 1996a, b, 2004) and to Wilcox (1955) and US Salinity (Richards 1954) diagrams were also prepared for the study of irrigation water quality.
Description of the study area
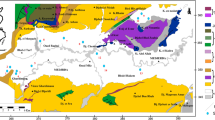
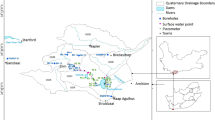
The deep Djeffara aquifers of the northern Gabes are located in south-eastern Tunisia and have an extension of 1,000 km2 comprising 36 km along the coast and are positioned approximately between the longitudes UTM X1 = 550,000 and X2 = 610,000 and latitudes UTM Y1 = 3740,000 and Y2 = 3780,000. The study area is limited by Zemlet El Beïdha Mountain in the north, Mediterranean Sea coast in the east, El Hamma faults to the west. The southern limit consists in Jebel Raguba, Jebel Monncef and Jebel Sidi Saleh (Fig. 1a). As representing a part of the Djeffara coastal plain of Tunisia, the region has undergone an arid to semi-arid climate changes marked by seasonal contrasting climatic variables. Also the area is influenced by dry/hot and humid air masses coming respectively from the desert and from the Mediterranean Sea (Kallel 2003). The rainfall average is approximately 180 mm/year. However, the monthly average temperature ranges between 12 °C in winter (January) and 28 °C in summer (August), and the average annual temperature is 20 °C. The precipitation period in a typical year varies between October and March with the dry period extending from April to September. The potential evapotranspiration estimated by Thornthwaite method is very high (2,800 mm/year) and largely exceeds the rainfall. This situation makes the groundwater resources quite delicate. The wadis of Gabes region have an intermittent flow regime, because the dry season is typically very long (6–8 months/year). The main wadis in the north Gabes are the El Hamma wadi, El Melah wadi, Gabes wadi and El Akarit wadi, which receive many important flow tributaries, in particular from the Dahar, Er Ragouba, Monncef and Dissa mountains in the south of the study area and the Koudiat El Hmmeïmet and Zemlet Beïdha mountains in the north. Surface water resources are insufficient for current domestic, industrial and agricultural needs of the region and therefore the groundwater constitutes the main water resource in southern Tunisia.
The Djeffara plain hosts a large number of wells with depths varying from 200 to 550 m. Most of these wells, which tap the multilayered confined aquifer of Gabes-north, contain dolomites and limestones of the Turonian and Lower Senonian, and fine to gravel grained sandstones of the Miocene. Some of the hydraulic deep wells are used for drinking purposes (7 %), only few for chemical industry (9 %), and approximately 84 % of the water is used for irrigation. Consequently, groundwater constitutes the main water resource in southern Tunisia. In 2008, the total water extraction in this aquifer is estimated by the local water authorities in Hamma–Chennchou and northern Gabes is 18 and 50 million m3/year (Fig. 2a, b), respectively (DGRE 2008). These exploitation rates, which are required to meet the increasing demands of the current agricultural practices, exceed the natural replenishment (30.5 Mm3/year, OSS 2003) of these basins and had led to depletion of the groundwater resources and degradation of their quality. The conditions in this aquifer system were originally artesian with numerous springs occurring across the plain (Fig. 2c, d). Actually, 96 of deep wells were drilled to meet the rapid growing demand for water. This over-pumping of the Djeffara aquifers has resulted in water-level declines ranging from 0.25 to 1 m/year during the past three decades (Abidi 2004).
Geological and hydrogeological settings
A representative west–east cross section of the study area is shown in Fig. 1b. The regional geology has been discussed by several authors (Busson 1970; Abdeljaouad 1983; Bouaziz 1995; Abbès et al. 1986). From a geological point of view (Fig. 3), the investigated area is characterized by outcrops ranging from the Lower Cretaceous to Quaternary units and by lateral lithostratigraphic variations, with an unconformity from Palaeocene to Oligocene. The catchment area consists of limestone, dolomite, gypsum, halite, clayey sand and conglomerates (Fig. 3).
The relation between stratigraphic and hydrogeologic units is presented in Fig. 3. As shown in Figs. 1b and 3, the CI aquifer is situated within the continental formations of the Lower Cretaceous (Neocomian, Barremian, Aptian and Albian) which is located to the west of the study region. The aquifer is formed by a complex succession of clastic sediments, differentiated into several units of detrital sediments and separated by clay-rich strata, with the maximum aquifer thickness exceeding 1,500 m (Edmunds et al. 2003). The Djeffara aquifer is hosted in the Upper Cretaceous (Turonian and Lower Senonian) and Tertiary formations (Miocene) which are located in the coastal plain. Tectonic movements have significantly influenced hydrodynamic functioning of these aquifers. Moreover, the northern part of Djeffara basin is strongly affected by a complex system of NE/SW faults crossed by NW/SE ones detected by geophysical survey. These geophysical faults provoked a lateral compartmentalization and connections between different aquiferous horizons. This is the case in the El Hamma–Chennchou where a hydraulic continuity between the Continental Intercalaire and the Djeffara aquifers has been recognized.
In the northern part of the basin (Djeffara of northern Gabes and El Hamma–Chennchou), Cretaceous and tertiary formations are characterized by lateral variation in depths and thickness. Moreover, in Zemlet el Beïda Mountain, Cenomanian and Turonian deposits are missing (Fig. 1a). Stratigraphic gaps in these areas reflect several factors such as the irregularity of the paleotopography, eustatic movements and tectonic instabilities (Castany 1954; Bouaziz 1995).
The Djeffara aquifer system is characterized by large changes in facies both in the vertical and lateral directions, and thickness of water-bearing horizons. In the north of Gabes, Djeffara waters are mainly hosted in the Miocene continental deposits with clays and sandy intercalation produced after the post-Cretaceous erosion (Bouaziz 1995; Benton et al. 2000). The thickness of these sand deposits increases from east (30 m) to west (>80 m). They are characterized by coarse and fine grain size. Permeability of the Miocene horizon ranges from 2 × 10−5 to 3 × 10−4 m/s, while transmissivities obtained from pumping tests ranges from 3 to 33.8 × 10−3 m2/s (Mekrazi 1975).
The principal aquifer of Gabes is hosted in the Senonian horizon represented by two stratigraphic units. The first one constitutes a fractured limestone horizon with a thickness of approximately 200 m, and the second is formed by marl and gypsum characterized by variable thickness up to 500 m. These water-bearing formations are strongly fissured, with the transmissivity ranging from 0.9 to 345 × 10−3 m2/s (Mamou 1990).
The Turonian carbonates constitute the principal aquifer formation in Hamma–Chennchou region. The aquifer consists of dolomites and fissured limestones, whose maximum thickness reach exceeds 50 m (Rouatbi 1967). Although its thickness is limited, the Turonian aquifer is characterized by high transmissivity, generally around 100 × 10−3 m2/s (Rouatbi 1967).
The northern part of Djeffara basin is largely affected by several normal faults in different directions as a result of tectonic activity (Bouaziz 1995). This extensive tectonic phase during the Upper Cretaceous caused lateral compartmentalization of Djeffara aquifers. All water-bearing formations of the Djeffara plain are hydraulically connected through the existing faults and semi-permeable strata.
The hydraulic continuity between the CI and Djeffara aquifers has been demonstrated through the differences in hydraulic heads and a set of boreholes showing concordant hydrogeologic, hydrodynamic and hydrochemistry parameters (Rouatbi 1967; ERESS 1972; Mamou 1990; OSS 2003). The CI aquifer of North Africa is one of the largest confined aquifers in the world (Castany 1982), comparable in scale to the Great Artesian Basin of Australia and covers some 600,000 km2. The hydraulic heads of the CI aquifer indicate W–E flow, in the direction of Gulf of Gabes, with an artesian pressure head of about 200 m above ground surface (OSS 2003). On the other hand, the Djeffara aquifers show a general W–E flow, from El Hamma region toward the Mediterranean Sea, in accordance with the plain structure (Fig. 4). Near El Hamma faults, the artesian pressure head is in the order of 50 m and is explained by the ascension of CI groundwater through El Hamma faults (OSS 2003). The piezometric gradient varies between 1 and 3 ‰.
Sampling and analytical methods
A total of 54 samples of groundwater from boreholes tapping CI aquifer (8) and Djeffara aquifers (46) (Tables 1, 2) have been collected in January 2008 (Fig. 5). For this study, sampling points were chosen according to their geographic and stratigraphic locations in a way that they represent hydrochemical types and hydrogeological conditions typical for the diverse sectors and different depths of the aquifer. Water samples were collected from pumping wells after minimum of several hours of pumping prior to sampling. This was done to remove groundwater stored in the well. Water samples were collected in clean polyethylene bottles. Temperature, pH and electrical conductivity (EC) were measured using a portable field kit. To prevent changes in chemical equilibrium and adsorption on the inner surface of the bottles, the samples were acidified with 1:1 extra pure HNO3 to 1 ‰ without disturbing the sample volume, and the final acidity of the samples during storage was around pH ≤ 2.0. Preservation and transportation of the water samples to the laboratory followed standard methods. The collected samples were brought to the laboratory and were filtered using 0.45 μm millipore filter paper and acidified with nitric acid (Ultrapure Merck) for cation analysis. Prior to laboratory analysis, the samples were stored below 4 °C. Chemical analyses (Cl−, SO4 2−, HCO3 −, F−, Ca2+, Mg2+, Na+ K+, Sr2+ and B+) were carried out in Tunisian Chemical group Laboratory (GCT).
Chloride analysis was carried out by the standard titration method, or the Mohr method. Water alkalinity is primarily attributed to the presence of bicarbonates (HCO3 −) since the pH of most water samples ranges between 6.74 and 8.5. Bicarbonate (HCO3 −) is estimated by titrating with HCl. Using BaCl2, the gravimetric method was used to estimate sulfate concentrations (SO4 2−). Calcium (Ca2+) was analyzed titrimetrically, using standard EDTA. Magnesium (Mg2+) was computed, taking the difference between TH (Ca2+ plus Mg2+) and Ca2+ values. Sodium (Na+) and potassium (K+) were measured by flame photometer. Fluoride (F−) is analyzed using a spectrophotometer. Strontium (Sr2+) and boron (B+) concentrations were measured by inductively coupled plasma–optical emission spectrometry (ICP-OES). All the water quality parameters are expressed in mg/l, except EC, T and pH. The analytical error as inferred from the balance between cations (Ca2+, Mg2+, Na+, K+, Sr2+ and B+) and anions (HCO3 −, Cl−, SO −24 and F−) is observed to be within the stipulated limit of ±5 % (Mandel and Shiftan 1981). Table 1 presents the groundwater characteristics data for sampling campaign during January 2008. Each parameter was compared to the standard desirable limit of that parameter stipulated for drinking water as prescribed by the WHO (1996a, b, 2004) for drinking and public health purposes.
