Abstract
Neurodegenerative diseases afflict a large number of persons worldwide, with the prevalence and incidence of dementia rapidly increasing. Despite their prevalence, clinical diagnosis of dementia syndromes remains imperfect with limited specificity. Conventional structural-based imaging techniques also lack the accuracy necessary for confident diagnosis. Multiparametric magnetic resonance imaging and molecular imaging provide the promise of improving specificity and sensitivity in the diagnosis of neurodegenerative disease as well as therapeutic monitoring of monoclonal antibody therapy. This educational review will briefly focus on the epidemiology, clinical presentation, and pathologic findings of common and uncommon neurodegenerative diseases. Imaging features of each disease spanning from conventional magnetic resonance sequences to advanced multiparametric methods such as resting-state functional magnetic resonance imaging and arterial spin labeling imaging will be described in detail. Additionally, the review will explore the findings of each diagnosis on molecular imaging including single-photon emission computed tomography and positron emission tomography with a variety of clinically used and experimental radiotracers. The literature and clinical cases provided demonstrate the power of advanced magnetic resonance imaging and molecular techniques in the diagnosis of neurodegenerative diseases and areas of future and ongoing research. With the advent of combined positron emission tomography/magnetic resonance imaging scanners, hybrid protocols utilizing both techniques are an attractive option for improving the evaluation of neurodegenerative diseases.
Key points
-
Neurodegenerative diseases represent a growing substantial burden of disease worldwide.
-
Clinical diagnosis and standard imaging techniques lack accuracy in diagnosing dementia syndromes.
-
Multiparametric magnetic resonance and molecular imaging are tools for evaluating neurodegenerative disease.
-
Combined positron emission tomography/magnetic resonance imaging protocols provide the ideal dementia appraisal.
Similar content being viewed by others
Background
Neurodegenerative disease (NDDs) including dementia syndromes represent a substantial burden of disease worldwide. Estimated global prevalence of all-cause dementia is 700 per 100,000 persons with the number of patients with dementia nearly doubling every five years [1]. Clinical diagnosis alone remains not entirely reliable with a median sensitivity of 87% and specificity of 58% [2]. Proper diagnosis is crucial to aid with prognostication and pharmacologic management of patients with NDDs, with disease modifying therapies an active field of research interest and recent US Food and Drug Administration approval of aducanumab, an IgG1 anti-amyloid-beta antibody targeting amyloid beta plaques, the first therapy of its kind available, though not yet approved in Europe [3,4,5,6]. Imaging is often applied to increase diagnostic confidence in the setting of a suspected NDD and in 2011 the US National Institute on Aging and the Alzheimer’s Association incorporated imaging biomarkers into the guidelines for diagnosis of Alzheimer’s disease (AD) [7]. Structural imaging methods such as magnetic resonance imaging (MRI) are often undertaken first, though limited sensitivity and specificity and high interobserver variability limit the applicability of these more commonly used methods [8]. Molecular imaging provides promise in its unique ability to visualize the spatial distribution of pathologic changes in NDDs and has been demonstrated to lead to increases in diagnostic certainty and provide therapeutic guidance. We aim to provide a brief review of the characteristics and epidemiology of NDDs, as this topic has been covered in detail elsewhere, and focus on their imaging features across multiple modalities, particularly advanced multiparametric MRI and molecular imaging.
Alzheimer’s disease
Alzheimer’s disease is the most common form of progressive dementia which accounts for up to 60% of cases in patients older than 65 years [9]. Alzheimer’s disease is pathologically defined by senile gray matter plaques consisting of neurotoxic deposits of extracellular amyloid–beta (Aβ) 42 protein, intracellular neurofibrillary tangles (NFTs) including the three repeat (3R) and four repeat (4R) tau isoforms, and decreased neuronal density from neuronal death. Neurofibrillary changes tend to progress in an orderly manner starting in the transentorhinal cortex and progressing through the medial temporal lobes to neocortical association areas in the frontal, parietal, and occipital lobes [10, 11]. Sporadic and familial forms have been defined with approximately 10% of patients related to presenilin 1 (PSEN1), presenilin 2 (PSEN2), and APOE*E4 genes. The clinical course of Alzheimer’s disease typically begins with a slow decline in memory followed by diminishing function in language, visuospatial, and executive abilities [9, 12]. Patients typically have poor recent memory; disorientation to time and place; impaired recall, recognition, object and space perception, conversational speech, and working memory; and word retrieval difficulty.
Structural imaging in the evaluation of AD is typically performed with volumetric T1-weighted imaging. Various scales and grading systems have been studied including those evaluating atrophy of the medial temporal lobes [13, 14]. Overall, these have shown adequate sensitivity and specificity, however, are limited by interobserver variation [8]. Automated segmentation methods have also shown the ability to discriminate between AD and controls [15, 16]. Image analysis software with automated volumetric segmentation of brain regions has also shown value for the prediction of development of AD from mild cognitive impairment (MCI), a condition characterized by decline in performance on standardized neurocognitive testing, demonstrating an area under the curve between 0.6 and 0.77 [17, 18]. Structural imaging is also valuable in following patients on monoclonal anti-Aβ antibody therapy, as pooled analysis reported approximately 40% of patients on aducanumab develop amyloid-related imaging abnormalities (ARIAs) (Fig. 1) [19].
A 67-year-old man with AD on anti-Aβ monoclonal antibody trial. T2 FLAIR image shows hyperintensity in the parasagittal left frontal lobe (oval in a), with no evidence of hyperintensity on DWI (b), and associated subcortical microhemorrhages (oval in c). None of these abnormalities were present prior to receiving antibody therapy (d–f), consistent with ARIA with edema and hemorrhage. Arterial spin labeling demonstrates hypoperfusion to the parietal and frontal lobes (g), with corresponding diffuse cortical uptake on florbetapir PET/CT typical of AD (h)
Advanced non-molecular imaging techniques have also been researched for the diagnosis of AD. Diffusion tensor imaging (DTI) has shown decreased fractional anisotropy (FA) and increased mean diffusivity (MD) in medial temporal lobe structures including the hippocampus, parahippocampal cingulum, uncinate fasciculus, and fornix as well as the posterior cingulate cortex (PCC), splenium of the corpus callosum, and superior longitudinal fasciculus [20, 21]. Magnetization transfer imaging (MTI), which relies on the exchange of magnetization between protons bound to macromolecules and free water, is another advanced MRI biomarker for evaluating AD, with values often reported as a magnetization transfer ratio (MTR) calculated by subtracting the signal of tissue prior to the pulse sequence by the signal following the pulse sequence then dividing by the signal prior to the pulse sequence [22]. Decreased MTR has been reported in multiple brain regions in AD including the locus coeruleus (LC), hippocampus, entorhinal cortex, precuneus, and global gray matter [22,23,24]. The proposed mechanism of decreased MTR in the LC is loss of neuromelanin and increased free water, with the LC potentially one of the earliest structures afflicted by NFTs [25, 26]. Decreased N-acetylaspartate (NAA)/myo-inositol ratio in the PCC may predict development of AD [27]. Arterial spin labeling (ASL) has been shown to be comparable to 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) in the diagnosis of AD, however, with lesser diagnostic performance in MCI [28]. Resting-state functional MRI (rsfMRI) has fairly consistently shown hypoconnectivity in the default mode network (DMN), which can be seen in earlier stages of the disease process, especially in impacted mutation carriers [29]. Decreased stiffness within the temporal and parietal lobes on magnetic resonance elastography (MRE) has been reported [30].
