Abstract
Background
Over the past few years, mesenchymal stromal cells (MSCs) have attracted a great deal of scientific attention owing to their promising results in the treatment of incurable diseases. However, there are several concerns about their possible side effects after direct cell transplantation, including host immune response, time-consuming cell culture procedures, and the dependence of cell quality on the donor, which limit the application of MSCs in clinical trials. On the other hand, it is well accepted that the beneficial effects of MSCs are mediated by secretome rather than cell replacement. MSC secretome refers to a variety of bioactive molecules involved in different biological processes, specifically neuro-regeneration.
Main body
Due to the limited ability of the central nervous system to compensate for neuronal loss and relieve disease progress, mesenchymal stem cell products may be used as a potential cure for central nervous system disorders. In the present study, the therapeutic effects of MSC secretome were reviewed and discussed the possible mechanisms in the three most prevalent central nervous system disorders, namely Alzheimer's disease, multiple sclerosis, and Parkinson's disease. The current work aimed to help discover new medicine for the mentioned complications.
Conclusion
The use of MSC-derived secretomes in the treatment of the mentioned diseases has encouraging results, so it can be considered as a treatment option for which no treatment has been introduced so far.
Similar content being viewed by others
Background
Neurodegenerative disorders can be the result of an injury to the brain and spinal cord neurons. When the body is unable to replace damaged neurons, structural damage and function failure lead to neuronal death. Consequently, dementia, mental dysfunction, and movement problems occur in neurodegenerative diseases, such as Parkinson’s disease (PD) [1], Alzheimer’s disease (AD) [2], and multiple sclerosis (MS) [3]. Although the exact pathophysiology of neurodegenerative diseases is unclear, several studies have suggested that oxidative stress [4], environmental pollution [5], aging [6], infection [7], chemical exposure [8], and immune dysregulation [9] play a role in the accumulation of misfolded proteins (such as tau, amyloid-β, α-syncline) [10] over time. Different types of pharmacotherapies were developed to treat more common neurodegenerative disorders, like AD [11] and PD [12]; among them, acetylcholinesterase inhibitors (donepezil and rivastigmine) and N-methyl-D-aspartate (NMDA) receptor agonists were found to be more effective, especially for AD [13]. Regarding PD, only one drug, Xadago safinamide, has received FDA approval [14]. Despite their ability to alleviate symptoms, these drugs cannot stop the disease progression [15]. The available drugs for MS (glatiramer acetate, cladribine, natalizumab, mitoxantrone, and ocrelizumab) are ineffective in accelerating tissue repair [16]; therefore, attempts have been made to find new effective therapeutic strategies, such as stem cell therapy, to treat neurodegenerative diseases [17, 18].
Mesenchymal stromal cells (MSCs) are cells with the capacity for self-renewal and differentiation into various cell lineages [19]. The International Society of Cell Therapy (ISCT) states that MSCs must satisfy three criteria to be labeled as MSCs; these are adipogenesis, chondrogenesis, and osteogenesis; plastic adhesion property; and positivity of CD90, CD105, and CD73 surface markers along with negativity for CD45, CD19, CD79a, CD34, and human leukocyte antigen (HLA)-DR surface markers. In contrast to other stem cell groups, the expression of CD34 is believed to be challenging [20], and no specific marker has been developed to identify MSCs [21]. Mesenchymal stromal cells can be derived from several sources, including bone marrow, adipose tissue, dental tissues, placenta, umbilical cord blood, Wharton's jelly, and the brain [22]. Despite meeting the abovementioned criteria, these mesenchymal stromal cells from different sources differ in several characteristics, such as their ability to differentiate into specific cell lineages, their cytokine secretion profiles, and surface markers [23, 24].
More than 2000 patients suffering from different stages of neurodegenerative diseases received MSCs, and most of them achieved promising results [25]. Nonetheless, there are several concerns about MSC-based cell therapy, including heterogeneity, potential side effects after allogeneic MSC transplantation, and the difficulties in choosing proper donors. Heterogeneity could be due to differences in MSC sources and cell culture methods [26, 27]. This fact makes it difficult to compare results from clinical trials that have chosen different sources and methods. From another point of view, it can be considered a favorable option in applying MSC to treat different conditions by manipulating the culture medium and choosing the appropriate source to achieve the optimum results.
Another concern is the host immune system's reaction after transplanting MSCs. The use of autogenic MSCs is considered an option to avoid this complication; however, it has certain limitations since the potential therapeutic effects of MSCs depend on the donor’s age and health [28]. Previous studies have revealed that MSCs obtained from patients with obesity [29] and inflammatory diseases did not have normal differentiation and proliferation capacity; therefore, they could not produce the expected therapeutic effects [30]. The last concern is about the growing data suggesting that MSC therapy may be ineffective, in particular for neural disorders. One reason may be the weak and transient benefit of MSC therapy in studies of central nerves system (CNS) disorders, such as ALS [31], MS [32], and stroke [33]. It could be the result of an ineffective transplantation method. After intravenous (IV) transfusion, MSCs are trapped in some organs, mostly in the lungs [34]. In most cases, they cannot cross the blood–brain barrier successfully. Applying a novel method of MSCs transplantation, such as intralesional, intranasal, intra-arterial, or the use of MSC’s secretome, solves this issue [35].
The proteins released by cells are a straightforward definition of the secretome. Secretomes derived from MSCs include soluble (cytokines and chemokine) and insoluble (extra vesicles) factors [36]. MSC-derived secretomes are composed of a protein-soluble fraction, like growth factors and cytokines, and a vesicular fraction, composed of microvesicles and exosomes. Insoluble components are released into the extracellular environment and participate in trafficking, adhesion, and endocrine signaling [37]. Exosomes' cargo consists of three categories: I. proteins, which include signaling peptides, heat-shock proteins, vesicular transport proteins, signal transduction proteins, cytoskeletal proteins, and cell metabolism enzymes [38]; II. lipids, which include ceramides, cholesterols, phosphatidylserines, and sphingomyelins; III. nucleic acids which include genomic DNA, mitochondrial DNA (mtDNA), transfer RNAs (tRNAs) [39], messenger RNAs (mRNAs), ribosomal RNAs (rRNAs), complementary DNA (cDNAs), and small and long non-coding RNA [40]. Numerous recent studies have highlighted the beneficial therapeutic effects associated with MSC transplantation to the CNS due to its higher paracrine activity than its cell differentiation capacity [41]. The presence of immune regulatory and neurotrophic factors in MSCs’ secretome plays a crucial role in their desirable effects on CNS disorders. Some of these neurotrophic factors can increase neuronal proliferation and survival, such as nerve growth factor (NGF), glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), NT-4, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), pigment epithelium-derived factor (PEDF), insulin-like growth factor (IGF)-1, IGF2, transforming growth factor-beta 1 (TGF-β1), interleukin (IL)-6, pigment epithelium-derived factor (PEDF), DJ-1, and cystatin-C (Cys-C) [42].
To obtain MSC-derived exosomes, first, MSCs cultivated in an exosome production medium, and then the supernatant undergoes the isolation methods. The lack of standard isolation and purification methods is the main obstacle in routing exosomes into the translational clinical application. Although exosome isolation is conventionally performed using ultracentrifugation (differential ultracentrifugation, density gradient centrifugation), the gold-standard method, other techniques have been developed to overcome the ultracentrifugation limitations. The alternative isolation techniques are based on isolation by size (ultrafiltration, sequential filtration, exosome isolation kits, size exclusion chromatography, flow field-flow fractionation, hydrostatic filtration dialysis), immunoaffinity capture (enzyme-linked immunosorbent assay, magneto-immunoprecipitation), exosome precipitation (polyethylene glycol precipitation, lectin induced agglutination), and microfluidic techniques (acoustic nano-filter, immuno-based microfluidic isolation). Each isolation method has some advantages and disadvantages. For instance, immunoprecipitation yields the most efficient recovery rate, while the acoustic nano-filter method gives the highest purity and requires the least sample volume and time. The exosome analysis is performed via different methods based on physical characteristics (electron microscopy, dynamic light scattering, nanoparticle tracking analysis, tunable resistive pulse sensing), chemical, biochemical, and compositional characteristics (immunodetection methods such as flow cytometry and western blotting, thermophoretic profiling, and mass spectrometry-based proteomic analysis) [43].
In this study, the effects of MSCs on cells on CNS injury were reviewed. The focus of the study was on secretome in three common diseases, namely Alzheimer’s disease, Parkinson’s disease, and MS. This was done to explore the therapeutic horizons concerning these conditions.
Main text
Mechanism of function
Different mechanisms are considered to explain the beneficial effects of MSC transplantation [44, 45]. In vivo studies have shown that autologous or exogenous MSCs could modify tissue structure and function by migrating to damaged areas, which involves several steps. Although the exact homing mechanism is not fully understood, there is a hypothesis based on its similarity to leukocyte migration via integrins, selectins, adhesion molecules (like VCAM-1), and G-protein signaling pathways [46]. Upon migration to the CNS, MSCs interact with surrounding tissues. This leads to neuronal differentiation and secretion of cytokines and growth factors involved in various mechanisms, such as neuroprotection, immunoregulation, angiogenesis, and inhibiting neuronal apoptosis [47]. Despite the beneficial features of MSCs, they show limited differentiation capacity compared to other types of stem cells, like embryonic pluripotent stem cells. It is not entirely accepted that MSCs can replace all lost neurons through neural differentiation. Therefore, improvement in neuronal survival following MSC administration is possibly due to the secretion of various neurotrophic factors by MSCs rather than differentiation. Secretion of neurotrophic factors can be increased by manipulating culture media [48]. Some priming protocols have been assessed in MSC culture media to induce particular alterations, leading to desirable changes [49]. For instance, Redondo-Castro et al. reported that preconditioning treatments of MSCs with inflammatory cytokines, like IL-1, prime them for a neurotrophic phenotype to release secretomes containing neurotrophic and anti-inflammatory cargos in the culture media supernatant []. Findings indicated that inducing MSCs to overexpress neurotrophic factors like GDNF and BDNF resulted in promising outcomes in PD and AD mouse models [50]. Moreover, using dynamic culturing conditions in computer-controlled bioreactors induces MSCs to produce a higher amount of neurotrophic factor, leading to a more effective cocktail for therapeutic applications in neurodegenerative disease [51, 52].