The total hardness (TH) in ppm (Todd 1980; Hem 1985; Ragunath 1987) was determined by the following Eq. 1:
where the concentrations of Ca2+ and Mg2+ are represented in mg/l.
The development and maintenance of successful irrigation projects not only supply irrigation water to the land but also control salt and alkali in the soil (Haritash et al. 2008). Salinity and indexes such as sodium absorption ratio (SAR), sodium percentage (%Na), residual sodium carbonate (RSC), residual sodium bicarbonate (RSBC) and permeability index (PI) are important parameters for determining the suitability of groundwater for agricultural uses (Srinivasa Gowd 2005; Raju 2007). The sodium adsorption ratio (SAR) values were calculated for each well by the following equation given by Richards (1954):
where the concentrations are expressed in meq/l.
The sodium percentage (%Na) was calculated using the formula given below (Todd and Mays 2005):
where all ionic concentrations are expressed in meq/l.
The RSC index is calculated by the following equation (Ragunath 1987):
where concentrations are reported in meq/l.
The RSBC, as defined by Gupta and Gupta (1987), is calculated by the following equation.
where concentrations are expressed in meq/l.
The permeability index (PI), as defined by Doneen (1964) and Ragunath (1987), is calculated by the following equation:
where all the ions are expressed in meq/l.
Results and discussion
In situ measurements interpretation
The groundwater samples belonging to the CI system are characterized by elevated temperatures that range between 57.3 and 65 °C (Table 1) indicating that the water in these wells is derived from the main horizons of the CI formations, as indicated in the cross section shown in Fig. 1b, without any significant leakage from the overlaying strata. The temperature of the Djeffara groundwater is widely varying, from 18 to 65.20 °C, independently of the borehole depth. The water temperature is higher (between 35 and 65 °C) in the tectonic zones of El Hamma and Chennchou regions, which points to some inflow of groundwater from the CI aquifer through the existing normal faults (Fig. 6a). The lowest values of temperature (lower than 35 °C), probably due to the influence of the atmosphere, characterize the shallowest wells (Table 1).
The values of pH of the CI groundwater samples range from 7.46 to 8.5 with an average of 7.92. However, in the Djeffara aquifers, pH values range from 6.74 to 8.3 (Fig. 6b; Table 1) with an average of 7.29. This shows that the groundwater of the study area is generally neutral. According to the WHO (2004), the range of desirable pH values of water prescribed for drinking purposes is 6.5–9.2. There are no water samples with pH values outside of the desirable ranges.
The measured conductivity of CI groundwater samples ranges from 3,680 to 10,500 μS/cm (Table 1), while the corresponding values for the Djeffara aquifers vary between 3,783 and 13,648 μS/cm (Fig. 6c). The large variation in EC is mainly attributed to geochemical processes prevailing in this region. The spatial distributions of the EC, the TDS and major ions over the study area are somewhat similar. They show an increase from the mountainous regions (recharge areas) toward Sabkhas area. The highest values were measured in the Miocene sands in the northern part of Gabes region and in the Senonian limestone of the El Hamma region and Ouedref-El Metouia Graben. These high conductivity measurements are correlated with the high total mineralization which varies largely between 2,800 and 10,100 mg/l.
The total dissolved solid (TDS) of the Continental Intercalaire groundwater ranges from 2,760 to 7,750 and 2,736 to 10,100 mg/l in Djeffara aquifers (Fig. 6d; Table 1). In Djeffara aquifers, this variation conforms partially with the main groundwater flow directions, indicating that the groundwater salinity is someway controlled by the residence time in the aquifer. Indeed, in the El Hamma region, high salinities (TDS higher than 4,000 mg/l) appear near El Hamma sebkha which relatively disturbs the evolution of the mineralization in the direction of the groundwater flow. This sudden increase of groundwater salinity suggests an influence of the high saline waters originating from El Hamma sabkhas. The increase of salinity (TDS exceeds 5,000 mg/l) in some points located in Ouedref-El Metouia and the El Meïda grabens is due to the influence of highly saline waters originating from El Hamma and Melah-M’Khcherma sebkhas and interaction with evaporitic formations outcrops in Koudiat El Hmmeïmet and Zemlet El Beïdha mountain.
The high salinity of Miocene sand aquifer in the north of Gabes increases through time (Mamou 1990). This can be explained by the high groundwater extraction rates leading to the formation of depression cone, which facilitates the leakage of saline water from local shallow saline drainage aquifer formed by return flow irrigation. The lowest values of salinity are measured in the Djeffara aquifers of the south part of the study area. This low salinity value (lower than 3,500 mg/l) reveals the dilution of the groundwater by the recharge coming from the fissured carbonate formation outcrops between El Hamma and Gabes.
The TDS in water is represented by the weight of residue left when a water sample has evaporated to dryness (Bahar and Reza 2009). The analytical results have been evaluated to ascertain the suitability of groundwater of the study area for drinking, by comparing with the specifications of TDS set to the standard guideline values as recommended by the WHO (1993). According to WHO specification, water containing <500 mg/l of dissolved solids is suitable for domestic use. Water containing more than 1,500 mg/l is maximum permissible (Table 3). Water containing more than 1,500 mg/l of dissolved solids is likely to contain enough of certain constituents to cause noticeable taste or make the water undesirable or unsuitable for drinking. Based on this classification, all ground water samples exceed the maximum allowable limits and thus indicate the unsuitability of water for drinking purposes (Table 3).
To ascertain the suitability of groundwater for any purposes, it is essential to classify the groundwater depending on their hydrochemical properties based on their TDS values (Davis and DeWiest 1966; Freeze and Cherry 1979), which are presented in Table 4. According to Freeze and Cherry (1979), the groundwater of the area is of brackish water type for all samples of CI groundwater and for 97.83 % of Djeffara aquifers, except for one sample from Djeffara aquifers that represents saline water type. Davis and DeWiest (1966) have also classified groundwater on the basis of TDS such as values up to 500 mg/l render the water desirable for drinking, whereas values ranging from 500 to 1,000 mg/l render the water permissible for drinking. Hence, classification of the groundwater of the study area based on TDS (Davis and DeWiest 1966) has been presented in Table 4. Based on this classification, all groundwater samples exceed the permissible limit for drinking.
The hardness values range from 1,187 to 1,603.59 mg/l with an average of 1,427.29 mg/l in the CI groundwater and from 1,233 to 3,173 mg/l with an average of 1,600 mg/l in the Djeffara aquifers (Fig. 6e; Table 5). Cation concentrations and ratios can trace water–rock interaction processes, such as mineral weathering and cation exchange (Han et al. 2009). In determining the suitability of groundwater for domestic and industrial purposes, some chemical analyses are made. The presence of carbonates and bicarbonates indicates temporary hardness; sulfates and chlorides of calcium and magnesium indicate permanent hardness. Temporary hardness is due to the presence of bicarbonates of calcium and magnesium and can be removed by boiling, whereas permanent hardness is attributed to other salts such as sulfate and chloride salts, which cannot be removed by boiling. Despite all these problems, hard water is not considered to be a health hazard. However, water hardness of more than 200 mg/l causes noticeable taste or makes the water undesirable or unsuitable for drinking and scale formation (ACF 1996). Water hardness has no known adverse effects; however, it causes more consumption of detergents at the time of cleaning, and some evidences indicate its role in heart disease (Schroeder 1960).
The classification of groundwater based on total hardness (Sawyer and McMcartly 1967) shows that all of the groundwater samples fall in the very hard water category (Table 6). The maximum allowable limit of TH for drinking is 500 mg/l and the most desirable limit is 100 mg/l as per the WHO international standard. In CI groundwater and Djeffara aquifers, 100 % of samples exceed permissible limit (Table 6). Groundwater exceeding the limit of 300 mg/l is considered to be very hard (Sawyer and McMcartly 1967). In our study, the classification of groundwater based on TH shows that all of the groundwater samples fall in the very hard water category (Table 6). Indeed, hardness values of all samples derived from the CI groundwater and Djeffara aquifers were found to be above the specified safe limit and cannot be used for domestic proposes because it coagulates soap lather. Also, waters are characterized by high concentrations of calcium, magnesium, sulfate and chloride. Hydrogenocarbonate anions are at a limited concentration which is, however, sufficient for the formation of large quantifies of scale: 40–50 tons per year for each well (Rosset et al. 1999).
Major elements geochemistry
In Djeffara aquifers, concentration of sodium and chloride varies, respectively, between 432 and 2,530, and 539 and 3,647 mg/l, during the considered period (Table 5). The concentration of sodium and chloride varies, respectively, between 410 and 2,074, and 646 and 2,414 mg/l in the CI groundwater. Na+ has different roles in the human body. It plays an important role in the function of the nervous system, membrane system, and excretory system (Ketata et al. 2011). According to the WHO guidelines, the maximum admissible limit for drinking water is 200 mg/l. In the study area, all groundwater samples exceed the maximum permissible limit for drinking water (Table 3). The chloride ion concentration in groundwater of the study area exceeds the maximum permissible limit for drinking water of 600 mg/l (Table 3) in almost all samples collected from CI groundwater and Djeffara aquifers. For both Na+ and Cl−, the highest concentration levels characterize the wells situated to the north part (11, 18, 19, 20, 21, 47, 48, 49, 51, 52 and 53) of the Djeffara aquifers, whereas the lowest ones characterize the wells situated to the south part (23, 26, 27, 28, 30 and 31). The spatial distribution of chloride and sodium concentrations illustrates a similar evolution as the salinity; they both decrease in the direction of the groundwater flow (Fig. 7a, b). Chloride and sodium are influenced by the contributions of water of the CI groundwater in El Hamma–Chennchou region (Na+ and Cl− concentrations can reach, respectively, 680 and 1,099) and intrusion of salt water from sabkhas.
Ca2+ concentration ranged between 300 and 702 mg/l in Djeffara aquifers (Table 5). It varied from 352 to 702 mg/l in CI groundwater. Such concentrations of calcium have no hazardous effect on human health. Knowing that the maximum permissible limit of calcium concentration set by the WHO for drinking water was specified as 200 mg/l (Table 3), it is observed that all of CI groundwater and Djeffara aquifer samples showed higher Ca2+ concentration than the permissible limit. The most elevated values were measured in wells 15, 18, 20, 21, 47, 48 and 49, F8, while the lowest levels were recorded in the well 26. The spatial distribution of calcium concentrations (Fig. 7c) decreased in the direction of groundwater flow, confirming the dominant influence of the infiltration waters charged in calcium (by change of the chalky and gypseous formations of Cretaceous). Calcium concentration is also influenced by the contribution of water of the CI groundwater in Hamma–Chennchou region (Ca2+ concentration vary between 352 and 480 m) and intrusion of salt water from sabkhas.