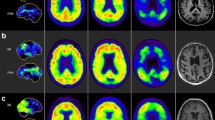
Recently, molecular imaging has shown promise in improving diagnostic confidence in AD. Current targets of clinically used radiotracers include glucose metabolism, amyloid beta plaques, and tau. Figure 2 demonstrates normal patterns of radiotracer activity for these agents. Patient preparation, dosing and kinetics, and normal and abnormal distribution of the commonly used radiotracers are described in Table 1.
Normal patterns of cerebral uptake across multiple radiotracers. Fluorodeoxyglucose PET/CT demonstrates high-grade uptake in the cortical and deep gray matter which should be greater than cerebellum (a). Cerebral blood flow imaging with early-frame or dynamic amyloid agents shows a similar pattern to FDG (b). Non-specific low-grade white matter activity with absence of gray matter activity is the normal pattern of delayed amyloid PET agents (c). Normal distribution of tau agents is low-grade gray and white matter activity with absence of elevated neocortical uptake and a variable amount of off-target binding, commonly involving the basal ganglia and choroid plexuses (arrows in d)
Fluorodeoxyglucose PET imaging has been used as a discriminatory tool for the diagnosis of AD since the early 2000s. Hypometabolism on FDG-PET scans has shown to be a reliable indicator of neuronal degeneration in AD [7]. A meta-analysis demonstrated excellent performance of FDG-PET in discriminating between AD and non-AD neurodegenerative syndromes with a sensitivity of 90% and specificity of 89% [31]. Additionally, FDG-PET has also shown signs of value in predicting the progression from amnestic type MCI to AD, especially when single-subject statistical parametric mapping (ss-SPM) was utilized, with a low probability of progression in three years with a negative study [32, 33]. Typical patterns of hypometabolism in MCI and early typical AD include the temporal lobes, parahippocampal gyri, PCC, and precuneus, with involvement of the precuneus and middle and inferior temporal gyri more characteristic of AD [34,35,36]. Advanced typical AD shows a fairly consistent pattern of diffuse hypometabolism involving the aforementioned areas as well as the parietal lobes and prefrontal cortex with relative sparing of the precentral gyrus, basal ganglia, and occipital cortex (Fig. 3), except in the posterior cortical atrophy (PCA, also known as Benson’s) clinical phenotype of Alzheimer’s dementia, where lateral occipital lobe atrophy and hypometabolism creates the “occipital tunnel” sign (Fig. 4) [37, 38]. Limbic-predominant AD tends to have more pronounced hypometabolism in the hippocampus and related mesial temporal lobe structures with additional involvement of the frontal cortex, while the limbic-sparing or cortical-predominant subtype involves similar regions to typical AD, with more prominent involvement of the frontal lobes and lesser involvement of the mesial temporal lobes as its name suggests [39]. Additional clinical AD phenotypes which can be discriminated on FDG-PET include the logopenic variant of primary progressive aphasia (lvPPA) which shows left/dominant hemisphere posterior perisylvian or parietal hypometabolism and the dysexecutive/behavioral variant which has similar temporoparietal hypometabolism to typical AD however with variable involvement of the PCC and frontal lobes [40, 41].
A 73-year-old man with AD, Mini Mental State Examination (MMSE) 23/30. Fluorodeoxyglucose PET/CT (a) demonstrates hypometabolism involving the bilateral parietal lobes (arrows) and precuneus and PCC (oval). Flortaucipir PET/CT (b) shows tau retention in the bilateral parietal lobes (arrows) and precuneus and PCC (oval), mirroring the regions of hypometabolism. Florbetapir PET/CT (c) demonstrates diffuse cortical uptake with “kissing hemispheres.” Arterial spin labeling (d) demonstrates hypoperfusion to the bilateral parietal lobes (arrows), similar to the FDG-PET/CT
A 76-year-old woman with history of memory loss. Fluorodeoxyglucose PET/MRI (a) demonstrates hypometabolism of the parietal lobes (arrows), precuneus, and PCC as well as the visual association centers in the lateral occipital lobes (arrows in b). Metabolic activity is preserved in the medial occipital lobes (“occipital tunnel sign,” white oval in b). Pattern of hypometabolism is most suggestive of posterior cortical atrophy
Along with qualitative assessment, semiquantitative assessment of FDG-PET can be performed by calculating the cerebral metabolic rate of glucose (CMRgluc), formally calculated by diving the plasma glucose level by a “lumped constant,” to correct for the varying affinities of FDG and glucose for the hexokinase transporter, multiplied by the rate of transfer of FDG from blood to brain [42]. As the method is invasive and requires numerous blood draws, other methods of calculation have been developed to approximate CMRgluc and standard uptake value of glucose (SUVgluc) [43, 44]. Studies have linked the degree of decreased glucose metabolism with the severity of cognitive impairment on standardized mental status examinations [45, 46].
Single-subject statistical parametric mapping can also be applied to the FDG-PET data set to better quantitate the degree of abnormality of an individual scan. This technique allows for voxel-based analysis through creation of a statistical map of an individual’s scan and comparing the uptake values on that scan to a series of control scans [47]. Multiple groups have documented increased diagnostic performance with ss-SPM of FDG-PET/CT [48, 49].