Immune dysfunctionality has been introduced as one of the main causes of neurodegenerative diseases such as AD, PD, and MS. In this light, glial cells’ hyperactivity and immune cells’ infiltration into the CNS lead to disease progression [3]. But the MSCs’ capacity to affect the proliferation and activation of all types of immune cells is another promising feature that assures their efficacy in neurodegenerative disorders [53]. MSCs are not immunosuppressive by nature, but they need a specific cytokine profile [54]. However, the exact mechanism is still under question. Some reports have indicated that it occurs directly through cell-cell contact and secreting soluble factors [55]. MSCs interrupt three pivotal phases of the immune response: antigen presentation, T cell activation and proliferation, and effectors’ responses [53], through modulating the behavior of macrophages, dendritic cells (DCs), natural killer cells (NK cells), B cells, and T cells. It happens mostly through secreting certain molecules, including indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), TGF-β1, HLA-G5, IL-10, and IL-6 [56]. Besides, the mediators secreted by MSCs comprise various cytokines and growth factors that mostly play an immunomodulatory role in the cell microenvironment. They also actively produce neurotrophic factors like BDNF, GDNF, VEGF, NT-3, NGF, and IGF, which induce endogenous neurogenesis and raise neuronal survival. Numerous studies have confirmed the neuro-protective effects of these growth factors [57, 58]. GDNF, as a well-known trophic factor, was reported to exhibit remarkable neuroprotective properties [59]. BDNF is the main promoting agent to axonal outgrowth in the CNS, since with its removal from the secretome, the effect was not observed anymore [60]. Moreover, several papers have introduced VEGF as another critical factor, which induces explicitly axonal outgrowth [61, 62]. This result was confirmed by Zhou et al. as they promoted neurogenesis and functional recovery following VEGF and BDNF co-overexpressed MSC administration to the cerebral ischemia model [63].
MSCs can impair antigen presentation by DCs through reducing surface markers such as CD-11c, major histocompatibility complex (MHC)-class II, and CD83, indirectly leading to the inhibition of adaptive immune cell activation [64]. Cross-talk with macrophages, which play an imperative role in CNS inflammation under the high level of inflammatory cytokines (IFN-γ and TNF-α), polarizes them toward anti-inflammatory phenotype (M2), unlike pro-inflammatory (M1), which can contribute to tissue regeneration by increasing arginase-1 and IL-10 levels [65]. Microglial cells, which are resident macrophages in CNS tissue, can cause inflammation (by secreting IL-6, NO, TNF-α, and IL-1β) and are related to different pathogens of neurodegenerative disorders [66]. MSCs, through secretion of TNF-α, stimulated protein 6 (TSG-6), suppress microglia activation [67], and exert M1 to M2 switch by activating the CX3CL1/CX3CR1 signaling pathway in these cells [68].
MSCs suppress lymphocytes via three vital mechanisms: firstly, reducing the proliferation of both T [69] and B [70] lymphocytes by arresting the cell cycle in the G0/G1 phase even after exposure to allogenic cells; secondly, reducing IFN-γ and IL-17 production by polarizing the Th0 toward Th2 shift rather than that toward Th1 and Th17; and lastly, indirect induction of Treg generation [71]. It has been shown that MSCs exert further Treg immune suppression activity, probably via IL-10 secretion [72]. It could be a reassuring option for treating CNS disorders with autoimmune origins, such as MS.
Other secretory products, like HLA-G5, TGF-β1, IDO, and PGE2, suppress the proliferation, cytokine secretion, and cytotoxicity of NK cells. They also inhibit the activity of NK cells as a bridge between innate and adaptive immune responses [73].
To sum up, the beneficial effect of MSCs on neurodegenerative disorders is mostly attributed to the secretion of several cytokines and growth factors involved in immune regulation and neuroprotection.
Secretome-based therapy in neurodegenerative diseases
Gnocchi first discovered the therapy based on MSC secretome in 2005 [44], which attracted scientific attention owing to addressing several concerns about the side effects following allogenic or autologous MSC transplantation. In addition to the risk of immune response and tumor genesis, culturing MSCs takes too much time for cell proliferation and may be associated with undesirable differentiation [45, 74]. On the other hand, MSC secretomes can be easily collected from commercial culture media without any invasive process [75]. Furthermore, in various types of diseases related to the damaged structure of different brain parts, administering culture media is effective. These reasons, along with the high prevalence of central nervous system disorders and their low regenerative potential, make these diseases one of the critical targets for MSC secretome-based therapy. Numerous studies have verified the neuroprotective and neurotrophic effects of MSC secretome based on this hypothesis [76]. Teixeira et al. reported that just a single administration of MSC secretome, even without cell transplantation, increased endogenous neuronal differentiation in the hippocampus region [77]. MSC-derived secretomes are flexible according to the state and site of the injury [78]. Therefore, they could be regulated by various pathological conditions similar to what may be observed in different CNS disorders.
In the following sections, the studies conducted on the effect of secretome-based therapies in the three main neurodegenerative diseases, including AD, PD, and MS, are reviewed (Table 1).
Alzheimer's disease (AD)
AD is a prevalent chronic disease of the central nervous system with multifactorial and relatively complex pathology. AD patients suffer from the gradual loss of brain abilities such as memory and cognitive function. It has been estimated that approximately 131 million people, even those under the age of 65, will be affected until 2050 worldwide.
AD was primarily discovered in 1907 by Alzheimer, a neuropathologist specialist [79], and has since been divided into sporadic (sAD) and familial (fAD) groups. In the fAD group, three common gene mutations were reported, namely presenilin 1 (PSEN1), presenilin 2 (PSEN2) β, and precursor protein (APP) [80]. In sAD, apolipoprotein E (APOE), nutrition, lifestyle, and aging are known as major risk factors. However, the exact underlying mechanism remains ambiguous. Any efficient drug or curative method has been investigated. Although some current treatment methods, including cholinesterase blockers and NMDA receptor antagonists, are effective in the early stages of the disease, they are not highly efficient after a while due to a high number of neuronal losses in the hippocampus, the inability of drugs to cross the blood–brain barrier (BBB), and the increasing side effects following long-term use [81].
The main pathological features in both fAD and sAD are chronic accumulation of amyloid-beta (Aβ) plaque and neurofibrillary tangles (NFT) enriched by hyperphosphorylated protein tau [74]. This accumulation leads to the hyperactivation of microglia (MG) and astrocytes (AC) as the most involved immune cells in AD pathology [82]. Brain neurons of AD patients are permanently susceptible to inflammatory conditions induced by these immune cells. Although opinions about the role of MG and AC are controversial, most researchers consider them immunostimulators after exposure to Aβ plaques by their toll-like surface receptors (TLRs) (TLR 2, TLR4, TLR6, and TLR9) and CD markers (CD36, CD14, and CD47) [83, 84]. Expression of these TLRs in different cells has been suggested as the initiation of the inflammatory response. Thus, increased TLR2 levels are considered a diagnostic marker for activated MGs [85], and TLR4 overexpressing neurons are more prone to degradation after releasing IL-1B, TNFα, and IL-17 [86]. Modulating the TLRs' stimulation would be a critical step to control MG activation and AC status. In this context, one paper showed that hypoxia-preconditioned adipocyte-derived MSC secretome injection to AD mouse models decreased TLR2 and TLR4 expression, similar to astrocyte inflammatory cytokines, such as IL-1 and TNFα, which increase hippocampus neuronal survival [87].
Secreting inflammatory cytokines by activated MGs and ACs plays an important role in disease progression, neurotoxicity, and cognitive dysfunction [88]. Using IL-17 neutralizing antibodies improved memory function in an animal model of AD by preventing pro-inflammatory mediators [89]. On the other hand, some types of secretory products are crucial factors that cause resistance to the accumulation of AB plaque in the brain. An interesting study examined the expression of inflammatory and anti-inflammatory cytokines in the brains of the resistance group. Comparing the control and AD groups showed that they expressed different cytokines, which prevented neurodegeneration and dementia despite the presence of AB plaque [90]. The resistance groups differed in the number of Aβ plaques and NTFs. Higher levels of IL-6, IL-1ra, IL-13, and IL-4 belonged to the highest rate group (HP), whereas the dominant cytokines in the lowest rate group (LP) were IL-10, IL-6, and IP10. Among them, IL-4, IL-13, and IL-10 shifted the activated MG to M2 form to modulate the inflammatory response. The M1 phenotype massively releases inflammatory cytokines, which deteriorate CNS damage [91]. Conversely, the phenotypical exchange of glial cells in AD has been reported in favor of the inflammatory process. This has been reported during aging, diabetes, obesity, and some other mentioned risk factors associated with immune disturbances [92].
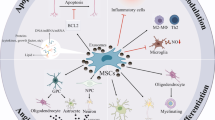
The MSC secretome comprises an array of bioactive molecules that ameliorate AD-related symptoms through different mechanisms (Fig. 1).
Graphical summary of different mechanisms conducted by MSCs secretome to hippocampal neuron protection. MSC products ameliorate neurodegeneration related to (A1) astrocyte and (M1) microglia, two major immune cells involved in the pathogenesis of Alzheimer’s disease. CX3CL1, IL- 4, IL-13, and IL-10 induced MGs switching from harmful phenotype M1 to protective form M2. Exosomes decrease the expression levels of IL-1a, IL-1β, and TNF-α; thus, MSCs can modulate the excessive inflammatory responses. The elevated secretion of CCL5 and ICAM-1 induces Aβ plaque degradation by increasing the level of protease enzyme (NEP). VEGF and FGF-2 can decrease neuronal apoptosis by changing the BAX/BCL-2 balance in favor of cell survival. IL: interleukin, TGF-β: transforming growth factor-beta, Fractalkine R: fractalkine receptor, TLR: Toll-like receptor, NEP: neprilysin, Aβ: amyloid beta, BAX: BCL-2-associated X, apoptosis regulator, BCL-2: B cell CLL/lymphoma 2. The images depicted in the figure are designed by authors
Transplantation of hypoxia-preconditioned MSC-exosomes to app/PS1 mouse models induced phenotypic exchanges in the lesion area by decreasing the expression levels of IL-1a, IL-1β, and TNF-α and increasing IL-4 and IL-10; thus, MSCs can modulate the excessive inflammatory responses caused by M1 MGs. Additionally, they suppressed the activation of astrocytes and decreased AB plaque deposition, leading to improved memory deficits and cognitive AD-related disorders [88]. In line with previous studies, MSC-derived CX3CL1 induced MGs switch from harmful phenotype M1 to protective form M2 through the fractalkine receptor, thereby protecting neurons against destructive effects following inflammatory cytokines release [93].