Magnesium concentration was found to be in the range of 65–345 and 69–96 mg/l for the studied samples collected from the Djeffara aquifers and CI groundwater, respectively (Table 5). In the Djeffara aquifers, 20 % of the samples exceeded the permissible limit of 150 mg/l, while all the CI groundwater samples showed a magnesium concentration within the permissible limit for drinking water (Table 3). The higher values were recorded for wells 18, 19, 20, 21, 46, 47, 48, 49, 50, 51, 52, 53 and 45, whereas the lower ones were recorded for wells 9, 10, 12, 13 and 16. The spatial distribution of Mg2+ correlates with the distribution of Ca2+, decreasing in the direction of the groundwater’s flow (Fig. 7d). Calcium and magnesium ions present in groundwater are particularly derived from leaching of limestone, dolomites, gypsum and anhydrites, whereas the calcium ions are also derived from cation exchange process (Garrels 1976; Haritash et al. 2008).
The carbonate and bicarbonate concentrations in groundwater are derived from carbonate weathering as well as dissolution of carbonic acid in the aquifers (Jeevanandam et al. 2006; Kumar et al. 2009). The contents in HCO3 − of the Djeffara aquifers of the northern Gabes vary from 79 to 183 mg/l (Table 5), with a maximum recorded for wells 18 and 45. It varied from 92 to 189 mg/l in the CI groundwater. As per the WHO guidelines, all samples show a bicarbonate concentration within the permissible limit of 240 mg/l for drinking water (Table 3, WHO 2004). Maps representing spatial distribution for bicarbonates concentrations (Fig. 7e) show a significant similar evolution as Ca2+ with a reduction of the contents in the direction of the groundwater’s flow.
Concentrations of K are relatively low compared to the concentrations of other cations, with values varying between 12 and 215 mg/l (Table 5). It varied from 34 to 70 mg/l in the CI groundwater. As per the WHO guidelines, all the CI groundwater and Djeffara aquifer samples exceed this permissible limit of 12 mg/l (Table 3). The highest concentrations have been recorded for boreholes 18 and 19. The maps elaborated here (Fig. 7) show that the concentration of potassuim are simular to those of salinity, chloride, sodium and calcium. Low concentrations of potassium in these waters would originate from alteration of the silicates which have low solubility rates. Potassium distribution is influenced by the chemical composition of the waters of infiltration and the waters from the CI groundwater, and intrusion of salt water from sabkhas.
The sulfate concentration of the waters in the Djeffara aquifers of the northern Gabes varied between 1,043 and 2,350 mg/l (Table 5). It varied from 925 to 1,992 mg/l in CI groundwater. Sulfate is unstable if it exceeds the maximum permissible limit of 400 mg/l (Table 3) and causes a laxative effect on humans, together with excess of magnesium in groundwater (Kumar et al. 2007; Arumugam and Elangovan 2009). This may result in gastrointestinal irritation in the human system. In the study area, all groundwater samples exceed the maximum permissible limit for drinking water (Table 3). The highest concentrations were measured for wells 18, 19, 21, 47, 48, 51, 52 and 53, while the lowest values are characteristic of wells 10, 14, 45 and 46. Spatial distribution maps show the same evolution with salinity (Fig. 7g). Since sulfates are the dominant anions, they control, along with Na+, Ca2+ and Cl−, the saline load of the waters of the Djeffara aquifers.
Water types
The method of geochemical graphic represented by Piper diagram (Piper 1944) has been widely used in groundwater studies to characterize a large number of water chemical data. Piper trilinear diagram was constructed using scientific software to display the relative concentrations of the different ions from individual water samples from the study area. This diagram reveals similarities and differences among groundwater samples because those with similar qualities will tend to plot together as groups (Todd and Mays 2005).
The Piper diagram (Fig. 8) shows similarity in major cation proportions in the Djeffara aquifers, intermediate between Na+ and Ca2+ content. Results of chemical analyses indicate enrichment in SO4 2− relative to Cl−, except of groundwater samples from Senonian limestones and Miocene sands in Ouedref-El Metouia and El Meïda grabens, respectively, which are depleted in SO4 2− with respect to Cl− ions. Consequently, a Piper diagram shows that the water facies, in Djeffara aquifers, gradually changes from the Na–Ca–SO4–Cl type to the Na–Cl–SO4 type along the flow lines, i.e., from west to east of the study area.
The Na–Ca–SO4–Cl type (corresponding to wells 9, 10, 14, 16, 22, 23, 24, 25, 26, 27, 28, 29, 30 and 31) occurs mainly in El Hamma–Chennchou and in the South-eastern part of the study area. The composition of this type is due to the mixtures of the two end members: CI water (average TDS = 3,500 mg/l) and recent recharge water (corresponding to wells 23, 26 and 27; TDS < 3,000 mg/l) from the recharge area toward El Hamma and Gabes (Mamou 1990; Trabelsi et al. 2009, 2011).
The Na–Ca–Cl–SO4 and Na–Cl type (which corresponds to wells 11, 18, 19, 20, 21, 47, 48, 49, 51, 52 and 53) waters are highly mineralized and are more influenced by the chemistry of the rocks with which the waters is contact. This high salinity level is explained by salt water intrusion from sabkhas and leakage with underlying aquifer with evaporite dissolution during fluxes ascent for high salinity.
Groundwater mineralization processes
In order to highlight different mechanisms that contribute to groundwater mineralization, principal components analysis (PCA) has been applied to physicochemical parameters corresponding to sampling made in January 2008. The chemical composition of the groundwater is characterized by major cations and anions such as Ca, Mg, Na, K, Cl, SO4, HCO3, Sr, B and F. The correlation matrix of physicochemical parameters is reported in Table 7. Cation concentrations and ratios can trace water–rock interaction processes, such as mineral weathering, cation exchange and anthropogenic input in the groundwater system (Han et al. 2009; Chan 2001).
A correlation analysis is a bivariate method, which simply exhibits how well one variable predicts the other. In this study, the relationship between various elements has been studied using the Spearman rank coefficient, which is based on the ranking of the data and not on their absolute values (Kurumbein and Graybill 1965; Kumar et al. 2006, 2007, 2009). The numerical level of the relationships represented by the coefficient of correlation varies between −1 and +1 according to the statistical significance of the estimated correlation. In general, the results show high correlations (>0.7) between some pairs of geochemical parameters, whereas a value between 0.5 and 0.7 shows moderate correlation (Giridharan et al. 2008). A negative coefficient indicates that the considered variables are evolving in opposite directions. The correlation matrices for 13 variables were prepared for CI groundwater and Djeffara aquifers (Table 7) using the computer program STATISTICA 7.1 (StatSoft) software.
The correlation matrix of the 13 variables analyzed (pH, temperature (T), TDS, Cl−, SO4 2−, HCO3 −, Na+, K+, Ca2+, Mg2+, Sr2+ F− and B+) given in Table 7 allows us to distinguish high correlation coefficients, which indicate several relevant hydrochemical relationships. Sulfate, chloride, sodium, magnesium and calcium are the main ions responsible for the increase of the salinity of the waters of Djeffara groundwater. A close correlation noted between the sulfate and calcium (r = 0.63 and 0.60), sulfate and magnesium (r = 0.73 and 0.78), chloride and sodium (r = 0.94 and 0.91), strontium and sulfate (r = 0.76 and 0.85), strontium and chloride (r = 0.72 and 0.88), and between boron and chloride (r = 0.76 and 0.75) indicates a dissolution of the sulfate and chloride evaporite rocks surrounding the aquifer and its substratum. The dissolution of sodium chloride salts induces an increase in the ionic strength of the water, which favors a greater dissolution of the sulfate salts and the enrichment of the waters by magnesium and calcium.
The relationship between Ca2+ and SO4 2− concentrations is characterized by a relatively low correlation coefficient (r = 0.6 and 0.63) and a SO4 2− ratio, in moles per liter, superior to 1. This indicates that calcium and sulfate are not involved in the same geochemical processes (Hounslow 1995). Actually, sulfates are mostly found in a dissolved form, whereas calcium can undergo a precipitation of calcite and/or a Ca/Na base exchange between clay minerals and water (Desbarats 2009).
The good correlation between SO4 2− and Mg2+ (r = 0.73 and 0.78) suggests that a part of the SO4 2− and Mg2+ may also be derived by the weathering of magnesium sulfate mineral (MgSO4). There is no relation between Ca2+ and HCO3 −, and correlation coefficient (r = −0.11 and −0.02) is not significant. This indicates that calcite may not be the source of Ca2+. Indeed, the bicarbonate concentration in groundwater is derived from carbonate weathering as well as dissolution of carbonic acid in the aquifers.
Finally, the high correlation values between concentrations of Ca2+ and Cl− (0.70) can hardly be related to any of the salinization processes recognized, and only possibly with secondary processes such as ionic exchange, which become more evident in the more salinized water as the Cl concentration increases.
R-mode factor analysis for extraction was carried out using program STATISTICA 7.1 (StatSoft) software and all the factors were plotted (Table 8; Fig. 9). Table 8 shows that the variables TDS, Cl−, SO4 2−, Ca2+, Na+, Sr2+ B+ and F− have high factor loadings in factor 1 (55.33 % (marked in bold)), whereas (HCO3 −, T, K+) have moderate and high factor loadings in factor 2 (16.80 %), respectively. Therefore, factor 1 describes the salinization factor. It represents the weathering of halite and evaporates minerals from the underlying geology. A high similarity is observed when comparing the Turonian factor to Tunisian chott one region and Turonian groundwater salinization factor’ in the western part of the study area (Kamel et al. 2008; Abid et al. 2010, 2011) and to processes of the evaporate dissolution rocks’ in Alto Guadalentín, SE Spain (Ceron et al. 2000) and in Bou-Areg, NE Morocco (Yaouti et al. 2009). The potential sources of the F− and B+ in the study area could be linked to natural origin. However, part of the F− and B+ is from natural F and B in the soil, released to the unsaturated zone due to intense irrigation and intrusion of salt water from sabkhas.