In comparison to FDG, Aβ selective radiotracers provide a disease-specific means of imaging the pathologic changes of AD in vivo. Carbon-11 (C-11) Pittsburgh compound B (PiB) was the first amyloid radiotracer used in human studies; however, the short half-life of C-11 limited its clinical applicability and fluorine-18 (F-18) labeled tracers were later produced [50]. Florbetaben (Neuraceq, Piramal, Mumbai), florbetapir (Amyvid, Eli Lilly, USA), and flutemetamol (Vizamyl GE Healthcare, USA) are commonly used F-18 labeled Aβ selective radiotracers, with third-generation tracers in development and preclinical trials. These tracers have also shown robust ability to distinguish AD from controls with pooled analysis demonstrating a sensitivity and specificity of 90% and 87% for florbetapir and 89% and 88% for florbetaben [51]. Amyloid imaging has shown added value to FDG-PET and clinical evaluation [52]. Qualitative interpretation of amyloid imaging is either considered positive (moderate to frequent amyloid plaque) or negative (no to sparse amyloid plaque) based on abnormal gray matter uptake on grayscale imaging in one or two regions for florbetaben and florbetapir respectively, or one region on rainbow color scale for flutemetamol [53,54,55]. Normal studies exhibit physiologic uptake within the white matter with the absence of uptake in the gray matter creating many named imaging signs, including the “diamond” of the white matter tracts of the orbitofrontal gyri, “cartoon hand” and “tree in winter” in the white matter of the frontal lobes, “double convex lens” involving the frontal and parietal parasagittal region, and the “temporo-occipital ridge” (Fig. 5) [56]. Abnormal regions demonstrate radiotracer activity within the gray matter, leading to blurring of the gray matter-white matter junction with most common sites including the precuneus, PCC, and lateral temporal and parietal lobes. The activity within the gray matter leads to the appearance of “kissing hemispheres” along the interhemispheric fissure, “tree in summer” when viewed in the coronal plane, and a “temporal plain” as opposed to a “temporo-occipital ridge” (Fig. 5) [9, 12, 56]. Even small amounts of amyloid plaques may be predictive of eventual development of AD [57]. Positron emission tomography amyloid imaging has shown similar diagnostic accuracy to cerebrospinal fluid (CSF) analysis, with the obvious advantage of negating an invasive procedure [58]. Amyloid imaging may also be used to follow patients on monoclonal antibody therapy to demonstrate clearance of Aβ plaques (Fig. 6).
Named signs of normal and abnormal patterns of amyloid activity on florbetapir PET/CT. Normal activity in the white matter of the frontal lobes leads to the “tree-in-winter sign” on coronal images, abnormal activity in the gray matter leads to the appearance of leaves known as the “tree-in-summer sign” (a, b). Absence of gray matter activity in the medial orbitofrontal lobes creates a diamond pattern of the white matter activity which is lost in abnormal studies (c, d). A similar phenomenon is seen at the cerebral convexities where the superior white matter tracts create the “double convex lens sign” (e) which is lost with abnormal gray matter activity morphing to the “kissing hemispheres sign” (f). Lastly, normal absence of gray matter activity in the temporal and occipital lobes creates a mountainous façade referred to as the “temporo-occipital ridge” (g), whereas abnormal gray matter activity leads to a more flat profile termed the “temporo-occipital plain” (h)
A 83-year-old male with typical AD on anti-Aβ monoclonal antibody clinical trial drug. Pre-treatment florbetapir PET/CT demonstrates substantial cortical amyloid burden with kissing hemispheres in midline (a). Following treatment there is significant reduction in amyloid uptake with visualization of corticomedullary differentiation reminiscent of a normal scan (b)
Semiquantitative analysis can also be performed for PET amyloid studies by calculating a standardized uptake value ratio (SUVr). This is achieved by first selecting a region of interest to determine baseline, most often the cerebellar gray matter as this rarely accumulates amyloid, and comparing the ratio of uptake in this region to specified regions of interest (ROIs) [59]. If the region demonstrates a SUVr above a predetermined threshold, it is considered positive for moderate to frequent amyloid plaque [60]. Currently there is debate in the literature about whether qualitative or semiquantitative interpretation of amyloid imaging is more efficacious [61, 62]. Additionally, as with FDG-PET, ss-SPM can be undertaken to increase diagnostic confidence. While the literature at the time is sparse, coupling these techniques with amyloid PET imaging has shown promising results in terms of diagnostic accuracy, with sensitivity and specificity near or surpassing 90% [63]. Dynamic imaging can also be performed immediately following the bolus to estimate cerebral blood flow, with a report suggesting decreased early time frame amyloid activity correlating with tau retention [64].
Tau selective radiotracers can also be utilized for disease-specific imaging of AD. Currently, the most widely used tau selective radiotracer is older-generation F-18 flortaucipir (Tauvid; Avid Radiopharmaceuticals, previously known as AV 1451 and T807) [65]. The limitation of this older-generation tau radiotracer is substantial off-target binding, with one study reporting 64% of the signal in amyloid negative healthy controls was due to off-target binding primarily from the basal ganglia structures and choroid plexuses [66]. Off-target binding can also occur in the muscles and secondary to monoamine oxidase (MAO) enzymatic activity in astrocytes in non-specified neuroinflammation. Newer-generation tau radiotracers including F-18RO-948 (previously RO69558948), F-18-MK6240, F-18-PI2620, and F-18-GTP1 are under investigation [67,68,69,70,71]. Carbon-11 labeled tau radiotracers are also being studied, although as with PiB, C-11 labeled radiotracers will inherently be limited by short half-life [68, 72]. Fluorine-18-RO-948, F-18-MK6240, and F-18-GTP1 have been shown to have greater affinity for tau than F-18 flortaucipir [70, 72, 73]. In contrast to amyloid imaging, tau imaging allows for better visualization of the topography of the pathologic changes of AD. The earliest regions of radiotracer uptake are in the mesial temporal lobes including the entorhinal cortices and hippocampi, progressing to the middle and inferior temporal lobes, parietal lobes including the angular and supramarginal gyrus, cingulate cortex, and dorsolateral frontal lobes, with groups showing promise of in vivo Braak staging using tau PET imaging [74, 75]. A recent study demonstrated a sensitivity of 92.3% to 100.0% and specificity ranging from 52.0 to 92.0% for identifying Braak stage V or VI disease at postmortem evaluation for F-18 flortaucipir (Fig. 7) [76]. Tau imaging may also differentiate between AD subtypes better than amyloid and closely mirrors cortical gray matter atrophy and FDG-PET hypometabolism [77,78,79]. As with amyloid radiotracers SUVr values can be calculated, again generally using the cerebellum for the reference value [80].
In vivo Braak staging on flortaucipir PET/CT. Activity confined to the entorhinal cortices and hippocampi falls into the Braak I-II stages (arrows in a), further progression in the temporal lobes and limbic cortices classifies stages III-IV (parahippocampal gyri; arrows in b), and neocortical activity defines stages V-VI (arrows in c)
Lastly, ongoing research is being performed to utilize molecular imaging for the evaluation of neuroinflammation and synaptic density in AD with a variety of C-11 and F-18 labeled radiotracers, none of which are currently widely used clinically. An 18 kDa translocator protein is the typical target for microglial activation with the most widely used tracer 11C-PK11195 [81]. Astrocytosis in AD is imaged by targeting MAO-B with 11C-deuterium-l-deprenyl (11C-DED) [82]. Studies have reported increased and no significant difference in microglial activation in AD patients when compared to controls [83,84,85,86]. Temporal changes in astrocytosis have been reported, with increased radiotracer activity in prodromal AD followed by a gradual decline in activity as the disease progresses which correlates with hypometabolism [87,88,89]. Uptake of radiotracers 11C-UCB-J and 18F-UCB-H, which bind to the synaptic glycoprotein 2, have been shown to be reduced in the hippocampi of patients with AD [90,91,92,93].
