Abstract
Streptococcus suis is a zoonotic agent that causes sepsis and meningitis in pigs and humans. S. suis infections are responsible for large economic losses in pig production. The lack of effective vaccines to prevent the disease has promoted the extensive use of antibiotics worldwide. This has been followed by the emergence of resistance against different classes of antibiotics. The rates of resistance to tetracyclines, lincosamides, and macrolides are extremely high, and resistance has spread worldwide. The genetic origin of S. suis resistance is multiple and includes the production of target-modifying and antibiotic-inactivating enzymes and mutations in antibiotic targets. S. suis genomes contain traits of horizontal gene transfer. Many mobile genetic elements carry a variety of genes that confer resistance to antibiotics as well as genes for autonomous DNA transfer and, thus, S. suis can rapidly acquire multiresistance. In addition, S. suis forms microcolonies on host tissues, which are associations of microorganisms that generate tolerance to antibiotics through a variety of mechanisms and favor the exchange of genetic material. Thus, alternatives to currently used antibiotics are highly demanded. A deep understanding of the mechanisms by which S. suis becomes resistant or tolerant to antibiotics may help to develop novel molecules or combinations of antimicrobials to fight these infections. Meanwhile, phage therapy and vaccination are promising alternative strategies, which could alleviate disease pressure and, thereby, antibiotic use.
Similar content being viewed by others
1 Introduction: the zoonotic pathogen Streptococcus suis
S. suis resides asymptomatically in the upper respiratory tract, i.e., the tonsils and the nasal cavities, the gut, and the genitals of pigs, as part of the normal microbiota. Piglets can be colonized via horizontal and vertical transmission, caused by nose-to-nose contact and nose-to-vagina contact during farrowing, respectively. The colonization rate can be up to 100%. However, S. suis can turn pathogenic when it penetrates mucosal barriers and accesses the bloodstream, joints, and the central nervous system, thereby causing a variety of symptoms such as bacteremia, endocarditis, arthritis, pneumonia, and sudden death [1]. The penetration of the epithelial mucosa and the evasion of innate immune defenses are essential steps for the invasion. For this, S. suis produces a large variety of virulence factors, including enzymes, such as proteases and DNases, and toxins, which all contribute to the evasion of the host immune system and to nutrient acquisition within the host [1]. For the invasion process, S. suis can additionally take advantage of the depression of mucosal immunity by respiratory viral infections, particularly by swine influenza virus and porcine reproductive and respiratory syndrome virus [2]. Thus, S. suis has been considered as a pathobiont [2].
The streptococcal swine disease resulting from S. suis infection is a major cause of mortality and economic losses in the pig production industry worldwide. It has been estimated that the incidence ranges ranged from 5 to 20%, but this largely varies between regions and farms. Notably, the disease is a leading cause of mortality in piglets aged 4–12 weeks, but it may also affect both younger and older pigs. About 70% of the cases where the infection reaches the nervous system end in death. Much of the economic losses are attributed to mortality, pig management and attempts to control infection, but the disease can also reduce weight gain and raise production costs.
Historically, antibiotics have been used to prevent S. suis cases, but this practice is nowadays prohibited in many countries. Also, vaccination is used to prevent infection, but its efficacy is limited. Only bacterins are applied in the field for immunization of piglets or sows. Bacterins are suspensions of whole killed bacteria prepared from invasive clones collected in certain farms. The protection provided by bacterins is strain specific and often unpredictable [3]. The main drawbacks associated with bacterins are: (i) high diversity of antigens produced by S. suis, (ii) antigenic variability of surface-exposed structures, and (iii) loss of the tertiary structure of many antigens during cell inactivation required for bacterin production. Therefore, bacterins are not universal and have limited effectiveness in preventing S. suis outbreaks. In this context, treatment of the disease is mainly based on antibiotic therapies combined with the use of bacterins to avoid the expansion of the disease, while prevention is limited to managing environmental conditions and, only in particular farms affected by certain clones, to the use of bacterins. The lack of an effective and universal vaccine formula to prevent or reduce the appearance of S. suis infections together with the high incidence and mortality of the infection has provoked the exhaustive use of antibiotics for a long time. In addition, S. suis is, as a commensal bacterium, exposed to antibiotics used for growth promotion, prophylaxis, and the treatment of other infectious diseases. All factors together have created a good scenario for the emergence of antimicrobial-resistant (AMR) S. suis isolates.
2 S. suis is a widespread superbug
Antibiotics used to treat S. suis infection are multiple, and they are from different classes, including β-lactams, aminoglycosides (usually combined with β-lactams), amphenicols, and fluoroquinolones. The pattern of antibiotic usage varies between countries, regions, and even farms, which largely influences the AMR profile of S. suis. AMR in S. suis was first reported around the 80 s of the previous century, and since then, the AMR rates increased over time globally. Figure 1 shows the historical development of AMR rates for different classes of antibiotics in S. suis isolates from Europe, Asia, and America, compiled by using publications and reports from the last 20 years. For simplicity, the rates of resistance were calculated for each class of antibiotics (including different antibiotics of the same family) even though, in some studies, resistance varied between different antibiotics of the same class. In Additional file 1, information regarding the methods used to measure antibiotic resistance and tolerance in S. suis is expanded.
Historical development of resistance of S. suis to different antibiotic classes in A Europe, B Asia, and C America (North and South America). The graphs include data reported between 1987 and 2021 in scientific articles published in National Library of Medicine under search criterium ¨antimicrobial resistance and Streptococcus suis¨, and the antibiotic surveillance data from Denmark (DANMAP) and France (RESAPATH). For panel A, a total of 19 articles and 18 reports (DANMAP, RESAPATH) were used, and totals of 13 and 8 articles were used for panels B and C, respectively. For simplicity, the averages of the percentage of resistance to antibiotics of the same class were calculated for each report. Please note that not all the surveillance systems are using the same Epidemiological cut-off value ECOFFs; we followed the criteria used by authors. The evolution of the multidrug-resistance rate worldwide is depicted in D, taking into consideration a total of 20 scientific articles. Multiresistance was considered when an isolate was resistant to at least three antibiotics of different classes as declared by authors. Also, the average of the antimicrobial-resistance rate of different reports in the same year was used. The X-axes show the year of isolation.
In Europe, the highest AMR rates were reported for lincosamides, macrolides, and tetracyclines, as is evident from numerous reports since 1998. Lincosamides, including lincomycin and clindamycin, together with streptogramin B and macrolides, often showed cross resistance due to their common mechanisms of action (discussed in the next section). They have been used largely in food production animals, particularly in intensive pig farming, to treat a variety of infectious diseases. This may explain the high resistance rates registered in S. suis isolates in last two decades (Figure 1A). For example, tylosin (Tylan) has been extensively used as growth promoter, and this could be related to high macrolide resistance rates observed. Also, notable differences were reported between countries over time. For example, macrolide (erythromycin)-resistance rates ranged from 29% in Norway in 1986 [4] to 56% in Denmark in 2020 [5], and lincosamide (lincomycin)-resistance levels were 40% in Denmark in 1995 [6], 61% in France in 2019 [7], and up to 87% in Spain in 2021 [8]. Together with lincosamides, tetracyclines were also among the most widely used antibiotics in the European pig industry because of their broad spectrum. As a consequence, many European countries reported tetracycline-resistance rates higher than 60% since the end of the 90 s, for example 68% in England in 2004 [9], 73% in France in 2011 [10], and 88% in Sweden in 2019 [11].
Because of the high resistance rates, the aforementioned antibiotics have been replaced by others, such as β-lactams of which the resistance rates have persisted low over time (Figure 1A). For example, resistance rates of less than 10% to β-lactams were reported in Denmark (1995) [6], Spain (2000) [12], Poland (2004) [9], The Netherlands (2014) [13], and, more recently, in Czech Republic (2020) [14]. These data indicate that the development of β-lactam resistance is rather modest in S. suis. Also, quinolones have been used as an alternative to tetracyclines. Reports indicate resistance rates of less than 10% in the last two decades in several European countries but with few exceptions (Figure 1A). Good examples are Belgium (2000) [15], The Netherlands (2014) [13], and Sweden (2019) [11], where the resistance rates remained well below 10% in this period. However, the high rates reported in France (18% in 2008) [16] and Spain (47% in 2020) [8] suggest that fluoroquinolone resistance may become problematic in the future. Other antibiotics used to treat S. suis infections in Europe were aminoglycosides (often combined with β-lactams), amphenicols, sulfonamides, and pleuromutilins. Reports have notified low or medium AMR rates. For example, aminoglycoside-resistance rates lower than 10% were continuously reported in France in 2002 [17], 2009 [18], 2014 [19], and 2017 [20]), but high rates were reported in Denmark in 2016 (up to 41%) [21], 2018 (up to 36%) [22], and in 2020 (44%) [5]. Sulfonamide-resistance rates showed large variation between countries in the same time period, ranging from 3% in The Netherlands in 2016 [13] to 21% in France in 2017 [20] or up to 94% (sulfadimethoxine) in Spain in 2021 [8]. For amphenicols, AMR rates remained very low in the last two decades, e.g. in Belgium (2013) [23], Denmark (2016) [21], and Czech Republic (2021) [14]. In contrast, AMR rates to pleuromutilins have been moderate, showing an increasing trend in recent years, with the highest reported rates in Denmark (24% in 2017) [24] and Czech Republic (31% in 2021) [14]. Overall, AMR rates to most antibiotics vary considerably between European countries. This could be related to differences in pig production, growth systems, or the political regulations concerning the usage of antibiotics. For instance, The Netherlands and France were big antibiotic consumers, followed by the United Kingdom, Czech Republic, Switzerland, Germany, and Denmark. In contrast, antibiotics were less frequently used in Finland, Sweden, and Norway, due to the less intensive pig industry [25].
In Asia, the AMR rates for tetracyclines, lincosamides, and macrolides are extremely high (Figure 1B), even higher than in Europe (Figure 1A). In general, resistance to tetracyclines is around 95%, showing little variation over the last two decades. Examples are 92% in China in 2005–2007 [26], 98% in Korea in 2010 [27], or up to 92% in Thailand in 2019 [28]. Notably, tetracycline-resistant S. suis isolates were common in both diseased and healthy pigs [26] as well as in diseased humans [29]. Many reports revealed increasing rates of resistance to lincosamides and macrolides since 2012. This is illustrated in reports from China notifying rates of resistance for macrolides in S. suis from up to 35% in isolates from the period 2005–2010 [30], to 68% in isolates from 2008 to 2010 [31], 87% in isolates from 2013 [32], and up to 97% in isolates from 2017 [33]. A concomitant increase in the rates of resistance to lincosamides was reported from 39% in the period 2005–2012 [34], to 98% in 2008–2010 [35], 96% in 2015 [32], and up to 100% in 2021 [36]. Recent studies in other Asian countries, such as Thailand (2019) [28] and India (2021) [37], notified similar AMR profiles. AMR rates for sulfonamides, aminoglycosides, and fluoroquinolones, which were moderate in Europe, ranged from medium to high in Asia (compare panels A and B in Figure 1). The rates varied between countries, but, in general, there was a notable increase since 2010 (Figure 1B). Thus, the rates of resistance for sulfonamides (trimethoprim/sulfamethoxazole) in earlier reports were 0% in Japan (isolates from 1987 to 1996) [38] or 16% in China (isolates from 2005 to 2012) [34], and they were higher after 2010, for instance 60% in Thailand in 2019 [28]. The resistance rates for aminoglycosides are higher than for sulfonamides with, e.g., reported rates in China ranging from 62% in 2012 [35] to 87% in 2019 [32], indicating an increasing trend. The reported resistance rates for fluoroquinolones remained below 40%, but they are also increasing, e.g., China showed 6% resistance in 2015 [34] and up to 37% in 2021 [39]. β-Lactams still showed the lowest resistance rates, although they were higher than in Europe, reaching values still below 15% for instance in China in 2014 [30] and in 2019 [32]. Yet, large differences between various β-lactam antibiotics were detected. In a study in Thailand (2019), AMR rates were found of around 20% for penicillin and ampicillin, 5% for cefotaxime and ceftiofur, and up to 35% for cephalexin [28]. For amphenicols, low resistance rates were reported, e.g., of 4% in China in 2015 [30] and in 2020 [33]. Curiously, a notable drop in resistance rates for aminoglycosides and fluoroquinolones was observed from 2018 on (Figure 1B). Altogether, these data show, in general, higher AMR rates in Asia than in Europe. This could be due to the massive use of antibiotics as growth promoters and the lack of regulation for the sales of antibiotics, which might have generated a selective pressure for resistance. Both practices also occurred in Europe, but to a lesser extent. For example, the use of antibiotics as growth promoters was banned at an early stage. Sweden and Denmark were the first countries that banished the use of antibiotics as growth promoters in 1986 and 2000, respectively, and this was followed by a general prohibition in the whole European Union (EU) in 2006. However, whereas, for example, Japan, Taiwan, and Hong Kong have similar restrictions, most Asian countries still use antimicrobials for this purpose with exceptions only for some specific antibiotics [40]. Few reports from Oceania revealed overall low resistance rates for β-lactams, amphenicols, fluoroquinolones, and sulfonamides, moderate rates for macrolides, and the highest rates for tetracyclines (almost 100%), with some differences between reports [41, 42]. In general, for most antibiotics, AMR rates were considerably lower in Oceania than in Asia.
In North America, AMR rates for tetracyclines, lincosamides, and macrolides were the highest, being > 80% for tetracyclines and > 50% for aminoglycosides and lincosamides in 2007. An example is Canada reporting rates of 89% and 97% for tetracyclines in S. suis isolates from 2005 [43] and 2019 [44], respectively, of 77% and 90% for macrolides (erythromycin) in isolates from 2007 to 2001 [45] and from 2013 to 2018 [46], respectively, and of 96% for lincosamides (clindamycin) in isolates from 2013 to 2018 [46]. In contrast, resistance rates for other antibiotic classes (β-lactams and amphenicols) have remained < 10% up to date (Figure 1C) [45, 47], while, in general, moderate rates (up to 25%) were reported for sulfonamides and pleuromutilins during the last two decades (Figure 1C) [43, 46]. Few data have been reported about aminoglycosides and fluoroquinolones, whose AMR rates varied between the different antibiotic types [48] over time [45, 48]. Very little data have been reported in South America. Low-medium AMR rates for β-lactams (up to 18%) and amphenicols (up to 14%) were reported, while higher rates for fluoroquinolones (up to 77%), aminoglycosides (up to 50%), sulfonamides (up to 100%), lincosamides (85%), macrolides (up to 66%), and tetracyclines (98%) were described in Brazil [49, 50].
Altogether, resistance against antibiotics in S. suis is a worldwide problem and, particularly, resistance rates are very high for tetracyclines, macrolides, and lincosamides. In contrast, resistance rates for β-lactams, fluoroquinolones, and amphenicols remain low, but recent reports suggest an increasing trend. This has been associated with the occurrence of multidrug resistance, i.e., simultaneous resistance to antimicrobials of at least three different classes, which increased over time (Figure 1D). Alarmingly, multidrug-resistant isolates have been recovered from diseased humans as reported by Hoa et al. [51], who showed multidrug resistance to tetracycline, erythromycin, and chloramphenicol in S. suis-infected patients and a significant increase in multidrug resistance from 6% in 1999 to 23% in 2006 and 2007. These data point out that S. suis is a globally disseminated bacterial ¨superbug¨.
3 Genetic basis of S. suis resistance to antibiotics
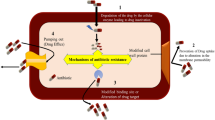
The mode of action of the above-mentioned antibiotics is class-dependent, as schematized in Figure 2. Overall, antibiotics obstruct vital biological processes, such as cell-wall synthesis, protein synthesis, or DNA replication, mainly by interfering with the activity of specific enzymes. Different classes of antibiotics can share similar targets but make use of different mechanisms. To counteract antibiotic activity, S. suis has developed various mechanisms of resistance that generally fall into four categories: (i) target mutation, often preventing the binding of an antibiotic to its target, (ii) enzymatic target modification, (iii) antibiotic modification, comprising degradation of the antibiotic or its modification, thereby preventing target binding, and (iv) export of antibiotics out of the cell by increased production of efflux pumps. AMR genes of S. suis and resistance and tolerance mechanisms have been reviewed previously [29, 52, 53]; here, we have updated the AMR list in Table 1. Often, accumulation of different genes and mechanisms enhance resistance. Below, the mode of action of the antibiotics used to treat S. suis infections and the reported resistance and tolerance mechanisms are discussed.
Overview of the mode of action of antibiotics used to treat S. suis infections. According to its target site, antibiotics can be classified as A inhibitors of peptidoglycan (PG) synthesis by blocking penicillin-binding proteins (PBPs) (β-lactams) or binding D-Ala-D-Ala in the peptide chain of the PG precursor lipid II (glycopeptides), B inhibitors of ribosome function that bind to the ribosome 50S (macrolides, amphenicols, lincosamides, and pleuromutilins) or 30S (tetracyclines and aminoglycosides) subunits, C inhibitors of folic acid synthesis including sulfonamides and trimethoprim, and D inhibitors of DNA transcription and replication by interfering with DNA gyrase or topoisomerase IV (quinolones). In A, the process of the synthesis and transport of PG precursors is indicated. The 30S and 50S ribosome subunits and the A, P, and E sites are indicated in B. The A site is the binding site for charged tRNA molecules during protein synthesis. Then, the tRNA moves to the P site and its cargo is then linked to the growing polypeptide chain. Thereafter, the tRNA is moved to the E site for exit. The route for folate synthesis is shown in C. In D, the requirement for DNA gyrase and topoisomerase IV in DNA transcription and replication processes is shown. Antibiotics are indicated with colored stars. A red circle indicates resulting inhibition of the cellular process. Abbreviations: PABA, p-aminobenzoic acid; pteridine, 7,8-dihydro-6-hydroxymethylpterin-pyrophosphate; UDP, uridine diphosphate.
3.1 Resistance to β-lactams
β-lactams comprise several large families of antibiotics, including penicillins. Penicillins share a thiazolidine ring attached to a β-lactam ring and a side chain (Figure 3). There are five classes of penicillins, including natural penicillins (penicillin G and V), penicillinase-resistant penicillins (methicillin), aminopenicillins (ampicillin), extended-spectrum penicillins (carbenicillin), and aminopenicillin/β-lactamase inhibitor combinations (amoxicillin/clavulanic acid). In addition, cephalosporins, carbapenems, and monobactams have a β-lactam ring and, therefore, they are also classified as β-lactams. Amoxicillin and other penicillins are nowadays broadly used to treat S. suis infections. β-Lactams blocks the activity of enzymes involved in cell-wall synthesis by binding of the β-lactam ring to the active site of the penicillin-binding proteins (PBPs) (Figure 2A) at the DD-transpeptidase domain [54]. As a result, new peptidoglycan (PG) precursors can be incorporated into the PG, as the antibiotics inhibit the transpeptidation reaction that establishes crosslinks between the PG polymers (Figure 2A), thus preventing the formation of a rigid cell wall and resulting in cell death. S. suis, as other streptococci, is generally susceptible to β-lactam antibiotics (Figure 1A–C).