Factor 2 (16.80 %) opposes Mg2+, HCO3 −, B+ and F− concentrations with temperature, pH, Ca2+, Na+ and K+ contents. This axis, basically representing the calco-carbonic parameters, describes factor dilution of groundwater by water recharge and/or mineralization by water–soil/rock interaction. The opposite evolution of Ca2+ and HCO3 − in factor 2 indicates that the increase of Ca2+ content in groundwater is essentially related to gypsum dissolution and cation exchange processes, and not to carbonate weathering. The positive loadings of pH values suggested that the major ion concentration is controlled by pH variations in the study area, whereas pH as a controlling factor rather than Mg2+, HCO3 −, F−, B+ has low factor loadings in factor 2, which indicates that they may be derived from rock–water interaction processes.
Several bivariate diagrams of major elements are used to precisely determine the origins of the referred ions and the processes that control their concentrations in groundwaters. The plots of Na+ versus Cl− and Ca2+ versus SO4 2− content (Fig. 10a–c) are shown. The Na+–Cl− relationship has often been used to identify mechanisms responsible for the origin of water salinity in arid and semi-arid regions (Magaritz et al. 1981; Dixon and Chiswell 1992; Sami 1992; Guendouz et al. 2002; Jalali 2009). The relationship between these two ions shows that the majority of points cluster along the halite dissolution line (line of slope 1, Fig. 10a, b). Observed depletion in Cl− content relative to Na+ concentration in some samples probably reflects the cation exchange reactions leading to adsorption of Ca2+ on clay minerals and simultaneous releasing of Na+ ions. Those samples in which Na+/Cl− molar ratios are higher than 1 (Fig. 10d) also show a deficit in Ca2+ with respect to SO4 2− ions (samples located above the line of slope 1, Fig. 10c). This remains in good agreement with a Ca2+–Na+ cation exchange theory leading to a softening of water (Hidalgo et al. 1995).
On the other hand, the SO4 2− versus Ca2+ plot (Fig. 10c) shows a more pronounced loss of Ca2+ with respect to SO4 2−. This may be the result of calcite precipitation controlled by gypsum dissolution which tends to maintain saturation or oversaturation with respect to calcium bearing minerals.
In some samples, a relative depletion of Na+ with respect to Cl− is noted (Fig. 10a, b). This probably reflects the cation exchange process where Ca2+ is removed from the aquifer exchangers and replaced by Na+. The referred cation exchange is confirmed through the relation characterized by a slope of −1 traced by the position of the samples (Garcia et al. 2001). In the absence of this exchange, all analytical points should lie close to the origin (McLean et al. 2000). Figure 10e shows the scatter plot for the relationship, which is characterized by a negative slope (−0.78), suggesting that cation exchange is probably a factor in the Djeffara aquifers. Likewise, 31 % of the samples plot within the negative ordinate and positive abscissa zone. This implies that Na+ and K+ are enriched relative to Ca2+ and Mg2+ in most of the aquifers. Thus, the cation exchange sites preferentially adsorb Ca2+ and Mg2+ and discharge Na+ and K+ (direct cationic exchange). However, 43 % of samples plot within the positive ordinate and negative abscissa zone. Thus, the cation exchange sites preferentially adsorb Na+ and K+ and discharge Ca2+ and Mg2+ (reverse cationic exchange). The rest of the samples (26 %) lie close to the origin (absence of cationic exchange).
Figure 11 shows the saturation indices of halite, gypsum, calcite and dolomite, calculated using WATEQ-F computer code (Plummer et al. 1976), as a function of (Na+ + Cl−), (Ca2+ + SO4 2−), (Ca2+ + Mg2+ + HCO3 −) and (Ca2+ + HCO3 −), respectively. Results of these calculations indicate that the water evolves from a state close to undersaturated with respect to gypsum and halite (Fig. 11a, b) and saturation with respect to calcite and dolomite toward an oversaturation (Fig. 11c, d). Once the system is saturated in calcite, the hydrochemical evolution is affected by the dissolution of gypsum. Interaction between groundwater and the gypsum layer would lead to the dissolution of gypsum (Qiyan and Baoping 2002). Common-ion effects tend to increase calcite deposition to keep the balance of calcite dissolution.
Trace elements geochemistry and fluoride
Strontium and strontium/calcium rate
The dosage of strontium is important because it indicates the influence of evaporite formations. In this study, the strontium concentration ranges between 4 and 6, and 4.97 and 23 mg/l for the studied samples collected from the CI groundwater and Djeffara aquifers, respectively (Table 5). In the study area, all groundwaters exceed the maximum permissible limit of 0.5 mg/l (WHO 2004) for drinking water (Table 3).
The groundwater acquires Sr during recharge and along its flow path as it interacts with Sr-bearing minerals such as celestite (SrSO4) associated with gypsum and strontianite (SrCO3), carbonate and clay minerals through adsorption and ion exchange reactions, accompanying calcium ion (Morgan-Jones and Eggboro 1981). In some waters, enrichment in Sr could be related to the time of residence of water in the aquifer, so that the ion is a good environmental tracer (Brondi et al. 1983). Molar concentration ratio Sr2+/Ca2+ is characteristic of evaporitic water if it is higher than 1 ‰ (Meybeck 1984). Besides, as each evaporitic set has its own Sr2+/Ca2+ ratio value, Sr2+ and Ca2+ contents of water that dissolve an evaporitic formation would enable an identification of underground flow and a determination of their origin (Hsissou et al. 1996). Strontium concentrations are relatively high in Djeffara aquifers, with values varying between 4.97 and 23 mg/l (Fig. 12a). Thanks to the ratio, different evaporitic origins can be distinguished (Fig. 13):
In the zone of hydraulic sill in Hamma and Chennchou, the Sr2+/Ca2+ ratio (GRI) varies from 4.97 to 6.5 ‰, strontium varies from 4 to 6.5 mg/l, and SO4 2− contents range between 1,043 and 1,476 mg/l. In this area, the mixing process is mainly through the vertical leakage of the deep Lower Cretaceous groundwater (Sr2+/Ca2+ = 5 ‰) replenishing the shallow Djeffara aquifers. In this area, there are some evaporitic layers in Jebel Aziza and Jebel Ragouba outcropping Cretaceous (Lower Cretaceous, Cenomanian and Lower Senonian).
In the eastern part of Chennchou and in coastal plain, the well waters (GRII) are characterized by a Sr2+/Ca2+ ratio which varies between 6.5 and 8.75 ‰, strontium varies between 6 and 8.32 mg/l and sulfates range between 1,050 and 1,400 mg/l. The high concentration of sulfates and strontium and Sr2+/Ca2+ ratio indicate that it is due to the leaching of Lower Cretaceous evaporites levels and marly gypsum of Lower Senonian which act as substratum of sandy Miocene aquifer and limestone of Lower Senonian aquifer, respectively.
In the northern part of study area, waters (GRIII) show the highest Sr2+/Ca2+ ratios (8.5–9.90), Sr2+ contents varying between 7 and 23 mg/l and SO4 2− contents ranging from 1,452 to 2,350 mg/l, are mainly located close to El Hamma and Melah-M’Khacherma sabkhas. These waters are distinct from the previous ones. In this zone, the salinity may have several origins: intrusion of saline water from El Hamma and Melah-M’khacherma sabkhas and dissolution of the evaporite formations of Lower Cretaceous and Lower Senonian which act as substratums of sandy Miocene aquifer and limestone of Lower Senonian aquifer, respectively in El Meïda and in Ouedref-El Metouia grabens.
Boron
Boron provides additional information on the groundwater evolution (Edmunds et al. 2003). High concentrations of boron sometimes occur in groundwater by anthropogenic activities (Giménez and Morell 1992) and interaction with evaporate levels (Rose et al. 2000; Sánchez-Martos et al. 2002; Hébrard et al. 2006).
The boron concentrations of the waters in the Djeffara aquifers vary between 0.42 and 2.3 mg/l (Table 5). In the study area, almost all CI groundwater samples show a boron concentration within the permissible limit for drinking, whereas all groundwater from Djeffara aquifers exceeds this limit (Table 3). The spatial distribution maps of the boron concentration are similar to those of salinity and chloride (Fig. 12b).
The boron values are higher near El Hamma and Melah-M’Khacherma Sabkhas (wells 11, 18, 19, 20, 21, 45, 46, 47, 48, 49, 50, 51, 52 and 53). An increase in B+ and Cl−, simultaneously, in waters in the Ouedref Metouia and Meïda grabens provides a good indicator for different origins of dissolved elements and water contributions as the area of the El Hamma and Melah-M’khacherma Sabkhas. The increase of boron concentration observed near Koudiat El Hmmeïmet in some wells (wells 45, 46, 47, 48, 49, 50, 51, 52 and 53) may be related to the thickness of the aquifer, or the lithological character of the formations in the recharge area. The B+ is probably from the evaporitic rocks leaching of the Mio-Pliocene and Quaternary formations outcropping in Koudiat El Hmmeïmet. In fact, this part of B+ is from natural B in the soil, released to the unsaturated zone due to intense cultivation in recharge area of sandy aquifer. The other samples reflecting boron released from evaporites or syn-depositional clay minerals are characterized by low TDS values (2,736–3,581 mg/l), relatively low concentrations of boron (0.42–0.9 mg/l) and Cl (525–860 mg/l), and high concentrations of SO4 (1,125–1,527 mg/l) and Na+ (400–668 mg/l).
Fluoride
Fluoride is one of the main trace elements in groundwater, which generally occurs as a natural constituent. In fact, fluoride related to groundwater has been studied intensively during the past decades (Roberston 1986, Zhaoli et al. 1989; Travi and Faye 1992; Hitchon 1995; Subba Rao 2003; Coetsiers et al. 2008). These studies show that fluoride is the result of water–rock interaction and leaching from minerals in various aquifers with different lithologies. Bedrocks containing fluoride minerals are generally responsible for high concentration of fluoride in groundwater (Handa 1975; Wenzel and Blum 1992; Bardsen et al. 1996). Subba Rao (2003) found that high fluoride concentrations in India are related to the evapotranspiration, long-term contact of waters in the weathered zone by virtue of its low hydraulic conductivity and stagnation of water in the aquifer zone caused by intrusive bodies, intensive and long-term irrigation, and heavy use of fertilizers.
During January 2008, the concentration of fluoride in the Djeffara groundwater varies between 0.57 and 2.84 mg/l (Table 5). The presence of low concentration of fluoride in majority of wells for drinking water in the study area is of minor concern, as all the samples are found to have a fluoride concentration within the permissible limit of 1.5 mg/l for drinking water (Table 3, WHO 2004), except one sample (sample No. 46).