Dementia with Lewy bodies
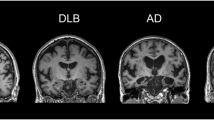
Dementia with Lewy bodies (DLB) is considered the second or third most common dementia, accounting for approximately 5% of all dementia cases in incidence studies, and up to approximately 20% of all dementia cases in prevalence studies [94]. Clinically DLB is defined by fluctuating cognition, visual hallucinations, rapid eye motion sleep behavior disorder, and parkinsonism [95]. Pathologically DLB is defined by loss of dopaminergic neurons in the substantia nigra with reduced striatal dopaminergic activity and neuronal inclusions of alpha-synuclein-positive Lewy bodies in the cerebral cortex, substantia nigra, and brainstem. These findings are indistinguishable from Parkinson’s disease (PD); however, the involvement of the cortex may be more pronounced in DLB and neuronal loss in the substantia nigra more prominent in PD [96]. In addition, there are often concomitant Aβ plaques and 3R/4R NFTs and over-expression of the APOE*E4 genotype, overlapping with the pathologic features of AD [97, 98].
As with AD, structural MRI plays a role in the assessment of DLB. T1 volumetric analysis of patients with DLB demonstrates atrophy of the frontal and temporal lobes and insular cortices with less pronounced involvement of the medial temporal lobe when compared to AD, hypothesized to be related to comparatively reduced NFT formation [99, 100]. Sparse partial least squares classification of cortical thickness based on T1-weighted imaging demonstrated good discrimination of DLB from AD with a sensitivity of 78% and specificity of 75% [101]. Atrophy of the midbrain, hypothalamus, and substantia innominate have also been shown to be useful in discriminating DLB from AD [102].
Advanced MRI techniques have also been reported to be of value in the diagnosis of DLB. Studies have shown decreased FA involving the cortical and subcortical regions including the parieto-occipital lobes, PCC, precuneus, inferior longitudinal fasciculus, caudate, putamen, and pons [103,104,105,106]. Abnormalities in FA in the parietal and occipital regions in DLB have been shown to not significantly change over time when compared to controls, corroborating the theory DLB is primarily associated with synaptic dysfunction rather than neuronal loss [107]. Absence of the “swallow tail sign,” hypointensity within nigrosome-1 on susceptibility-weighted imaging, has been reported to have a sensitivity of 63–93% and a specificity of 79–87% in discrimination DLB from other forms of dementia (Fig. 8) [108, 109]. A similar finding was demonstrated with postmortem MTI with lower signal in the substantia nigra pars compacta in patients with DLB and PD, potentially secondary to neuromelanin loss [110]. Regions of decreased contrast-to-noise ratio in the LC on MTI have been shown to relate to symptoms in PD [111]. Similar to AD, rsfMRI exhibits decreased connectivity in the default mode and executive networks and additionally the visual networks including the medial occipital network [112, 113].
A 82-year-old female with history of rigidity, bradykinesia, hallucinations, and dementia. Axial susceptibility-weighted imaging demonstrates loss of the normal hyperintense signal in nigrosome-1 (arrows), the so-called loss of “swallow tail sign” (a). The normal hyperintense signal in nigrosome-1 is shown in (b) (arrows)
Molecular imaging also plays a crucial role in the diagnosis and potentially response to therapy of DLB. Dopamine transporter (DAT) imaging has been a target for DLB, specifically the imaging of the density of the presynaptic striatal neurons to assess for degeneration in presynaptic parkinsonian syndromes. Single-photon emission computed tomography (SPECT) imaging with iodine-123 N-ω-fluoropropyl-2β-carbomethoxy-3β-[4-iodophenyl] nortropane (I-123-FP-CIT, DaTSCAN™, GE Healthcare), a cocaine analogue with high affinity for the dopamine and serotonin transporters allowing for in vivo evaluation of presynaptic striatal neuronal degeneration, was initially used clinically for discriminating PD from essential tremor on the basis of excellent reported specificity [114]. As PD and DLB share the same pathologic basis, I-123-FP-CIT has shown similar value in the diagnosis of DLB, with a diagnostic accuracy around 90% in discriminating from other dementia syndromes; however, DAT imaging is not reliable for discriminating DLB from PD-related MCI or dementia [115, 116]. Single-photon emission computed tomography imaging with I-123-Metaiodobenzylguanidine to assess for cardiac postganglionic sympathetic denervation observed in DLB is reported to have similar sensitivity and specificity to I-123-FP-CIT SPECT, although pre-existing cardiac disease and diabetes mellitus can lead to false positives [117, 118].
Positron emission tomography imaging can also be performed to evaluate for DLB. Decreased metabolic activity in the occipital, temporoparietal, and prefrontal cortices with relative sparing of the pre- and post-central gyri and medial temporal lobes has been reported in DLB [119]. Additionally, there is relatively preserved FDG uptake within the PCC, with surrounding hypometabolic activity in the cuneus and precuneus and parietal lobes, creating the so-called cingulate island sign, reported to have excellent specificity of up to 100% in differentiating AD from DLB when present (Fig. 9) [120]. The “occipital tunnel sign” is also seen in DLB, which is characterized by hypometabolism in the visual association cortex of the lateral occipital lobes and preserved metabolism in the medial occipital lobes [38]. Other studies have demonstrated less promising results for FDG-PET in differentiating DLB from AD, indicating the value of a multimodality assessment [115].
A 73-year-old man with memory loss and visuospatial processing deficits. Fluorodeoxyglucose PET/CT demonstrates hypometabolism in the bilateral parietal lobes including the bilateral precuneus with preserved metabolism of the PCC (cingulate island sign, oval in a). Also present is hypometabolism of the bilateral lateral occipital lobes with preserved medial occipital lobe activity (occipital tunnel sign, asterisk in b). Single-subject statistical parametric mapping (c) demonstrates parietal-occipital hypometabolism reiterating the occipital tunnel (arrow bottom left panel) and posterior cingulate island (arrow bottom right panel), with areas of blue progressing to purple representing further negative deviation from the mean uptake values of controls
As DLB can pathologically demonstrate Aβ plaques and NFTs, both amyloid and tau tracers can be of value in the imaging of DLB. The majority of DLB patients show amyloid uptake, with a similar pattern to AD involving the dorsolateral frontal lobes, parietal lobes including the precuneus, and temporal lobes, with greater amyloid deposition in the occipital lobes in DLB [121, 122]. Multiple groups have reported the degree of amyloid burden may be related to cognitive decline in DLB and helpful in differentiating DLB from PD-related dementia (PDD) [122,123,124]. Combined amyloid and dopamine terminal PET imaging was reported to have high accuracy in diagnosing dementia subtype when compared to pathologic examination [125]. Tau PET imaging is less well studied in DLB, with typical areas of increased uptake involving the inferolateral temporal lobes, parietal lobes including the precuneus, and occipital lobes, with decreased degree of uptake and involvement of the medial temporal lobes when compared to AD, with DLB again showing greater abnormality compared to PDD [126, 127]. Attempts at developing a radiotracer to target alpha-synuclein, the pathologic hallmark of DLB, have not been successful at the current time [128,129,130].