There are three main mechanisms of resistance to β-lactam antibiotics comprising (i) reduced access to PBPs, (ii) reduced PBP binding affinity, and (iii) destruction of the antibiotic through β-lactamases [55]. In Gram-positive bacteria, loss of β-lactam sensitivity is mostly driven by alterations in the transpeptidase domains of PBPs that decrease the affinity of the enzyme for β-lactams. There are several pbp genes in streptococci [56], coding for PBP1a, PBP1b, PBP2a, PBP2b, PBP2x, and PBP3. Three of them are critical for β-lactam susceptibility or resistance, i.e., PBP1a, PBP2b, and PBP2x. This matches with the essential role of some of these enzymes. PBP2b and PBP2x are members of elongosome and the divisome complex, respectively, and are essential for bacterial survival. PBP1a, PBP1b and PBP2a are redundant, and they can be depleted individually. PBP3 and PBP1a are dispensable, but their depletion causes morphological changes and loss of fitness. This is also the case with the double mutants pbp1a/pbp1b and pbp1b/pbp2a, but, in contrast, a mutant lacking pbp1a and pbp2a is not viable. As PBP1a, PBP2b, and PBP2x are key targets for β-lactam antibiotics [56], they are often examined to assess the genetic origin of resistance to these drugs. Resistance to β-lactams in streptococci is primarily caused by mutations within the pbp2x and pbp2b genes [57]. These mutations confer a modest level of resistance, while they can confer high-level resistance in combination with mutations within pbp1a [58, 59]. Interestingly, a sequential order of mutations has been observed in several streptococcal species. In S. pneumoniae, mutations within pbp2x and/or pbp2b, depending on the specific antibiotic used, are the first event to occur [60], and they are followed by mutations in pbp1a. It has been proposed that the secondary mutations, besides increasing antibiotic resistance, are selected to compensate for a fitness cost resulting from the pbp2b mutations [61]. Recent work in S. suis exploring the origin of β-lactam resistance also reached similar conclusions [62]. Mutations occur first in pbp2b and then in pbp2x (Table 1). Analysis of β-lactam-resistant mutants of S. pneumoniae selected in the laboratory revealed that point mutations in pbp genes suffice to confer β-lactam resistance, while analysis of resistant clinical isolates showed a mosaic of sequences that may diverge in about 20% compared to susceptible isolates (reviewed in Hakenbeck et al. [63]). It is entirely possible that the resistance alleles contain many substitutions that are compensatory mutations that alleviate the fitness costs of the resistance mutations. Remarkably, a study exploring 2528 invasive S. pneumoniae isolates related β-lactam resistance levels to sequence signatures in the pbp1a, pbp2b, and pbp2x genes [64], which has generated a classification system of PBP types for pneumococci. Thus, PBP types identified based on pbp gene sequences are related to particular Minimal Inhibitory Concentrations (further described in Additional file 1). Such a system has not yet been proposed for S. suis, but it could help to classify the increasing β-lactam-resistant isolates (Figure 1A–C).
Apart from mutations in pbp1a, pbp2b, and pbp2x, mutations in pbp1b, pbp2a, and pbp3 have been described in 10 β-lactam-resistant S. suis isolates [65]. As the corresponding PBPs are no key targets for β-lactam antibiotics, the contribution of these mutations to β-lactam resistance remains to be clarified. Presumably, they contribute to compensate for the fitness cost of mutations within pbp1a, pbp2x, and/or pbp2b. In addition, mutations in the mraY gene, which codes for the enzyme that transfers N-acetylmuramyl-pentapeptide-1-phosphate to undecaprenyl phosphate to generate the PG precursor lipid I, have been detected in 62 penicillin-resistant S. suis isolates [62] (Table 1). As MraY is not a target for the β-lactams, probably also these mutations are compensatory mutations. Besides, mutations in the two-component regulatory system CiaRH [66], the glycosyltransferase CpoA [67], and the cell-wall muropeptide-branching enzyme MurM [68] confer or increase resistance to different β-lactams in S. pneumoniae. Some of them, i.e., mutations affecting CiaRH and CpoA, occur independently of pbp mutations. The CiaRH system is constituted of the histidine kinase CiaH and the response regulator CiaR. Recognition of an external stimulus by the surface-exposed region of CiaH triggers CiaH autophosphorylation, and the phosphoryl group is then transferred to CiaR. Phosphorylation of CiaR activates gene expression by interacting with the regulatory regions of the target genes. In streptococci, CiaRH negatively regulates competence development and expression of virulence factors, and it triggers biofilm formation [69]. CiaRH also controls the level of the lipid carrier for the transport of PG precursors. Certain mutations in CiaH enhance phosphorylation of CiaR, resulting in more lipid-linked murein precursors that counteract cell damage caused by the antibiotics. Mutations in CiaH have not been reported in β-lactam-resistant S. suis isolates yet, but the CiaRH system is highly conserved in streptococci [69]. CpoA is a glycosyltransferase that transfers a galactose residue to monoglucosyl-diacylglycerol, which is the lipid anchor for lipoteichoic acids [70]. Point mutations in cpoA might increase the production of lipoteichoic acids that could counteract the damage caused by β-lactams on the cell wall. Streptococcal PG can be heterogeneously composed of branched and unbranched subunits that generate indirect and direct cross-linking bridges in the cell wall, respectively. Penicillin-resistant S. pneumoniae strains contain higher levels of indirect crosslinking in the cell wall compared with penicillin-susceptible isolates [71]. Probably, alterations in PBPs that result in antibiotic resistance may alter the specificity of PBPs for branched lipid II over unbranched lipid II. MurN and MurM are part of the biosynthetic route for branched PG precursors. Both are non-essential aminoacyl-tRNA-dependent ligases that add amino acids to the lysine in the third-position of the pentapeptide stem of lipid II [72]. Particularly, MurM can attach either L-serine or L-alanine at the first position of the dipeptide bridge. MurM from penicillin-resistant S. pneumoniae preferentially incorporates L-alanine, while MurM from penicillin-sensitive strains preferentially incorporates L-serine. Deletion of the murM gene in penicillin-resistant pneumococci depletes indirect crosslinks from the PG and results in the loss of penicillin resistance [73]. Thus, MurM is necessary for high levels of resistance but not sufficient. Also, mutations within murM enhance antibiotic resistance [68]. This might result in new species of branched peptides with superior binding to mutated PBPs. As the cpoA and murM genes are present in S. suis genomes, mutations in these genes could possibly also contribute to β-lactam resistance, but, so far, they have not been reported yet.
3.2 Resistance to the macrolide, lincosamide, and streptogramin (MLS) group
MLS antibiotics exert their antimicrobial action by binding to the 23S rRNA of the 50S subunit of the bacterial ribosome causing dissociation of peptidyl-tRNAs (Figure 2B). Consequently, MLS block translation. This group is composed of three chemically different molecules.
Macrolides consist of a macrocyclic lactone ring of 12, 14, 15, or 16 atoms (see erythromycin in Figure 3). The ring is linked to side chains, including specific sugar residues, which are varying between macrolide types. Macrolides block ribosome function by binding to the exit tunnel through which the nascent proteins leave the ribosome. The bound macrolide stalls the ribosome when it needs to polymerize specific amino-acid sequences generally called macrolide arrest motifs (MAMs) [74]. Thus, when a protein lacks MAMs, it can be synthesized in the presence of macrolides. MAM sequences vary for each macrolide type, which influences its antibiotic activity. The broader the variety of MAMs a macrolide recognizes, the higher the chance it stops translation. Also, the macrolide structure impacts the affinity and dynamics of drug-ribosome interaction, which correlates with the bacteriostatic or bactericidal activity of each macrolide.
There are three lincosamides approved for veterinary use: lincomycin, pirlimycin, and clindamycin. Lincomycin consists of a trans-N-methyl-4-n-L-proline linked via a peptide bond with 6-amino-6,8-dideoxy-1-thio-D-erythro-α-D-galactopyranoside (Figure 3). It is dedicated to use in companion animals, and it can also be applied to pigs, particularly in the case of infectious arthritis such as caused by S. suis. In contrast to macrolides, lincosamides directly inhibit the peptidyl transferase center of the ribosome.
Streptogramins include two different classes of molecules, called type A and type B. Type A streptogramins are polyketide-amino acid hybrids linked to the N-terminal side of an oxazole ring derived from serine. They inhibit protein synthesis by blocking substrate attachment to both the A and P sites of the ribosome (Figure 2B). Type B streptogramins are peptides cyclized through an ester bond between the carboxyl group of the C-terminal phenylglycine and the hydroxyl group of a threonine residue. They inhibit peptide bond formation during elongation by causing incorrect positioning of the peptidyl tRNA at the P site of the ribosome, particularly upon incorporation of proline or basic amino acids.
Resistance to MLS antibiotics is generated by various mechanisms. One of the most widespread mechanisms is methylation of the ribosome at the macrolide-binding site, i.e., the adenine at position 2058 (A2058) located in the variable region of the 23S rRNA in E. coli, by erythromycin-resistance methyltransferases encoded by erm genes. Erm enzymes add one or two methyl groups to the N6 amino group of A2058, thereby preventing the formation of a key hydrogen bond between A2058 and the desosamine sugar at C5 of the macrolide. The acquisition of MLS resistance by ribosome methylation reduces bacterial fitness [75]. However, methylation can be regulated translationally or transcriptionally. About 40 erm genes have been reported, which are grouped in 14 erm classes. The classes found in S. suis are listed in Table 1. The erm(B) class is the most common macrolide-resistance determinant in S. suis [76], while the erm(G) [77] and erm(T) classes [78] are less frequently found [62]. Indeed, ribosome methylation by Erm(B) confers a high level of resistance not only to macrolides but also to lincosamides and streptogramin B. This phenotype is called a macrolide-lincosamide-streptogramin B resistance phenotype (often referred to as MLS(B)). In contrast to other methylases that require the presence of specific drugs for induction of their synthesis, the synthesis of this methylase is constitutive, which leads to the development of resistance to all MLS(B) drugs. Cfr is a methyltransferase that methylates nucleotide A2503 of the 23S rRNA causing combined resistance to MLS, amphenicols, and pleuromutilins (known as PhLOPSA phenotype) [79]. The A2503 residue is present in the overlapping binding sites of the mentioned antibiotics, and its methylation interferes with the position and binding of the drugs. Thus, Cfr prevents the binding of antibiotics to the ribosome. The cfr gene has been found in various Gram-positive species, such as Staphylococcus aureus [80], Bacillus spp. [81], and Enterococcus spp. [82], where it is located on plasmids or on the chromosome. The cfr gene was first identified in a florfenicol-resistant S. suis isolate from a healthy pig in China in a routine surveillance study [83]. In S. suis strain 10, it was found to be present on a 100-kb plasmid, where it was flanked by two copies of the insertion sequence ISEnfa5 [84], probably contributing to its dissemination.
Drug-inactivating enzymes can also mediate resistance to various MLS antibiotics. Examples are the phosphorylation of the 2′-hydroxyl group of the amino sugar and hydrolysis of the macrocyclic lactone exerted by phosphotransferases and esterases, respectively, or the chemical modification of lincosamide with either phosphate or adenylate groups. Lincosamide nucleotidyltransferases inactivate only lincosamides. They comprise members of the lnu (previously lin) gene family, of which different types were identified, i.e., lnu(A), lnu(B), lnu(C), lnu(D), lnu(E), and lnu(F), most of which have also been identified in S. suis isolates (Table 1).
Besides their modification, also their active export can confer resistance to various MLS antibiotics in S. suis (Table 1). However, in contrast to resistance mediated by target-modifying enzymes, the resistance conferred by specific pumps is often specific for each antibiotic type. An example is the Mef(E)/Mel system that confers resistance to macrolides. Mef(E) is a protein of 405 amino-acid residues that belongs to the major facilitator superfamily (MFS) and that expels macrolides from cells by using the proton-motive force as the energy source. Mel, a.k.a. Msr(D), is a homolog of ATP-binding cassette (ABC) transporter proteins but misses membrane-spanning domains [85]. These proteins are called ABC-F proteins and contain two ATP-binding cassettes separated by a linker of about 80 amino-acid residues. Presumably, Mel displaces ribosome-bound macrolides after which it may transfer them to Mef(E) for efflux [86]. As such, Mef(E) and Mel operate as a two-component efflux pump [85, 87]. Several Mef(E) variants have been described, i.e. Mef(A) and Mef(I). They share around 90% of sequence identity with Mef(E) and, therefore, they are not always distinguished in the literature. In S. suis, mef(A) and/or mef(E) have been identified [88]. Lincosamide resistance can be conferred by the ABC-F proteins of the Vga and Lsa families, but, unlike in the case of Mel, there are no analogues of Mef(E) protein involved. Thus, Vga and Lsa could primarily function as ribosome-protection proteins (RPP). Genes encoding the ABC-F proteins Lsa(E) and Vga(F), which confer resistance to lincosamides, streptogramins A, and pleuromutilins, have been detected in S. suis [89]. Finally, mutations affecting ribosomal components could potentially also confer MLS(B) resistance in S. suis. Substitutions of A2058, A2059, and C2611 in the 23S rRNA (nucleotide numbering according to E. coli) [90] and mutations in ribosomal proteins L4 and L22 generate macrolide resistance. L4 and L22 are ribosomal proteins with domains on the surface of the ribosome and at the exit tunnel near the macrolide-binding site [91]. Such substitutions in the 23 rRNA and in the L4 and L22 proteins have been described in S. pneumoniae [92], but not yet in S. suis.
3.3 Resistance to amphenicols and pleuromutilins
Like MLS antibiotics, amphenicols and pleuromutilins bind to the 23S rRNA of the 50S subunit of the bacterial ribosome, thereby blocking translation (Figure 2B). They are chemically different from MLS (see examples in Figure 3) but, nevertheless, they share overlapping binding sites.
Amphenicols comprise a series of molecules with a monocyclic core. The first molecule of this class was chloramphenicol isolated from Streptomyces venezuelae. However, chloramphenicol produced serious side effects and, therefore, it was replaced by synthetic analogues. Thiamphenicol is a chloramphenicol derivative that contains a methylsulfonyl group instead of a p-nitro group. Florfenicol is a fluorinated derivative of thiamphenicol with a fluorine group replacing a hydroxyl group at C3 (Figure 3). Florfenicol is approved for use in food production animals and has been used to treat S. suis infections. Pleuromutilins contain a diterpene structure. The first antimicrobial pleuromutilin was isolated from Pleurotus mutilus [93]. Later, synthetic analogues were produced, including tiamulin and valnemulin, which were approved exclusively for veterinary use in food production animals [94]. Tiamulin (Figure 3) is broadly used to treat S. suis.
As for MLS antibiotics, amphenicol and pleuromutilin resistance can be acquired by several routes, including target-site modification, enzymatic inactivation of the antibiotics, and active efflux (Table 1). Some of these mechanisms are common to amphenicols, MLS, and/or pleuromutilins, because of their similar mechanism of action (Table 1). For example, the substitution of guanine at position 2032 in the 23S rRNA confers resistance to chloramphenicol, clindamycin, and pleuromutilins, and the substitution of guanine at position 2576 confers resistance to chloramphenicol and clindamycin (reviewed in Schwarz et al. [95]).
Production of chloramphenicol O-acetyltransferases (Cats) inactivates amphenicols by transferring an acetyl group from acetyl-S-coenzyme A to the C3 or C1 positions of the amphenicol molecule generating mono- or di-acetylated derivatives, which lack antimicrobial activity [96]. However, they can not inactivate florfenicol, due to the presence of a fluorine instead of a hydroxyl group at the C3 position (Figure 3). There are two types of Cats, called CatA and CatB. CatA can be further classified into 22 different groups based on their percentage of indentity; they are broadly distributed in Gram-positive and Gram-negative bacteria. CatB can be classified in five subtypes; they are related to acetyltransferases involved in streptogramin A resistance. As compared to CatA enzymes, CatB confers lower MICs. CatA-encoding genes have been described in S. suis [62, 97], but their prevalence is rather low. This could be explained by the inactivity of these enzymes against florfenicol or by the prohibition of the use of chloramphenicol for food-production animals because of toxicity issues. This catA gene and an upstream-located optrA gene, another amphenicol-resistance determinant, are flanked by two IS1216 elements, allowing their co-mobilization [97]. Pleuromutilin-inactivating enzymes have not been described so far.
Amphenicols and pleuromutilins can be expelled from the bacterial cells using broad-spectrum exporters or specific transporters. A specific amphenicol exporter described in Gram-positive bacteria is FexA. FexA is an MFS exporter. Its expression is inducible with chloramphenicol or florfenicol, and it confers resistance to both antibiotics. The fexA gene was first found in a florfenicol-resistant S. suis isolate, located on a ∼100-kb plasmid, designated pStrcfr, together with a cfr gene [84]. However, its distribution and activity are controversial. In most of the studies analyzing AMR determinants in S. suis, fexA is not reported. For example, in a recent screening, 14% of 148 Australian S. suis isolates showed florfenicol resistance, but only one carried a fexA gene. In contrast, in a recent study in Spain, a fexA gene was found in 25% of the tested isolates but, curiously, it was not related to florfenicol resistance [8]. A possible explanation is that the detected fexA is a non-functional variant. In fact, a fexA variant that confers resistance only to chloramphenicol was detected in a canine S. pseudintermedius isolate [98]. This is caused by two mutations, Gly33Ala and Ala37Val, both of which are critical for substrate recognition and, therefore, the encoded transporter is inactive for florfenicol. This new variant is not distinguished by PCR from the wild type, which could explain the lack of association between the presence of fexA and florfenicol resistance in the Spanish isolates. However, considering that chloramphenicol is prohibited for use in Spain since more than a decade ago, this new fexA variant, which appears to be widespread within recent S. suis isolates, might be associated with the export of other AMR determinants. Thus, the role of fexA in amphenicol resistance in S. suis remains unclear. Also, the ABC-F RPP variants Lsa(E) and Vga(F) confer resistance to pleuromutilins [99], and both have been detected in S. suis [89].