A high concentration of inflammatory products is commonly believed a sign of MGs and ACs shifting to a harmful form that compromises Aβ clearance. However, it seems that it could not be applied to all inflammatory cytokines [94]. Multiple studies have found TGF-β, IL-6, and other inflammatory cytokines accumulating around Aβ plaques in the brains of AD patients [2]. According to researchers, these two cytokines protect neurons by increasing plaque clearance, which results in improved cognitive and behavioral disorders [29]. IL-6, a pleiotropic cytokine expressed at a high rate in both resistance groups (HP and IP), was reported to be protective and increase neuronal survival. Contrary to normal conditions, IL-6 concentration was shown to be significantly increased during disease progression [95]. However, data in this context are controversial since IL-6, as a well-known inflammatory mediator, might either initiate a harmful cascade leading to cerebral damage or facilitate the healing process via raising angiogenesis and other protective mechanisms [96]. In this regard, Yang et al. indicated reduced autophagy in hippocampal neurons induced by endogenous MSC-derived IL-6 by inhibiting the AMPK/mTOR pathway via the gp130-IL-6R receptor complex. They revealed that MSC-derived IL-6 significantly decreases Beclin 1 and LC3 II, autophagy-associated proteins (Fig. 97) [98]. It was also reported that this cytokine could decrease the proliferation of activated astrocytes by affecting the AMPK/mTOR signaling pathway [99]. Further investigation is needed to clarify the reciprocal role of inflammatory cytokines in AD.
MSC-derived IL-6 suppresses the autophagy pathway in injured hippocampal neurons in Alzheimer’s disease through the downregulation of the AMPK (mitogen-activated protein kinase) signaling pathway. The AMPK signaling pathway is a downstream target of IL-6. IL-6 binds to the gp130-IL-6R receptor complex and induces a downregulation in the AMPK signaling pathway. IL-6 can induce mTOR, a pivotal factor in the autophagic signaling pathway, in an AMPK-dependent and STAT3-independent manner, leading to decreasing autophagy-associated proteins like Beclin 1 and LC3 II. The images depicted in the figure are designed by authors
Lee et al. injected bone marrow (BM)-derived MSCs into the hippocampal region of AD mice and realized that BM-derived MSCs could recruit activated MG cells to the inflamed area after exposure to Aβ deposition. The elevated secretion of CCL5, a chemoattractant factor, induced the recruitment of alternatively activated MG. Alternatively activated MGs attenuated memory impairment and decreased Aβ plaques through the secretion of IL-4 and neprilysin (NEP) [100]. Several beneficial effects of IL-4 were previously reported in AD, including improved spatial learning and increased Aβ degradation in phagocytic cells [101].
MSCs secretome can indirectly contribute to Aβ plaque degradation through the NEP enzyme. Neprilysin is a well-known protease enzyme that has an inverse relationship with the Aβ level. Recently, it has become a new target in the search for novel AD therapies. Cultured MSC-derived secretomes have the potential to regulate neprilysin’s activity. In vitro studies have revealed that MSC-derived soluble intracellular adhesion molecule-1 (ICAM-1) [99] and CCL5 [100] induce Aβ plaque degradation by increasing the level of NEP through alternative activation of MG cells. However, there is little knowledge about this enzyme and it needs further investigation.
MSC secretome not only contains NEP, but can also decrease the toxic effects and neuronal apoptosis caused by Aβ. MSC-derived secretome significantly reduced neuronal apoptosis by changing the BAX/BCL-2 balance in favor of cell survival. Aβ plaques induce neuronal apoptosis either directly by upregulating the Bax protein [102] or indirectly through increasing ROS-induced oxidative stress [103]. Dental pulp stem cell (DPSC)-derived secretomes can counteract Bax expression in neuroblastoma cell lines through upregulation of the anti-apoptotic protein Bcl-2. Scientists believed that it could be related to the expression of RANTES, Fractalkine, VEGF, and fibroblast growth factor-2 (FGF-2), which can promote Bcl-2 expression. Interestingly, it has also been proven that DPSCs secrete these cytokines and growth factors at higher concentrations than other common sources of MSC, like bone marrow and adipocytes [76]. Based on the aforementioned data, manipulating some factors aiming to upregulate Bcl-2 expression would be a promising strategy to develop more efficient therapies.
In summary, AD neuropathology is a relatively complex disease and requires multi-target therapy approaches. The use of MSCs with neuroprotection potential, potent immune modulation, and anti-amyloidogenic activities could be considered a multi-approach alternative for AD model therapy. MSC secretome immunomodulatory properties are partially regulated by MG and AC activity, either by regulating their proliferation potential and phenotypic changes from neurodegenerative to neuroprotective states, or by regulating TLR expression. However, further clinical trials are required to explain which one is precisely responsible for the observed therapeutic outcomes and optimize this option.
Multiple sclerosis (MS)
MS is one of the prevalent neurodegenerative disorders affecting approximately 2.5 million people aged 20–40 years [103]. It is caused by the immune system attack through T helper reactive cells (TCD4 +) and antibodies against the lipoprotein of nerve fibers, due to genetic and environmental backgrounds. Chronic degeneration of myelin sheath leads to a progressive disability in patients. MS is divided into three stages according to clinical symptoms [104]. The most common type is relapse-remitting (RR) MS which occurs at an approximate rate of 85%-90% and is characterized by exacerbation and relative remission cycles [105]. The second phase is defined as "secondary progressive" (SP) and characterized by aggravated symptoms without remission periods, affecting 50–60% of MS patients. The third phase is defined as "primary progressive" (PP) and presented in about 15% of MS patients and is characterized by more severe clinical symptoms and chronic progressive disability with or without exacerbation episodes. Both SP and RR are more severe and frequent in women than in men [106].
Injury of the blood–brain barrier followed by infiltration of immune cells into the brain and spinal cord occurs in the early stages of MS [107]. Nevertheless, the exact reason behind the immune reaction against the nerve fibers remains unclear to date. The major pathological cause is a disturbance of the balance between T cell populations (TCD4 + and Treg) and antigen-presenting cells (microglia) in favor of inflammation following neuronal degeneration. The major subset of autoreactive CD4 + cells (Th1, Th17) seems to be critically involved in MS pathogenesis and its experimental animal model, experimental autoimmune encephalomyelitis (EAE) [108]. Hence, transferring Th17/Th17 cells to healthy rats causes EAE. Th1 and Th17 negatively affect immune tolerance, leading to inflammatory damage in MS, Th17 by producing inflammatory cytokines such as IL-17a, IL-17F, IL-21, IL-22, and Th1 by secreting TNF-α and IFN-γ [109]. Thus, these cytokines are abundantly expressed in the inflammation area. Although the healing process of neurons occurs naturally, it is not enough to compensate for neuronal loss and prevent disease progression. The current treatment methods (anti-inflammatory drugs, antioxidants, steroidal hormones, and corticosteroids) are available only for RR patients and reduce the number of relapses through immune system suppression [17, 110]. Nonetheless, they cannot stop degradation and replace lost neurons. Moreover, in the case of using this kind of therapies the potential side effects, such as secondary autoimmune disease and increased risk of infection, should be seriously considered [111].
As mentioned previously, the role of immune cells is undeniable in MS pathogenesis. Accordingly, targeting these immune cells and recovering from immune hemostasis can be an effective and promising strategy for treating this disease. MSC-derived secretome can justify the unbalanced immune response in MS through different mechanisms. These factors also exhibit neuroprotective effects that inhibit neuronal degeneration after the disease's progression. The following paragraphs describe different mechanisms induced by MSC secretome (Fig. 3).
Graphical summary of immunomodulation and neuroprotective effects of MSCs in MS. MSC-derived secretome justifies the unbalanced immune response through secretion of different cytokines and growth factors. These products inhibit the proliferation, differentiation, and migration of Th1 and Th17 to the CNS and increase the Th0 differentiation to Treg instead. MSC secretome also comprises an array of neurotrophic factors which induce endogenous neurogenesis; this compensates lost neurons. MMP9: matrix metalloproteinases9, TIMP: tissue inhibitor of metalloproteinase, IL: interleukin, IFN-γ: interferon-gamma, PGE2: prostaglandin E2, IDO: indoleamine 2,3-dioxygenase. HGF: hepatocyte growth factor, BDNF: brain-derived neurotrophic factor, GDNF: glial cell-derived neurotrophic, BFGF: basic fibroblast growth factor, IGF: insulin-like growth factors, VEGF: vascular endothelial growth factor, KYN: kynurenine, FOXP3: forehead box P3, STAT5: signal transducer and activator of transcription 5, C-met: hepatocyte growth factor receptor, BBB: blood–brain barrier. The images depicted in the figure are designed by authors
MSC-derived cytokines can induce Th0 differentiation to other T cell subsets. IL-10 and TGF-β are competent representatives of these cytokines. Following investigations of the underlying mechanisms, it was observed that co-cultured BM-MSC with CD62Lhigh CD44low CD4 + CD25low T cell population reduced differentiation of Th0 toward Th17 through downregulation of the RORγT-related signaling pathway. Researchers have demonstrated that the increase in IL-10 secretion might play a key role in this action, so that IL-10 neutralization could significantly restore the Th17 population [112]. Svobodova et al. co-cultured the alloantigen-stimulated spleen cells with TGF-β releasing MSCs to examine the effect of MSC-derived TGF-β on the naïve T cell differentiation. They confirmed that TGF-β reciprocally regulated Th17 development and its secretory cytokine, IL-17, as well as two crucial transcription factors, Foxp3 and RORγT []. As a result, inducing Th0 differentiation into the Treg cell population instead of Th17 is known as another chief function of the mentioned cytokines, resulting in changing the Treg/Th17 ratio in favor of tissue hemostasis. Tregs also suppress the DC-induced Th17 differentiation [113].
Inhibition of T cell proliferation is another critical mechanism induced by MSC mediators. HGF exerts its indirect T cell anti-proliferative role by regulating c-met receptors in antigen-presenting cells, especially DCs [76]. However, the direct suppression effect of this receptor on Th17 remains unclear. In addition to the HGF functional assessment, another study designed by Bai et al. demonstrated improved memory deficit and functional recovery following the infusion of HGF-induced MSC secretome to EAE mouse models. Their results revealed that HGF is a pleiotropic cytokine with neurotrophic and immunomodulatory effects [114]. PGE2, as another potent immunomodulatory product of MSC, can prevent the Th1 and Th17 proliferation and secretion of their inflammatory cytokines [115]; meanwhile, it could be controversial depending on lymphocyte maturation status [116]. IDO specifically released by human MSCs, exerts an anti-inflammatory role by inducing the production of kynurenine, a toxic metabolite against T cell proliferation and apoptosis [117].