The spatial distribution of fluorine (Fig. 12c) shows that the highest concentration levels characterize the wells located in the northern part of the study area (wells 18, 19, 21, 45, 46, 47, 48, 49, 50, 51, 52, 53), where intense cultivation is applied in the recharge area of sandy aquifer. These contents decrease in the direction of groundwater flow. Fluorine may essentially be from a natural origin. Limestone (chalky) and marly gypsum formations can contain significant quantities of fluorine which can be liberated by the water–rock interaction. However, a part of the F− is from natural F in the soil, released to the unsaturated zone due to intense cultivation.
Irrigation purpose
Quality of groundwater is of paramount importance for irrigation in arid and semi-arid regions. Crop cultivation mainly depends on groundwater supply for irrigation in several arid and semi-arid areas in the world, e.g. in Tunisia. In fact, the suitability of groundwater for irrigation depends on the effects of constituent minerals of water on both the plant and soil. Excessive amounts of dissolved ions in irrigation water affect plants and agricultural soil, thus reducing the productivity (Ravikumar et al. 2010). The high salt content in irrigation water causes an increase in soil solution osmotic pressure. The chemical composition is directly affecting either the growth of plants and disrupts their metabolism. Also, it affects soil structure, permeability and aeration which indirectly affect plant growth. The important chemical constituents that affect the suitability of water for irrigation are the total concentration of dissolved salts, concentration of other elements that may be toxic to plants, relative proportion of bicarbonate to calcium, magnesium and relative proportion of sodium to calcium (Haritash et al. 2008). Water quality problems in irrigation include salinity and alkalinity. The important chemical constituents that affect the suitability of water for irrigation (Table 9) can be utilized to verify the suitability as follows:
-
Salinity and indexes such as SAR (Eq. 1).
-
Sodium hazard expressed as percent sodium of total cations (%Na, Eq. 2).
-
Bicarbonate hazard or (HCO3) concentration as related to the concentration of calcium and magnesium such as RSC (Eq. 3) and RSBC (Eq. 4).
-
Boron hazard (concentration of boron or other elements) that may be toxic.
-
Permeability index (PI, Eq. 5).
Electrical conductivity
Electrical conductivity is a good measure of salinity hazard to crops as it reflects the TDS in groundwater. The primary effect of high EC water on crop productivity is the incapability of the plant to compete with ions in the soil solution for water. The higher EC, the less water is available to plants (Tank and Chandel 2009). The US Salinity Laboratory (1954) classified ground waters on the basis of electrical conductivity (Table 9). The EC classification indicates that 33.3 % of CI groundwater and 14.64 % of Djeffara aquifers are doubtful for irrigation; and for CI groundwater and Djeffara aquifers, respectively, 66.7 and 85.36 % of collected samples are unsuitable for irrigation. This indicates leaching and dissolution of salts in deep Djeffara aquifer of Gabes.
Total dissolved solids (TDS)
To ascertain the suitability of groundwater for any purposes, the TDS should be below 500 mg/l (Catroll 1962; Freeze and Cherry 1979). Based on Freeze and Cherry (1979), all groundwater samples collected from the CI groundwater and 97.83 % of those collected from the Djeffara aquifers are brackish in nature, except one sample from the Djeffara aquifers showing saline water type indicating high dissolved load in water (Table 4). Based on Davis and DeWiest (1966) classification (Table 4), 37.5 % of groundwater samples collected from the CI groundwater and 8.7 % of those collected from the Djeffara aquifers one can be used for irrigation purpose. High TDS in most of the groundwater clearly indicate the unsuitability of groundwater for irrigation purposes.
Total hardness (TH)
Hard water is a potential problem since the calcium and magnesium can combine with bicarbonate to form insoluble calcium and magnesium carbonate salts. These salts can affect media pH and reduce the amount of sodium available to a plant (Robbins 2010). According to United States Department of Agriculture method (USDA) specification of TH, up to 200 mg/l is the upper limit for water irrigation. The high TH values (Table 4) reveal that all the sampling sites are unsuitable for irrigation.
Chloride classification
Stuyfzand (1989) classified water on the basis of Cl− ion concentration into eight divisions as shown in Table 9. Based on this classification, the groundwater of the area is of brackish water type for 33.3 % of the CI groundwater and for 58.53 % of the Djeffara aquifers. The rest of the samples represent brackish–salt water type.
Sodium adsorption ratio (SAR)
High concentration of sodium in water produces harmful effects changing soil properties and reducing soil permeability (Kelley 1951; Domenico and Schwartz 1990; Todd and Mays 2005). Thus, sodium concentration is essential to classify the water for irrigation purposes. SAR is an important parameter for determining the suitability of groundwater for irrigation because it is a measure of alkali/sodium hazard to crops (Subramani et al. 2005). Indeed, it indicates the degree of which irrigation water tends to enter cation exchange reactions in soil. Sodium replacing adsorbed calcium and magnesium is a hazard causing damage to the soil structure, making it compact and impervious (Raju 2007).
Based on SAR classification (Richards 1954), it is observed that 66.7 % of groundwater samples collected from CI groundwater and 95.12 % of those collected from the Djeffara aquifers fall into the category of waters excellent for irrigation purposes in almost all soils (Table 9, Eq. 2). Therefore, the SAR value implies that most of the groundwater is suitable for irrigation. The spatial distribution maps of SAR values in groundwater is presented in Fig. 14a for the Djeffara aquifers. A more detailed analysis for the suitability of water for irrigation can be made by plotting the SAR and electrical conductivity (Fig. 15) data on the US Salinity Laboratory (USSL) diagram (Richards 1954).
Plots of calculated values of SAR and EC of groundwater samples (after Richards 1954; January 2008)
Based on salinity hazard, the types of water can be classified as C1, C2, C3, C4 and as S1, S2, S3, or S4 based on sodium hazard. The significance and interpretation of quality ratings on the USSL diagram is summarized as: (1) Low salinity hazard class (C1) can be used for irrigation of most crops and the majority of soils. However, some leaching is required, but this occurs under normal irrigation practices except in soils of extremely low permeability. (2) Medium salinity hazard class (C2) can be used if a moderate amount of leaching occurs. (3) High salinity hazard class (C3) is satisfactory for plants having moderate salt tolerance, on soils of moderate permeability with leaching. (4) High salinity hazard class (C4), (5) extremely high salinity water (C5) and (6) water where the salinity hazard is in excess (C6) cannot be used in soils with restricted drainage. Even with adequate drainage, special management for salinity control is required, and crops with good salt tolerance should be selected. Such areas need special attention as far as irrigation is concerned (Ravikumar et al. 2010).
Low sodium water (S1) can be used for irrigation on almost all soils with little danger of developing harmful levels of exchangeable Na+ (Ketata et al. 2011). Medium sodium water (S2) presents an appreciable sodium hazard in certain fine-textured soils, especially poorly leached soils (Subba Rao 2006). Waters belonging to this class are classified as excellent for irrigation and can be used safely on coarse-textured or organic soils having good permeability and may be used to irrigate salt-tolerant and semi-tolerant crops under favorable drainage conditions. High sodium waters (S3) are usually doubtful/fair poor for irrigation and cannot be used on clayey soils of low permeability. Waters belonging to this class may produce harmful levels of exchange sodium in most soils and will require special soils management such as good drainage and leaching and addition of organic matter. Very high sodium water (S4) is usually unsuitable for irrigation, except at low and perhaps medium salinity (Haritash et al. 2008).
USSL diagram
The USDA classification for salinity (C) and sodicity hazards (S) in the Djeffara aquifers is shown in Table 10 and Fig. 15. All of the wells fall within the very high salinity hazard zone, which shows a substantial amount of the total dissolved salts in the water. Following the USDA classification system, the water from all groundwater samples fall in the salinity class C4, including all three sodicity classes. On the other hand, nearly 95 % of the wells have SAR values below 15, which is of a medium quality for irrigation waters. The percentage of sodium is not very high in the groundwater throughout the CI groundwater and Djeffara aquifers, but the overall salt contents are very high. The combined EC/SAR classification locates the Gabes water in the C4S2, C4S3 and C4S4 classes with 61.13, 27.27 and 11.5 % of the wells, respectively. Such water is rated as highly and very highly saline for irrigation, i.e., not suitable for irrigating most crops except the tolerant ones. Special measures to control the salinity hazard are needed, including leaching and adequate drainage. The sodicity hazard is of less magnitude, with water from 61.23 % of the wells in the S2 class, 27.27 % in the S3 class and 11.5 % in the S4 class. All water from wells may cause infiltration problems, especially with heavy textured and poorly drained salts.
Water from about 61.23 % of the wells corresponds to medium sodium water (S2) that may be of an appreciable sodium hazard in finely textured soils with a high cation exchange capacity. This water could be used for coarsely textured soils with good permeability. Water from 38.77 % of the wells corresponds to water with a high sodium content that produces harmful levels of exchangeable sodium in most soils and requires special soil amendments. From this classification, it is clear that the groundwater in the Djeffara aquifers is unfit for irrigation.
Percent sodium (%Na)
Sodium content is usually expressed in terms of percentage of sodium or soluble-sodium percentage (%Na). The percent sodium is calculated by methods of Wilcox (1948) by Eq. (3). This method has been used to classify and understand the basic character of the chemical composition of groundwater. Indeed, the suitability of groundwater for irrigation depends on the mineralization of water and its effect on plants and soil.
Based on the percentage of Na as per Wilcox (Table 11), 66.7 % of CI groundwater is within the permissible, whereas 33.3 % is doubtful for irrigation. In the Djeffara aquifers, 9.75 % of samples are good for irrigation, 87.8 % is within permissible limit, whereas 2.45 % is doubtful for irrigation. Spatial variation of percentage of sodium is shown in Fig. 14b for the Djeffara aquifers. The high percentage of Na in most of the groundwater indicates its unsuitability for irrigation.
Wilcox (1948) proposed a method for rating irrigation waters, based on percentage of sodium and electrical conductivity. The significance and interpretation of quality ratings based on Wilcox (1948) diagram can be summarized as follows: (1) excellent to good, (2) good to permissible, (3) permissible to doubtful, (4) doubtful to unsuitable and unsuitable. Data of the groundwater samples of the studied area are summarized in Table 9 and show that all samples of the Djeffara aquifers fall above the unsuitable categories indicating their unsuitability for irrigation. Indeed, this method is not applicable for the water of Djeffara aquifers. The norms proposed by Wilcox (1948) appear very severe for the Djeffara groundwater of the northern Gabes. When the concentration of Na+ is high in irrigation water, sodium tends to be absorbed by clay particles, displacing Mg2+ and Ca2+ ions. This exchange process of Na+ in water for Ca2+ and Mg2+ in soil reduces the permeability and eventually results in soil with poor internal drainage (Subramani et al. 2005; Kumar et al. 2007, Ravikumar et al. 2009). Hence, air and water circulation are restricted during wet conditions and such soil is usually hard when dry (Collins and Jenkins 1996; Salesh et al. 1999).