In recent years, optrA has emerged as a determinant of resistance to amphenicols and oxazolidinones, a class of antibiotics that inhibit translation by binding the ribomome P site. Initial studies characterized OptrA as an ABC transporter [100], but later work showed that it is an RPP protein of the ABC-F family [86]. The optrA gene was frequently detected in S. suis isolates from China with a prevalence ranging from 11% [33, 97] to 38% [83]. An initial genetic analysis of publicly accessible S. suis genome sequences revealed that optrA was flanked downstream by an IS1216E element in five out of six genomes. In one genome, it was located together with several resistance genes on a genetic segment flanked on either side by IS1216E, and in two genomes, it was located within pathogenicity islands and conjugative elements (described in Sect. 4). In the remaining genomes, the optrA gene was integrated in a large prophage genome [101]. A later study also located an optrA gene on a 40-kb plasmid [33]. Hence, these analyses suggest that optrA was acquired by horizontal gene transfer (HGT), probably from Enterococcus [100], and spread further among S. suis via mobile genetic elements (MGE, described in next section). A cfr gene (discussed above) also confers resistance to amphenicols and pleuromutilins and, as optrA, it was found in MGEs [84]. Its prevalence is rather low (< 1%) as compared with optrA (38%) [83], suggesting that optrA is a more relevant determinant for AMR in S. suis.
3.4 Resistance to tetracyclines
Tetracyclines comprise a family of broad-spectrum antibiotics with activity against Gram-positive and Gram-negative bacteria. Their structures share four rings linked to various substituents including amine, chloride, or hydroxyl groups (see doxycycline in Figure 3). Tetracyclines bind to the 16S rRNA in the 30S ribosomal subunit (Figure 2B), arresting translation by sterically interfering with the docking of aminoacyl-tRNA during elongation [102]. Thus, as MLS, amphenicols, and pleuromutilins, tetracyclines also inhibit translation, but their binding site is different. Uptake of tetracycline into the cytoplasm can be mediated by passive diffusion or active transport.
Resistance to tetracyclines can be attributed to different mechanisms, including (i) active drug export, (ii) ribosome protection, and (iii) drug inactivation. Target-site modifications have, so far, only been reported in other bacteria. For example, the substitutions C1054T and T1062G/A in the 16S rRNA conferred resistance to tigecycline in S. pneumoniae, and the resistance level was incremental with the number of the four genomic copies for the 16S rRNA that acquired the mutations [103]. Besides, mutations in genes for ribosomal proteins can result in tetracycline resistance. For example, mutations in the rpsJ gene resulting in substitutions or deletions within residues 53–60 of the 30S ribosomal subunit protein S10 conferred tetracycline and tigecycline resistance. Also, mutations in the rpsC gene resulting in Lys4Arg and His157Asp substitutions in ribosomal protein S3 were associated with reduced tigecycline susceptibility in S. pneumoniae [103].
Apart from target-site mutations, protection to tetracyclines can be generated by RPPs of the ABC-F family. These are GTPases that release the bound tetracycline from the ribosome. RPPs have structural similarity to elongation factors EF-G and EF-Tu. Conformational changes induced by RPPs promote the formation of the EF-Tu-GTP-aminoacyl-tRNA ternary complex, which allows translation to proceed in the presence of tetracycline [104]. There are currently 12 reported RPP genes; some of them can protect against multiple drugs, such as tetracycline, minocycline, and doxycycline, while others are more specific and do not inhibit the activity of some tetracyclines, such as tigecycline and other glycylcyclines. The best characterized RPPs are Tet(O) and Tet(M), which share about 75% of sequence similarity. The corresponding genes are frequently identified in tetracycline-resistant S. suis isolates worldwide [8, 39], and new variants were recently identified [62]. Interestingly, autoinducer 2 (AI-2), which stimulates gene expression for biofilm formation and increases growth rates, upregulates the expression of Tet(M) in S. suis [105]. Other RPPs are those encoded by tet(S), tet(44) and tet(W), which were identified in S. suis isolates from Asia [51], America [62] and United kingdom [62].
The most common tetracycline-specific efflux pumps are MFS transporters [106]. These pumps extrude tetracyclines from the cells at the expense of the proton-motive force and are classified in seven different groups based on amino-acid sequence similarities and the number of transmembrane segments [107]. Clinically, the most prevalent pumps are members of either group 1 or group 2. The group 2 pumps are present in Gram-positive bacteria and include Tet(K) and Tet(L); both of them, together with tet(B), and tet(40), were identified in S. suis (Table 1).
3.5 Resistance to sulfonamides and trimethoprim
Sulfonamides are polar molecules which contain a sulfonyl group connected to an amine group. More than 5000 derivatives have been developed, of which sulfathiazole (Figure 3), sulfamethazine, and sulfadiazine are the main ones used in veterinary medicine. Sulfonamides act as inhibitors of the enzyme dihydropteroate synthase. This enzyme catalyzes the conversion of p-aminobenzoic acid and 7,8-dihydro-6-hydroxymethylpterin-pyrophosphate into dihydropteroate, a precursor in the folate synthesis pathway (Figure 2C). Folates are important cofactors required to produce amino acids and nucleotides. Thus, as this is an essential process for growing bacteria, sulfonamides have a broad spectrum of activity against Gram-positive and Gram-negative microorganisms.
To the best of our knowledge, the mechanisms of resistance to sulfonamides have not been described so far in S. suis. In other pathogenic streptococci, the main mechanism of resistance correlates with mutations in conserved regions of folP, the gene that codes for dihydropteroate synthase. Specific alterations in the amino-acid sequence of the enzyme result in the loss of affinity for sulfonamides [108]. These mutations seem to occur spontaneously at various positions within the gene. For example, spontaneous sulfonamide-resistant S. pneumoniae mutants obtained in laboratory contained a 6-nucleotide duplication resulting in alteration of the tertiary structure of the enzyme [109], whereas several clinical resistant isolates also contained oligonucleotide duplications at different positions [110] or single nucleotide substitutions resulting in Ile100Leu or Glu20Asp substitutions in the enzyme [111]. Diverse studies on clinical sulfonamide-resistant Streptococcus pyogenes and Streptococcus mutans isolates reported a variety of mutations in the folP gene [112]. An alternative mechanism is tandem gene duplication. Studies in Streptococcus agalactiae isolates revealed a fourfold tandem amplification of a chromosomal DNA fragment carrying all five genes required for dihydrofolate biosynthesis including folP, which led to sulfonamide resistance [113]. Interestingly, trimethoprim, is a competitive inhibitor of dihydrofolate reductase (encoded by dhfr gene) that is a part of the folate production pathway (Figure 2). However, trimethoprim is a 2, 4-diamino-5–3´-trimethoxybenzyl pyrimidine (Figure 3) that belongs to diaminopyrimidines group, therefore is not a sulfonamide drug. It is often co-administrated with sulfamethoxazole because of the synergic activity of both antibiotics on the same pathway. Mutations in S. suis dhfr and its promoter were associated with reduced susceptibility to trimethroprim, as well as horizontal acquisition of transmissible trimethoprim-insensitive dhfr genes [62] (Table 1), as found in other streptococcus species.
3.6 Resistance to aminoglycosides
Aminoglycosides are antibiotics composed of a core structure of amino sugars linked to a dibasic aminocyclitol by a glycosidic bond (Figure 3). They are polycationic structures that bind to the negatively charged components of the bacterial membrane such as teichoic acids and phospholipids or, in Gram-negative bacteria, lipopolysaccharides. This activity causes magnesium displacement, a process that enhances membrane permeability and facilitates antibiotic entry. Some aminoglycosides are still active against Streptococcus and Enterococcus spp., e.g. tobramycin (Figure 3), but only at higher concentrations as compared to other bacteria because of natural resistance. They inhibit protein synthesis by binding to the A-site on the 16S rRNA (Figure 2B). Aminoglycoside-ribosome interaction causes codon misreading, resulting in incorrect assembling of amino acids. Different aminoglycosides have different ribosome specificities.
Aminoglycoside resistance is caused by different mechanisms, including enzymatic modification of the antibiotic, target-site modification via an enzyme, and efflux. Aminoglycoside-resistance mutations altering the ribosome have not been reported in S. suis. Aminoglycoside-modifying enzymes are widespread. Aminoglycoside modifications include acetylation, phosphorylation, and adenylation at different positions in the aminoglycoside [114]. Modification decreases the affinity of the drug for its target. Aminoglycoside-modifying enzymes comprise three families, i.e., aminoglycoside N-acetyltransferases (AACs), aminoglycoside O-nucleotidyltransferases (ANTs), and aminoglycoside O-phosphotransferases (APHs). The families are further divided into subtypes according to the position on the aminoglycoside that is modified. ANTs transfer AMP from ATP to the hydroxyl groups at positions 2″, 3″, 4’, 6’, or 9’ of the aminoglycoside. Several genes coding for ANTs have been discovered in aminoglycoside-resistant S. suis isolates. Examples include ant1 [62], ant(6´)-Ia [115, 116], ant(6´)-Ib [62], and ant(9´)-Ia [116] (Table 1). AACs comprise a large group of enzymes that acetylate amino groups at different positions on the aminoglycoside. There are four subclasses of AACs based on the position of the amino groups that are modified. The aac(6´) gene was identified in various multidrug-resistant S. suis isolates from Asia with high MIC values for aminoglycosides [115, 117]. It is often fused to other genes coding for different aminoglycoside-modifying enzymes, for example APHs (Table 1), thus generating bifunctional enzymes. APHs catalyze the ATP-dependent phosphorylation of hydroxyl groups on aminoglycosides [118]. In S. suis, several genes only coding for an APH variant, such as aph(3´)-IIIa and aph(6)-Ia, have been reported [88, 119] (Table 1).
3.7 Resistance to glycopeptides
Glycopeptides are a group of glycosylated cyclic or polycyclic peptides (Figure 3). They act by binding the D-alanyl-D-alanine terminus of cell wall precursors (Figure 2A), thus preventing their incorporation into the PG strands. Representative examples are vancomycin, teicoplanin, telavancin, dalbavancin, and oritavancin. These antibiotics are exclusively used in humans, but ovoparcin (Figure 3) has been used as growth promoter in pig production. Structural differences in these antibiotics have implications for their mechanism of action. For example, vancomycin has higher affinity for the PG precursors than teicoplanin, which is based on dimer formation. In contrast, teicoplanin interacts with the lipid bilayer of the bacterial membrane resulting in its localization near the lipid II substrate. However, these antibiotics can also select for resistance traits that could be transferred to human pathogens via food chain.
Glycopeptides are large molecules that cannot cross the outer membrane in Gram-negative bacteria through porins and, therefore, Gram-negative bacteria are intrinsically resistant to glycopeptides. In contrast, Gram-positive bacteria are susceptible. The main glycopeptide-resistance mechanism developed by Gram-positive bacteria is the production of substrate-modifying enzymes that reduce the affinity of the substrates for the antibiotics. By their action, the carboxy-terminal D-alanine residue of PG precursors is replaced by either D-lactate or D-serine [120]. There are several classes of cell-wall modification systems that confer resistance to glycopeptides. They are encoded by gene clusters referred to as van. The vanA, vanB, vanD, vanM, and vanF clusters code for enzymes that replace the terminal D-Ala by D-lactate, whereas the clusters vanC, vanE, vanG, and vanL code for enzymes that replace the terminal D-Ala by D-Ser. Several van genes were identified in S. suis (Table 1), including the vanG operon [121] and vanZ gene [122]. The presence of a vanG operon results in an intermediate level of resistance to vancomycin [123]. The vanG operon is composed of several genes: (i) vanG, which encodes a D-Ala-D-Ser ligase, (ii) vanX and vanY, which encode a putative D,D-peptidase and D,D-carboxypeptidase, respectively, (iii) vanT, which codes for a serine racemase, and (iv) vanR and vanS, whose products constitute a two-component regulatory system that controls expression the operon. The vanZ gene is usually located within the vanA gene cluster, but this is not the case in S. suis and Clostridioides difficile (previously called Clostridium difficile) [124]. The function of the VanZ protein remains unknown, but it increases teicoplanin resistance in Enterococcus faecium and C. difficile, and has no impact on vancomycin resistance [124, 125].
3.8 Resistance to quinolones
Quinolones are molecules composed of a basic bicyclic core, which may contain a fluorine atom (fluoroquinolones), usually at the C6 position, and various other substitutions (see enrofloxacin and norfloxacin in Figure 3). Depending on the core structure, they can be classified into four groups: (i) monocyclic, (ii) bicyclic, (iii) tricyclic, and (iv) tetracyclic derivatives, and, based on the position of the fluorine atom, each group can be subdivided into subgroups. Quinolones are derivatives of the synthetic nalidixic acid, first reported in 1962. Since then, nalidixic acid analogues were elaborated and optimized over time [126]. Examples of the second generation are norfloxacin (Figure 3) and ciprofloxacin. The third and fourth generations encompass fluoroquinolones with broader activity, efficacy, and lower resistance development than previous analogues. Relevant antibiotics are levofloxacin (third generation) and moxifloxacin (fourth generation), which are more active against Gram-positive bacteria than their predecessors [127]. Of the different quinolones generated, ciprofloxacin, norfloxacin, and enrofloxacin (Figure 3) have been and are broadly used to treat S. suis infections.
Quinolones target bacterial type II topoisomerases (Figure 2D), i.e., DNA gyrase and topoisomerase IV. These enzymes have crucial functions by catalyzing the interconversion of topological forms of DNA [128]. Although their working mechanism is similar, their biological roles are related but not identical. In contrast to topoisomerase IV, DNA gyrase generates negative supercoils into DNA, and it removes the torsional stress generated during replication and transcription from the DNA. Also, topoisomerase IV removes torsional stress, but, in addition, it mediates the separation of the two daughter chromosomes (decatenation) after DNA replication [129]. Both enzymes are composed of two subunits forming hetero-tetrameric complexes, i.e., GyrA and GyrB in the case of gyrase and ParA and ParC in the case of topoisomerase IV. For their activity, both enzymes have ATP-dependent DNA cleaving and ligating activity [128]. Quinolones intercalate between DNA bases at the DNA cleavage-ligation site, thereby inhibiting ligation. Thus, bacterial exposition to quinolones increases the concentration of cleaved DNA leading to cell death. Quinolones have different preferences for their targets. In Gram-negative bacteria, quinolones have higher affinity for DNA gyrase than for topoisomerase IV. However, in some Gram-positive bacteria, including S. pneumoniae, topoisomerase IV rather than gyrase is the primary target. Furthermore, in S. pneumoniae, the quinolones nature also determines the affinity for the target [130].
Quinolone resistance is grouped in several categories, including (i) alteration of quinolone targets, (ii) production of antibiotic-modifying enzymes, and (iii) enhanced production of efflux pumps. In Streptococcus species, including S. suis, two main mechanisms have been described: mutations at the target site and enhanced production of efflux pumps (Table 1). Quinolone resistance is often associated with chromosomal mutations in the gyrase- and/or topoisomerase IV-encoding genes. Combinations of mutations in both enzymes yield high levels of resistance. In S. suis, mutations in gyrA, gyrB, parC, and parA have been described and related to quinolone resistance (Table 1) [62, 88, 131]. The most frequent mutations occur in gyrA at position Ser81 and, less frequently, at position Glu85. Also, structural analysis identified amino acids involved in the binding of a quinolone via a Mg2+ ion by forming hydrogen bonds to water molecules that coordinate the Mg2+ ion. Thus, their substitution interferes with quinolone binding [132]. Curiously, these amino-acid residues are highly conserved within the bacterial kingdom, although their involvement in protein function remains unclear. To a lesser extent, quinolone-resistance mutations are found also at positions Ser79 and Asp83 in ParC of S. suis [62, 88]. This is in contrast to other Gram-positive bacteria, where mutations in parC are first to occur [133]. These data suggest that DNA gyrase is the main target for the quinolones used to treat S. suis. However, several isolates have mutations in both genes [88], probably as a mechanism to increase resistence levels.
Another mechanism of resistance to fluoroquinolones in Gram-positive bacteria is the increased production of efflux pumps. In Gram-positive bacteria, several members of the MFS, the multiple antibiotic- and toxin-extrusion (MATE), and the ABC-transporter families recognize quinolones as substrates [134]. In S. suis, the ABC transporter SatAB has been reported to be involved in this function [135]. SatAB exports norfloxacin and ciprofloxacin [131, 135]. On the chromosome, the satA and satB genes are organized in an operon. The regulation of satAB expression is complex and controlled by different molecules. SatR, a MarR-family regulator, acts as a repressor of the operon [136]. Besides, the operon is regulated by the quorum-sensing system LuxS/AI-2 in S. suis [137]. Particularly, AI-2 upregulates the expression of the sat genes, thus increasing efflux pump production, leading to increased quinolone resistance [137]. SatAB is homologous to pneumococcal PatAB, an ABC transporter that provides resistance to norfloxacin, ciprofloxacin, and levofloxacin [138]. The expression of PatAB is upregulated by exposition of the bacteria to quinolones [139]. Thus, it is expected that SatAB of S. suis is also regulated by quinolones, but this hypothesis requires experimental evidence. Besides, the gene with locus tag SS2069 was found to be upregulated in quinolone-resistant S. suis isolates carrying the anticipated mutations in the type II topoisomerases [131]. SS2069 codes for an extracellular protein that is part of an ABC transporter. This extracellular location is difficult to conciliate with a direct role in the export of quinolones out of the cells and, thus, its role in quinolone resistance remains enigmatic. In pneumococci, quinolone export is also driven by PmrA [140], an MFS-type efflux pump that exports a variety of substrates including norfloxacin, ethidium bromide, and acriflavine. A pmrA gene is also present in S. suis genomes (i.e., SSU1222 in P1/7 genome), but its contribution to quinolone resistance remains to be demonstrated.
The production of enzymes that alter the antibiotic targets or the quinolones has been identified in other bacteria, particularly in Gram-negative bacteria. Interestingly, a variant of the aminoglycoside acetyltransferase AAC(6´)-Ib generating a moderate resistance to ciprofloxacin has been identified in Gram-negative bacteria [141]. This enzyme inactivates ciprofloxacin by N-acetylation. This is not surprising, as AAC belongs to a superfamily of enzymes that modify a large variety of substrates. Remarkably, aac(6´) variants have been identified in various multidrug-resistant S. suis isolates from Asia with high MIC values for aminoglycosides [115, 117] (Table 1). In Gram-negative bacteria, the aac(6´)-Ib gene is transferred by plasmids, but this is not a usual mechanism in Gram-positive bacteria. However, the wide distribution of aac genes in S. suis genomes leads us to speculate that these enzymes could undergo adaptation to modify other antibiotics such as quinolones.