MSC-derived cytokines inhibit T cell migration through the BBB. In addition to preventing the antigen presentation to T cells and limiting their proliferation capacity, MSC secretomes have other functional methods to inhibit neuronal degradation and disease progression in MS. Autoreactive T cells can break the BBB and migrate to the CNS neurons using matrix metalloproteinase (MMP)-9, a well-known proteolytic enzyme involved in the MS and EAE pathogenesis, which lyses the myelin sheath [118]. Considering these data, inhibiting MMPs is a potential concept to develop a novel therapeutic method for neuroinflammatory diseases, especially MS [119]. Researchers have indicated that MSC secretomes could relieve disease severity by preventing MMP activity [120, 121]. In this regard, another study introduced MSC-secreted tissue inhibitors of metalloproteinase (TIMPs) as a potent inhibitor of MMP [122].
The immunomodulatory effect of MSCs can be partly explained by inflammasome inactivation. NALP3 is a well-known inflammasome involved in various autoimmune diseases, including MS [122]. This elucidates the therapeutic effect of PDLSC-derived secretome in MS, acting similarly to the conventional immunosuppressant drugs, such as interferon-beta (IFN-β), and suppressing the NALP3 inflammasome and the NFkβ signaling pathway via secreting cytokines such as IL-10 and TGF-β [123]. The inhibitory effect of IL-10 on activated macrophages via inhibiting NALP3 was previously confirmed [124]. It was reported that the main effect of IFN-β is immunomodulation and enhancement of BDNF levels [125].
In conclusion, MSCs can induce neuroprotection and endogenous neurogenesis at injury sites by secreting neurotrophic mediators to improve neuronal survival. Since the levels of neurotrophic factors are dramatically reduced in the CNS of MS patients, increasing their rate or at least maintaining their physiologic level seems to be a valuable therapeutic option [126].
Parkinson's disease (PD)
Following AD, PD is known to be the most prevalent neurodegenerative disease, discovered by James Parkinson in 1817. It affects approximately 1%-3% of the population above the age of 60 [1]. Among different factors involved in PD pathogenies, including disruption of the protein cleanup pathway, mitochondrial dysfunction, oxidative stress, and genetic mutation, the accumulation of α-synuclein protein in the Lewy bodies (LBs) is the most well-known pathological feature in the disease development [127]. Following damage to the mitochondria, the toxic effect of LBs induces apoptosis in dopaminergic (DA) neurons, specifically in the substantia nigra [128]. The progressive degeneration of DA neurons develops several symptoms of motor dysfunction, including postural instability, bradykinesia, and rigidity [129].
The current accepted standard therapy for PD is levodopa (L-DOPA). Though it alleviates the major symptoms, its dosage should be increased due to the inability to replace DA neurons, thus increasing its side effect. Additionally, its short half-life necessitates the use of other treatment methods [130]. To the best of our knowledge and according to the US National Institutes of Health website (http://www.clinicaltrials.gov), there are just two studies that reported cell-based treatment for PD. Li et al. reported that after fetal mesencephalic dopaminergic neuron transplantation to two PD patients, the neurons survived for over 10 years. However, it was revealed that the newly engrafted neurons were also affected by pathological conditions following α-synuclein accumulation in LBs [131]. In contrast, in seven patients who had BM-MSC autologous engraftment to the sub-lateral ventricular zone via surgery, three of them showed significant improvements, and two others had their medicine dosage reduced; no evidence of tumor growth was observed in the MRI after 12–36 month. Other papers focused mainly on animal models of PD [132]. As the preparation step of stem cell therapy is time-consuming and the procedures for keeping the engrafted cells alive in the transplantation zone are difficult to manage, secretome-based therapy for PD has attracted remarkable attention over the past few years.
The findings of several studies on animal models revealed that MSC-derived secretomes hold significant potential for PD treatment. Different strategies, such as inducing neuronal differentiation, increasing proliferation, raising density, and increasing neuronal viability, were reported by different research groups as having effectively improved motor dysfunctions. The discovery of the regenerative and protective effects of MSC secretomes on DA neurons has attracted a great deal of scientific attention to this new therapeutic aspect. Teixeira et al. revealed that injecting secretomes into rats’ dentate gyrus enhanced the endogenous proliferation of hippocampal neurons after seven days. They incubated human umbilical cord perivascular cells-derived secretomes with human telencephalon neural progenitor cells for five days and reported that neuronal differentiation and density increased in both mature and immature cells. NGF and FGF-2 levels increased simultaneously in that region [77]. Sakane and Miyamoto introduced CHD2 as an important modulator molecule in DA neuron differentiation and proliferation through the regulation of the Wnt-β-catenin signaling pathway [133].
Some evidence has confirmed that MSC secretome-mediated functional recovery in DA neurons. In mouse models of PD, DA neurons progressively degenerated, and accordingly, motor coordination was impaired. The researcher verified the motor performance improvement following injecting the MSC-derived secretomes compared to the control group. However, its effects gradually decreased as time passed, probably due to local consumption. They also showed that the tyrosine hydroxylase (TH) + neurons increased in the test groups compared to those in the control group [57]. The increased number of TH + neurons could be considered one of the factors involved in improving functional balancing. Following the detection of possible contributing factors in this action, Fábio and Teixeira introduced 21 proteins, namely BDNF, VEGF, IL-6, GDNF, cystatin-C, porcine epidermal growth factor (PEGF), galectin-1, heat-shock protein (HSP)-27, TRX1, UCHL1, semaphorin 7a (SEMA 7A), stromal cell-derived factor (SDF)-1, clustrin, CypA, CypA, CypC, DJ-1, cadherin (CDH)-2, PRDX1, UBE3A, and MMP2 by mass spectrometry [134]. Similarly, Cerri et al. confirmed GDNF, BDNF, VEGF, and IL-6 as the most effective molecules in restoring the functional balance of the dopaminergic system [135].
Strong evidence has emphasized the effect of neuronal growth factors on neuronal viability following secretome therapy. It was reported that BDNF expression is reduced in the substantia nigra pars compacta (SNC) in the early stages of PD [136]. Researchers have also introduced this molecule as an essential factor in the development and plasticity of DA neurons preventing neurodegeneration and increasing neuron viability and survival [137, 138]. Hence, knocking down the BDNF gene is associated with increased susceptibility and neuronal loss in DA neurons [139]. GDNF is another neurotrophic molecule involved in the viability and survival of DA neurons [140] through upregulating Bcl-x and Bcl-2 anti-apoptotic proteins [141]. As a potent antioxidant, GDNF inhibits ROS-mediated degeneration by increasing antioxidant enzyme activity [142]. IL-6, a scavenger of superoxide radicals, upregulates ROS activity, contributing to DA neurons’ protection [143]. In addition, IL-6 exerted protective effects on DA neurons against MPP + -mediated toxicity [144]. Thus, knocking down the IL-6 gene makes DA neurons more sensitive to methyl-phenyl-tetrahydropyridine neurotoxicity [145].
Several growth factors produced after secretome therapy have been recognized as having either a direct or indirect neuroprotective role in Parkinson’s disease. In addition to increasing neuronal viability, BDNF and GDNF can directly protect the dopaminergic system [146]. The indirect protection is provided by other growth factors like FGF-2 and EGF [147]. Furthermore, certain VEGF subtypes exert protective effects in a dose-dependent manner through both mentioned mechanisms. In response to acute damages to the dopaminergic system, another important neurotrophic molecule, called PDEF [148, 149], has a better functional outcome and easier delivery compared to other therapeutic methods using secreted molecules like GDNF [150]. PDEF exerts its effect by inducing the NF-kB signaling pathway. NF-kB, as a transcription factor, increases the expression of other neurotrophic molecules, such as BDNF and GDNF [], thereby indirectly increasing neuronal viability and survival.
The aforementioned data shed light on the fact that secretomes can relatively compensate for neuronal loss in PD without requiring further cell transfer. Comparing MSC engraftment and secretome to the animal models of PD showed that the secretome is more efficient in increasing the number and density of TH + neurons in the striatum and neural progenitor cells area, which is probably the cause of the low survival rate of the MSCs in these regions [151].
Preclinical studies and clinical trials
The therapeutic effects of MSC therapy and MSC-derived exosomes in animal models of neurodegenerative diseases are presented in Tables 1 and 2, respectively. While the preclinical results are promising, the safety and efficacy of MSC-derived secretomes in humans have not been confirmed yet.
Over the past decade, the therapeutic potential of MSC therapy in neurodegenerative diseases has been evaluated in various clinical trials. Table 3 presents all the clinical trials of MSC therapy in AD, PD, and MS patients listed in the US National Institutes of Health Clinical Trials Database (www.clinicaltrials.gov) till September 2022. Although various phase I and II clinical trials were conducted to assess the safety and efficiency of MSC therapy in neurodegenerative disease, the results should be warranted by phase III trials.
Despite all preclinical studies demonstrating the efficiency of MSC-derived exosomes in neurodegenerative diseases, their clinical utility has yet to be demonstrated. There are just phase I and II clinical trials initiated to evaluate the safety and efficacy of exosomes secreted from allogeneic adipose tissue-derived MSC in AD patients (NCT04388982).
Conclusion
MSC-derived secretomes can be used as a promising therapeutic approach in the treatment of neurodegenerative disorders for which no treatment has yet been introduced. This cell-free strategy can solve complications due to MSCs’ migration to the injury site and their differentiation. Because MSCs' beneficial properties are dependent on their ability to deliver their content, MSC-derived secretomes can be as effective as MSCs transplantation in activating the pro-survival and anti-apoptotic signals, leading to improved tissue neuron regeneration. Indeed, MSC-derived secretomes serve as an information transporter from MSCs to neurons.
Availability of data and materials
Not applicable.
Abbreviations
- CNS:
-
Central nervous system
- MS:
-
Multiple sclerosis
- AD:
-
Alzheimer's disease
- NMDA:
-
N-Methyl-D-aspartate
- ISCT:
-
International Society of Cell Therapy
- ALS:
-
Amyotrophic lateral
- IV:
-
Intravenous
- NGF:
-
Nerve growth factor
- GDNF:
-
Glial cell-derived neurotrophic factor
- BDNF:
-
Brain-derived neurotrophic factor
- NT:
-
Neurotrophin
- VEGF:
-
Vascular endothelial growth factor
- HGF:
-
Hepatocyte growth factor
- FGF:
-
Fibroblast growth factor
- PEDF:
-
Pigment epithelium-derived factor
- IGF:
-
Insulin-like growth factor
- TGF-β:
-
Transforming growth factor-beta
- IL:
-
Interleukin
- IDO:
-
Indoleamine 2,3-dioxygenase
- PGE2:
-
Prostaglandin E2
- HLA:
-
Human leukocyte antigen
- MHC:
-
Major histocompatibility complex
- IFN-γ:
-
Interferon γ
- TNF-α:
-
Tumor necrosis factor-α
- TSG-6:
-
TNF-α-stimulated protein 6
- NK:
-
Natural killer cells
- BBB:
-
Blood–brain barrier
- Aβ:
-
Amyloid-beta
- NFT:
-
Neurofibrillary tangles
- IFN-β:
-
Interferon-beta
- MG:
-
Microglia
- AC:
-
Astrocytes
- TLR:
-
Toll-like receptor
- CCL5:
-
Chemokine ligand 5
- ICAM-1:
-
Intracellular adhesion molecule-1
- MMP:
-
Matrix metalloproteinase
- LB:
-
Lewy body
- TH:
-
Tyrosine hydroxylase
References
Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):1–12.