The morphological study of soils by Hatira et al. (2007) showed the predominance of fine sand and silt conferring very important filtering capacity. In this study, all studied profiles are filled by gypsum. Indeed, concentrations of gypsum ranged between 2 and 61 %. Also, carbonate of calcium is present in all studied soils with an average value of 5.5 %, and more than 10 %. In fact, the soil features are presently dominated by the evolution of salinization and water-saturation processes either partially or totally affecting the soil profile. The visible salt deposits and soil salinization processes are generated by the salinity of water irrigation. These processes are strongly influenced by the weak soil leaching mechanisms, on the one hand, and the poor drainage schemes, on the other hand.
Residual sodium carbonate
In addition to the SAR and %Na, the excess sum of carbonate and bicarbonate in groundwater over the sum of calcium and magnesium also influences the suitability of groundwater for irrigation. The RSC was calculated to determine the hazard effects of carbonate and bicarbonate on the quality of groundwater for agricultural and irrigation purposes (Eaton 1950). The excess sum of carbonate (CO3 2−) and bicarbonate (HCO3 −) in groundwater over the sum of calcium (Ca2+) and magnesium (Mg2+) also influences the suitability of groundwater for irrigation because in waters having high concentration of bicarbonate (HCO3 −), there is a tendency for calcium and magnesium to precipitate as the water in the soil becomes more concentrated. An excess quantity of sodium bicarbonate and carbonate is considered to be detrimental to the physical properties of soils, as it causes dissolution of organic matter in the soil, which in turn leaves a black stain on the soil surface on drying (Ravikumar et al. 2010). Therefore, land irrigated with such water becomes infertile owing to deposition of sodium carbonate (Eaton 1950).
According to the US Department of Agriculture, waters having >2.5 (meq/l) RSC are unsuitable for irrigation, 1.25–2.5 are marginal and <1.25 are safe waters. The increase of RSC in groundwater is significantly harmful for plant growth (Krishna Kumar et al. 2009). The groundwater in the study area is classified on the basis of RSC, and the results are presented in Table 9 for the CI groundwater and Djeffara aquifers (Fig. 14c). The calculated values (Ragunath 1987, Eq. 4) reveal that all the sampling sites are good for irrigation purpose.
Residual sodium bicarbonate
Gupta and Gupta (1987) classified water on the basis of “RSBC”. RSBC was calculated for each well by Eq. (5) given by Gupta and Gupta (1987) and classified as satisfactory (<5 meq/l), marginal (5–10 meq/l) and unsatisfactory (>10 meq/l). The calculated values (Ragunath 1987; Gupta and Gupta 1987, Eq. 5) of “RSBC” are presented in Table 5b for the CI groundwater and Djeffara aquifers.
The RSBC of CI groundwater ranged from −21.66 to −15.96 meq/l with an average value of −19.56 meq/l and the corresponding values in Djeffara aquifers (Fig. 14d) ranged from −32.03 to −12.78 meq/l with an average −19.23 meq/l (Table 5b). According to the RSBC values, all groundwater samples collected were found to be satisfactory (<5 meq/l) according to the criteria set by Gupta and Gupta (1987). The RSBC values are <5 meq/l and are therefore considered safe for irrigation purposes.
Boron
Scofield (1936) and McCarthy and Ellery (1994) proposed a table of permissible boron limits to assess water quality using five class (1–5) and three levels of crop tolerance (sensitive, semi-tolerant and tolerant). The proposed limits of boron concentration in irrigation water and the total number of groundwater samples of the study area representing the boron classes are presented in Table 9. Based on this classification, it is observed that all of groundwater samples collected from the CI groundwater and 14 samples (34.15 %) of those collected from the Djeffara aquifers are found to be excellent for tolerant and semi-tolerant crops based on boron concentration. However, 15 samples (36.6 %) and 12 samples (29.25 %) were found to be permissible and unsuitable for semi-sensitive crops. Three samples from CI groundwater and 14 samples (34.41 %) from Djeffara aquifers were found to be excellent for semi-sensitive and tolerant crops. The remaining samples reveal that 17 samples (41.5 %) were found to be good, and 6 samples (14.7 %) belongs to the permissible category, and 4 samples (9.70 %) fall in the doubtful category for semi-sensitive and tolerant crops.
Permeability index (PI)
The permeability index also indicates whether groundwater is suitable for irrigation. The soil permeability is affected by the long-term use of irrigation water as influenced by Na+, Ca2+, Mg2+ and HCO3 − contents of the soil. Doneen (1964) and Ragunath (1987) used a criterion for assessing the suitability of water for irrigation based on permeability index and accordingly, water can be classified as class I, II and III. Class I and II water are categorized as good for irrigation with 75 % or more of maximum permeability. Class III water is unsuitable with 25 % of maximum permeability.
The PI of CI groundwater ranges from 47.72 to 73.94 % with an average value of 57.55 % and the corresponding values (Table 5b) in Djeffara aquifers ranged from 38.57 to 73.94 % with an average of 48.20 % (Table 5b; Fig. 14e). According to the permeability index values, all of groundwater samples comes under class II (Table 9), suggesting the suitability of groundwater for irrigation. The calculated values reveal that all the sampling sites are good for irrigation purpose.
Conclusion
The Djeffara aquifer system is one of the most important groundwater resources in Tunisia. It is the main water source for the local population and agriculture needs. Therefore, deciphering the mechanisms responsible for observed chemistry and assessing the water quality in this system, pursued in the framework of this study, are of great practical importance. Simultaneous analysis of both major and trace elements allowed better understanding of the hydrodynamic functioning and assessing the water quality in the Djeffara aquifer system. The Piezometric map (Fig. 4) and the geochemical data (Tables 1, 2) point to the significant role of carbonate and sandy outcrops of the Cretaceous and Miocene, respectively, located in the southern and northern parts of study area in local recharge of this aquifer system. Stratigraphic, tectonic and hydraulic conditions seem to be important factors controlling the geochemical evolution of groundwater in the study area. The principal changes in chemical composition of Djeffara aquifer system result from mixing with CI water of deeper circulation. Closer analysis of available chemical data reveals the importance of dissolution/precipitation processes as well as cation exchange reactions in evolution of groundwater chemistry. In general, mineralization of groundwater increases along the flow path from El Hamma region toward the sabkhas and Mediterranean Sea. The Na+/Cl− molar ratio suggests that the dissolution of halite in evaporites within the Miocene and Cretaceous sequences is the main source of these ions in groundwater. The values of this ratio, >1, observed in some of the wells may indicate the occurrence of cation exchange reaction releasing Na+ into the water with simultaneous removal of Ca2+. The existence of such exchange is confirmed by the balance between the excess of (Na+ + K+) over the Cl− ions and excess of (Ca2+ + Mg2+) over the (HCO3 − + SO4 2−) anions (Fig. 10e). Chemical data indicate also the importance of gypsum dissolution as a factor controlling groundwater chemistry. The source of the salinity as shown from the Sr2+/Ca2+ ratios is mainly derived from evaporitic formations of Cretaceous, except in the Ouedref Metouia/El Meïda graben where the influence of both the salt water from sabkhas and water leaching evaporitic formations of Cretaceous is evident. What is more, the molar ratio Sr2+/Ca2+ allows the distinguishing of the different evaporitic influences in this region.
The spatial distribution of fluoride and boron shows that highest concentration of these elements characterizes the waters of the northern part of study area, with a reduction in the flow direction. Fluorine and boron may essentially come from a natural origin. Limestone, marly formations and gypsum can contain significant quantities of fluorine and boron which can be released by water–rock interaction.
With limited supply by the recent infiltration waters, the increased exploitation of wells can lead to further deterioration of the quality of groundwater resources available for the population in this area.
Assessment of water samples according to exceeding the permissible limits prescribed by WHO for drinking purposes indicated that groundwater in study area is chemically unsuitable for drinking uses. High TH and TDS at a number of subareas clearly indicate the unsuitability of groundwater for drinking and irrigation purposes. Therefore, the water of these wells must be desalinised before use for drinking.
The suitability of groundwater for irrigation was evaluated based on the irrigation quality parameters like boron, SAR, %Na, RSC, RSBC, and permeability index. Among these parameters, boron and SAR imply that the water samples fall in excellent to permissible, excellent to doubtful, excellent to good for irrigation purpose. RSC and RSBC values specify that water samples belong to good classes. The high EC value and percentage of Na in most of the groundwater indicate its unsuitability for irrigation. However, permeability index recommends that the water samples from the Djeffara aquifers, belonging to class 2, are suitable for irrigation. According to the US Salinity Classification, in deep Djeffara aquifers of the northern Gabes, 56.52 % fall in the C4–S2 category, 32.61 % fall in the C4–S3 category and 10.87 % fall in the C4–S4 category, indicating high salinity/high sodium and medium salinity/high sodium type and there is a need for better drainage to overcome salinity problems. From this classification, it is clear that the groundwater in the Djeffara aquifers is unfit for irrigation. Water that is not suitable based on the above classification may be suitable in well-drained soils.
The following remedial measures can be helpful (Subba Rao 2003) in the Gabes-north region: (1) drip irrigation should be preferred over traditional irrigation practices, such as flooding to prevent weathering and leaching and also to reduce water consumption and evaporation; (2) application of calcium chlorite, gypsum and organic manures to cause de-alkalization and deflocculation, and to produce more permeable soil supporting plant expansion; (3) cultivation of sodium salt-tolerant crops and (4) using judicious fertilizers to obtain higher crop yields should be based on crop requirements and soil characteristics.
Groundwater quality in the study area reaches alarming situation so that stringent monitoring and control measures are necessary to ensure sustainable safe use for the resource. In this strategy, Tunisia is involved in a series of geological, hydrogeological and hydrochemical investigations that will provide technical support for developing policies to help preserve groundwater availability and protect water quality, also, assess the origins and the evolution of salinity in the water.
Finally, based on this studies, recommendations have been made to the local authorities to adopt conjunctive use of surface water with groundwater to stringently monitor and control low groundwater quality regions to ensure sustainable safe use of the resource.