Vascular dementia
Vascular dementia (VaD) is considered the second or third most common dementia, varying with DLB by source [131]. There are multiple subtypes of vascular dementias, attributed to small vessel and large vessel etiologies as well as acquired and inherited conditions, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Clinical presentation is heterogeneous, with the most prominent feature decline in frontal lobe tasks including executive function and attention with verbal memory less impacted [132]. VaD also has a strong correlation with neuropsychiatric symptoms including depression [133].
Structural MRI has long held a central role in the evaluation of VaD. Two or more large territory, three or greater lacunar infarcts, or strategically placed infarcts have been considered sufficient imaging evidence for VaD by various guidelines [134, 135]. Involvement of greater than 25% of a cerebral hemisphere with white matter abnormalities/hyperintensities (WMHs) can be suggestive of subcortical VaD, although there is interobserver variation in making the diagnosis [136, 137]. Dilated Virchow-Robins spaces have also been correlated with the degree of severity of subcortical VaD [138]. Diffusion tensor imaging may predict the structural changes in VaD even prior to development of WMHs and better predicts decline in cognition with reduced FA and increased MD reported in the centrum semiovale and anterior periventricular white matter [139, 140]. Reduced FA and increased MD in the inferior fronto-occipital fascicles, forceps minor, genu, and splenium of the corpus callosum, and the superior longitudinal fasciculus have shown value in discriminating VaD from AD [141]. Early studies of rsfMRI demonstrate dysfunction involving the DMN [142].
Molecular imaging has a developing role in the evaluation of VaD. Hypometabolism in VaD is pronounced in structures spared in early AD including the anterior cingulate cortex, deep gray nuclei, primary cortices, and middle temporal gyrus, with Kerrouche et al. reporting 100% accuracy in separating VaD from AD [143, 144]. Amyloid imaging is less helpful in discriminating between VaD and AD, with at least a quarter of VaD patients demonstrating amyloid uptake and those that are PiB positive have similar distribution to AD [145,146,147]. Positive amyloid imaging, however, may predict worse cognitive function in VaD [146, 148]. While it is known tau deposition occurs following cerebral ischemia (Fig. 10), reports of tau tracers in the evaluation of VaD are lacking [149].
A 76-year-old female with symptomatic left carotid stenosis (70% by North American Symptomatic Carotid Endarterectomy Trial criteria) who presented with right homonymous hemianopia compatible with a left posterior cerebral artery stroke. Arterial spin labeling demonstrates hypoperfusion throughout the left cerebral hemisphere (a) and CTA demonstrates a fetal left posterior cerebral artery supplied by the left internal carotid artery (b). Florbetaben PET/CT demonstrates no to sparse amyloid plaque (c). Flortaucipir PET/CT prior to the event demonstrates no areas of cortical tau retention (d). Flortaucipir PET/CT following the event (e) demonstrates focal tau retention in the left posterior cerebral artery territory (arrows), likely as a response to cerebral ischemia
Frontotemporal dementia
Frontotemporal dementia (FTD) or frontotemporal lobar degeneration (FTLD) is the second most common NDD in patients under 65 years of age, only behind AD [150]. The estimated prevalence is approximately 1–5 in 100,000 people [151, 152]. There is an equal predilection of the disease between men and women, most commonly diagnosed between the ages of 45 and 65; however, patients can be diagnosed as early as the second to third decade [150]. The most common subtype is the behavioral variant (bvFTD), characterized by social disinhibition, obsessive behaviors, and hyperorality, as well as the aptly named semantic (svPPA) and nonfluent/agrammatic primary progressive aphasias (nfPPA). Three distinct pathologic subtypes are described; first Pick reported argyrophilic cytoplasmic inclusions (of predominantly 3R tau) within cortical neurons leading to ballooning of the cells and eventually gliosis, later TAR DNA-binding protein 43 (TDP-43) and fused-in-sarcoma (FUS) pathologic variants were described [153, 154]. Heritable forms make up at least 10% of cases, including those with microtubule-associated protein tau (MAPT), Chromosome 9 Open Reading Frame 72 (C9ORF72), and Progranulin mutations [150, 155].
As with the other neurodegenerative syndromes, volumetric T1-weighted imaging to assess for patterns of atrophy is commonly applied in FTD. Behavioral variant FTD tends to demonstrate marked atrophy involving the ventromedial prefrontal and insular cortices and anterior temporal lobes (Fig. 11), svPPA in the left inferior temporal lobe including the insular gyrus, and nfPPA in the posterior frontal, temporal, and parietal lobes [156]. Resting-state functional MRI has most consistently reported disruption of the salience network and frontoinsular and executive connections [157,158,159]. One study showed patients with FTD exhibit hypoperfusion on ASL in the frontal lobes and anterior cingulate cortex, with the hypoperfusion within the anterior cingulate cortex helping to differentiate FTD from AD [160]. Decreased stiffness by MRE in the temporal lobes has been reported [30].
A 69-year-old female with odd behaviors including riding a children’s tricycle and impulsivity. Coronal volumetric T1-weighted images with proprietary automated segmentation software demonstrate volume loss in the frontal and temporal lobes (a). Volumetric quantitation identifies multiple regions in the frontal lobes in less than the fifth percentile of volume for age, including the medial orbital frontal lobes, typical of bvFTD (b)
Molecular imaging plays a crucial and ever-growing role in the evaluation of FTD. Unsurprisingly, hypometabolism corresponding to regions of atrophy in the frontal lobes, including the ventromedial region and anterior temporal lobes has been frequently reported in FTD (Fig. 12) [161, 162]. Fluorodeoxyglucose PET is also an important tool in distinguishing the PPA variants with ss-SPM demonstrating hypometabolism in the left or bilateral temporal poles, middle and inferior temporal gyri, and insula in svPPA and a more heterogeneous pattern of decreases in the inferior temporal gyrus, anterior cingulate cortex, and insula with sparing of the amygdala and hippocampi in nfPPA (Fig. 13) [40, 163]. Initial work on tau-labeled tracers in FTD has primarily been in patients harboring the MAPT mutation as they are known to have tau-related pathology; however, TDP-43-related syndromes have also demonstrated uptake with the basal and medial frontal lobes, inferior and lateral temporal lobes and temporal poles, and anterior cingulate cortex with the most involved regions varying by disease subtype [164, 165]. One study demonstrated co-localization between tau binding and microglial activation [166]. Lack of uptake of amyloid labeled radiotracers can differentiate FTD from AD [167]. Table 2 describes the common imaging features of Typical AD, DLB, VaD, and FTD.