Twohig D, Nielsen HM. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):1–19.
Zéphir H. Progress in understanding the pathophysiology of multiple sclerosis. Revue Neurol. 2018;174(6):358–63.
Singh E, Devasahayam G. Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection. Mol Biol Rep. 2020;47(4):3133–40.
Costa LG, Cole TB, Dao K, Chang Y-C, Coburn J, Garrick JM. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther. 2020;210:107523.
Choi JG, Kim SY, Jeong M, Oh MS. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol Ther. 2018;182:56–69.
Zimmer A, Youngblood A, Adnane A, Miller BJ, Goldsmith DR. Prenatal exposure to viral infection and neuropsychiatric disorders in offspring: a review of the literature and recommendations for the COVID-19 pandemic. Brain Behav Immun. 2021;91:756–70.
Khan J, Salhotra S, Goswami P, Akhter J, Jahan S, Gupta S, et al. Bisphenol A triggers axonal injury and myelin degeneration with concomitant neurobehavioral toxicity in C57BL/6J male mice. Toxicology. 2019;428:152299.
Cervellati C, Trentini A, Pecorelli A, Valacchi G. Inflammation in neurological disorders: the thin boundary between brain and periphery. Antioxid Redox Signal. 2020;33(3):191–210.
Skovronsky DM, Lee VMY, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol Mech Dis. 2006;1:151–70.
Matesanz AI, Caballero AB, Lorenzo C, Espargaro A, Sabaté R, Quiroga AG, et al. Thiosemicarbazone derivatives as inhibitors of amyloid-β aggregation: effect of metal coordination. Inorg Chem. 2020;59(10):6978–87.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):1–21.
Kulshreshtha A, Piplani P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol Sci. 2016;37(9):1403–35.
Giossi R, Carrara F, Mazzari M, Re FL, Senatore M, Schicchi A, et al. Overall efficacy and safety of safinamide in Parkinson’s disease: a systematic review and a meta-analysis. Clin Drug Investig. 2021;41:321–39.
Ellis JM, Fell MJ. Current approaches to the treatment of Parkinson’s disease. Bioorg Med Chem Lett. 2017;27(18):4247–55.
Dobson R, Giovannoni G. Multiple sclerosis–a review. Eur J Neurol. 2019;26(1):27–40.
Cohen JA, Imrey PB, Planchon SM, Bermel RA, Fisher E, Fox RJ, et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler J. 2018;24(4):501–11.
Comi G, Radaelli M, Soelberg SP. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389(10076):1347–56.
Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, et al. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: a new horizon of stem cell therapy. J Cell Physiol. 2020;235(12):9230–40.
Viswanathan C, Kulkarni R, Bopardikar A, Ramdasi S. Significance of CD34 negative hematopoietic stem cells and CD34 positive mesenchymal stem cells–a valuable dimension to the current understanding. Curr Stem Cell Res Ther. 2017;12(6):476–83.
Lin C-SS, Xin Z-CC, Dai J, Lue TF. Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histol Histopathol. 2013;28(9):1109.
Hashemi Goradel N, Darabi M, Shamsasenjan K, Ejtehadifar M, Zahedi S. Methods of liver stem cell therapy in rodents as models of human liver regeneration in hepatic failure. Adv Pharm Bull. 2015;5(3):293–8.
Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep. 2020;10(1):1–15.
Mathew SA, Naik C, Cahill PA, Bhonde RR. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci. 2020;77(2):253–65.
Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–48.
Mo M, Wang S, Zhou Y, Li H, Wu Y. Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell Mol Life Sci. 2016;73(17):3311–21.
Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H, et al. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013;3(2):433–7.
Timari H, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Pashoutan Sarvar D, Aqmasheh S. The effect of mesenchymal stem cell-derived extracellular vesicles on hematopoietic stem cells fate. Adv Pharm Bull. 2017;7(4):531–46.
Yang M, Sun W, Xiao L, He M, Gu Y, Yang T. Mesenchymal stromal cells suppress hippocampal neuron autophagy stress induced by hypoxic-ischemic brain damage: the possible role of endogenous IL-6 secretion. Neural Plast. 2020. https://doi.org/10.1155/2020/8822579.
Pachón-Peña G, Serena C, Ejarque M, Petriz J, Duran X, Oliva-Olivera W, et al. Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl Med. 2016;5(4):464–75.
Syková E, Rychmach P, Drahorádová I, Konrádová Š, Růžičková K, Voříšek I, et al. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647–58.
Abramowski P, Krasemann S, Ernst T, Lange C, Ittrich H, Schweizer M, et al. Mesenchymal stromal/stem cells do not ameliorate experimental autoimmune encephalomyelitis and are not detectable in the central nervous system of transplanted mice. Stem Cells Dev. 2016;25(15):1134–48.
Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, et al. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015;6(1):1–15.
Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transpl Proc. 2007;39(2):573–6.
Santamaria G, Brandi E, Vitola P, Grandi F, Ferrara G, Pischiutta F. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021;28(1):203–18.
Beer L, Mildner M, Ankersmit HJ. Cell secretome based drug substances in regenerative medicine: when regulatory affairs meet basic science. Ann Transl Med. 2017;5(7):170.
Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231.
Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33(5):419–40.
Gorabi AM, Kiaie N, Barreto GE, Read MI, Tafti HA, Sahebkar A. The therapeutic potential of mesenchymal stem cell–derived exosomes in treatment of neurodegenerative diseases. Mol Neurobiol. 2019;56(12):8157–67.
McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7.
Gomes ED, Mendes SS, Assunção-Silva RC, Teixeira FG, Pires AO, Anjo SI. Co-transplantation of adipose tissue-derived stromal cells and olfactory ensheathing cells for spinal cord injury repair. Stem Cells. 2018;36(5):696–708.
Gugliandolo A, Mazzon E. Dental mesenchymal stem cell secretome: an intriguing approach for neuroprotection and neuroregeneration. Int J Mol Sci. 2022;23(1):456.
Vilaça-Faria H, Salgado AJ, Teixeira FG. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease? Cells. 2019;8(2):118.
Fan X-L, Zhang Y, Li X, Fu Q-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771–94.
Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells. 2019;8(5):467.
Mansoor SR, Zabihi E, Ghasemi-Kasman M. The potential use of mesenchymal stem cells for the treatment of multiple sclerosis. Life Sci. 2019;235:116830.
Kim H-J, Lee J-H, Kim S-H. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27(1):131–8.
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Therapy. 2017;8(1):1–11.
Li Q, Wang Y, Deng Z. Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem cell Res Therapy. 2013;4(3):1–3.
Rahbaran M, Zekiy AO, Bahramali M, Jahangir M, Mardasi M, Sakhaei D, Thangavelu L, Shomali N, Zamani M, Mohammadi A, Rahnama N. Therapeutic utility of mesenchymal stromal cell (MSC)-based approaches in chronic neurodegeneration: a glimpse into underlying mechanisms, current status, and prospects. Cell Mol Biol Lett. 2022;27(1):1–36.
Teixeira FG, Panchalingam KM, Assunção-Silva R, Serra SC, Mendes-Pinheiro B, Patrício P, Jung S, Anjo SI, Manadas B, Pinto L, Sousa N. Modulation of the mesenchymal stem cell secretome using computer-controlled bioreactors: impact on neuronal cell proliferation, survival and differentiation. Sci Rep. 2016;6(1):1–14.
Teixeira FG, Panchalingam KM, Anjo SI, Manadas B, Pereira R, Sousa N, Salgado AJ, Behie LA. Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell Res Therapy. 2015;6(1):1–14.
Liang X, Ding Y, Zhang Y, Tse H-F, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–59.
Chen G, Ye Y, Cheng M, Tao Y, Zhang K, Huang Q. Quercetin combined with human umbilical cord Mesenchymal stem cells regulated tumour necrosis factor-α/interferon-γ-stimulated peripheral blood mononuclear cells via activation of toll-like receptor 3 Signalling. Front Pharmacol. 2020;11:499.
Wan YM, Qiang LZ, Zhou Q, Liu C, Wang MJ, Wu HX. Mesenchymal stem cells alleviate liver injury induced by chronic-binge ethanol feeding in mice via release of TSG6 and suppression of STAT3 activation. Stem Cell Res Ther. 2020;11(1):1–13.
Caffi V, Espinosa G, Gajardo G, Morales N, Durán MC, Uberti B. Pre-conditioning of equine bone marrow-derived mesenchymal stromal cells increases their immunomodulatory capacity. Front Vet Sci. 2020;7:318.
Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, et al. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s disease. Stem Cells Transl Med. 2017;6(2):634–46.
Whone AL, Kemp K, Sun M, Wilkins A, Scolding NJ. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012;1431:86–96.
Lavigne EG, Buttigieg D, Steinschneider R, Burstein ES. Pimavanserin promotes trophic factor release and protects cultured primary dopaminergic neurons exposed to MPP+ in a GDNF-dependent manner. ACS Chem Neurosci. 2021;12:2088–98.
Martins LF, Costa RO, Pedro JR, Aguiar P, Serra SC, Teixeira FG, et al. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci Rep. 2017;7(1):1–13.
Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, et al. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70(5):951–65.
Theis, Verena, and Carsten Theiss. Vascular endothelial growth factor and neurodevelopment. Factors Affecting Neurodevelopment. Academic Press; 2021;237–46.
Zhou L, Lin Q, Wang P, Yao L, Leong K, Tan Z, et al. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co-overexpressing BDNF and VEGF in a rat model of cardiac arrest-induced global cerebral ischemia. Cell Death Dis. 2017;8(5):e2774.
Dokic MJ, Tomic ZS, Colic JM. Cross-talk between mesenchymal stem/stromal cells and dendritic cells. Curr Stem Cell Res Ther. 2016;11(1):51–65.
Chung E, Son Y. Crosstalk between mesenchymal stem cells and macrophages in tissue repair. Tissue Eng Regener Med. 2014;11(6):431–8.