References
Abbès C, Abdeljouad S, Ben Ouezdou H, Zargouni F (1986) Carte. géologique d’El Hamma à 1:100 000. Feuille n°74, Office National des Mines, Tunis
Abdeljaouad S (1983) Etude sédimentologique et structure de la partie est de la chaine nord des Chotts. Thèse de Doctorat. Univ. de Tunis El Manar. Tunisie
Abid K, Trabelsi R, Zouari K, Abidi B (2010) Caractérisation hydrogéochimique de la nappe du Continental Intercalaire (sud tunisien). Hydrol Sci J 54(3):526–536
Abid K, Zouari K, Dulinski M, Chkir N, Abidi B (2011) Hydrologic and geologic factors controlling groundwater geochemistry in the Turonian aquifer (southern Tunisia). Hydrogeol J 19:415–427
Abidi B (2004) Caractéristiques hydrodynamiques et géochimiques de la Djeffara de Gabés. DGRE 2004
ACF (Action Contre la Faim) (1996) La qualité et les analyses d’eau. ACF, France
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. Hydrogeol J 241:91–103
Aghazadeh N, Mogaddam AA (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess 176:183–195
Al-Bassam AM, Al-Rumikhani YA (2003) Integrated hydrochemical method of water quality assessment for irrigation in arid areas: application to the Jilh aquifer, Saudi Arabia. J Afr Earth Sc 36:345–356
Al-Shaibani AM (2008) Hydrogeology and hydrochemistry of a shallow alluvial aquifer, western Saudi Arabia. Hydrogeol J 16:155–165
Anku YS, Banoeng-Yakubo AEB, Daniel AE, Asiedu K, Sandow A, Yidana EM (2009) Water quality analysis of groundwater in crystalline basement rocks, Northern Ghana. Environ Geol 58(5):989–997
Arumugam K, Elangovan K (2009) Hydrochemical characteristics and groundwater quality assessment in Tirupur Region, Coimbatore District, Tamil Nadu, India. Environ Geol 58:1509–1520
Ayenew T, Demlie M, Wohnlich S (2008) Hydrogeological framework and occurrence of groundwater in the Ethiopian aquifers. J Afr Earth Sc 52(3):97–113
Bahar MM, Reza MS (2009) Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environ Earth Sci. doi:10.1007/s12665-009-0427-4
Bardsen A, Bjorvatn K, Selvig KA (1996) Variability in fluoride content of subsurface water reservoirs. Acta Odontol Scand 54:343–347
Benton MJ, Bouaziz S, Buffetaut E, Martill D, Ouaja M, Soussi M, Trueman C (2000) Dinosaurs and other fossil vertebrates from fluvial deposits in the lower cretaceous of Southern Tunisia. Palaeolgeogr Palaeoclimatol Palaeoecol 157:227–246
Bouaziz S (1995) Etude de la tectonique cassante dans la plateforme et l’Atlas Sahariens (Tunisie Meridionale): évolution des paleochamps de contraintes et implications géodynamiques. Thèse de Doctorat. Univ. de Tunis El Manar. Tunisie
Boughriba M, Melloul A, Zarhloule Y, Ouardi A (2006) Extension spatiale de la salinisation des ressources en eau et modèle conceptuel des sources sale′es dans la plaine des Triffa (Maroc nord-oriental). CR Geosci 338(11):768–744
Brondi M, Fidelibus MD, Gragnani R, Tulipano L (1983) Hydrochemical study and distribution of some trace elements in the most important coastal springs and groundwater of the Apulian Region (Southern Italy). Geol Appl Idrogeol XVII(II):65–80
Busson G (1970) Le Mésozoïque saharien, tômes 1 et 2. Centre National de Recherche Scientifique, Paris
Custodio E, Bruggeman GA (1987) Groundwater problems in coastal areas. UNESCO. Studies and reports in hydrology
Castany G (1954) L’accident sud tunisien et ses relations avec l’accident sud-atlasique. C R Acad Sci 238:1–184
Castany G (1982) Bassin se′dimentaire du Sahara septentrional (Algérie-Tunisie)-Aquifères du Continental intercalaire et du Complexe Terminal. Bulletin Bureau Recherches Géologiques Minières (BRGM). Série 2.3. 127–147
Catroll D (1962) Rain water as a chemical agent of geological process: a view. USGS Water Supply 1533:18–20
Ceron JC, Jimenez-Espinosa R, Pulido-Bosch A (2000) Numerical analysis of hydrogeochemical data: a case study (Alto Guadalentõan, southeast Spain). Appl Geochem 15:1053–1067
Chan HJ (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol (Amsterdam) 253:194–210. doi:10.1016/S00221694(01)00481-4
Coetsiers M, Kilonzo F, Walraevens K (2008) Hydrochemistry and source of high fluoride in groundwater of the Nairobi area, Kenya. Hydrol Sci J 53(6):1230–1240
Collins R, Jenkins A (1996) The impact of agricultural land use on stream chemistry in the middle Hills of Himalaya, Nepal. J Hydrol 185(71):86
Dassi L (2010) Use of chloride mass balance and tritium data for estimation of groundwater recharge and renewal rate in an unconfined aquifer from North Africa: a case study from Tunisia. Environ Earth Sci 60(4):861–871
Dassi L, Zouari K, Peter Seiler K, Faye S, Kamel S (2005) Flow exchange between the deep and shallow groundwaters in the Sbeitla synclinal basin (Tunisia): An isotopic approach. Environ Geol 47(4):501–511
Davis SN, DeWiest RJ (1966) Hydrogeology. Wiley, New York
Desbarats AJ (2009) On elevated fluoride and boron concentrations in groundwaters associated with the Lake Saint-Martin Impact Structure, Manitoba. Appl Geochem 24:915–927. doi:10.1016/j.apgeochem.2009.02.016
DGRE (Direction Générale des Ressources en Eau) (2008). Annuaires d’exploitation de la nappe profonde 1950–2008. Ministère de l’Agriculture, Tunis
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland, Australia. Hydrogeol J 130:299–338
Djabri L, Rouabhia A, Hani A, Lamouroux Ch, Pulido-Bosch A (2007) Origin of water salinity in a lake and coastal aquifer system. Environ Geol 54(3):565–573
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York, pp 410–420
Doneen LD (1964) Notes on water quality in agriculture. Published as a water science and engineering paper, 4001, Department of water science and engineering, University of California
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Edmunds WM, Guendouz A, Mamou A, Moulla A, Shand P, Zouari K (2003) Groundwater evolution in the Continental Intercalaire aquifer of southern Algeria and Tunisia: trace element and isotopic indicators. Appl Geochem 18:805–822
Elango L, Kannan R, Senthil K (2003) Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram District, Tamil Nadu, India. Environ Geol 10(4):157–166
ERESS (1972) Etude des ressources en eau du Sahara Septentrional. UNESCO, Paris
Fehdi Ch, Aek Rouabhia, Baali F, Boudoukha A (2009) The hydrogeochemical characterization of Morsott-El Aouinet aquifer, Northeastern Algeria. Environ Geol 58(7):1611–1620
Freeze RA, Cherry JA (1979) Groundwater. Printice-Hall, New Jersey
Garcia MG, Del Hidalgo M, Blesa MA (2001) Geochemistry of groundwater in the alluvial plain of Tucumán province Argentina. Hydrogeol J 9(6):597–610
Garrels RM (1976) A survey of low temperature water mineral relations. In: Interpretation of environmental isotope and hydrogeochemical data in groundwater hydrology: Vienna, International Atomic Energy Agency, pp 65–84
Ghanmi M, Potfaj M, Ben Youssef M, Zargouni F (1984) Carte géologique d’Oglet Mertaba à 1:100 000. Feuille no. 82. Office nationale des mines. Tunisie
Giménez E, Morell I (1992) Utilisació del boro como indicatoro de contaminación en la Plana de Castellón. Hidrogeologica y Recursos Hidráulicos XVI(IV):285–292
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environ Monit Assess 143:161–178
Graniel CE, Morris LB, Carrillo Rivera JJ (1999) Effect of urbanization on groundwater resources of Merida, Yucatan, Mexico. Environ Geol 37(4):303–312. doi:10.1007/s002540050388
Guendouz A, Moulla AS, Edmunds WM, Zouari K, Shand P, Mamou A (2002) Hydrogeochemical and isotopic evolution of water in the Complex Terminal aquifer in the Algerian Sahara. Engineering Sciences Faculty, Blida, Algeria, pp 483–495
Guler C, Thyne GD (2004) Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian Wells—Owens Valley area, southeastern California, USA. Hydrogeol J 285:177–198
Guler C, Thyne GD, McCray JE, Turner AK (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10:455–474
Gupta SK, Gupta IC (1987) Management of saline soils and water. Oxford and IBM Publ. Co, New Delhi
Hamzaoui-Azaza F, Ketata M, Bouhlila R, Gueddari M, Ribeiro L (2011) Hydrogeochemical characteristics and assessment of drinking water quality in Zeus–Koutine aquifer, southeastern Tunisia. Environ Monit Assess 174:283–298
Hamzaoui-Azaza F, Ameur M, Bouhlila R, Gueddari M (2012) Geochemical characterization of groundwater in a Miocene Aquifer, Southeastern Tunisia. Environ Eng Geosci 18(2):159–174
Han D, Liang X, Jin M, Currell MJ, Han Y, Song X (2009) Hydrogeochemical indicators of groundwater flow systems in the Yangwu River Alluvial Fan, Xinzhou Basin, Shanxi, China. Environ Manage 44:243–255
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Groundwater 13(3):275–281
Haritash AK, Kaushik CP, Kaushik A, Kansal A, Kumar YA (2008) Suitability assessment of groundwater for drinking, irrigation and industrial use in some North Indian villages. Environ Monit Assess 145:397–406
Hatira A, Baccar L, Grira M, Gallali T (2007) Analyse de sensibilité du système oasien et mesures de sauvegarde de l’oasis de Métouia (Tunisie). Rev Sci Eau 20(1):59–69
Hébrard O, Pistre S, Cheynet N, Dazy J, Batiot-Guilhe C, Seidel JL (2006) Origine des eaux des émergences karstiques chlorurées du Languedoc-Roussillon. C R Geosci 338(2006):703–710
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. USGS, Water Supply paper no. 2254, pp 117–120, 264
Hidalgo MC, Cruz-Sanjulián J, Sanroma A (1995) Evolución geoquímica de las aguas subterráneas en una cuenca sedimentaria (acuífero de Baza-Caniles, Granada, España). Tierra Tecnol 20:39–48
Hitchon B (1995) Fluoride in formation waters. Alberta Basin, Canada. Appl Geochem 10:357–367
Hounslow AW (1995) Water quality data: analysis and interpretation. CRC/Lewis, Washington
Hsissou Y, Chauve P, Mania J, Mangin A, Bakalowicz M, Gaiz A (1996) Caracte’risation des eaux de l’aquife`re turonien du bassin du Tadla (Maroc) par le rapport des concentrations molaires Sr2+/Ca2+. Hydrogeol J 183:445–451
Jalali M (2007) Salinization of groundwater in arid and semiarid zones: An example from Tajarak, western Iran. Environ Geol 52(6):1133–1149