A 68-year-old man who presented to the memory care clinic with progressive personality changes and behavioral disturbances including violent outbursts and memory loss. Fluorodeoxyglucose PET/CT demonstrates marked frontal (a) and temporal (b) hypometabolism with relatively preserved parietal and occipital lobe activity including relative sparing of the PCC and precuneus. Single-subject statistical parametric mapping (c) from a similar patient demonstrates a similar pattern, with anterior greater than posterior cingulate hypometabolism, a hallmark of FTD, again with areas of blue progressing to purple representing further negative deviation from the mean uptake values of controls
A 58-year-old man with notable agrammatism, impaired comprehension and repetition of syntactically complex sentences, and spared single word comprehension, Montreal Cognitive Assessment (MoCA) 16/30. T1-weighted volumetric image (a) demonstrates asymmetric atrophy of the left perisylvian and insular cortices (arrow). Fluorodeoxyglucose PET/MRI demonstrates hypometabolism in this region (arrow in b). Single-subject statistical parametric mapping of the FDG-PET/MRI (c) demonstrates the asymmetric left greater than right hypometabolism involving the frontal and temporal operculum and parietal lobes with sparing of the PCC and precuneus (as before, areas of blue progressing to purple represent further negative deviation from the mean uptake values of controls). Combining the clinical and imaging data, a diagnosis of nfPPA was rendered
Parkinson’s disease and parkinsonian syndromes
Parkinsonian disorders are a heterogeneous group of syndromes sharing the clinical features of extrapyramidal symptoms and bradykinesia and common pathology related to alpha-synuclein and tau with degeneration of the nigrostriatal pathway. These conditions afflict at least 1% of the worldwide population greater than 70 years of age [168]. The differences between Parkinson’s disease and DLB have been previously discussed in the DLB section. Three of the most relevant parkinsonian syndromes will be reviewed; multisystem atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD).
Multisystem atrophy is a neurodegenerative disorder with multiple subtypes characterized clinically by parkinsonism with varying cerebellar, autonomic, and pyramidal dysfunction sharing common alpha-synucleinopathy pathology [169]. Two main subtypes exist, one with dominant parkinsonian features (MSA-P), and one with dominant cerebellar dysfunction (MSA-C) [170]. On structural 1.5 T MRI, MSA-P has been reported to have a characteristic appearance of a lateral rim of T2/proton density-weighted hyperintensity adjacent to the putamen and T2 hypointense signal involving the dorsolateral putamen, although the value of this finding has been questioned at higher field strengths (Fig. 14) [171, 172]. The hallmark imaging feature seen in up to 80% of patients with MSA-C is cruciform T2 hyperintense signal within the basis pontis, the so-called hot cross bun sign, although this imaging finding can also be present in spinocerebellar ataxia syndromes (Fig. 15) [173]. Additional structural imaging features of MSA-C include atrophy and T2 hyperintense signal within the pons, medulla, middle cerebellar peduncles, and cerebellar hemispheres [174, 175]. Diffusion tensor imaging exhibits widespread microstructural alterations when compared to controls including increased MD and reduced FA in the superior, middle, and inferior cerebellar peduncles and in the corona radiata and commissural fibers [176]. Patterns of reduced MTR have been shown to help differentiate MSA from PD and PSP [177]. Hypoconnectivity in the DMN, sensorimotor network, visual association cortices, and cerebellum has been reported in MSA-C [178]. Within the same study hypoperfusion of the cerebellum was exhibited on ASL imaging [178]. On FDG-PET MSA is distinguished by hypometabolism involving the bilateral putamina and cerebellar hemispheres, with the cerebellar hypometabolism reported in both MSA-C and MSA-P, with a sensitivity of 76% and specificity of 98% for the diagnosis by visual interpretation (Fig. 14) [179]. While MSA shows reduced uptake within the putamina on I-123-FP-CIT SPECT scans, this was not shown to correlate with disease severity [180].
A 76-year-old man with a history of memory impairment and parkinsonism including gait and proprioceptive dysfunction. Fluorodeoxyglucose PET/CT demonstrates hypometabolism in the right greater than left cerebellar hemispheres (a) and bilateral striatum (b), best appreciated on ss-SPM (areas of blue and purple representing negative deviation in uptake from controls, d). Iodine-123-FP-CIT scan shows left greater than right primarily putaminal presynaptic neuronal degeneration (c). Findings are compatible with MSA-P. Axial T2 FLAIR image of a separate patient demonstrates the lateral putaminal rim sign of hyperintensity (arrows in e)
Progressive supranuclear palsy is clinically defined by parkinsonism with vertical supranuclear gaze palsy and prominent postural instability with falls within the first year of onset and pathologically by 4R tau isoform NFTs in the basal ganglia, diencephalon, and brainstem [181, 182]. As with MSA, characteristic patterns of brainstem atrophy are present including reduced anterior–posterior dimension of the midbrain and widening of the interpeduncular cistern leading to the “mickey mouse sign” on axial and the “hummingbird sign” on sagittal images (Fig. 16) [183, 184]. Reduced FA in the posterior frontal lobes and cerebellar peduncles was reported to have a specificity of 91–96% and sensitivity of 85–95% in differentiating PSP from DLB [185]. Hypoconnectivity on rsfMRI has been documented of the lateral visual, auditory, cerebellar, and insular networks [186]. Typical findings of FDG-PET include hypometabolism of the brainstem and midline frontal cortex with a reported sensitivity of 60% and specificity of 96% by visual interpretation with significant augmentation of sensitivity when ss-SPM is added [179]. 18-Fluorine-PI-2620, which selectively binds to the 4R tau isoform, has shown promise as a biomarker in PSP, though further evaluation is warranted [187]. Single-photon emission computed tomography imaging of pre- and postsynaptic striatal neuronal degeneration shows similar patterns to and is not able to reliably differentiate PD from PSP [188].