Xu Y, Jin MZ, Yang ZY, Jin WL. Microglia in neurodegenerative diseases. Neural Regener Res. 2021;16(2):270.
Liu Y, Zeng R, Wang Y, Huang W, Hu B, Zhu G. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res. 2019;1724:146422.
Li A, Zhao J, Fan C, Zhu L, Huang C, Li Q. Delivery of exogenous proteins by mesenchymal stem cells attenuates early memory deficits in a murine model of Alzheimer’s disease. Neurobiol Aging. 2020;86:81–91.
Liang C, Jiang E, Yao J, Wang M, Chen S, Zhou Z. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology. 2018;23(1):44–9.
Magatti M, Masserdotti A, Bonassi Signoroni P, Vertua E, Stefani FR, Silini AR. B Lymphocytes as targets of the immunomodulatory properties of human amniotic mesenchymal stromal cells. Front Immunol. 2020;11:1156.
Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015;11(2):280–7.
Lim JY, Im KI, Lee ES, Kim N, Nam YS, Jeon YW. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep. 2016;6(1):1–13.
Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41:653–64.
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–8.
Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J. Conditioned medium of human menstrual blood-derived endometrial stem cells protects against MPP+-induced cytotoxicity in vitro. Front Mol Neurosci. 2019;12:80.
Luarte A, Bátiz LF, Wyneken U, Lafourcade C. Potential therapies by stem cell-derived exosomes in CNS diseases: focusing on the neurogenic niche. Stem Cells Int. 2016. https://doi.org/10.1155/2016/5736059.
Teixeira FG, Carvalho MM, Neves-Carvalho A, Panchalingam KM, Behie LA, Pinto L, et al. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev Rep. 2015;11(2):288–97.
Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20(5):531–6.
Liu AKL. Stem cell therapy for Alzheimer’s disease: hype or hope? Biosci Horiz Int J Stud Res. 2013. https://doi.org/10.1093/biohorizons/hzt011.
Filadi R, Pizzo P. Defective autophagy and Alzheimer’s disease: is calcium the key? Neural Regen Res. 2019;14(12):2081.
Botchway BOA, Moore MK, Akinleye FO, Iyer IC, Fang M. Nutrition: review on the possible treatment for Alzheimer’s disease. J Alzheimer’s Dis. 2018;61(3):867–83.
Ahmad MH, Fatima M, Mondal AC. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: rational insights for the therapeutic approaches. J Clin Neurosci. 2019;59:6–11.
Dansokho C, Heneka MT. Neuroinflammatory responses in Alzheimer’s disease. J Neural Transm. 2018;125(5):771–9.
Ries M, Sastre M. Mechanisms of Aβ clearance and degradation by glial cells. Front Aging Neurosci. 2016;8:160.
Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–83.
Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114(1):13–27.
Mehrabadi S, Motevaseli E, Sadr SS, Moradbeygi K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR) 2 and TLR4 expression in model of Alzheimer’s disease rats. Behav Brain Res. 2020;379:112362.
Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27(4):663–77.
Cristiano C, Volpicelli F, Lippiello P, Buono B, Raucci F, Piccolo M. Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br J Pharmacol. 2019;176(18):3544–57.
Barroeta-Espar I, Weinstock LD, Perez-Nievas BG, Meltzer AC, Chong MST, Amaral AC. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol Dis. 2019;121:327–37.
Yao K, Bing ZH. Microglial polarization: novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology. 2020;28(1):95–110.
Kinuthia UM, Wolf A, Langmann T. Microglia and inflammatory responses in diabetic retinopathy. Front Immunol. 2020;11:564077.
Giunti D, Parodi B, Usai C, Vergani L, Casazza S, Bruzzone S, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem cells. 2012;30(9):2044–53.
Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24(2):548–59.
Zheng C, Zhou XW, Wang JZ. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 2016;5(1):1–15.
Chen JH, Ke KF, Lu JH, Qiu YH, Peng YP. Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1–42-induced Alzheimer’s disease model rats. PLoS One. 2015;10(2):e0116549.
Spooren A, Kolmus K, Laureys G, Clinckers R, Keyser J, Haegeman G. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67(1–2):157–83.
Escrig A, Canal C, Sanchis P, Fernández-Gayol O, Montilla A, Comes G. IL-6 trans-signaling in the brain influences the behavioral and physio-pathological phenotype of the Tg2576 and 3xTgAD mouse models of Alzheimer’s disease. Brain Behav Immun. 2019;82:145–59.
He M, Shi X, Yang M, Yang T, Li T, Chen J. Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR signaling to inhibit the proliferation of reactive astrocytes induced by hypoxic-ischemic brain damage. Exp Neurol. 2019;311:15–32.
Lee JK, Schuchman EH, Jin HK, Bae J. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid β ameliorates Alzheimer’s disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells. 2012;30(7):1544–55.
Tang RH, Qi RQ, Liu HY. Interleukin-4 affects microglial autophagic flux. Neural Regen Res. 2019;14(9):1594–602.
Feng MG, Liu CF, Chen L, Feng WB, Liu M, Hai H. MiR-21 attenuates apoptosis-triggered by amyloid-β via modulating PDCD4/PI3K/AKT/GSK-3β pathway in SH-SY5Y cells. Biomed Pharmacother. 2018;101:1003–7.
Kanamaru T, Kamimura N, Yokota T, Iuchi K, Nishimaki K, Takami S. Oxidative stress accelerates amyloid deposition and memory impairment in a double-transgenic mouse model of Alzheimer’s disease. Neurosci Lett. 2015;587:126–31.
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet Lond Engl. 2018;391:1622–36.
Iwanowski P, Losy J. Immunological differences between classical phenothypes of multiple sclerosis. J Neurol Sci. 2015;349(1–2):10–4.
Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389(10076):1357–66.
Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–68.
Wagner CA, Roqué PJ, Goverman JM. Pathogenic T cell cytokines in multiple sclerosis. J Exp Med. 2020;217(1).
Li J-F, Zhang D-J, Geng T, Chen L, Huang H, Yin H-L, et al. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23:113–22.
Iacobaeus E, Kadri N, Lefsihane K, Boberg E, Gavin C, Törnqvist Andrén A, et al. Short and long term clinical and immunologic follow up after bone marrow mesenchymal stromal cell therapy in progressive multiple sclerosis—a phase i study. J Clin Medi. 2019;8(12):2102.
Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5(9):e00362.
Qu X, Liu X, Cheng K, Yang R, Zhao RCH. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol. 2012;40(9):761–70.
Gao W-X, Sun Y-Q, Shi J, Li C-L, Fang S-B, Wang D, et al. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther. 2017;8(1):1–16.
Hübel J, Hieronymus T. HGF/Met-signaling contributes to immune regulation by modulating tolerogenic and motogenic properties of dendritic cells. Biomedicines. 2015;3(1):138–48.
Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15(6):862–70.
Maseda D, Ricciotti E, Crofford LJ. Prostaglandin regulation of T cell biology. Pharmacol Res. 2019;149:104456.
Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206(3):535–48.
Zhang Y, Dong H, Seeburg DP, Wojtkiewicz GR, Waterman P, Pulli B, et al. Multimodal molecular imaging demonstrates myeloperoxidase regulation of matrix metalloproteinase activity in neuroinflammation. Mol Neurobiol. 2019;56(2):954–62.
Chelluboina B, Nalamolu KR, Mendez GG, Klopfenstein JD, Pinson DM, Wang DZ, et al. Mesenchymal stem cell treatment prevents post-stroke dysregulation of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Cell Physiol Biochem. 2017;44(4):1360–9.
Kay AG, Long G, Tyler G, Stefan A, Broadfoot SJ, Piccinini AM, et al. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Sci Rep. 2017;7(1):1–11.
Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226(2):385–96.
Hou B, Zhang Y, Liang P, He Y, Peng B, Liu W, et al. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020;11(5):1–16.
Soundara Rajan T, Giacoppo S, Diomede F, Bramanti P, Trubiani O, Mazzon E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int J Immunopathol Pharmacol. 2017;30(3):238–52.
Gurung P, Li B, Malireddi RKS, Lamkanfi M, Geiger TL, Kanneganti T-D. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci Rep. 2015;5(1):1–10.
Karmand Z, Hartung HP, Neuhaus O. Interferon beta-1a induces expression of brain-derived neurotrophic factor in human T lymphocytes in vitro and not in vivo. Future Neurol. 2020;15(1):FNL38.
Banitalebi E, Ghahfarrokhi MM, Negaresh R, Kazemi A, Faramarzi M, Motl RW. Exercise improves neurotrophins in multiple sclerosis independent of disability status. Mult Scler Related Disord. 2020;43:102143.
Shalash AS, Hamid E, Elrassas HH, Bedair AS, Abushouk AI, Khamis M. Non-motor symptoms as predictors of quality of life in Egyptian patients with Parkinson’s disease: a cross-sectional study using a culturally adapted 39-item Parkinson’s disease questionnaire. Front Neurol. 2018;9:357.
Manne S, Kondru N, Jin H, Anantharam V, Huang X, Kanthasamy A. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov Disord. 2020;35(2):268–78.
Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J Neurochem. 2016;139:318–24.
Shen T, Pu J, Si X, Ye R, Zhang B. An update on potential therapeutic strategies for Parkinson’s disease based on pathogenic mechanisms. Expert Rev Neurother. 2016;16(6):711–22.
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–3.
Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62–70.
Sakane F, Miyamoto Y. N-cadherin regulates the proliferation and differentiation of ventral midbrain dopaminergic progenitors. Dev Neurobiol. 2013;73(7):518–29.
Teixeira FG, Vilaça-Faria H, Domingues AV, Campos J, Salgado AJ. Preclinical comparison of stem cells secretome and levodopa application in a 6-hydroxydopamine rat model of Parkinson’s disease. Cells. 2020;9(2):315.
Cerri S, Greco R, Levandis G, Ghezzi C, Mangione AS, Fuzzati-Armentero MT. Intracarotid infusion of mesenchymal stem cells in an animal model of Parkinson’s disease, focusing on cell distribution and neuroprotective and behavioral effects. Stem Cells Transl Med. 2015;4(9):1073–85.
Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25(26):6251–9.
Zhang, N.; Kang, T.; Xia, Y.; Wen, Q.; Zhang, X.; Li, H.; Hu, Y.; Hao, H.; Zhao, D.; Sun, D.; et al. Effects of salvianolic acid B on survival, self-renewal and neuronal differentiation of bone marrow derived neural stem cells. Eur. J. Pharmacol. 2012, 697, 32–39
Yang T, Nie Z, Shu H, Kuang Y, Chen X, Cheng J. The role of BDNF on neural plasticity in depression. Front Cell Neurosci. 2020;14:82.