4 Transfer of antibiotic-resistance genes
S. suis is considered a reservoir of AMR genes, which are shared among S. suis clones and can also be transferred to other bacterial species by HGT [142, 143]. HGT can be mediated by MGEs during conjugation process or simply by DNA fragments by transformation process. MGEs are DNA elements that can be transferred between cells and/or within a genome. Many MGEs harbor genes for mobility, i.e., integration, excision, and conjugation. Genes involved in separate functions of the mobilization process are often clustered together and, thus, MGEs show a modular organization. In addition, MGEs can carry a diversity of genes for metabolic pathways, virulence factors, symbiosis, interbacterial competition, and/or AMR. Such genes confer an advantage to the host in a particular niche, which favors the selection of clones that acquired the MGE [144]. Clearly, S. suis prefers to share its AMR genes via MGEs. This was illustrated in a recent study which revealed that all AMR genes identified in 214 genomes of drug-resistant S. suis isolates of 26 different serotypes were located on MGEs [145].
Most MGEs use well-described mechanisms for their mobilization, such as type I and type II transposons, insertion sequences, plasmids, prophages, and chromosomal integrative elements transferring by conjugation. The latter elements include (i) integrative and conjugative elements (ICEs), which are autonomous in transfer and integration, (ii) integrative and mobilizable elements (IMEs), which are autonomous for excision and integration but not for transfer, (iii) elements that are autonomous for transfer but not for integration (i.e., deviating from an ICE). More recently, unconventional MGEs have been discovered, but the mechanisms for their mobility have not been identified yet. Regardless of the presence of genes related to mobilization and integration, MGEs contain genetic features, such as an unusual G + C content or codon usage, that indicate that they were acquired by HGT. Anyway, MGEs can be exchanged between bacteria by different mechanisms, including conjugation, transformation and transduction [146]. Other mechanisms implicated in HGT involve the release of membrane vesicles or elongated membranous structures named nanotubes.
4.1 Conjugation
Conjugation is the most frequently used mechanism of AMR-gene transfer in S. suis. Two categories of chromosomal elements can be transferred by conjugation, i.e., ICEs and IMEs [147]. ICEs contain all genetic information needed to mediate their autonomous excision, conjugation, and chromosome integration [147]. They are also known as conjugative transposons. They can also carry genes that mediate heavy-metal resistance, AMR, and/or biofilm formation, amongst others [146]. ICEs are excised from the chromosome by site-specific recombination at the att sites (attL and attR), a process mediated by tyrosine and serine recombinases or DDE transposases. After excision from the chromosome, ICEs are circularized and then transferred to a recipient cell by conjugation. To achieve this, the donor and the recipient must establish intimate contact, which is mediated by pili and/or adhesins at the cell surface. Then, the DNA is transferred by a conjugation apparatus that comprises a relaxase, called MOB, a mating-pair formation system, which is constituted by a membrane-spanning multi-protein complex, known as type IV secretion system (T4SS), and a coupling protein located at the inner side of the membrane [144]. The relaxase binds to the origin of transfer, oriT, on the ICE, cleaves one strand, and forms a covalent bond with the 5´ end of the cleaved strand. The coupling protein binds the DNA bound-relaxase to the T4SS. Rolling-circle replication displaces the cleaved strand, and the relaxase and the single strand are actively transferred from the donor through the T4SS to the recipient, where the complementary strand is synthesized. Finally, ICEs can be integrated into the recipient chromosome at various sites, often located in tRNA genes but also in various house-keeping genes [144]. IMEs undergo autonomous excision and integration but, in contrast to ICEs, they are not equipped with a full set of genes for conjugation. Even so, IMEs can contain genes for a relaxase or even a coupling protein and other conjugation-related gene can be present. Nevertheless, they parasitize on conjugative elements for mobility. In fact, many IMEs are integrated into ICEs. Even so, these elements correspond to different categories of MGEs since their transfer functions are genetically unrelated or only distantly related.
ICEs frequently mediate the transfer of AMR genes in S. suis and are responsible for multidrug-resistant phenotypes. Table 2 lists the characteristics of some representative ICEs found in S. suis and harboring AMR genes (reviewed in Dechêne-Tempier et al. [53]). They are broadly distributed among S. suis genomes. In silico analysis of 214 S. suis genomes revealed the presence of 242 complete ICEs and 135 derivative ICEs, i.e., ICEs containing truncated genes for mobilization [145]. The families of ICEs described in S. suis genomes are Tn916 [148], Tn5252 [148], Tn1549 [145], TnGBS2 [145], TnGBS1 [145], ICESt3 [145], and vanG [145]. Most of them encode a canonical relaxase of the MOBp family associated with a coupling protein of the VirD4 family. S. suis ICEs are mostly integrated in house-keeping genes, including rplL, rumA, mutT, a luciferase-like monooxygenase gene (SSU0468, llmO), and rbgA, among others, or in non-coding sequences, as well as in other MGEs (see Table 2). The integration sites vary between ICE families, and there may be more than one insertion site for each family (Table 2). AMR genes are present in several S. suis ICE families, but they are most frequently found in ICEs of the Tn5252 family [145]. AMR genes have so far not been detected in ICE families TnGBS1 and ICESt3 carried by S. suis. A large variety and different combinations of AMR genes can be found in a single ICE (Table 2). It has been demonstrated that ICEs can transfer AMR genes between S. suis strains and also to other streptococci, such as S. pneumoniae and S. agalactiae [148], and even to bacteria of other genera. In fact, many ICEs are found in several different bacterial species (Table 2). An example is ICESsD9, which carries the erythromycin- and tetracycline-resistance determinants erm(B) and tet(O) (Table 2), respectively, and which was transferred between S. suis and Enterococcus faecalis [149]. Also, ICESsu32457 (Table 2) of S. suis strain 32,457 was transferable to S. agalactiae strain RF12 in vitro. Importantly, ICESsu32457 recombined with S. agalactiae ICESa2603 generating a hybrid island that was transferable to S. pyogenes strains [150]. Obviously, the formation of ICE hybrids can facilitate the accumulation of AMR genes and the generation of multidrug-resistant strains. Interestingly, ICESsuSC216 of S. suis strain SC216 and a tandem ICE designated tandem ICESsuSC317 of strain SC317, which is composed of two different consecutive ICEs, both contain an optrA gene (oxazolidinone/amphenicol resistance) flanked by two IS1216 elements [97]. Both ICEs belong to Tn5252 family, but they are inserted at different locations. The optrA gene is also located on the prophage ΦSC181 (Table 2) in S. suis strain SC181 associated with a cat gene (chloramphenicol resistance) and an araC-like transcriptional regulatory gene [97], and flanked by two IS1216 elements. Inverse PCR assays revealed that IS1216 elements can recombine and form circular intermediates. Additional examples of ICEs carrying AMR genes in S. suis are listed in Table 2. Together, these observations demonstrate that AMR genes can move between various MGEs of a single strain and that independent ICEs can recombine to form tandems or hybrids. Both phenomena could explain, in part, the mosaic of AMR genes found in ICEs (Table 2), but, also importantly, they can facilitate the dissemination of many AMR genes between strains, thereby stimulating the emergence of multidrug-resistant S. suis strains. Supporting this notion are studies in S. suis strains carrying ICESsuBSB6, which is composed of two regions, ARGR1 and ARGR2, both containing AMR genes. The ARGR1 region carries six resistance determinants conferring resistance to macrolides, aminoglycosides, and tetracyclines (Table 2) and shows high similarity to the island ICESsu32457 and to E. faecalis plasmid pEF418. The ARGR2 region only possesses the glycopeptide-resistance operon vanG, and it is similar to the vanG1 island of E. faecalis BM4518 and the vanG2 island of S. agalactiae GBS-NM [121].
S. suis IMEs show more diversity than ICEs. IMEs are highly abundant in streptococcal species. Actually, IME-related elements (n = 457) were more prevalent than ICE-related elements (n = 377) in a large panel of S. suis genomes [145]. They can harbor canonical relaxases of the MOBC, MOBV, and MOBQ families or putative non-canonical relaxases with various domains [145]. On the S. suis chromosome, IMEs can be integrated in various genes (Table 2), including the tRNA-Leu- and tRNA-Asn-encoding genes, a putative peptidyl-prolyl isomerase-encoding gene (PPI), SNF2, and the house-keeping genes rpsI, rpmG, guaA, and traG, amongst others (Table 2). Some of these genes are located within ICEs of the Tn5252 family (e.g. PPI and SNF2). As ICEs, IMEs can carry AMR genes. Notably, 80% of the AMR genes in 247 S. suis genomes were present within the IMEs, whilst 20% were within the ICEs, which indicates the importance of IMEs as antibiotic-resistance gene dispersers. Most of these IMEs are carried by ICEs, mainly of the Tn5252 family (for further information [53]).
4.2 Transformation
Streptococcus species can acquire genes by direct uptake of extracellular DNA (eDNA). This process requires transport of the DNA into the cell and its integration into the chromosome by homologous recombination. The genes involved are regulated by quorum sensing. S. suis employs the ComRS system. The comS gene encodes a precursor peptide that is proteolytically processed into the pheromone XIP, which is secreted into the extracellular environment. ComR is a cytoplasmic transcriptional activator. When XIP accumulates in the environment at high cell density, it is taken up into the cells by an oligopeptide-permease (Opp), where it binds, and thereby activates ComR. Activated ComR induces the expression of sigX, which encodes an alternative sigma factor, and its regulon [151]. SigX stimulates the transcription of the competence genes encoding the DNA-uptake machinery, known as transformasome, which is a T2SS-like machine and consists of a pilus, an endonuclease called EndA, and the DNA transport proteins (ComEA, EC, FA). Based on experimental evidence in the pneumococcus, S. suis pili probably function as DNA receptor on the cell surface [152]. After binding the pilus, the pilus retracts, and ssDNA crosses the membrane through the transformasome.
There are three types of ComRS systems in streptococci [151], referred to as type I to type III. The distinction is based on differences in the sequence of the XIP produced and in the C-terminal domain of ComR with which XIP interacts [153]. Each XIP is specific to a group of S. suis strains [154], as cognate XIP-ComR interaction is required to activate the system [153]. S. suis elements for transformation share high similarities with those of other streptococcal species [154]. Natural transformation is stimulated by environmental stresses, such as starvation or the presence of antibiotics, as well as the presence of active porcine or human sera [154].
Natural transformation requires chromosomal DNA release. It is assumed that eDNA is released by bacterial cell lysis facilitated by the expression of autolysins, such as LytA, LytB, and AtlI. These enzymes are located at the cell surface and cleave covalent bonds in the cell wall. The cell wall consists of a network composed of polymeric chains of N-acetylglucosamine-β-(1,4)-N-acetylmuramic acid interconnected via short peptides bound to the lactyl group of N-acetylmuramic acid. LytA is an N-acetylmuramoyl-L-alanine amidase that hydrolyzes the cell wall by cleaving the lactyl-amide bond. LytA does not cause cell lysis during normal growth [155], but it induces cell lysis during stationary phase or when cell-wall synthesis is disrupted by antibiotic treatment or nutrient depletion. LytB is an endo-β-N-acetylglucosaminidase that cleaves the β(1,4) glycosidic bond between N-acetylglucosamine and N-acetylmuramic acid. LytB has a role during bacterial cell division, acting as a chain-dispersing enzyme during the separation of daughter cells [156]. AtlI contains a catalytic N-acetylmuramoyl-L-alanine amidase domain and induces bacterial autolysis. SigX controls a competence-induced cell-lysis mechanism called fratricide. Competent cells stimulate lysis of non-competent cells in the same ecological niche, which leads to the release of chromosomal DNA into the milieu. In this process, the murein hydrolase CrfP is an important player. CrfP is exported to the extracellular milieu and lyses S. suis [157]. An immunity protein encoded by the comM gene is produced by competent cells and protects them from their own lysins [158]. Apart from PG hydrolases, small peptide bacteriocins, called suisins if produced by S. suis, mediate antagonistic activities to different bacteria, ultimately leading to lysis and DNA release. The two-peptide suisin CibAB participates in this process. Because the DNA released from lysed cells can be taken up by competent attacker cells, the rate of gene transfer is greatly increased. Three suicin synthesis clusters have been described, i.e., those for suicins 65, 90–1330, and 3908 [159,160,161]. Interestingly, they can be located on ICEs. Thus, suicins can provide an advantage to ICE-carrying strains by inactivating competitors, but they can also enhance the uptake of novel AMR genes by stimulating the presence of eDNA.
The exchange of AMR genes in S. suis by natural transformation has experimentally been demonstrated. Recently, Yu et al. [162] identified the genomic island SsuSC128 in S. suis strain SC128. This non-mobilizable island carries tet(L), tet(M), and catA8 conferring a tigecycline- and chloramphenicol-resistance phenotype [162]. SsuSC128 was introduced into the genome of S. suis strain P1/7 by natural transformation, and the resulting transformants showed an increased MIC to tigecycline [162]. Thus, natural competence could mediate the transfer of non-mobilizable elements. This mechanism could be relevant, for example, for the acquisition of pbp gene variants that confer resistance to β-lactams. It is assumed that gene exchange by transformation has contributed significantly to the increasing incidence of penicillin-resistant S. pneumoniae [163, 164], and this is probably also the case in S. suis. In this context, the acquisition of a pbp2x gene of S. pneumoniae by a Streptococcus mitis strain has been reported [165]. Thus, exchange of pbp sequences could quickly generate β-lactam-resistant strains by introducing multiple simultaneously occurring substitutions in a single gene. This could explain the increasing β-lactam-resistance rates in S. suis reported in various countries over time (Figure 1). However, the rates of β-lactam resistance increase only slightly as compared to the rates of resistance to tetracyclines or lincosamides (Figure 1), the genetic determinants for which are located on conjugative elements (Table 2). This supports the notion that AMR gene transfer via transformation occurs less frequently than via conjugation. This difference in frequency could explain the large differences in resistance rates for different antibiotic families (Figure 1). Also, S. suis genomes contain AMR genes located on defective ICEs and IMEs whose genes involved in excision and/or conjugation are truncated. Up to 40 ICEs and 45 IME derivatives that lack a relaxase gene cluster were found in 215 S. suis genomes [145]. Therefore, these elements are not mobilizable by conjugation. The abundance and spread of these elements in S. suis genomes of different lineages could be explained by alternative transfer systems, such as natural transformation.
4.3 Transduction
Transduction is the mechanism by which DNA is transferred between bacteria via bacteriophages (a.k.a. phages). Transduction can take place by three mechanisms, called generalized, specialized, and lateral transduction. In generalized transduction, bacterial DNA can be randomly packed into new phage particles during the lytic cycle and then be transferred into a new host. Some MGEs hijack the phage DNA-packing machinery for their own transfer [166]. In specialized transduction, only bacterial DNA directly adjacent to the prophage is packed into new virus particles as result of anomalous prophage excision. In lateral transduction, phage DNA replication is initiated in the integrated prophage and results in the amplification also of flanking host DNA. The amplified DNA is packed while still integrated in the chromosome until the virus capsule is full. In this way, long fragments of the host genome, including MGEs or independent AMR genes, are packed into phage particles and transferred to a new host. The exchange of AMR genes occurs at higher frequencies by lateral transduction than by generalized and specialized transduction [167].
The ubiquity and high abundance of phages in nature suggests that transduction occurs at high frequency. Table 2 lists prophages described in S. suis carrying AMR genes. Phages are often specific for a certain species or even for strains of a species, as the phage receptor and bacterial defense mechanisms can vary. Nevertheless, the exchange of AMR genes between different streptococcal species by transduction has been reported. There is in vitro evidence of transfer of bacteriophage Φm46.1 between S. suis and S. pyogenes. Interestingly, the transfer occurs in both directions, but the transfer rate is higher from S. suis to S. pyogenes than vice versa (8.5 × 10–4 versus 2.3 × 10–9) [168], again supporting the notion that S. suis is a reservoir of AMR genes. This phage is one of the most frequently mobile elements found in S. suis isolates and carries the resistance genes mef(A) and tet(O) [169], conferring resistance to erythromycin and tetracycline, respectively. It also improves S. suis fitness and leads to overexpression of atlI, although the latter effect was not observed in other Streptococcus species [168]. S. suis strain SC181 contains a Φm46.1-like prophage (Table 2), named ΦSC181, which carries many AMR genes including, for example, an optrA gene [97] (Table 2). ΦSsu1135/10 was found in S. suis isolates from Brazil [170] (Table 2). This phage carries aph(3’)-IIIa, ant(6’)-Ia, and erm(B) resistance genes [170], indicating that it can spread several AMR genes turning infected bacteria into multidrug resistant strains. Another phage, named ΦSsUD.1, contains tet(W), aph(3’)-III, ant(6’)-Ia, aphA3, aadE, sat4, and erm(B) [170], conferring a macrolide–aminoglycoside–streptothricin-resistance phenotype [171], as well as a cadC/cadA cadmium-efflux cassette [142]. All these bacteriophages are like each other and are found in different species of Streptococcus. Together, these data support the notion that AMR genes can also be spread in S. suis by transduction and that this mechanism can contribute to a rapid acquisition of a multidrug-resistant phenotype.
4.4 Alternative HGT mechanisms
Extracellular vesicles are membrane-derived lipid bilayers released into the milieu by Gram-negative and Gram-positive bacteria. They often show a typical spherical morphology of 20 to 300 nm in diameter. The composition of extracellular vesicles of Gram-positive bacteria resembles essentially that of the cytoplasmic membrane; however, they are enriched in anionic phospholipids and may contain a variable composition of lipoproteins [172]. Extracellular vesicles can encapsulate different molecules, including cytosolic proteins, secreted proteins, and nucleic acids. Proteins can be localized in the vesicle lumen, integrated into the vesicle membrane, or membrane anchored and exposed at the vesicle surface. Genetic material can be present in the vesicle lumen or externally associated with the membrane. Extracellular vesicles are used to interact with eukaryotic and prokaryotic cells in the environment without establishing direct cell-to-cell contact. They can deliver their cargo to eukaryotic cells through three pathways, (i) endocytosis, (ii) membrane fusion, and (iii) clathrin-dependent endocytosis. In many pathogenic bacteria, extracellular vesicles can act as carriers of virulence factors, such as toxins [173], or of factors mediating evasion of the host immune system [174]. They can also participate in interbacterial interactions [175] by carrying quorum-sensing molecules [176] or toxins directed to other bacteria [177], and by stimulating the formation of bacterial associations, such as biofilms [178]. Extracellular vesicles can also deliver chromosomal DNA or plasmids, and, indeed, they have been associated with HGT in Gram-negative bacteria, including in AMR gene transfer. For example, Acinetobacter baumannii can acquire resistance to carbapenem antibiotics via extracellular vesicles harboring the blaOXA-24 gene [179]. In contrast to transformation, where bacterial lysis is required, vesicles are released from live bacteria, which can modulate vesicle production according to environmental conditions [180]. Furthermore, in contrast to free DNA involved in transformation, vesicle-encapsulated DNA is protected against extracellular nucleases, which can be present in host tissues. Thus, this process of HGT confers a particular advantage for the dissemination of genes and constitutes an alternative and more secure way of dispersing AMR genes in vivo. Although this mechanism has yet to be proven in Gram-positive bacteria, DNA is an abundant constituent of extracellular vesicles of these bacteria [181]. Thus, AMR-gene transfer via extracellular vesicles could occur in Gram-positive bacteria, a hypothesis that requires experimental confirmation.