Jalali M (2009) Geochemistry characterization of groundwater in an agricultural area of Razan, Hamadan, Iran. Environ Geol 56:1479–1488
Jeevanandam M, Kannan R, Srinivasalu S, Rammohan V (2006) Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district, South India. Environ Monit Assess 132(1):263–274. doi:10.1007/s10661-006-9532-y
Kallel R (2003) Hydrologie de la Jeffara tunisienne. Rapport interne. CRDA de Gabes
Kamel S, Younes H, Chkir N, Zouari K (2008) Geochemical and isotopic investigation of the aquifer system in the Djerid–Nefzoua basin, southern Tunisia. Environ Geol 49:59–170
Kelley WP (1951) Alkali soils: their formation properties and reclamations. Reinhold, New York
Ketata M, Hamzaoui F, Gueddari M, Bouhlila R, Ribeiro L (2011) Hydrochemical and statistical study of groundwaters in Gabes south deep aquifer (southeastern Tunisia). J Phys Chem Earth 36:187–196
Khazaei E, Stednick JD, Sanford WE, Warner JW (2006) Hydrochemical changes over time in the Zahedan aquifer, Iran. Environ Monit Assess 114:123–143
Krishna Kumar S, Rammohan V, Dajkumar Sahayam J, Jeevanandam M (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ Monit Assess 159:341–351
Krishnakumar S (2004) Quality characterization and geochemical characteristics of lower Gadilum River Basin, Tamil Nadu, India. Dissertation, University of Madras
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 5(553):574
Kumar M, Kumari K, Singh UK, Ramanathan AL (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57:873–884
Kurumbein WC, Graybill FA (1965) An introduction to statistical models in geology. McGraw-Hill, New York
Magaritz M, Nadler A, Koyumdjisky H, Dan N (1981) The use of Na/Cl ratio to trace solute sources in a semiarid zone. Water Resour Res 17:602–608
Mamou A (1990) Caractéristiques, Evaluation, Gestion des ressources en eau du Sud Tunisien. PhD thesis, University of Paris South, Paris
Mandel S, Shiftan ZL (1981) Groundwater resources investigation and development. Academic Press Inc, New York
McCarthy TS, Ellery WN (1994) The effect of vegetation on soil and ground water chemistry and hydrology of islands in the seasonal swamps of the Okavango Fan, Botswana, Botswana. Hydrogeol J 154:169–193
McLean W, Jankowski J, Lavitt N et al (2000) Groundwater quality and sustainability in an alluvial aquifer, Australia. In: Sililo O (ed) Groundwater, past achievement and future challenges. Balkema, Rotterdam, pp 567–573
Mekrazi AF (1975) Contribution à l’étude géologique et hydrogéologique de la région de Gabes Nord. PhD thesis, University of Bordeaux, France
Meybeck M (1984) Les fleuves et le cycle géochimique des éléments. Etat Thesis, Univ Paris VI
Morgan-Jones M, Eggboro MD (1981) The hydrogeochemical of the Jurassic limestones in Gloucestershire, England. Q J Eng Geol (London) 14:25–29
OSS (Observatoire Sahara et Sahel) (2003) Système aquifère du Sahara septentrional: gestion commune d’un bassin transfrontière. Review article. OSS, Tunisia
Piper AM (1944) A graphical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Plummer LN, Jones BF, Truesdell AH (1976) WATEQF, a Fortan IV version of WATEQ, a computer program for calculating chemical equilibrium of natural waters. US Geol Surv Water Resour Invest 76:13
Qiyan F, Baoping H (2002) Hydrogeochemical simulation of water-rock interaction under water flood recovery in Renqiu Oilfield, Hebei Province, China. Chin J Geochem 21:156–162
Ragunath HM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol 52:1067–1074
Ravikumar P, Venkatesharaju K, Somashekar RK (2009) Major ion chemistry and hydrochemical studies of groundwater of Bangalore South Taluk, India. Environ Monit Assess 163:643–653
Ravikumar P, Somashekar RK, Angami M (2010) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ Monit Assess 173(1–4):459–487
Richards LA (1954) Diagnostics and improvement of saline and alkaline soils. U.S. Dept. of Agriculture hand book no. 60. U.S. Salinity Laboratory, Washington, DC
Risacher F, Alonso H, Salazar C (2003) The origin of brines and salts in Chilean salars: a hydrochemical review. Earth Sci Rev 63:249–293
Robbins J (2010) Irrigation water for greenhouses and nurseries. U.S. Dept. of Agriculture, and County Governments Cooperating no. 6061. Agriculture and Natural Resources, University of Arkansas, Division of Agriculture
Rose EF, Chaussidon M, France-Lanord C (2000) Fractionation of boron isotopes during erosion processes: the example of Himalayan rivers. Geochim Cosmochim Acta 64:397–408
Rosset R, Douville S, Ben Amor M, Walha K (1999) L’inhibition de l’entartrage par les eaux géothermales du sud tunisien. Étude sur site. Rev Sci Eau 12(4):753–764
Rouabhia A, Baali F, Fehdi Ch, Kherici N, Djabri L (2009) Hydrochemical and isotopic investigation of a sandstone aquifer groundwater in a semiarid region, El Ma El Abiod, Algeria. Environ Geol 57(8):1699–1705
Rouatbi R (1967) Contribution à l’étude hydrogéologique du Karst en terre de Gabès Sud. PhD thesis, Montpellier
Salesh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. Hydrogeol J 139:27–48
Sánchez-Martos F, Pulido-Bosch A, Molina-Sánchez L, Vallejos-Izquierdo A (2002) Identification of the origin of salinization in groundwater using minor ions (lower Andarax, southeast Spain). Sci Total Environ 297:43–58
Sawyer GN, McMcartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York
Schroeder HA (1960) Relations between hardness of water and death rates from certain chronic and degenerative diseases in the United States. J Chronic Dis 12:586–591
Scofield CS (1936). The salinity of irrigation water. Annual Report, Smithsonian Institute, pp 275–287
Srinivasa Gowd S (2005) Assessment of groundwater quality for drinking and irrigation purpose: a case study of Peddavanka watershed, Anantapur District, Andhra Pradesh, India. Environ Geol 48:702–712
Stuyfzand PJ (1989) Nonpoint source of trace element in potable groundwater in Netherland. In: Proceedings of the 18th TWSA Water Working, Testing and Research Institute. KIWA, Nieuwegein
Subba Rao N (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur District, Andhra Pradesh, India. Hydrol Sci J 48:835–847
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49:413–429
Subba Rao N, Gurunadha Rao VVS, Gupta CP (1998) Groundwater pollution due to discharge of industrial effluents in Venkatapuram area, Visakhapatnam, Andhra Pradesh, India. Environ Geol 33(4):289–294
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Tank DK, Chandel CPS (2009) A hydrochemical elucidation of the groundwater composition under domestic and irrigated land in Jaipur city. Environ Monit Assess. doi:10.1007/s10661-009-0985-7
Tayfur G, Kirer T, Baba A (2008) Groundwater quality and hydrogeochemical properties of Torbali Region, Izmir, Turkey. Environ Monit Assess 146:157–169
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Todd DK, Mays LW (2005) Groundwater hydrology, 3rd edn. Wiley, New York
Trabelsi R, Zaïri M, Smida H, Ben Dhia H (2005) Salinisation des nappes côtières: cas de la nappe nord du Sahel de Sfax, Tunisie. C R Acad Sci 337(5):515–524
Trabelsi R, Zairi Z, Ben Dhia H (2007) Groundwater salinization of the Sfax superficial aquifer, Tunisia. Hydrogeol J 15(7):1341–1355
Trabelsi R, Kacem A, Zouari K, Rozanski K (2009) Quantifying regional groundwater flow between Continental Intercalaire and Djeffara aquifers in southern Tunisia using isotope methods. Environ Geol 58(1):171–183
Trabelsi R, Abid K, Zouari K (2011) Geochemistry processes of the Djeffara palaeogroundwater (Southeastern Tunisia). Quat Int 3:1–13
Travi Y, Faye A (1992) Fluoride in Paleocene aquifers in Sengal. An example of the contamination of a confined aquifer by its roof zone, aggravated by intensive exploitation. In: Proceedings of XXIII IAH Congress on Aquifer Overexploitation of Canary Islands and Spain, pp 171–174
Umar R, Sami Ahmad M (2000) Groundwater quality in part of Central Ganga Basin, India. Environ Geol 39(6):673–678. doi:10.1007/s002540050480
Vengosh A, Kloppmann W, Marie A, Livshitz Y, Gutierrez A, Banna M, Guerrot C, Pankratov I, Ranan H (2005) Sources of salinity and boron in the Gaza Strip: natural contaminant flow in the southern Mediterranean Coastal aquifer. Water Resour Res 41:W01013. doi:10.1029/2004WR003344
Wen XH, Wu AEY, Andwu AEJ (2008) Hydrochemical characteristics of groundwater in the Zhangye Basin, northwestern China. Environ Geol 55(8):1713–1724
Wenzel WW, Blum WEH (1992) Fluoride speciation and mobility in fluoride contaminated soil and minerals. J Soil Sci 153:357–364
WHO (1993) Guidelines for drinking water quality, recommendations, vol 1, 2nd edn. WHO, Geneva
WHO (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. E&FN Spon, London
WHO (2004) Guidelines for drinking water quality: training pack. WHO, Geneva
Wilcox LV (1948) The quality of water for irrigation uses. U.S. Dept. Agri. Tech. Bull. 962. USDA, Washington, DC
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Circular 969, Washington, DC
World Health Organization (WHO) (1996) Guidelines for drinking water quality. Health criteria and other supporting information, vol 2, 2nd edn. WHO, Geneva
Yaouti FEl, Mandour AEl, Khattach D, Benavente J, Kaufmann O (2009) Salinization processes in the unconfined aquifer of Bou-Areg (NE Morocco): a geostatistical, geochemical, and tomographic study. Appl Geochem 24:16–31
Zhaoli S, Mi Z, Minggao T (1989) The characteristics of fluoride in groundwater of north China and significance of fluorite–water interaction to fluorine transportation. In: Proceedings of 6th international symposium on water rock interaction, Malvern (UK Balkema), pp 801–804
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Alaya, M.B., Saidi, S., Zemni, T. et al. Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ Earth Sci 71, 3387–3421 (2014). https://doi.org/10.1007/s12665-013-2729-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2729-9