Hou L, Chen W, Liu X, Qiao D, Zhou FM. Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front Aging Neurosci. 2017;9:358.
Sun S, Zhang Q, Li M, Gao P, Huang K, Beejadhursing R. GDNF promotes survival and therapeutic efficacy of human adipose-derived mesenchymal stem cells in a mouse model of Parkinson’s disease. Cell Transplant. 2020;29:0963689720908512.
Cao JP, Niu HY, Wang HJ, Huang XG, Gao DS. NF-κB p65/p52 plays a role in GDNF up-regulating Bcl-2 and Bcl-w expression in 6-OHDA-induced apoptosis of MN9D cell. Int J Neurosci. 2013;123(10):705–10.
Chao CC, Lee EHY. Neuroprotective mechanism of glial cell line-derived neurotrophic factor on dopamine neurons: role of antioxidation. Neuropharmacology. 1999;38(6):913–6.
Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254.
Akaneya Y, Takahashi M, Hatanaka H. Interleukin-1β enhances survival and interleukin-6 protects against MPP+ neurotoxicity in cultures of fetal rat dopaminergic neurons. Exp Neurol. 1995;136(1):44–52.
Bolin LM, Strycharska-Orczyk I, Murray R, Langston JW, Monte D. Increased vulnerability of dopaminergic neurons in MPTP-lesioned interleukin-6 deficient mice. J Neurochem. 2002;83(1):167–75.
Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci. 2020;21(3):1170.
Casper D, Blum M. Epidermal growth factor and basic fibroblast growth factor protect dopaminergic neurons from glutamate toxicity in culture. J Neurochem. 1995;65(3):1016–26.
Yasuda T, Fukuda-Tani M, Nihira T, Wada K, Hattori N, Mizuno Y. Correlation between levels of pigment epithelium-derived factor and vascular endothelial growth factor in the striatum of patients with Parkinson’s disease. Exp Neurol. 2007;206(2):308–17.
Meng X, Huang A, Khan A, Zhang L, Sun X, Song H. Vascular endothelial growth factor-loaded poly-lactic-co-glycolic acid nanoparticles with controlled release protect the dopaminergic neurons in Parkinson’s rats. Chem Biol Drug Des. 2020;95(6):631–9.
Falk T, Gonzalez RT, Sherman SJ. The Yin and Yang of VEGF and PEDF: multifaceted neurotrophic factors and their potential in the treatment of Parkinson’s disease. Int J Mol Sci. 2010;11(8):2875–900.
Mendes-Pinheiro B, Anjo SI, Manadas B, Silva JD, Marote A, Behie LA, et al. Bone marrow mesenchymal stem cells’ secretome exerts neuroprotective efects in a Parkinson’s disease rat model. Front Bioeng Biotechnol. 2019;7:294.
Gong Y, Yan Y, Ma T, Gong K, Ao Q, Zhang X. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regener Res. 2014;9(8):798–805
Kim D, Lee D, Chang E, et al. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer's disease model. Stem Cells Develop. 2015;24(20):2378–2390
Xie ZH, Liu Z, Zhang XR, Yang H, Wei LF, Wang Y, Xu SL, Sun L, Lai C, Bi JZ, Wang XY. Wharton's Jelly-derived mesenchymal stem cells alleviate memory deficits and reduce amyloid-β deposition in an APP/PS1 transgenic mouse model. Clin Exp Med. 2016, 16(1),89–98. https://doi.org/10.1007/s10238-015-0375-0
Nakano, M., Kubota, K., Kobayashi, E. et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep 10:10772
Park SE, Lee J, Chang EH, Kim JH, Sung JH, Na DL, Chang JW. Activin A secreted by human mesenchymal stem cells induces neuronal development and neurite outgrowth in an in vitro model of Alzheimer's disease: neurogenesis induced by MSCs via activin A. Arch Pharm Res. 2016;39(8):1171–9
Lee NK, Park SE, Kwon SJ, Shim S, Byeon Y, Kim JH, Na DL, Chang JW. Agouti Related Peptide Secreted Via Human Mesenchymal Stem Cells Upregulates Proteasome Activity in an Alzheimer's Disease Model. Sci Rep. 2017;7:39340
Zheng XY, Wan QQ, Zheng CY, Zhou HL, Dong XY, Deng QS, Yao H, Fu Q, Gao M, Yan ZJ, Wang SS, You Y, Lv J, Wang XY, Chen KE, Zhang MY, Xu RX. Amniotic Mesenchymal Stem Cells Decrease Aβ Deposition and Improve Memory in APP/PS1 Transgenic Mice. Neurochem Res. 2017;42(8):2191–2207
Naaldijk Y, Jäger C, Fabian C, Leovsky C, Blüher A, Rudolph L, Hinze A, Stolzing A. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43(4):299–314
Kim DH, Lim H, Lee D, Choi SJ, Oh W, Yang YS, Oh JS, Hwang HH, Jeon HB. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer's disease model. Sci Rep. 2018;8(1):354
Zhao Y, Chen X, Wu Y, Wang Y, Li Y, Xiang C. Transplantation of Human Menstrual Blood-Derived Mesenchymal Stem Cells Alleviates Alzheimer's Disease-Like Pathology in APP/PS1 Transgenic Mice. Front Mol Neurosci. 2018;11:140
Park BN, Kim JH, Lim TS, Park SH, Kim TG, Yoon BS, Son KS, Yoon JK, An YS. Therapeutic effect of mesenchymal stem cells in an animal model of Alzheimer’s disease evaluated by β-amyloid positron emission tomography imaging. Aust N Z J Psychiatry. 2020;54(9):883–91.
Lim H, Lee D, Choi W, Choi S, Oh W, Kim D. Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces aberrant tau phosphorylation in an Alzheimer disease model. Stem Cells Int. 2020;2020:8878412.
Nakano M, Kubota K, Kobayashi E, et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772.
Santamaria G, Brandi E, Vitola P, Grandi F, Ferrara G, Pischiutta F, Vegliante G, Zanier ER, Re F, Uccelli A, Forloni G, de Rosbo NK, Balducci C. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021;28(1):203–18.
Park, Kim H, Kwon S, et al. Exposure of mesenchymal stem cells to an Alzheimer’s disease environment enhances 10 Stem Cells Internationaltherapeutic effects. Stem Cells Int. 2021;2021:6660186.
Kurte M, Bravo-Alegria J, Torres A, Carrasco V, Ibanez C, Vega-Letter AM, Fernandez-O’Ryan C, Irarrazabal CE, Figueroa FE, Fuentealba RA, et al. Intravenous administration of bone marrow-derived mesenchymal stem cells induces a switch from classical to atypical symptoms in experimental autoimmune encephalomyelitis. Stem Cells Int. 2015;2015:140170.
Glenn JD, Smith MD, Kirby LA, Baxi EG, Whartenby KA. Disparate Effects of Mesenchymal Stem Cells in Experimental Autoimmune Encephalomyelitis and Cuprizone-Induced Demyelination. PLoS One. 2015;10(9).
El-Akabawy G, Rashed LA. Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a non-immune model of demyelination. Ann Anat. 2015;198:11–20. https://doi.org/10.1016/j.aanat.2014.12.002.
Anderson P, Gonzalez-Rey E, O’Valle F, Martin F, Oliver FJ, Delgado M. Allogeneic Adipose-Derived Mesenchymal Stromal Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Regulating Self-Reactive T Cell Responses and Dendritic Cell Function. Stem Cells Int. 2017;2017:2389753.
Mahfouz MM, Abdelsalam RM, Masoud MA, Mansour HA, Ahmed-Farid OA, Kenawy SA. The neuroprotective effect of mesenchymal stem cells on an experimentally induced model for multiple sclerosis in mice. J Biochem Mol Toxicol. 2017. https://doi.org/10.1002/jbt.21936.
Cedola A, Bravin A, Bukreeva I, et al. X-Ray Phase Contrast Tomography Reveals Early Vascular Alterations and Neuronal Loss in a Multiple Sclerosis Model. Sci Rep. 2017;7:5890. https://doi.org/10.1038/s41598-017-06251-7.
Marzban M, Mousavizadeh K, Bakhshayesh M, Vousooghi N, Vakilzadeh G, Torkaman-Boutorabi A. Effect of Multiple Intraperitoneal Injections of Human Bone Marrow Mesenchymal Stem Cells on Cuprizone Model of Multiple Sclerosis. Iran Biomed J. 2018;22:312–21.
Kurte M, Luz-Crawford P, Vega-Letter AM, Contreras RA, Tejedor G, Elizondo-Vega R, Martinez-Viola L, Fernandez-O’Ryan C, Figueroa FE, Jorgensen C, et al. IL17/IL17RA as a novel signaling axis driving mesenchymal stem cell therapeutic function in experimental autoimmune encephalomyelitis. Front. Immunol. 2018;9:802.
Rivera FJ, de la Fuente AG, Zhao C, Silva ME, Gonzalez GA, Wodnar R, Feichtner M, Lange S, Errea O, Priglinger E, O’Sullivan A, Romanelli P, Jadasz JJ, Brachtl G, Greil R, Tempfer H, Traweger A, Bátiz LF, Küry P, Couillard-Despres S, Franklin RJM, Aigner L. Aging restricts the ability of mesenchymal stem cells to promote the generation of oligodendrocytes during remyelination. Glia. 2019;67(8):1510–25. https://doi.org/10.1002/glia.23624.
Barati S, Kashani IR, Tahmasebi F, Mehrabi S, Joghataei MT. Effect of mesenchymal stem cells on glial cells population in cuprizone induced demyelination model. Neuropeptides. 2019;75:75–84.
Barati S, Ragerdi Kashani I, Moradi F, Tahmasebi F, Mehrabi S, Barati M, Joghataei MT. Mesenchymal stem cell mediated effects on microglial phenotype in cuprizone-induced demyelination model. J Cell Biochem. 2019;120(8):13952–64. https://doi.org/10.1002/jcb.28670.
Xin Y, Gao J, Hu R, Li H, Li Q, Han F, He Z, Lai L, Su M. Changes of immune parameters of T lymphocytes and macrophages in EAE mice after BM-MSCs transplantation. Immunol Lett. 2020;225:66–73.
Liu Y, Ma Y, Du B, Wang Y, Yang GY, Bi X. Mesenchymal Stem Cells Attenuated Blood-Brain Barrier Disruption via Downregulation of Aquaporin-4 Expression in EAE Mice. Mol Neurobiol. 2020;57(9):3891–901. https://doi.org/10.1007/s12035-020-01998-z.