Many streptococcal species, including S. suis, were reported to produce extracellular vesicles. As compared with the cytoplasmic membrane, vesicles produced by S. pneumoniae are enriched in lipoproteins and short-chain fatty acids [182]. In some streptococcal species, the protein composition of the vesicles varies and also deviates from that of the cytoplasmic membrane as it contains only few membrane proteins [183]. The production and size of extracellular vesicles vary between strains and also fluctuate depending on the growth conditions [184]. For example, the extracellular vesicles of S. pneumoniae strain R6 are 130–160 nm in diameter [185], while those of strain TIGR44 are between 25 and 250 nm [183]. The extracellular vesicles of S. suis strain P1/7 range in diameter up to 130 nm [186]. Proteomic analysis of these vesicles identified up to 46 different proteins, including cytoplasmic, membrane/cell wall-associated, and secreted proteins; nine of them were virulence factors. The sorting mechanisms used to load the protein cargo of these vesicles have not been elucidated. Also in pathogenic streptococci, extracellular vesicles have an important role during bacterial infection. In pneumococci, they stimulate proinflammatory immune responses and antigen presentation by eukaryotic cells [185], and they activate C3b deposition and the formation of the membrane attack complex. Furthermore, they bind factor H and decrease bacterial opsonophagocytosis [183]. Extracellular vesicles of S. suis degrade neutrophil extracellular traps (NETs), which contributes to the evasion of the immune response, and they activate the nuclear factor-kappa B signaling pathway in monocytes and macrophages, which increases the permeability of the blood brain barrier [186]. So far, there are no reports of gene transfer mediated by extracellular vesicles in S. suis or any other Gram-positive bacteria. However, genetic material has been reported to be associated with streptococcal vesicles. Actually, in S. mutants, a major contributor to human dental caries, extracellular vesicles form an active route for the delivery of eDNA to the extracellular matrix (ECM) of the biofilm [181]. Interestingly, β-lactamase-positive Moraxella catarrhalis produce extracellular vesicles that carry β-lactamase, which protects S. pneumoniae against β-lactams [187]. Very likely, S. suis may also benefit of antibiotic-degrading enzymes present in extracellular vesicles produced by other AMR bacteria. Yet, this mechanism must be demonstrated.
5 Role of S. suis biofilms in antimicrobial resistance and microbial persistence
The formation of biofilms increases tolerance and resistance of bacteria to antimicrobials. This feature of biofilms is caused by several mechanisms [188] that vary between bacterial species and even between strains and ultimately depends on (i) the dynamics of biofilm formation, (ii) biofilm architecture, and (iii) ECM composition.
5.1 Biofilm-mediated antibiotic tolerance
Biofilm formation is initiated by the binding of planktonic bacteria to a substratum, an activity mediated by long appendices that protrude from the cell surface, including flagella and fimbriae or pili. S. suis can produce peritrichous and flexible fimbriae [189, 190], but they do not produce flagella. In the three major human streptococcal pathogens, i.e., S. agalactiae, S. pyogenes, and S. pneumoniae, a role for pili in biofilm formation has been reported, but their function has not been examined yet in S. suis. After binding to the substratum is established, shorter surface-exposed structures, such as fibrinogen-binding proteins, establish additional contacts and stabilize the binding. The proteins contain particular motifs that mediate adherence to different host structures or promote interbacterial interactions [191]. However, the binding of cell-surface-exposed proteins to the substratum or to the ECM can be occluded in many bacteria by capsule. This was also demonstrated for S. suis [192]. Thus, the loss of capsule enhances biofilm formation and, consequently, antibiotic tolerance. After establishing intimate contact with the substratum, bacteria proliferate to form microcolonies, i.e., small aggregates of bacteria originating from the same parental cell, and secrete ECM that stabilizes cell-to-cell and cell-substratum interactions. Microcolonies can interact with each other to form macrocolonies. At the end, the spatial organization of the biomass on the substratum defines the biofilm architecture, which varies during biofilm development. In general, mature biofilms are more resistant to antibiotics than younger biofilms [193] and, thus, the level of tolerance to antibiotics varies according to biofilm dynamics.
Electron microscopic examination of S. suis biofilms formed on abiotic surfaces revealed interconnected aggregates of different sizes with well-defined intervenient spaces in between [192, 194]. These spaces could constitute channels for the exchange of molecules and resources within the biofilm community. This organization of the biomass generates gradients of dispersion that affect the distribution of antibiotics within the biofilms. Cells within clusters are exposed to lower antibiotic concentrations than those located in the upper layers of the biofilm. The dispersion gradients also limit the availability of nutrients and oxygen for bacteria and generate excess of waste products in the deeper layers. This causes starvation and hypoxia, which force bacteria to slow down their metabolism and growth, thereby entering into a quiescent state [195]. As a result, bacteria within a biofilm are metabolically heterogeneous. Quiescent-state bacteria are less susceptible to antibiotics because crucial processes targeted by antibiotics are stopped. Hence, binding of antibiotics to their targets does not exert bactericidal activity [196]. Nutrient starvation also activates the stringent response, a mechanism that reprograms bacterial metabolism. To save energy and nutrients, the stringent response decreases RNA synthesis [197]. It is signalled by the alarmone (p)ppGpp [197, 198]. In S. suis, (p)ppGpp is synthesized by two different proteins, RelA and RelQ [197, 199]. This system seems to play a key role in S. suis pathogenesis, since it regulates the expression of genes presumably coding for virulence factors, such as cps2, a member of the capsule operon, and eno, which encodes the adhesin enolase, amongst others. Together, down regulation of capsule synthesis and over expression of adhesins, improve the adhesion to and invasion of host epithelial cells [200]. The response induced by (p)ppGpp helps S. suis to evade phagocytosis by host macrophages [199], presumably by promoting biofilm formation [198]. The involvement of the stringent response in antibiotic tolerance and resistance has been shown in many bacteria (reviewed in Hobbs and Boraston [201]). The mechanisms behind seems to be related to the upregulation, downregulation, or mutation of stress-responsive genes [201]. In S. suis, the activity of pyruvate dehydrogenase, which is encoded by the pdh gene and whose expression is upregulated by (p)ppGpp, affects adhesion to and invasion of host epithelial tissues as well as biofilm formation and stress response [202, 203]. Pyruvate dehydrogenase activity is suspected to increase antibiotic tolerance by increasing biofilm production [202].
Variations in the biofilm structure influence antibiotic tolerance and resistance. In many bacteria, large inter-strain variations in biofilm structure have been observed, and they appear to be a consequence of variation in the expression of surface-exposed proteins or ECM components. Presumably, this occurs also in S. suis. Metagenomic analysis of 375 S. suis genomes revealed that the species is genetically and phenotypically highly heterogeneous [204], and this could be translated in variation in biofilm-forming capacities. In fact, the biofilm-forming capacity of 46 clinical S. suis isolates showed high variability even between strains of the same serotype, while comparison of biofilm architecture also revealed large differences [194]. Thus, it is probable that the antibiotic-tolerance capacity in S. suis is strain dependent.
The ECM composition impacts antibiotic efficacy. ECM may contain extracellular polysaccharides, DNA and RNA, proteins, membrane vesicles, and cell debris. ECM components can interact with certain antibiotics, thereby affecting antibiotic effectivity. Extracellular polysaccharides are relevant constituents of the ECM from many bacterial species. Their nature is diverse, including linear or branched polysaccharides, and varies between bacterial species. Notably, the presence of unstructured polysaccharides, a.k.a. slime, in the ECM can strongly affect biofilm tolerance to antibiotics. An example is alginate produced by P. aeruginosa, which protects biofilms from aminoglycosides [205]. Alginate is negatively charged and, thus, it may interact with positively charged antibiotics. The production of extracellular polysaccharides was independently demonstrated in biofilms of S. suis by two research groups using microscopy. The presence of extracellular polysaccharides was visualized in biofilm cells, but not in planktonic cells of S. suis strain NJ-3 by FITC-ConA staining [194]. Slime was also visualized in biofilms of S. suis strain 95–8242 using Congo red staining and scanning electron microscopy [205]. Remarkably, biofilms of S. suis strain 95–8242 were 1000 times more tolerant to penicillin G and ampicillin than planktonic cells of this strain, but such differences were much smaller in the case of S. suis strain AAH4, which does not produce extracellular polysaccharides [205]. S. suis exopolysaccharides could interact with penicillin G and ampicillin and, thus, deplete antibiotic activity, a hypothesis that has not been explored. However, the production and the structure of these polysaccharides seem to vary between strains, consistent with the large variability in the genes coding for polysaccharide synthesis. In addition to exopolysacharides, eDNA is a relevant component of the ECM in many bacteria, and it is definitively more conserved than the exopolysaccharides. eDNA plays an important role in promoting and modulating biofilm development by participating in the adhesion to the substratum and in the structural integrity of biofilms. Also, eDNA can enhance resistance to antibiotics. In Staphylococcus epidermidis, eDNA enhances tolerance to vancomycin about 100-fold [206]. The binding constant of vancomycin to DNA is 100-fold higher than that to its target [206] and, thus, eDNA may trap this antibiotic in the ECM. The presence of eDNA in the ECM of biofilms was reported for several pathogenic streptococci, including S. pneumoniae [207], S. mutants [208], and S. intermedius [209]. For S. suis, only biofilms formed in the presence of neutrophils [210] have been reported to be sensitive to DNase. In this special case, eDNA is released from neutrophils to entrap bacteria in NETs, an immune defence mechanism. eDNA can also be released from bacteria by a specific DNA secretion system, but such system is not present in S. suis. So far, there is no direct evidence that S. suis actively secretes DNA. Alternatively, eDNA can be released from extracellular vesicles or by bacterial autolysis, which is enhanced by overproduction of autolysins that form part of the cell wall synthesis machinery. Two S. suis autolysins (AtlAss and AtlI) have been reported to contribute to biofilm formation [211, 212]. Also, antibiotics can promote the release of eDNA and increase biofilm production and antibiotic resistance. Indeed, subinhibitory concentrations of amoxicillin, lincomycin, and oxytetracycline were reported to enhance biofilm formation in S. suis [213]. Yet, experimental evidence is required to demonstrate that eDNA contributes to the ECM of S. suis as has been described for other streptococci. Yet, another component of the ECM of S. suis biofilms is fibrinogen. Fibrinogen is a mammalian protein present in blood and host tissues. Fibrinogen binds to fibrinogen-binding proteins exposed at the bacterial cell surface, resulting in cell aggregation [214], which ultimately stimulates biofilm formation. This can be relevant during bacteraemia, in which fibrinogen can generate bacterial aggregates that are more resistant to phagocytosis and allow bacteria to adhere to organs and tissues, causing endocarditis or arthritis. Interestingly, fibrinogen-stimulated S. suis biofilms showed higher minimal bactericidal concentrations to penicillin G than planktonic cells [214].
Intercellular communication contributes to antibiotic tolerance and resistance of biofilms through multiple mechanisms. In general, biofilms formed by mutants in quorum-sensing systems are less tolerant to antibiotics as has been reported for P. aeruginosa [215] and S. aureus [216], amongst others. In S. suis, quorum sensing also regulates biofilm formation and influences thereby antibiotic tolerance. Addition of autoinducer-2 (AI-2) (2 µM) to S. suis cultures increased biofilm formation [217], while deletion luxS, involved in the synthesis of AI-2, reduced biofilm formation in the presence of fibrinogen [218]. This is in agreement with a reduced expression of fibrinogen-binding proteins in the luxS mutant [218]. Thus, considering that the biofilm biomass and architecture are important factors for antibiotic tolerance, AI-2 considerably contributes to the development of tolerance. In contrast, high concentrations of AI-2 (> 5 µM) reduced biofilm formation and growth in S. suis [217]. High concentrations of AI-2 trigger a signalling cascade that leads to the expression of the tet(M) gene on Tn916, resulting in resistance to tetracycline [105], which is an antibiotic-resistance mechanism independent of the tolerance generated by the biofilm. Expression of tet(M) is also activated by tetracycline at concentrations below the minimal bactericidal concentration [105], conditions that can also be met inside biofilms. In addition, LuxS/AI-2 is involved in fluoroquinolone resistance by regulating the synthesis of efflux pump SatAB [135, 137]. Together, these data show that quorum sensing directly contributes to antibiotic resistance by inducing expression of AMR genes and indirectly to antibiotic tolerance by regulating biofilm formation and biofilm biomass. Apart from quorum sensing, other intercellular communication systems have been described in S. suis. As described above, some S. suis strains produce suicins that target competing bacteria [161, 219]. Killed target bacteria release intracellular components, including DNA, which can be taken up by transformation but also contribute to biofilm formation and antibiotic tolerance. To summarize, biofilm formation in S. suis causes tolerance to antibiotics through biofilm architecture, ECM composition, and intercellular communication.
5.2 Biofilm-mediated antibiotic resistance
Biofilm formation drastically enhances HGT and, consequently, the transfer of resistance genes. This is caused by several mechanisms. Firstly, biofilms can be formed by a diversity of bacterial species that establish close proximity. S. suis can form mixed biofilms with other species, as was shown, for example, in vitro with Actinobacillus pleuropneumoniae [220], another pathogen of the upper and lower respiratory tract of pigs. Such mixed biofilms increased the expression of virulence factors in both species and enhanced antibiotic resistance. Secondly, the reduced mobility of cells in biofilms and the high bacterial density favour interbacterial interactions. This facilitates the conjugation process, which is extensively used by S. suis for gene transfer. Conjugative transposons of the Tn916 family carrying genes coding for tetracycline resistance were shown to be transferred within multispecies biofilms of oral bacteria [221]. Thirdly, the presence of eDNA and extracellular vesicles within the ECM facilitates the transformation process. Transformation and the presence of extracellular vesicles have been demonstrated in S. suis. This could facilitate the dispersion of mutant alleles, such as those for DNA gyrase and PBPs, or of genes coding for antibiotic-degrading enzymes.
Biofilm formation enhances the mutation rate through multiple mechanisms. Exposure to agents that elicit oxidative stress increases the mutation rate. Oxidative stress is related to the generation of reactive oxygen species, which cause direct DNA damage and mutations. At sublethal doses of antibiotics, as can be present within the biofilm mass, the production of reactive oxygen species promotes antibiotic resistance by triggering the production of efflux pumps. Increased mutation rate can be a consequence of the activation of the SOS response. The SOS response is activated when bacterial DNA is damaged, a condition that occurs at high frequency within certain parts of the biofilm. RecA binds to damaged DNA and stimulates self-cleavage and, thereby, inactivation of the repressor LexA. This promotes the expression of SOS genes, the products of which repair the DNA, enhance mutagenesis, and slow down bacterial growth until the DNA is fixed.
The SOS response, especially in combination with a lack of amino acids such as lysine, leucine, and cysteine, induces a high tolerance to ofloxacin [222]. Interestingly, the SOS response enhances the transfer of ICEs [223], which, as described above, is a prime mechanism in S. suis for the exchange of resistance genes. Mutations can occur in genes coding for the mismatch repair system, which increases the mutation rate then by about 100- to 1000-fold. This hypermutator phenotype has been reported in several pathogenic bacteria, including P. aeruginosa, Haemophilus influenzae, and S. aureus [224], but not yet in S. suis. If the mutation rate is indeed increased in S. suis biofilms, resistance to β-lactams, fluoroquinolones, and aminoglycosides could be enhanced by favouring mutations in PBP-encoding genes, the parC and gyrA genes, and the rpsL gene, which codes for 16S rRNA. Also, the enhanced mutation rate may increase the production of efflux pumps or alter the specificity of enzymes of the folate-synthesis pathway. However, an early study in S. mutans proved that the accumulation of mutations in biofilms was associated with natural selection rather than with a high mutation rate [225]. This raises the question if hypermutability is favoured within S. suis biofilms. Overall, mechanisms of biofilm-mediated antibiotic resistance in S. suis involve increased HGT and intercellular communication.
6 Concluding remarks
For more than three decades, antibiotics have been used to control S. suis infections. Undoubtably, they have saved thousands of pigs and even human lives, particularly in view of the expansion of the intensive pig production industry. Their efficacy has, however, declined by the successive acquisition of resistances. High AMR rates (> 80%) to lincosamides, macrolides, and tetracyclines were reported over the world (Figure 1, panels A-C). Even further, this has been accompanied by a rapid development of multidrug resistance over time (Figure 1D). The genetic origin of AMR has been related to mutations in the target site, the production of target-protective and antibiotic-degrading or -modifying enzymes, and of antibiotic exporters (Table 1), whose genes are spread by HGT. Massive metagenomic analysis of resistant isolates showed the location of most of the corresponding AMR genes in MGEs, such as ICEs, IMEs, and derivatives (Table 2). These elements can carry genes for autonomous conjugation and integration of the MGE into the bacterial chromosome. Conjugation seems a very efficient way for gene dispersion in S. suis, as judged by the massive presence of MGEs in S. suis genomes of different genetic lineages. Examination of streptococcal genomes showed a higher accumulation of these elements in S. suis than in other pathogenic Streptococcus species. Therefore, S. suis is considered as an AMR gene reservoir capable of accumulating and transferring MGEs at high frequency. Remarkably, some MGEs carry AMR genes for several different antibiotics leading to a rapid increase of multidrug-resistant strains. Yet, resistance to β-lactams, quinolones, and, in some regions, amphenicols remains low (Figure 1, panels A–C). Therefore, they are the first choice of drug to treat streptococcal swine disease in many countries nowadays. The slow development of resistance to these antibiotics contrasts to that reported for lincosamides, macrolides, and tetracyclines (Figure 1, panels A–C). This is in part due to a lower consumption of these antibiotics and/or their mechanism of resistance. For example, the main mechanism of β-lactam resistance is polymutation in pbp genes [62, 65]. S. suis could also transfer pbp resistance alleles by HGT, but, as they are not located in MGEs, gene transfer must be mediated by natural transformation (or other methods alternative to conjugation and transduction). Natural transformation is an inducible mechanism triggered by the quorum-sensing system, diverse environmental factors, and, possibly, unknown host factors [154, 226]. Ultimately, it depends on ComR-XIP interaction [153], which is strain specific. Thus, these possible limitations could explain in part the low rates of resistance to certain antibiotics.
Clearly, S. suis infection requires alternatives to conventional antibiotics. The understanding of the mode of action of antibiotics, as well as of the mechanisms of resistance, can help in designing novel molecules or combinations of molecules to counteract the bacterial resistance mechanisms. Different platforms for the discovery of novel molecules or the improvement of the current ones have been proposed [227], and they should be urgently exploited. However, this report illustrates that S. suis can rapidly acquire resistance to antimicrobials, which results in a lack of economic appeal for pharmaceutical companies to invest in the development of new molecules, which could be out of the market within a few years. An alternative approach is the exploitation of plant extracts that contain bactericidal components as part of the native immune system of plants. Some examples are extracts obtained from red thyme, common thyme, oregano, and cinnamon, which showed bactericidal activity in vitro against virulent strains of S. suis [228]. Particularly, these extracts could be presented to piglets as feed additives, thereby preventing S. suis colonization, a crucial step in the infection process. However, natural extracts exhibit drawbacks for their application in vivo, including toxicity at the required concentrations or activity against beneficial commensal flora. Their application is poorly regulated by law, and their presence in the final meat product is unknown. Phage therapy, i.e., the use of phages that kill bacteria, is becoming again popular these years. Phages are easy to produce, specific, self-controlled, and they do not generate side effects in the host. Several phages that kill S. suis have been isolated [229]. Yet, phages have several disadvantages, i.e., they are strain specific, and bacteria can easily develop resistance, for instance by altering the phage receptors by mutation. Moreover, while phage receptors are fully available in planktonic cells, their accessibility in biofilms is compromised by the ECM, preventing the phages of reaching their targets. Also, the ECM of biofilms contains phage receptors that compete with those located at the bacterial cell surface. To overcome these limitations of phage therapy, phage-produced enzymes, required for membrane destabilization and bacterial lysis, have been proposed to treat S. suis infections [230, 231]. Positive results of this approach have been shown in experimental infections of laboratory animals. Yet, their high production costs remain a major concern.
Vaccination could also be a solution. Alternatives to bacterins have been proposed, for example live-attenuated or subunit-based vaccines. Several live-attenuated vaccines have been studied, including the use of mutants auxotrophic for aromatic amino acids [232], or mutants lacking virulence factors [233, 234]. In general, live-attenuated vaccines show better efficacy than bacterins because the native structure of antigens is retained, and the immune system is better stimulated. However, toxicity of certain components is a drawback. Also, considering the variety of HGT mechanisms in S. suis discussed here, there is a risk that the attenuated strain turns virulent by acquiring genes from other circulating strain in the pigs. In contrast to live attenuated vaccines, subunit-based vaccines are safe because of the purity of the antigens. The capsule has been studied as a vaccine candidate for specific serotypes, but it elicits a T-cell-independent response that is very limited in piglets. In human vaccines against S. pneumoniae, conjugation of the capsule to protein antigens carrying T-cell epitopes makes them more immunogenic and capable of eliciting T- and B-cell responses. Yet, this strategy greatly increases the cost of a porcine vaccine, particularly when multiple capsules must be included to elicit cross-protection against different serotypes, at least against the most prevalent ones (n = 9) [235]. Subunit vaccines based on proteins constitute a more promising approach as they can elicit T-cell-dependent responses. Many antigens were proposed as putative vaccine candidates and, indeed, some elicited protective immune responses against the homologous strain in experimental animals. Some examples include Antigen One (Sao), enolase (Eno), peptidase SsPepO, DNA nuclease SsnA, and IgA1 protease, as discussed in [3]. Results are promising but not enough to guarantee complete coverage of the circulating S. suis strains. Also, some antigens elicit a protective response in mice but not in pigs [3]. This suggests that better adjuvants could be very helpful to increase the efficacy of such vaccines. However, despite many trials and few patents, there are no commercial vaccine(s) on the market to effectively prevent the disease caused by the superbug S. suis.
Abbreviations
- AACs:
-
N-acetyltransferases
- ABC:
-
ATP-binding cassette
- AI-2:
-
autoinducer-2
- AMR:
-
antimicrobial resistance
- ANTs:
-
aminoglycoside O-nucleotidyltransferases
- APHs:
-
aminoglycoside O-phosphotransferases
- Cats:
-
chloramphenicol O-acetyltransferases
- ECM:
-
extracellular matrix
- eDNA:
-
extracellular DNA
- EU:
-
European Union
- HGT:
-
horizontal gene transfer
- ICEs:
-
integrative and conjugative elements
- IMEs:
-
integrative and mobilizable elements
- MAMs:
-
macrolide arrest motifs
- MATE:
-
multiple antibiotic and toxin extrusion
- MFS:
-
major facilitator superfamily
- MGE:
-
mobile genetic elements
- MLS:
-
macrolides, lincosamide, streptogramin
- MLS(B) :
-
macrolides, lincosamide, streptogramin B
- Opp:
-
oligopeptide permease
- PBPs:
-
penicillin-binding proteins
- PG:
-
peptidoglycan
- RPP:
-
ribosome-protection proteins
- T4SS:
-
type IV secretion system
References
Segura M, Calzas C, Grenier D, Gottschalk M (2016) Initial steps of the pathogenesis of the infection caused by Streptococcus suis: fighting against nonspecific defenses. FEBS Lett 590:3772–3799
Vötsch D, Willenborg M, Weldearegay YB, Valentin-Weigand P (2018) Streptococcus suis–the “two faces” of a pathobiont in the porcine respiratory tract. Front Microbiol 9:480
Segura M (2015) Streptococcus suis vaccines: candidate antigens and progress. Expert Rev Vaccines 14:1587–1608
Wasteson Y, Høie S, Roberts MC (1994) Characterization of antibiotic resistance in Streptococcus suis. Vet Microbiol 41:41–49
DANMAP (2020) Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark
Aarestrup FM, Jorsal SE, Jensen NE (1998) Serological characterization and antimicrobial susceptibility of Streptococcus suis isolates from diagnostic samples in Denmark during 1995 and 1996. Vet Microbiol 60:59–66
Resapath (2019) French surveillance network for antimicrobial resistance in bacteria from diseased animals. Annual report
Petrocchi-Rilo M, Martínez-Martínez S, Aguarón-Turrientes Á, Roca-Martínez E, García-Iglesias M-J, Pérez-Fernández E, González-Fernández A, Herencia-Lagunar E, Gutiérrez-Martín C-B (2021) Anatomical site, typing, virulence gene profiling, antimicrobial susceptibility and resistance genes of Streptococcus suis isolates recovered from pigs in Spain. Antibiotics 10:707
Hendriksen RS, Mevius DJ, Schroeter A, Teale C, Meunier D, Butaye P, Franco A, Utinane A, Amado A, Moreno M (2008) Prevalence of antimicrobial resistance among bacterial pathogens isolated from cattle in different European countries: 2002–2004. Acta Vet Scand 50:28
Resapath (2011) French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin. Annual report
Werinder A, Aspán A, Backhans A, Sjölund M, Guss B, Jacobson M (2020) Streptococcus suis in Swedish grower pigs: occurrence, serotypes, and antimicrobial susceptibility. Acta Vet Scand 62:36
Vela AI, Moreno MA, Cebolla JA, González S, Latre MV, Domínguez L, Fernández-Garayzábal JF (2005) Antimicrobial susceptibility of clinical strains of Streptococcus suis isolated from pigs in Spain. Vet Microbiol 105:143–147
van Hout J, Heuvelink A, Gonggrijp M (2016) Monitoring of antimicrobial susceptibility of Streptococcus suis in the Netherlands, 2013–2015. Vet Microbiol 194:5–10
Matiašovic J, Nedbalcová K, Žižlavský M, Fleischer P, Pokludová L, Kellnerová D, Nechvátalová K, Šimek B, Czanderlová L, Zouharová M (2021) Streptococcus suis isolates—serotypes and susceptibility to antimicrobials in terms of their use on selected repopulated Czech pig farms. Pathogens 10:1314
Martel A, Baele M, Devriese LA, Goossens H, Wisselink HJ, Decostere A, Haesebrouck F (2001) Prevalence and mechanism of resistance against macrolides and lincosamides in Streptococcus suis isolates. Vet Microbiol 83:287–297
Resapath (2008) Surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin. Results 2008
Vachee A, Varon E, Jouy E, Meunier D (2008) Antibiotics susceptibility of Streptococcus and Enterococcus: data of Onerba network. Pathol Biol (Paris) 57:240–244
Resapath (2009) French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin. Annual report
Resapath (2014) French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin. Annual report
Resapath (2017) French surveillance network for antimicrobial resistance in bacteria from diseased animals. Annual report
DANMAP (2016) Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark
DANMAP (2018) Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark
Callens BF, Haesebrouck F, Maes D, Butaye P, Dewulf J, Boyen F (2013) Clinical resistance and decreased susceptibility in Streptococcus suis isolates from clinically healthy fattening pigs. Microb Drug Resist 19:146–151
DANMAP (2017) Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark
Grave K, Torren-Edo J, Mackay D (2010) Comparison of the sales of veterinary antibacterial agents between 10 European countries. J Antimicrob Chemother 65:2037–2040
Zhang C, Ning Y, Zhang Z, Song L, Qiu H, Gao H (2008) In vitro antimicrobial susceptibility of Streptococcus suis strains isolated from clinically healthy sows in China. Vet Microbiol 131:386–392
Oh SI, Jeon AB, Jung BY, Byun JW, Gottschalk M, Kim A, Kim JW, Kim HY (2017) Capsular serotypes, virulence-associated genes and antimicrobial susceptibility of Streptococcus suis isolates from pigs in Korea. J Vet Med Sci 79:780–789
Yongkiettrakul S, Maneerat K, Arechanajan B, Malila Y, Srimanote P, Gottschalk M, Visessanguan W (2019) Antimicrobial susceptibility of Streptococcus suis isolated from diseased pigs, asymptomatic pigs, and human patients in Thailand. BMC Vet Res 15:5
Seitz M, Valentin-Weigand P, Willenborg J (2016) Use of antibiotics and antimicrobial resistance in veterinary medicine as exemplified by the swine pathogen Streptococcus suis. Curr Top Microbiol Immunol 398:103–121
Huang J, Shang K, Kashif J, Wang L (2015) Genetic diversity of Streptococcus suis isolated from three pig farms of China obtained by acquiring antibiotic resistance genes. J Sci Food Agric 95:1454–1460
Chen L, Song Y, Wei Z, He H, Zhang A, Jin M (2013) Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J Vet Med Sci 75:583–587
Zhang B, Ku X, Yu X, Sun Q, Wu H, Chen F, Zhang X, Guo L, Tang X, He Q (2019) Prevalence and antimicrobial susceptibilities of bacterial pathogens in Chinese pig farms from 2013 to 2017. Sci Rep 9:9908
Zhang C, Zhang P, Wang Y, Fu L, Liu L, Xu D, Hou Y, Li Y, Fu M, Wang X (2020) Capsular serotypes, antimicrobial susceptibility, and the presence of transferable oxazolidinone resistance genes in Streptococcus suis isolated from healthy pigs in China. Vet Microbiol 247:108750
Zhang C, Zhang Z, Song L, Fan X, Wen F, Xu S, Ning Y (2015) Antimicrobial resistance profile and genotypic characteristics of Streptococcus suis capsular type 2 isolated from clinical carrier sows and diseased pigs in China. Biomed Res Int 2015:284303
Li LL, Liao XP, Sun J, Yang YR, Liu BT, Yang SS, Zhao DH, Liu YH (2012) Antimicrobial resistance, serotypes, and virulence factors of Streptococcus suis isolates from diseased pigs. Foodborne Pathog Dis 9:583–588
Wang X, Sun J, Bian C, Wang J, Liang Z, Shen Y, Yao H, Huang J, Wang L, Zheng H (2021) The population structure, antimicrobial resistance, and pathogenicity of Streptococcus suis cps31. Vet Microbiol 259:109149
Rajkhowa S, Rajesh JB (2021) Virulence associated gene profiling and antimicrobial resistance pattern of Streptococcus suis isolated from clinically healthy pigs from North East India. Lett Appl Microbiol 73:392–397
Kataoka Y, Yoshida T, Sawada T (2000) A 10-year survey of antimicrobial susceptibility of Streptococcus suis isolates from swine in Japan. J Vet Med Sci 62:1053–1057
Tan MF, Tan J, Zeng YB, Li HQ, Yang Q, Zhou R (2021) Antimicrobial resistance phenotypes and genotypes of Streptococcus suis isolated from clinically healthy pigs from 2017 to 2019 in Jiangxi Province, China. J Appl Microbiol 130:797–806
Maron DF, Smith TJS, Nachman KE (2013) Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob Health 9:48
O’Dea MA, Laird T, Abraham R, Jordan D, Lugsomya K, Fitt L, Gottschalk M, Truswell A, Abraham S (2018) Examination of Australian Streptococcus suis isolates from clinically affected pigs in a global context and the genomic characterisation of ST1 as a predictor of virulence. Vet Microbiol 226:31–40
Riley CB, Chidgey KL, Bridges JP, Gordon E, Lawrence KE (2020) Isolates, antimicrobial susceptibility profiles and multidrug resistance of bacteria cultured from pig submissions in New Zealand. Animals 10:1427
Glass-Kaastra SK, Pearl DL, Reid-Smith RJ, McEwen B, Slavic D, Fairles J, McEwen SA (2014) Surveillance of antimicrobial resistance in clinical isolates of Pasteurella multocida and Streptococcus suis from Ontario swine. Can J Vet Res 78:241–249
Arndt ER, Farzan A, MacInnes JI, Friendship RM (2019) Antimicrobial resistance of Streptococcus suis isolates recovered from clinically ill nursery pigs and from healthy pigs at different stages of production. Can Vet J 60:519
Athey TBT, Teatero S, Takamatsu D, Wasserscheid J, Dewar K, Gottschalk M, Fittipaldi N (2016) Population structure and antimicrobial resistance profiles of Streptococcus suis serotype 2 sequence type 25 strains. PLoS One 11:e0150908
Aradanas M, Poljak Z, Fittipaldi N, Ricker N, Farzan A (2021) Serotypes, virulence associated factors, and antimicrobial resistance of Streptococcus suis isolates recovered from sick and healthy pigs determined by whole genome sequencing. Front Vet Sci 8:742345
Hayer SS, Rovira A, Olsen K, Johnson TJ, Vannucci F, Rendahl A, Perez A, Alvarez J (2020) Prevalence and time trend analysis of antimicrobial resistance in respiratory bacterial pathogens collected from diseased pigs in USA between 2006–2016. Res Vet Sci 128:135–144
Okwumabua O, Peterson H, Hsu HM, Bochsler P, Behr M (2017) Isolation and partial characterization of Streptococcus suis from clinical cases in cattle. J Vet Diagn 29:160–168
Soares TCS, Paes AC, Megid J, Ribolla PEM, Paduan KdS, Gottschalk M (2014) Antimicrobial susceptibility of Streptococcus suis isolated from clinically healthy swine in Brazil. Can J Vet Res 78:145–149
Matajira CEC, Moreno LZ, Poor AP, Gomes V, Dalmutt AC, Parra BM, Oliveira CHd, Barbosa MRF, Sato MIZ, Calderaro FF (2020) Streptococcus suis in Brazil: Genotypic, virulence, and resistance profiling of strains isolated from pigs between 2001 and 2016. Pathogens 9:31
Hoa NT, Chieu TTB, Nghia HDT, Mai NTH, Anh PH, Wolbers M, Baker S, Campbell JI, Chau NVV, Hien TT (2011) The antimicrobial resistance patterns and associated determinants in Streptococcus suis isolated from humans in southern Vietnam, 1997–2008. BMC Infect Dis 11:6
Wang Y, Wang Y, Sun L, Grenier D, Yi L (2018) Streptococcus suis biofilm: regulation, drug-resistance mechanisms, and disinfection strategies. Appl Microbiol Biotechnol 102:9121–9129
Dechêne-Tempier M, Marois-Créhan C, Libante V, Jouy E, Leblond-Bourget N, Payot S (2021) Update on the mechanisms of antibiotic resistance and the mobile resistome in the emerging zoonotic pathogen Streptococcus suis. Microorganisms 9:1765
Gordon E, Mouz N, Duee E, Dideberg O (2000) The crystal structure of the penicillin-binding protein 2x from Streptococcus pneumoniae and its acyl-enzyme form: implication in drug resistance. J Mol Biol 299:477–485
Fisher JF, Mobashery S (2016) β-Lactam resistance mechanisms: Gram-positive bacteria and Mycobacterium tuberculosis. Cold Spring Harb Perspect Med 6:a025221
Briggs NS, Bruce KE, Naskar S, Winkler ME, Roper DI (2021) The pneumococcal divisome: dynamic control of Streptococcus pneumoniae cell division. Front Microbiol 12:737396
Kocaoglu O, Tsui HCT, Winkler ME, Carlson EE (2015) Profiling of β-lactam selectivity for penicillin-binding proteins in Streptococcus pneumoniae D39. Antimicrob Agents Chemother 59:3548–3555
Grebe T, Hakenbeck R (1996) Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob Agents Chemother 40:829–834
Dahesh S, Hensler ME, Van Sorge NM, Gertz RE Jr, Schrag S, Nizet V, Beall BW (2008) Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to β-lactam antibiotics. Antimicrob Agents Chemother 52:2915–2918
Krauß JAN, van der Linden M, Grebe T, Hakenbeck R (1996) Penicillin-binding proteins 2x and 2b as primary PBP targets in Streptococcus pneumoniae. Microb Drug Resist 2:183–186
Albarracín Orio AG, Piñas GE, Cortes PR, Cian MB, Echenique J (2011) Compensatory evolution of pbp mutations restores the fitness cost imposed by β-lactam resistance in Streptococcus pneumoniae. PLoS Pathog 7:e1002000
Hadjirin NF, Miller EL, Murray GGR, Yen PLK, Phuc HD, Wileman TM, Hernandez-Garcia J, Williamson SM, Parkhill J, Maskell DJ (2021) Large-scale genomic analysis of antimicrobial resistance in the zoonotic pathogen Streptococcus suis. BMC Biol 19:191