Gramlich OW, Brown AJ, Godwin CR, Chimenti MS, Boland LK, Ankrum JA, Kardon RH. Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis. Transl Vis Sci Technol. 2020;9(8):16. https://doi.org/10.1167/tvst.9.8.16.
Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Neurochem. 2008;107(1):141–51. https://doi.org/10.1111/j.1471-4159.2008.05589.x.
Bouchez G, Sensebé L, Vourc’h P, Garreau L, Bodard S, Rico A, Guilloteau D, Charbord P, Besnard JC, Chalon S. Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem Int. 2008;52(7):1332–42. https://doi.org/10.1016/j.neuint.2008.02.003.
Chao YX, He BP, Tay SSW. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinfammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J Neuroimmunol. 2009;216(1):39–50.
Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res. 2010;1311:12–27. https://doi.org/10.1016/j.brainres.2009.11.041.
Wolff EF, Gao XB, Yao KV, Andrews ZB, Du H, Elsworth JD, Taylor HS. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J Cell Mol Med. 2011;15(4):747–55. https://doi.org/10.1111/j.1582-4934.2010.01068.x.
Cerri S, Greco R, Levandis G, Ghezzi C, Mangione AS, Fuzzati-Armentero M-T, Bonizzi A, Avanzini MA, Maccario R, Blandini F. Intracarotid infusion of mesenchymal stem cells in an animal model of parkinson’s disease, focusing on cell distribution and neuroprotective and behavioral efects. Stem Cells Transl Med. 2015;4(9):1073–85.
Wolff EF, Mutlu L, Massasa EE, Elsworth JD, Eugene Redmond D, Jr Taylor HS. Endometrial stem cell transplantation in MPTP- exposed primates: an alternative cell source for treatment of Parkinson’s disease. J Cell Mol Med. 2015;19(1):249–56. https://doi.org/10.1111/jcmm.12433.
Park BN, Kim JH, Lee K, Park SH, An YS. Improved dopamine transporter binding activity after bone marrow mesenchymal stem cell transplantation in a rat model of Parkinson’s disease: small animal positron emission tomography study with F-18 FP-CIT. Eur Radiol. 2015;25(5):1487–96. https://doi.org/10.1007/s00330-014-3549-3.
Salama M, Sobh M, Emam M, Abdalla A, Sabry D, El-Gamal M, Lotfy A, El-Husseiny M, Sobh M, Shalash A. Efect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson’s disease. Exp Ther Med. 2017;13(3):976–82.
Chen D, Fu W, Zhuang W, Lv C, Li F, Wang X. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson’s disease. J Neurosci Res. 2017;95(3):907–17. https://doi.org/10.1002/jnr.23879.
Yang C, Qiu Y, Qing Y, Xu J, Dai W, Hu X, Wu X. Synergistic efect of electric stimulation and mesenchymal stem cells against Parkinson’s disease. Aging. 2020;12(16):16062–71.
Ghahari L, Safari M, Rahimi Jaberi K, Jafari B, Safari K, Madadian M. Mesenchymal stem cells with granulocyte colony-stimulating factor reduce stress oxidative factors in Parkinson’s disease. Iran Biomed J. 2020;24(2):89–98.
Park H, Chang KA. Therapeutic potential of repeated intravenous transplantation of human adipose-derived stem cells in subchronic MPTP-induced Parkinson’s disease mouse model. Int J Mol Sci. 2020;21:21.
Jalali MS, Sarkaki A, Farbood Y, Azandeh SS, Mansouri E, Ghasemi Dehcheshmeh M, Saki G. Transplanted Wharton’s jelly mesenchymal stem cells improve memory and brain hippocampal electrophysiology in rat model of Parkinson’s disease. J Chem Neuroanat. 2020;110.
Wang SS, Jia J, Wang Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppresses iNOS Expression and Ameliorates Neural Impairment in Alzheimer’s Disease Mice. J Alzheimers Dis. 2018;61(3):1005–13. https://doi.org/10.3233/JAD-170848.
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32(2):654–68. https://doi.org/10.1096/fj.201700600R.
Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem Res. 2018;43(11):2165–77. https://doi.org/10.1007/s11064-018-2641-5.
Elia CA, Tamborini M, Rasile M, Desiato G, Marchetti S, Swuec P, Mazzitelli S, Clemente F, Anselmo A, Matteoli M, et al. Intracerebral Injection of Extracellular Vesicles from Mesenchymal Stem Cells Exerts Reduced Aβ Plaque Burden in Early Stages of a Preclinical Model of Alzheimer’s Disease. Cells. 2019;8:1059.
Zhao W, Zhang H, Yan J, Ma X. An experimental study on the treatment of diabetes-induced cognitive disorder mice model with exosomes deriving from mesenchymal stem cells (MSCs). Pak J Pharm Sci. 2019;32:1965–70.
Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, MárquezAguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen Res. 2019;14:1626–34.
Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen Res. 2019;14:1626–34.
Wang X, Yang G. Bone marrow mesenchymal stem cells-derived exosomes reduce Aβ deposition and improve cognitive function recovery in mice with Alzheimer’s disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int. 2021;45(4):775–84. https://doi.org/10.1002/cbin.11522.
Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q, Li Q, Gu X, Chen H, Jiang G, et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J Control Release. 2020;327:688–702.
Losurdo M, Pedrazzoli M, D’Agostino C, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. STEM CELLS Trans Med. 2020;9(9):1068–84.
Hassan R, Rabea AA, Ragae A, Sabry D. The prospective role of mesenchymal stem cells exosomes on circumvallate taste buds in induced Alzheimer’s disease of ovariectomized albino rats: (Light and transmission electron microscopic study). Arch Oral Biol. 2020;110.
Li Z, Liu F, He X, Yang X, Shan F, Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol. 2019;67:268–80.
Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto A, Calle EN, Crescitelli R, Liao W, Pham V, Yin Y, Jayaraman J, Lakey JRT, Walsh CM, Van Keuren-Jensen K, Lotvall J, Zhao W. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019;13(6):6670–88. https://doi.org/10.1021/acsnano.9b01004.
Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, Manadas B, Behie LA, Sousa N, Salgado AJ. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s disease. Stem Cells Transl Med. 2017;6(2):634–46.
Chierchia A, Chirico N, Boeri L, Raimondi I, Riva GA, Raimondi MT, Tunesi M, Giordano C, Forloni G, Albani D. Secretome released from hydrogel-embedded adipose mesenchymal stem cells protects against the Parkinson’s disease related toxin 6-hydroxydopamine. Eur J Pharma Biopharm. 2017;121:113–20.
Mendes-Pinheiro B, Anjo SI, Manadas B, Da Silva JD, Marote A, Behie LA, Teixeira FG, Salgado AJ. Bone marrow mesenchymal stem cells’ secretome exerts neuroprotective efects in a Parkinson’s disease rat model. Front Bioeng Biotechnol. 2019;7:294.
Giampà C, Alvino A, Magatti M, Silini AR, Cardinale A, Paldino E, Fusco FR, Parolini O. Conditioned medium from amniotic cells protects striatal degeneration and ameliorates motor defcits in the R6/2 mouse model of Huntington’s disease. J Cell Mol Med. 2019;23(2):1581–92.
Chen HX, Liang FC, Gu P, Xu BL, Xu HJ, Wang WT, Hou JY, Xie DX, Chai XQ, An SJ. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020;11:288.
Teixeira FG, Vilaça-Faria H, Domingues AV, Campos J, Salgado AJ. Preclinical comparison of stem cells secretome and levodopa application in a 6-hydroxydopamine rat model of Parkinson’s disease. Cells. 2020;9(2):315.
Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modifed adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol Ther Nucleic Acids. 2021;23:1334–44.
Xue C, Li X, Ba L, Zhang M, Yang Y, Gao Y, Sun Z, Han Q, Zhao RC. MSC-derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson’s disease. Aging Dis. 2021;12(5):1211–22.
Marques CR, Pereira-Sousa J, Teixeira FG, Sousa RA, Teixeira-Castro A, Salgado AJ. Mesenchymal stem cell secretome protects against alpha-synuclein-induced neurodegeneration in a Caenorhabditis elegans model of Parkinson’s disease. Cytotherapy. 2021;23(10):894–901.
Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front Pharmacol. 2016;7:231.
Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. In Seminars in immunopathology, vol. 33. 2011; Springer-Verlag, pp. 419–40
Gorabi AM, Kiaie N, Barreto GE, Read MI, Tafti HA, Sahebkar A. The Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol Neurobiol. 2019;56(12):8157–67.
McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: Mechanisms of Uptake. J Circ Biomark. 2015;4:7.
Li Q, Wang Y, Deng Z. Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem Cell Res Ther. 2013;4(3):1–3.
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8(1):79.
Rahbaran M, Zekiy AO, Bahramali M, Jahangir M, Mardasi M, Sakhaei D, Thangavelu L, Shomali N, Zamani M, Mohammadi A, Rahnama N. Therapeutic utility of mesenchymal stromal cell (MSC)-based approaches in chronic neurodegeneration: a glimpse into underlying mechanisms, current status, and prospects. Cell Mol Biol Lett. 2022;27(1):56. https://doi.org/10.1186/s11658-022-00359-z.
Teixeira FG, Panchalingam KM, Assunção-Silva R, Serra SC, Mendes-Pinheiro B, Patrício P, Jung S, Anjo SI, Manadas B, Pinto L, Sousa N, Behie LA, Salgado AJ. Modulation of the Mesenchymal Stem Cell Secretome Using Computer-Controlled Bioreactors: Impact on Neuronal Cell Proliferation. Survival and Differentiation Sci Rep. 2016;6:27791. https://doi.org/10.1038/srep27791.
Teixeira FG, Panchalingam KM, Anjo SI, Manadas B, Pereira R, Sousa N, Salgado AJ, Behie LA. Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome? Stem Cell Res Ther. 2015;6(1):133. https://doi.org/10.1186/s13287-015-0124-z.
Acknowledgements
All authors read and approved the final manuscript.
Funding
This article was partly funded by Tabriz Stem Cell Research Center at the Tabriz University of Medical Sciences, Iran (Grant/Award Number: 63912). (The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.)
Author information
Authors and Affiliations
Contributions
MG made the literature review and wrote the manuscript draft, ER edited the manuscript draft scientifically, MM reviewed and edited the manuscript, BF contributed to writing the manuscript draft, and PA and KS supervised the data collection, wrote the manuscript draft, and finalized the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghasemi, M., Roshandel, E., Mohammadian, M. et al. Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials. Stem Cell Res Ther 14, 122 (2023). https://doi.org/10.1186/s13287-023-03264-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03264-0