Hakenbeck R, Brückner R, Denapaite D, Maurer P (2012) Molecular mechanisms of β-lactam resistance in Streptococcus pneumoniae. Future Microbiol 7:395–410
Li Y, Metcalf BJ, Chochua S, Li Z, Gertz RE Jr, Walker H, Hawkins PA, Tran T, Whitney CG, McGee L (2016) Penicillin-binding protein transpeptidase signatures for tracking and predicting β-lactam resistance levels in Streptococcus pneumoniae. mBio 7:e00756-16
Bamphensin N, Chopjitt P, Hatrongjit R, Boueroy P, Fittipaldi N, Gottschalk M, Kerdsin A (2021) Non-penicillin-susceptible Streptococcus suis isolated from humans. Pathogens 10:1178
Guenzi E, Gasc AM, Sicard MA, Hakenbeck R (1994) A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol Microbiol 12:505–515
Grebe T, Paik J, Hakenbeck R (1997) A novel resistance mechanism against beta-lactams in Streptococcus pneumoniae involves CpoA, a putative glycosyltransferase. J Bacteriol 179:3342–3349
Smith AM, Klugman KP (2001) Alterations in MurM, a cell wall muropeptide branching enzyme, increase high-level penicillin and cephalosporin resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother 45:2393–2396
He LY, Le YJ, Guo Z, Li S, Yang XY (2021) The role and regulatory network of the CiaRH two-component system in streptococcal species. Front Microbiol 12:693858
Seo HS, Cartee RT, Pritchard DG, Nahm MH (2008) A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J Bacteriol 190:2379–2387
Garcia-Bustos J, Tomasz A (1990) A biological price of antibiotic resistance: major changes in the peptidoglycan structure of penicillin-resistant pneumococci. Proc Natl Acad Sci USA 87:5415–5419
Filipe SR, Pinho MG, Tomasz A (2000) Characterization of the murMN operon involved in the synthesis of branched peptidoglycan peptides in Streptococcus pneumoniae. J Biol Chem 275:27768–27774
Filipe SR, Severina E, Tomasz A (2001) The role of murMN operon in penicillin resistance and antibiotic tolerance of Streptococcus pneumoniae. Microb Drug Resist 7:303–316
Davis AR, Gohara DW, Yap MNF (2014) Sequence selectivity of macrolide-induced translational attenuation. Proc Natl Acad Sci USA 111:15379–15384
Gupta P, Sothiselvam S, Vázquez-Laslop N, Mankin AS (2013) Deregulation of translation due to post-transcriptional modification of rRNA explains why erm genes are inducible. Nat Commun 4:1984
Chang B, Wada A, Ikebe T, Ohnishi M, Mita K, Endo M, Matsuo H, Asatuma Y, Kuramoto S, Sekiguchi H (2006) Characteristics of Streptococcus suis isolated from patients in Japan. Jpn J Infect Dis 59:397
Syrogiannopoulos GA, Grivea IN, Fitoussi F, Doit C, Katopodis GD, Bingen E, Beratis NG (2001) High prevalence of erythromycin resistance of Streptococcus pyogenes in Greek children. Pediatr Infect Dis J 20:863–868
Camilli R, Del Grosso M, Iannelli F, Pantosti A (2008) New genetic element carrying the erythromycin resistance determinant erm(TR) in Streptococcus pneumoniae. Antimicrob Agents Chemother 52:619–625
Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B (2006) The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob Agents Chemother 50:2500–2505
Kehrenberg C, Cuny C, Strommenger B, Schwarz S, Witte W (2009) Methicillin-resistant and-susceptible Staphylococcus aureus strains of clonal lineages ST398 and ST9 from swine carry the multidrug resistance gene cfr. Antimicrob Agents Chemother 53:779–781
Zhang W-J, Wu C-M, Wang Y, Shen Z-Q, Dai L, Han J, Foley SL, Shen J-Z, Zhang Q (2011) The new genetic environment of cfr on plasmid pBS-02 in a Bacillus strain. J Antimicrob Chemother 66:1174–1175
Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du X-D, Dai L, Zhang W, Zhang Q, Shen J (2012) First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother 56:1650–1654
Huang J, Sun J, Wu Y, Chen L, Duan D, Lv X, Wang L (2019) Identification and pathogenicity of an XDR Streptococcus suis isolate that harbours the phenicol-oxazolidinone resistance genes optrA and cfr, and the bacitracin resistance locus bcrABDR. Int J Antimicrob Agents 54:43–48
Wang Y, Li D, Song L, Liu Y, He T, Liu H, Wu C, Schwarz S, Shen J (2013) First report of the multiresistance gene cfr in Streptococcus suis. Antimicrob Agents Chemother 57:4061–4063
Ambrose KD, Nisbet R, Stephens DS (2005) Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrob Agents Chemother 49:4203–4209
Sharkey LKR, Edwards TA, O’Neill AJ (2016) ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio 7:e01975
Zhang Y, Tatsuno I, Okada R, Hata N, Matsumoto M, Isaka M, Isobe K-i, Hasegawa T (2016) Predominant role of msr(D) over mef(A) in macrolide resistance in Streptococcus pyogenes. Microbiology 162:46–52
Gurung M, Tamang MD, Moon DC, Kim S-R, Jeong J-H, Jang G-C, Jung S-C, Park Y-H, Lim S-K (2015) Molecular basis of resistance to selected antimicrobial agents in the emerging zoonotic pathogen Streptococcus suis. J Clin Microbiol 53:2332–2336
Huang J, Ma J, Shang K, Hu X, Liang Y, Li D, Wu Z, Dai L, Chen L, Wang L (2016) Evolution and diversity of the antimicrobial resistance associated mobilome in Streptococcus suis: a probable mobile genetic elements reservoir for other streptococci. Front Cell Infect Microbiol 6:118
Vester B, Douthwaite S (2001) Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob Agents Chemother 45:1–12
Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JHD (2005) Structures of the bacterial ribosome at 3.5 A resolution. Science 310:827–834
Bozdogan B, Bogdanovich T, Kosowska K, Jacobs MR, Appelbaum PC (2004) Macrolide resistance in Streptococcus pneumoniae: clonality and mechanisms of resistance in 24 countries. Curr Drug Targets Infect Disord 4:169–176
Bryskier A (ed) (2005) Mutilins. Antimicrobial Agents Antibacterials and Antifungals. Wiley, Hoboken, pp 1239–1241
Novak R, Shlaes DM (2010) The pleuromutilin antibiotics: a new class for human use. Curr Opin Investig Drugs 11:182–191
Schwarz S, Shen J, Kadlec K, Wang Y, Michael GB, Feßler AT, Vester B (2016) Lincosamides, streptogramins, phenicols, and pleuromutilins: mode of action and mechanisms of resistance. Cold Spring Harb Perspect Med 6:a027037
Murray IA, Shaw WV (1997) O-Acetyltransferases for chloramphenicol and other natural products. Antimicrob Agents Chemother 41:1–6
Shang Y, Li D, Hao W, Schwarz S, Shan X, Liu B, Zhang S-M, Li X-S, Du X-D (2019) A prophage and two ICESa2603-family integrative and conjugative elements (ICEs) carrying optrA in Streptococcus suis. J Antimicrob Chemother 74:2876–2879
Gómez-Sanz E, Kadlec K, Feßler AT, Zarazaga M, Torres C, Schwarz S (2013) A novel fexA variant from a canine Staphylococcus pseudintermedius isolate that does not confer florfenicol resistance. Antimicrob Agents Chemother 57:5763–5766
Wendlandt S, Lozano C, Kadlec K, Gómez-Sanz E, Zarazaga M, Torres C, Schwarz S (2013) The enterococcal ABC transporter gene lsa(E) confers combined resistance to lincosamides, pleuromutilins and streptogramin A antibiotics in methicillin-susceptible and methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 68:473–475
Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T (2015) A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190
Huang J, Chen L, Wu Z, Wang L (2017) Retrospective analysis of genome sequences revealed the wide dissemination of optrA in Gram-positive bacteria. J Antimicrob Chemother 72:614–616
Pioletti M, Schlünzen F, Harms J, Zarivach R, Glühmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C (2001) Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J 20:1829–1839
Lupien A, Gingras H, Leprohon P, Ouellette M (2015) Induced tigecycline resistance in Streptococcus pneumoniae mutants reveals mutations in ribosomal proteins and rRNA. J Antimicrob Chemother 70:2973–2980
Dönhöfer A, Franckenberg S, Wickles S, Berninghausen O, Beckmann R, Wilson DN (2012) Structural basis for TetM-mediated tetracycline resistance. Proc Natl Acad Sci USA 109:16900–16905
Liu B, Yi L, Li J, Wang Y, Mao C, Wang Y (2020) Autoinducer-2 influences tetracycline resistance in Streptococcus suis by regulating the tet(M) gene via transposon Tn916. Res Vet Sci 128:269–274
Chopra I, Roberts M (2001) Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260
Thaker M, Spanogiannopoulos P, Wright GD (2010) The tetracycline resistome. Cell Mol Life Sci 67:419–431
Maskell JP, Sefton AM, Hall LMC (2001) Multiple mutations modulate the function of dihydrofolate reductase in trimethoprim-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 45:1104–1108
Lopez P, Espinosa M, Greenberg B, Lacks SAt, (1987) Sulfonamide resistance in Streptococcus pneumoniae: DNA sequence of the gene encoding dihydropteroate synthase and characterization of the enzyme. J Bacteriol 169:4320–4326
Padayachee T, Klugman KP (1999) Novel expansions of the gene encoding dihydropteroate synthase in trimethoprim-sulfamethoxazole-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 43:2225–2230
Manyahi J, Moyo S, Aboud S, Langeland N, Blomberg B (2020) High rate of antimicrobial resistance and multiple mutations in the dihydrofolate reductase gene among Streptococcus pneumoniae isolated from HIV-infected adults in a community setting in Tanzania. J Glob Antimicrob Resist 22:749–753
Gt S, Ringertz S, Sköld O (1998) Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of its chromosomal dihydropteroate synthase. Antimicrob Agents Chemother 42:1062–1067
Brochet M, Couvé E, Zouine M, Poyart C, Glaser P (2008) A naturally occurring gene amplification leading to sulfonamide and trimethoprim resistance in Streptococcus agalactiae. J Bacteriol 190:672–680
Ramirez MS, Tolmasky ME (2010) Aminoglycoside modifying enzymes. Drug Resist Updat 13:151–171
Wang S, Zhang D, Jiang C, He H, Cui C, Duan W, Hu S, Wang J, Cai X (2021) Strain characterization of Streptococcus suis serotypes 28 and 31, which harbor the resistance genes optrA and ant(6)-Ia. Pathogen 10:213
Liang P, Wang M, Gottschalk M, Vela AI, Estrada AA, Wang J, Du P, Luo M, Zheng H, Wu Z (2021) Genomic and pathogenic investigations of Streptococcus suis serotype 7 population derived from a human patient and pigs. Emerg Microbes Infect 10:1960–1974
Du F, Lv X, Duan D, Wang L, Huang J (2019) Characterization of a linezolid-and vancomycin-resistant Streptococcus suis isolate that harbors optrA and vanG operons. Front Microbiol 10:2026
Krause KM, Serio AW, Kane TR, Connolly LE (2016) Aminoglycosides: an overview. Cold Spring Harb Perspect Med 6:a027029
Yongkiettrakul S, Wongsurawat T, Jenjaroenpun P, Acheampong DA, Srimanote P, Maneerat K, Visessanguan W, Nookaew I (2021) Genome sequences of antibiotic-resistant Streptococcus suis strains isolated from human patients and diseased and asymptomatic pigs in Thailand. Infect Genet Evol 87:104674
Pootoolal J, Neu J, Wright GD (2002) Glycopeptide antibiotic resistance. Annu Rev Pharmacol Toxicol 42:381–408
Huang J, Chen L, Li D, Wang M, Du F, Gao Y, Wu Z, Wang L (2018) Emergence of a vanG-carrying and multidrug resistant ICE in zoonotic pathogen Streptococccus suis. Vet Microbiol 222:109–113
Lai L, Dai J, Tang H, Zhang S, Wu C, Qiu W, Lu C, Yao H, Fan H, Wu Z (2017) Streptococcus suis serotype 9 strain GZ0565 contains a type VII secretion system putative substrate EsxA that contributes to bacterial virulence and a vanZ-like gene that confers resistance to teicoplanin and dalbavancin in Streptococcus agalactiae. Vet Microbiol 205:26–33
Depardieu F, Bonora MG, Reynolds PE, Courvalin P (2003) The vanG glycopeptide resistance operon from Enterococcus faecalis revisited. Mol Microbiol 50:931–948
Woods EC, Wetzel D, Mukerjee M, McBride SM (2018) Examination of the Clostridioides (Clostridium) difficile VanZ ortholog, CD1240. Anaerobe 53:108–115
Arthur M, Depardieu F, Molinas C, Reynolds P, Courvalin P (1995) The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 154:87–92
Millanao AR, Mora AY, Villagra NA, Bucarey SA, Hidalgo AA (2021) Biological effects of quinolones: a family of broad-spectrum antimicrobial agents. Molecules 26:7153
Bush NG, Diez-Santos I, Abbott LR, Maxwell A (2020) Quinolones: mechanism, lethality and their contributions to antibiotic resistance. Molecules 25:5662
Levine C, Hiasa H, Marians KJ (1998) DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim Biophys Acta 1400:29–43
Champoux JJ (2001) DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70:369–413
Pan XS, Fisher LM (1998) DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother 42:2810–2816
Yao J, Shang K, Huang J, Ran W, Kashif J, Wang L (2014) Overexpression of an ABC transporter and mutations of GyrA, GyrB, and ParC in contributing to high-level ciprofloxacin resistance in Streptococcus suis type 2. Biosci Trends 8:84–92
Wohlkonig A, Chan PF, Fosberry AP, Homes P, Huang J, Kranz M, Leydon VR, Miles TJ, Pearson ND, Perera RL (2010) Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat Struct Mol Biol 17:1152–1153
Ostrer L, Khodursky RF, Johnson JR, Hiasa H, Khodursky A (2019) Analysis of mutational patterns in quinolone resistance-determining regions of GyrA and ParC of clinical isolates. Int J Antimicrob Agents 53:318–324
Hooper DC, Jacoby GA (2016) Topoisomerase inhibitors: fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med 6:a025320
Escudero JA, San Millan A, Gutierrez B, Hidalgo L, La Ragione RM, AbuOun M, Galimand M, Ferrándiz MJ, Domínguez L, de la Campa AG (2011) Fluoroquinolone efflux in Streptococcus suis is mediated by SatAB and not by SmrA. Antimicrob Agents Chemother 55:5850–5860
Escudero JA, San Millan A, Montero N, Gutierrez B, Ovejero CM, Carrilero L, González-Zorn B (2013) SatR is a repressor of fluoroquinolone efflux pump SatAB. Antimicrob Agents Chemother 57:3430–3433
Wang Y, Liu B, Li J, Gong S, Dong X, Mao C, Yi L (2019) LuxS/AI-2 system is involved in fluoroquinolones susceptibility in Streptococcus suis through overexpression of efflux pump SatAB. Vet Microbiol 233:154–158
Garvey MI, Baylay AJ, Wong RL, Piddock LJV (2011) Overexpression of patA and patB, which encode ABC transporters, is associated with fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 55:190–196
El Garch F, Lismond A, Piddock LJV, Courvalin P, Tulkens PM, Van Bambeke F (2010) Fluoroquinolones induce the expression of patA and patB, which encode ABC efflux pumps in Streptococcus pneumoniae. J Antimicrob Chemother 65:2076–2082
Piddock LJV, Johnson MM, Simjee S, Pumbwe L (2002) Expression of efflux pump gene pmrA in fluoroquinolone-resistant and-susceptible clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 46:808–812
Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC (2006) Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88
Palmieri C, Varaldo PE, Facinelli B (2011) Streptococcus suis, an emerging drug-resistant animal and human pathogen. Front Microbiol 2:235
Huang J, Liang Y, Guo D, Shang K, Ge L, Kashif J, Wang L (2016) Comparative genomic analysis of the ICESa2603 family ICEs and spread of erm(B)-and tet(O)-carrying transferable 89K-subtype ICEs in swine and bovine isolates in China. Front Microbiol 7:55
Bellanger X, Payot S, Leblond-Bourget N, Guédon G (2014) Conjugative and mobilizable genomic islands in bacteria: evolution and diversity. FEMS Microbiol Rev 38:720–760
Libante V, Nombre Y, Coluzzi C, Staub J, Guédon G, Gottschalk M, Teatero S, Fittipaldi N, Leblond Bourget N, Payot S (2020) Chromosomal conjugative and mobilizable elements in Streptococcus suis: Major actors in the spreading of antimicrobial resistance and bacteriocin synthesis genes. Pathogens 9:22
Johnson CM, Grossman AD (2015) Integrative and conjugative elements (ICEs): what they do and how they work. Annu Rev Genet 49:577–601
Burrus V, Pavlovic G, Decaris B, Guédon G (2002) The ICESt1 element of Streptococcus thermophilus belongs to a large family of integrative and conjugative elements that exchange modules and change their specificity of integration. Plasmid 48:77–97
Holden MTG, Hauser H, Sanders M, Ngo TH, Cherevach I, Cronin A, Goodhead I, Mungall K, Quail MA, Price C, Rabbinowitsch E, Sharp S, Croucher NJ, Chieu TB, Mai NT, Diep TS, Chinh NT, Kehoe M, Leigh JA, Ward PN, Dowson CG, Whatmore AM, Chanter N, Iversen P, Gottschalk M, Slater JD, Smith HE, Spratt BG, Xu J, Ye C et al (2009) Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One 4:e6072
Huang K, Song Y, Zhang Q, Zhang A, Jin M (2016) Characterisation of a novel integrative and conjugative element ICESsD9 carrying erm(B) and tet(O) resistance determinants in Streptococcus suis, and the distribution of ICESsD9-like elements in clinical isolates. J Glob Antimicrob Resist 7:13–18
Marini E, Palmieri C, Magi G, Facinelli B (2015) Recombination between Streptococcus suis ICESsu32457 and Streptococcus agalactiae ICESa2603 yields a hybrid ICE transferable to Streptococcus pyogenes. Vet Microbiol 178:99–104
Shanker E, Morrison DA, Talagas A, Nessler S, Federle MJ, Prehna G (2016) Pheromone recognition and selectivity by ComR proteins among Streptococcus species. PLoS Pathog 12:e1005979
Laurenceau R, Péhau-Arnaudet G, Baconnais S, Gault J, Malosse C, Dujeancourt A, Campo N, Chamot-Rooke J, Le Cam E, Claverys J-P (2013) A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog 9:e1003473
Fontaine L, Wahl A, Fléchard M, Mignolet J, Hols P (2015) Regulation of competence for natural transformation in streptococci. Infect Genet Evol 33:343–360
Zaccaria E, van Baarlen P, de Greeff A, Morrison DA, Smith H, Wells JM (2014) Control of competence for DNA transformation in Streptococcus suis by genetically transferable pherotypes. PLoS One 9:e99394
Hirst RA, Gosai B, Rutman A, Guerin CJ, Nicotera P, Andrew PW, O’Callaghan C (2008) Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J Infect Dis 197:744–751
Fernebro J, Andersson I, Sublett J, Morfeldt E, Novak R, Tuomanen E, Normark S, Normark BH (2004) Capsular expression in Streptococcus pneumoniae negatively affects spontaneous and antibiotic-induced lysis and contributes to antibiotic tolerance. J Infect Dis 189:328–338
Zhu Y, Ma J, Zhang Y, Zhong X, Bai Q, Dong W, Pan Z, Liu G, Zhang C, Yao H (2021) CrfP, a fratricide protein, contributes to natural transformation in Streptococcus suis. Vet Res 52:50
Håvarstein LS, Martin B, Johnsborg O, Granadel C, Claverys JP (2006) New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol Microbiol 59:1297–1307
LeBel G, Vaillancourt K, Frenette M, Gottschalk M, Grenier D (2014) Suicin 90–1330 from a nonvirulent strain of Streptococcus suis: a nisin-related lantibiotic active on gram-positive swine pathogens. Appl Environ Microbiol 80:5484–5492
Vaillancourt K, LeBel G, Frenette M, Fittipaldi N, Gottschalk M, Grenier D (2015) Purification and characterization of suicin 65, a novel class I type B lantibiotic produced by Streptococcus suis. PLoS ONE 10:e0145854
Vaillancourt K, LeBel G, Frenette M, Gottschalk M, Grenier D (2015) Suicin 3908, a new lantibiotic produced by a strain of Streptococcus suis serotype 2 isolated from a healthy carrier pig. PLoS ONE 10:e0117245
Yu R, Zhang Y, Xu Y, Schwarz S, Li X-S, Shang Y-H, Du X-D (2021) Emergence of a tet(M) variant conferring resistance to tigecycline in Streptococcus suis. Front Vet Sci 8:709327
Hakenbeck R (1995) Target-mediated resistance to β-lactam antibiotics. Biochem Pharmacol 50:1121–1127
Chi F, Nolte O, Bergmann C, Ip M, Hakenbeck R (2007) Crossing the barrier: evolution and spread of a major class of mosaic pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. Int J Med Microbiol 297:503–512
Rieger M, Mauch H, Hakenbeck R (2017) Long persistence of a Streptococcus pneumoniae 23F clone in a cystic fibrosis patient. mSphere 2:e00201-17
Calero-Cáceres W, Méndez J, Martín-Díaz J, Muniesa M (2017) The occurrence of antibiotic resistance genes in a Mediterranean river and their persistence in the riverbed sediment. Environ Pollut 223:384–394
Chen J, Quiles-Puchalt N, Chiang YN, Bacigalupe R, Fillol-Salom A, Chee MSJ, Fitzgerald JR, Penadés JR (2018) Genome hypermobility by lateral transduction. Science 362:207–212
Giovanetti E, Brenciani A, Morroni G, Tiberi E, Pasquaroli S, Mingoia M, Varaldo PE (2015) Transduction of the Streptococcus pyogenes bacteriophage Φm46.1, carrying resistance genes mef(A) and tet(O), to other Streptococcus species. Front Microbiol. 5:746
Brenciani A, Bacciaglia A, Vignaroli C, Pugnaloni A, Varaldo PE, Giovanetti E (2010) Φm46. 1, the main Streptococcus pyogenes element carrying mef(A) and tet(O) genes. Antimicrob Agents Chemother 54:221–229
Zheng H, Du P, Qiu X, Kerdsin A, Roy D, Bai X, Xu J, Vela AI, Gottschalk M (2018) Genomic comparisons of Streptococcus suis serotype 9 strains recovered from diseased pigs in Spain and Canada. Vet Res 49:1
Cochetti I, Vecchi M, Mingoia M, Tili E, Catania MR, Manzin A, Varaldo PE, Montanari MP (2005) Molecular characterization of pneumococci with efflux-mediated erythromycin resistance and identification of a novel mef gene subclass, mef(I). Antimicrob Agents Chemother 49:4999–5006
Coelho C, Brown L, Maryam M, Vij R, Smith DFQ, Burnet MC, Kyle JE, Heyman HM, Ramirez J, Prados-Rosales R (2019) Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J Biol Chem 294:1202–1217
Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE (2003) Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35
Fernandez-Moreira E, Helbig JH, Swanson MS (2006) Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes. Infect Immun 74:3285–3295
Kim Y, Edwards N, Fenselau C (2016) Extracellular vesicle proteomes reflect developmental phases of Bacillus subtilis. Clin Proteomics 13:6
Mashburn LM, Whiteley M (2005) Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425
Arenas J, Catón L, van den Hoeven T, de Maat V, Cruz Herrero J, Tommassen J (2020) The outer-membrane protein MafA of Neisseria meningitidis constitutes a novel protein secretion pathway specific for the fratricide protein MafB. Virulence 11:1701–1715
Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, Hanawa T, Kamiya S (2009) Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol 9:197
Rumbo C, Fernández-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, Mosquera A, Chaves F, Bou G (2011) Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother 55:3084–3090
Sabra W, Lünsdorf H, Zeng AP (2003) Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology 149:2789–2795
Liao S, Klein MI, Heim KP, Fan Y, Bitoun JP, Ahn S-J, Burne RA, Koo H, Brady LJ, Wen ZT (2014) Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol 196:2355–2366
Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martín-Peña R, González-Reyes JA, Jiménez-Munguía I, Gómez-Gascón L, Fernández J, Luque-García JL, García-Lidón C (2014) Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics 106:46–60
Codemo M, Muschiol S, Iovino F, Nannapaneni P, Plant L, Wai SN, Henriques-Normark B (2018) Immunomodulatory effects of pneumococcal extracellular vesicles on cellular and humoral host defenses. mBio 9:e00559-18
Olaya-Abril A, González-Reyes JA, Rodríguez-Ortega MJ (2021) Approaching in vivo models of pneumococcus–host interaction: insights into surface proteins, capsule production, and extracellular vesicles. Pathogens 10:1098
Mehanny M, Koch M, Lehr CM, Fuhrmann G (2020) Streptococcal extracellular membrane vesicles are rapidly internalized by immune cells and alter their cytokine release. Front Immunol 11:80
Haas B, Grenier D (2015) Isolation, characterization and biological properties of membrane vesicles produced by the swine pathogen Streptococcus suis. PLoS One 10:e0130528
Schaar V, Nordström T, Mörgelin M, Riesbeck K (2011) Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother 55:3845–3853
Uruén C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J (2021) Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 10:3
Jacques M, Gottschalk M, Foiry B, Higgins R (1990) Ultrastructural study of surface components of Streptococcus suis. J Bacteriol 172:2833–2838
Garibaldi M, Rodríguez-Ortega MJ, Mandanici F, Cardaci A, Midiri A, Papasergi S, Gambadoro O, Cavallari V, Teti G, Beninati C (2010) Immunoprotective activities of a Streptococcus suis pilus subunit in murine models of infection. Vaccine 28:3609–3616
Chuzeville S, Auger J-P, Dumesnil A, Roy D, Lacouture S, Fittipaldi N, Grenier D, Gottschalk M (2017) Serotype-specific role of antigen I/II in the initial steps of the pathogenesis of the infection caused by Streptococcus suis. Vet Res 48:39
Tanabe S-I, Bonifait L, Fittipaldi N, Grignon L, Gottschalk M, Grenier D (2010) Pleiotropic effects of polysaccharide capsule loss on selected biological properties of Streptococcus suis. Can J Vet Res 74:65–70
Muñoz Egea MC, García Pedrazuela M, Mahillo Fernandez I, Esteban J (2016) Effect of antibiotics and antibiofilm agents in the ultrastructure and development of biofilms developed by nonpigmented rapidly growing mycobacteria. Microb Drug Resist 22:1–6
Dawei G, Liping W, Chengping L (2012) In vitro biofilm forming potential of Streptococcus suis isolated from human and swine in China. Braz J Microbiol 43:993–1004
Lewis K (2007) Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56
Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS (2003) Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323
Zhang T, Zhu J, Wei S, Luo Q, Li L, Li S, Tucker A, Shao H, Zhou R (2016) The roles of RelA/(p)ppGpp in glucose-starvation induced adaptive response in the zoonotic Streptococcus suis. Sci Rep 6:27169
Zhang T, Zhu J, Xu J, Shao H, Zhou R (2020) Regulation of (p)ppGpp and its homologs on environmental adaptation, survival, and pathogenicity of Streptococci. Front Microbiol 11:1842
Zhu J, Zhang T, Su Z, Li L, Wang D, Xiao R, Teng M, Tan M, Zhou R (2016) (p)ppGpp synthetases regulate the pathogenesis of zoonotic Streptococcus suis. Microbiol Res 191:1–11
Zhu J, Zhang T, Su Z, Feng L, Liu H, Xu Z, Wu Y, Gao T, Shao H, Zhou R (2019) Co-regulation of CodY and (p)ppGpp synthetases on morphology and pathogenesis of Streptococcus suis. Microbiol Res 223:88–98
Hobbs JK, Boraston AB (2019) (p)ppGpp and the stringent response: an emerging threat to antibiotic therapy. ACS Infect Dis 5:1505–1517
Wang Y, Wang Y, Liu B, Wang S, Li J, Gong S, Sun L, Yi L (2019) pdh modulate virulence through reducing stress tolerance and biofilm formation of Streptococcus suis serotype 2. Virulence 10:588–599
Yi L, Fan Q, Wang Y, Mao C, Li J, Jin M, Zhang X, Ding K, Wang Y (2021) Evaluation of immune effect of Streptococcus suis biofilm-associated protein PDH. Vet Microbiol 263:109270
Weinert LA, Chaudhuri RR, Wang J, Peters SE, Corander J, Jombart T, Baig A, Howell KJ, Vehkala M, Välimäki N (2015) Genomic signatures of human and animal disease in the zoonotic pathogen Streptococcus suis. Nat Commun 6:6740
Grenier D, Grignon L, Gottschalk M (2009) Characterisation of biofilm formation by a Streptococcus suis meningitis isolate. Vet J 179:292–295
Doroshenko N, Tseng BS, Howlin RP, Deacon J, Wharton JA, Thurner PJ, Gilmore BF, Parsek MR, Stoodley P (2014) Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob Agents Chemother 58:7273–7282
Moscoso M, García E, López R (2006) Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol 188:7785–7795
Petersen FC, Tao L, Scheie AA (2005) DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J Bacteriol 187:4392–4400
Petersen FC, Pecharki D, Scheie AA (2004) Biofilm mode of growth of Streptococcus intermedius favored by a competence-stimulating signaling peptide. J Bacteriol 186:6327–6331
Ma F, Yi L, Yu N, Wang G, Ma Z, Lin H, Fan H (2017) Streptococcus suis serotype 2 biofilms inhibit the formation of neutrophil extracellular traps. Front Cell Infect Microbiol 7:86
Ju CX, Gu HW, Lu CP (2012) Characterization and functional analysis of atl, a novel gene encoding autolysin in Streptococcus suis. J Bacteriol 194:1464–1473
Zhang Y, Zhong X, Lu P, Zhu Y, Dong W, Roy S, Hejair HMA, Pan Z, Ma J, Yao H (2019) A novel autolysin AtlASS mediates bacterial cell separation during cell division and contributes to full virulence in Streptococcus suis. Vet Microbiol 234:92–100
Waack U, Nicholson TL (2018) Subinhibitory concentrations of amoxicillin, lincomycin, and oxytetracycline commonly used to treat swine increase Streptococcus suis biofilm formation. Front Microbiol 9:2707
Bonifait L, Grignon L, Grenier D (2008) Fibrinogen induces biofilm formation by Streptococcus suis and enhances its antibiotic resistance. Appl Environ Microbiol 74:4969–4972
Bjarnsholt T, Jensen PØ, Burmølle M, Hentzer M, Haagensen JAJ, Hougen HP, Calum H, Madsen KG, Moser C, Molin S (2005) Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151:373–383
Yarwood JM, Bartels DJ, Volper EM, Greenberg EP (2004) Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 186:1838–1850
Wang Y, Yi L, Zhang Z, Fan H, Cheng X, Lu C (2014) Biofilm formation, host-cell adherence, and virulence genes regulation of Streptococcus suis in response to autoinducer-2 signaling. Curr Microbiol 68:575–580
Wang Y, Zhang W, Wu Z, Zhu X, Lu C (2011) Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence. Vet Microbiol 152:151–160
Sun Y, Veseli IA, Vaillancourt K, Frenette M, Grenier D, Pombert J-F (2019) The bacteriocin from the prophylactic candidate Streptococcus suis 90–1330 is widely distributed across S. suis isolates and appears encoded in an integrative and conjugative element. PLoS One 14:e0216002
Wang Y, Gong S, Dong X, Li J, Grenier D, Yi L (2020) In vitro mixed biofilm of Streptococcus suis and Actinobacillus pleuropneumoniae impacts antibiotic susceptibility and modulates virulence factor gene expression. Front Microbiol 11:507
Hannan S, Ready D, Jasni AS, Rogers M, Pratten J, Roberts AP (2010) Transfer of antibiotic resistance by transformation with eDNA within oral biofilms. FEMS Microbiol Immunol 59:345–349
Bernier SP, Lebeaux D, DeFrancesco AS, Valomon A, Soubigou G, Coppée J-Y, Ghigo J-M, Beloin C (2013) Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet 9:e1003144
Beaber JW, Hochhut B, Waldor MK (2004) SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 427:72–74
Driffield K, Miller K, Bostock JM, O’Neill AJ, Chopra I (2008) Increased mutability of Pseudomonas aeruginosa in biofilms. J Antimicrob Chemother 61:1053–1056
Banas JA, Miller JD, Fuschino ME, Hazlett KRO, Toyofuku W, Porter KA, Reutzel SB, Florczyk MA, McDonough KA, Michalek SM (2007) Evidence that accumulation of mutants in a biofilm reflects natural selection rather than stress-induced adaptive mutation. Appl Environ Microbiol 73:357–361
Ferrando ML, Gussak A, Mentink S, Gutierrez MF, van Baarlen P, Wells JM (2021) Active human and porcine serum induce competence for genetic transformation in the emerging zoonotic pathogen Streptococcus suis. Pathogens 10:156
Guzmán-Trampe S, Ceapa CD, Manzo-Ruiz M, Sánchez S (2017) Synthetic biology era: Improving antibiotic’s world. Biochem Pharmacol 134:99–113
de Aguiar FC, Solarte AL, Tarradas C, Luque I, Maldonado A, Galán-Relaño Á, Huerta B (2018) Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiologyopen 7:e00613
Tang F, Lu C (2015) Lytic phages and prophages of Streptococcus suis-a review. Wei Sheng Wu Xue Bao 55:389–394
Gilmer DB, Schmitz JE, Thandar M, Euler CW, Fischetti VA (2017) The phage lysin PlySs2 decolonizes Streptococcus suis from murine intranasal mucosa. PLoS One 12:e0169180
Wang Z, Ma J, Wang J, Yang D, Kong L, Fu Q, Cheng Y, Wang H, Yan Y, Sun J (2019) Application of the phage lysin Ply5218 in the treatment of Streptococcus suis infection in piglets. Viruses 11:715
Fittipaldi N, Harel J, D’Amours B, Lacouture S, Kobisch M, Gottschalk M (2007) Potential use of an unencapsulated and aromatic amino acid-auxotrophic Streptococcus suis mutant as a live attenuated vaccine in swine. Vaccine 25:3524–3535
Li M, Cai RJ, Li CL, Song S, Li Y, Jiang ZY, Yang DX (2017) Deletion of ssnA attenuates the pathogenicity of Streptococcus suis and confers protection against serovar 2 strain challenge. PLoS One 12:e0169791
Li Z, Chang P, Xu J, Tan C, Wang X, Bei W, Li J (2019) A Streptococcus suis live vaccine suppresses streptococcal toxic shock-like syndrome and provides sequence type-independent protection. J Infect Dis 219:448–458
Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M (2014) Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect 3:e45
Ahmed W, Neubauer H, Tomaso H, El Hofy FI, Monecke S, Abdeltawab AA, Hotzel H (2020) Characterization of staphylococci and streptococci isolated from milk of bovides with mastitis in Egypt. Pathogens 9:381
Bojarska A, Molska E, Janas K, Skoczyńska A, Stefaniuk E, Hryniewicz W, Sadowy E (2016) Streptococcus suis in invasive human infections in Poland: clonality and determinants of virulence and antimicrobial resistance. Eur J Clin Microbiol Infect Dis 35:917–925
Zhao Q, Wendlandt S, Li H, Li J, Wu C, Shen J, Schwarz S, Wang Y (2014) Identification of the novel lincosamide resistance gene lnu(E) truncated by ISEnfa5-cfr-ISEnfa5 insertion in Streptococcus suis: de novo synthesis and confirmation of functional activity in Staphylococcus aureus. Antimicrob Agents Chemother 58:1785–1788
Yu R, Xu Y, Schwarz S, Shang Y, Yuan X, Zhang Y, Li D, Du X-D (2022) erm(T)-mediated macrolide-lincosamide resistance in Streptococcus suis. Microbiol Spectr 10:e0165721
Chander Y, Oliveira SR, Goyal SM (2011) Identification of the tet(B) resistance gene in Streptococcus suis. Vet J 189:359–360
Escudero JA, San Millan A, Catalan A, de la Campa AG, Rivero E, Lopez G, Dominguez L, Moreno MA, Gonzalez-Zorn B (2007) First characterization of fluoroquinolone resistance in Streptococcus suis. Antimicrob Agents Chemother 51:777–782
Shaw KJ, Rather PN, Hare RS, Miller GH (1993) Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev 57:138–163
Novick RP, Clowes RC, Cohen SN, Curtiss R 3rd, Datta N, Falkow S (1976) Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev 40:168–189
Palmieri C, Magi G, Mingoia M, Bagnarelli P, Ripa S, Varaldo PE, Facinelli B (2012) Characterization of a Streptococcus suis tet(O/W/32/O)-carrying element transferable to major streptococcal pathogens. Antimicrob Agents Chemother 56:4697–4702
Guérillot R, Da Cunha V, Sauvage E, Bouchier C, Glaser P (2013) Modular evolution of TnGBSs, a new family of integrative and conjugative elements associating insertion sequence transposition, plasmid replication, and conjugation for their spreading. J Bacteriol 195:1979–1990
Funding
This work received funding from Gobierno de Aragón (Department of Science, University and Knowledge Society) for the development of I + D + i projects in priority lines (Grant agreement LMP58_21) and from Ministerio de Ciencia e Innovación/Agencia Española de Investigacion MCIN/AEI/10.13039/501100011033 and, as appropriate, by “ERDF A way of making Europe”, by the “European Union” or by the “European Union NextGenerationEU/PRTR¨ (Grant agreement PID2020-114617RB-I00).
Author information
Authors and Affiliations
Contributions
Conceptualization, JA; Methodology, CU, CG, JA. Writing—Original draft preparation, CU, CG, LF, JA; Writing—Review and editing, CU, CG, LF, JT, JA; Visualization, CU, CG, JA; Supervision, JA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Uruén, C., García, C., Fraile, L. et al. How Streptococcus suis escapes antibiotic treatments. Vet Res 53, 91 (2022). https://doi.org/10.1186/s13567-022-01111-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-022-01111-3