Sagittal T1-weighted image in the midline coned to the brainstem demonstrates midbrain atrophy creating the so-called hummingbird sign of PSP (a). Axial T1-weighted image at the level of the midbrain demonstrates decrease in the AP dimension of the midbrain and widening of the interpeduncular cistern; the so-called morning glory sign (b)
Corticobasal degeneration is a specific form of the corticobasal syndrome (CBS) defined by extrapyramidal symptoms which are often asymmetric including an “alien limb” phenomenon with later onset loss of multidomain cognitive functioning with preserved episodic memory and poor response to levodopa therapy [189, 190]. Pathologically CBD is primarily a 4R tauopathy which demonstrates ballooned neurons in many areas of brain including the primary cortices as well as the cingulate gyrus, amygdala, insular cortex, and claustrum [191]. Typical structural MRI findings include asymmetric atrophy of the posterior frontal and parietal lobes contralateral to the patient’s symptoms with associated atrophy of the contralateral cerebral peduncle and subcortical T2 FLAIR hyperintensity with relative preservation of basal ganglia volume and signal [174, 175, 192]. Reduced FA has been reported in the pre- and post-central gyri, cingulum, and supplementary motor area [193]. Resting-state functional MRI has documented hypoconnectivity of the lateral visual and auditory networks and hyperconnectivity of the salience and executive control networks [186]. Fluorodeoxyglucose PET shows similar findings with hypometabolism involving the primary cortices and additionally basal ganglia contralateral to the affected side [179, 194, 195]. Early research on Tau tracers for CBD has shown mixed results with 18-F-AV-1451, 18-F-PI-2620 may be a more appropriate tracer given its affinity for the 4R tau isoform [196,197,198]. The role of amyloid labeled tracers is uncertain given the overlap of CBS secondary to other dementias and imperfect tau isoform selectivity of current radiotracers (Fig. 17) [198]. Iodine-123-FP-CIT SPECT demonstrates asymmetric decreased striatal uptake with less disproportionate involvement of the putamen when compared to other Parkinsonian syndromes [199, 200]. Table 3 describes the common imaging features of parkinsonian syndromes.
A 60-year-old female with cognitive decline (MoCA 19/30) and limb apraxia. Flortaucipir PET/CT (a) demonstrates tau retention in the bilateral perirolandic regions (arrow indicating left precentral gyrus). Florbetapir PET/CT (b) demonstrates radiotracer activity in the bilateral perirolandic regions (arrow indicating left precentral gyrus). Final diagnosis was CBS secondary to underlying AD, given the abnormal amyloid binding
Miscellaneous syndromes
Cerebral amyloid angiopathy
Cerebral amyloid angiopathy (CAA) is a disease of Aβ deposition in the walls of primarily the small cortical and leptomeningeal arteries and arterioles favoring the posterior regions [201, 202]. Approximately 10–20% of intracerebral hemorrhage at autopsy may be attributed to CAA with up to 80% of patients with CAA demonstrating concurrent AD pathology [203]. The updated Boston criteria 2.0, which includes findings on structural MRI of at least two either lobar or subcortical hemorrhagic lesions or scattered superficial siderosis or one of the aforementioned hemorrhagic lesions with greater than 20 perivascular spaces or white matter hyperintensities in one hemisphere without evidence of deep hemorrhagic lesions or evidence of other cause, was reported to have 64.5% sensitivity and 95% specificity for the diagnosis of typical CAA [204]. There are two rarer subtypes of CAA, inflammatory CAA, characterized by lobar edema with overlying leptomeningeal enhancement and subcortical microhemorrhages without diffusion restriction and amyloidoma, characterized by a solitary enhancing mass with surrounding edema (Fig. 18) [205]. One study demonstrated amyloid PET has good sensitivity to discriminate CAA from controls, however, with modest specificity (sensitivity of 91% and specificity of 55%) [206]. The poor specificity of amyloid PET for the diagnosis of CAA is attributed to the overlap with AD pathology; the posterior predominance of CAA has led to reports of the occipital-to-posterior cingulate or global ratio as a possible means of discrimination from AD [207, 208]. Fluorodeoxyglucose PET has demonstrated similar findings with decrease in SUVr ratio of the occipital lobe relative to the PCC in patients with CAA compared to AD [209]. As with AD, early reports of tau agents in CAA have demonstrated increased binding correlated with worsening cognition, which was not demonstrated with amyloid agents [210].
A 81-year-old female with a gradual decline in short term memory and depression. Susceptibility-weighted image demonstrates numerous subcortical microhemorrhages (a). Fluorodeoxyglucose PET/MRI (b) demonstrates relatively normal cerebral metabolism. Later she presented with an acute worsening of symptoms including new right-side weakness and dysphasia. Magnetic resonance imaging demonstrated T2 FLAIR hyperintensity within the left frontal lobe (c) without restricted diffusion (d). Final diagnosis was amyloid beta-related angiitis. Cognitive function improved following administration of steroids. Fluorodeoxyglucose PET/MRI suggested against concurrent AD. Images from a separate patient demonstrate occipito-temporal subcortical microhemorrhages (e) with diffuse amyloid uptake on florbetapir PET/CT, a pattern suggesting concurrent CAA with AD (f)
Chronic traumatic encephalopathy
Chronic traumatic encephalopathy (CTE) is a tauopathy secondary to repetitive mild traumatic brain injury more recently described in the literature with clinical features of decreased attention and memory, affective disturbances, psychosis, and gait and speech difficulties [211, 212]. Four progressive pathologic stages of the disease are defined with perivascular hyperphosphorylated tau NFTs beginning in the dorsolateral frontal cortices and spreading to the temporal and parietal lobes and deep nuclei with sparing of the calcarine cortex except in severe cases [213]. Amyloid plaques are not considered a feature of the disease [211]. Structural imaging shows an exceedingly higher than expected number of patients with a cavum septum pellucidum, felt to be related to shear forces due to a CSF fluid wave from trauma [213, 214]. Additionally, generalized cerebral white and gray matter volume loss has been reported [214, 215]. Reduced FA in white matter tracts including the superior and inferior longitudinal fasciculus, corona radiata, cerebral peduncle, uncinate fasciculus, and anterior thalamic radiations as well as the ventral striatum has been described [216,217,218]. Tau radiotracers are reported to have increased uptake in the frontotemporal lobes including the medial temporal lobes with frontal-lobe predominant involvement of the sulcal depths (Fig. 19) [219, 220]. Areas of hypometabolism on FDG-PET generally mirror the regions of tau retention [215, 220]. Variable positivity has been seen with amyloid tracers [219].
A 70-year-old man with history of military combat and traumatic brain injury with MCI, MoCA 26/30. Flortaucipir PET/CT demonstrates regions of tau retention in the sulcal depths of the parasagittal frontal cortices (arrows in a and b). Tau retention is also noted in the bilateral mesial temporal lobes (arrows in c). Florbetapir PET/CT (d) demonstrates no significant cortical amyloid uptake involving the parietal lobes. Given the findings on PET/CT memory impairment was diagnosed as related to CTE
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized primarily by upper and lower motor neuron degeneration leading to clinical signs of progressive weakness, muscle atrophy, and fasciculations starting in the limbs [221]. The pathologic hallmark of the disease is ubiquitinated TDP-43 inclusions [222]. T2 hyperintensity involving the corticospinal tracts (CSTs) was one of the earliest reported imaging features of ALS; however, studies have demonstrated inconsistency in this finding [223,224,225]. Reduced FA on DTI and NAA on MR spectroscopy in the CSTs has been consistently reported [226, 227]. Increased iron deposition quantified by T2* imaging has been shown in the motor cortex of patients with ALS [228]. Resting-state functional MRI shows decreased connectivity in the motor network [229]. Fluorodeoxyglucose PET imaging has demonstrated hypometabolism in the motor/perirolandic and frontal cortices as well as the occipital lobes with a sensitivity of 94.8–95.4% and specificity of 80–82.5% for the diagnosis [230, 231]. Binding in the CSTs of 2-([1E,3E]-4-[6-([11C]methylamino)pyridinyl]buta-1,3-dienyl)benzo[d]thiazol-6-ol ([11C]PBB3), a tau radiotracer, was shown to correlate with upper motor neuron signs in a patient with ALS/parkinsonian dementia complex overlap; however, extensive reports of tau agents are lacking at the current time, although an attractive target given the pathologic findings of TDP-43 in ALS [222, 232].
Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder characterized clinically by chorea, dementia, and psychosis, genetically by CAG trinucleotide repeat expansion on chromosome 4, and pathologically by atrophy in the striatum with involvement of other subcortical and cortical regions at higher stages [233]. Structural imaging shows striking atrophy of the striatum with ex vacuo dilation of the frontal horns of the lateral ventricles (so-called box-shaped ventricles), which is predictive of symptom onset (Fig. 20) [234]. Studies have overall demonstrated increased FA, felt to be related to selective neuronal degeneration (i.e. as selected tracts are damaged the remaining fibers demonstrate more organization), and increased MD in the basal ganglia [235]. Additional regions with reduced FA and increased MD include the CSTs and corpus callosum [235]. Hypometabolism in the striatum as well as the frontal and temporal lobes has been reported, with striatal hypometabolism a potential marker of time to symptom onset [236]. Decreased striatal dopamine receptor binding, most commonly utilizing [11C]raclopride-PET, has been exhibited in HD patients [237]. Other research targets have included adenosine, cannabinoid, and gamma-aminobutyric acid (GABA) receptors (diminished in HD), microglial activation (increased), phosphodiesterase 10A enzymatic activity (decreased), and synaptic vesicle protein 2A expression (decreased), all of which are not yet utilized clinically [238]. To date, a radiotracer targeting the Huntington protein is not available; however, this would obviously be an attractive target.
Creutzfeldt–Jakob disease
Creutzfeldt–Jakob disease (CJD) is a rapidly progressive uniformly fatal prion-driven NDD afflicting approximately 1–2 persons per million worldwide, with three subtypes, the sporadic subtype representing the majority of cases [239]. Characteristic MRI features include hyperintensity on T2 or diffusion-weighted imaging (DWI) involving the cortex (cortical ribboning) and basal ganglia with involvement of the thalamus (“hockey stick sign”) less commonly reported in the sporadic subtype and more common in variant CJD (Fig. 21) [240]. T1 hyperintensity in the globus pallidus, thought to be related to accumulation of misfolded proteins, may also be observed, even without DWI changes [241]. Decreased NAA on MR spectroscopy has been reported and is likely related to neuronal death [242]. Asymmetric hypometabolism in the frontal and parietal cortices with or without decreased activity in the basal ganglia has been demonstrated with FDG-PET (Fig. 21) [243, 244]. Sporadic case reports have shown no significant amyloid and F-18 flortaucipir retention with one case demonstrating uptake of 18F-THK5351, felt to be related to off-target binding of MAO-B activation in astrocytosis and correlating to the areas of signal abnormality [245,246,247,248].
A 64-year-old female with three months of progressive functional decline, failure to thrive, and weakness. Diffusion-weighted imaging (a) demonstrates hyperintensity in the right caudate head and lentiform nucleus (arrow) and the bilateral thalami with an L-shaped or “hockey stick” configuration (asterisks). Fluorodeoxyglucose PET/MRI (b) demonstrates corresponding asymmetric hypometabolism in the right lentiform nucleus (arrow) and bilateral thalami (asterisks). Cerebrospinal fluid 14-3-3 protein was positive and pathological examination of the brain was consistent with CJD
Pseudodementia
Technically a non-NDD, pseudodementia was first described in 1961 by Kiloh as a condition of apparent dementia most commonly to a secondary mental illness such as depression leading to memory loss of recent and remote events and inattention [249, 250]. Often a geriatric depression scale is performed with initial memory care clinic consultation to exclude concomitant mental illness driving dementia symptoms [251]. Molecular imaging provides an excellent method to discriminate non-NDDs/pseudodementia from NDDs (Fig. 22) [252, 253]. Table 4 describes the common imaging features of miscellaneous NDDs.
A 65-year-old female with reported gradual decline in short term memory and depressive symptoms, initial MoCA 25/30. Florbetapir PET/CT demonstrates normal white matter distribution with no regions of abnormal cortical uptake, compatible with no to sparse amyloid plaque (a). Flortaucipir PET/CT demonstrates no cortical tau retention with off-target binding in the bilateral choroid plexuses and basal ganglia (b). Score on MoCA improved to 29/30 on antidepressant therapy. Final diagnosis was pseudodementia related to major depressive disorder
Conclusion
A wide variety of advanced multiparametric MRI and molecular imaging techniques are now available to increase diagnostic confidence of neurodegenerative syndromes, with ongoing research to thrust more of these techniques into a wider clinical role. Combined PET/MRI is an attractive imaging modality to provide a comprehensive workup of NDDs in a single imaging session. Ultimately, these techniques may be of use in selecting for and following up patients on monoclonal antibody therapy.
Availability of data and materials
Not applicable.
Abbreviations
- 11C-DED:
-
11C-deuterium-l-deprenyl
- 3R:
-
Three repeat
- 4R:
-
Four repeat
- AD:
-
Alzheimer’s disease
- ARIAs:
-
Amyloid-related imaging abnormalities
- ASL:
-
Arterial spin labeling
- Aβ:
-
Amyloid-beta
- C-11:
-
Carbon-11
- C9ORF72:
-
Chromosome 9 open reading frame 72
- CAA:
-
Cerebral amyloid angiopathy
- CBD:
-
Corticobasal degeneration
- CBS:
-
Corticobasal syndrome
- CJD:
-
Creutzfeldt–Jakob disease
- CMRgluc:
-
Cerebral metabolic rate of glucose
- CTE:
-
Chronic traumatic encephalopathy
- CTSs:
-
Corticospinal tracts
- DAT:
-
Dopamine transporter
- DLB:
-
Dementia with Lewy bodies
- DMN:
-
Default mode network
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- F-18:
-
Fluorine-18
- FA:
-
Fractional anisotropy
- FDG-PET:
-
2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography
- FTD:
-
Frontotemporal dementia
- FTLD:
-
Frontotemporal lobar degeneration
- FUS:
-
Fused-in-sarcoma
- I-123-FP-CIT, DaTSCAN:
-
Iodine-123 N-ω-fluoropropyl-2β-carbomethoxy-3β-[4-iodophenyl] nortropane
- LC:
-
Locus coeruleus
- lvPPA:
-
Logopenic variant primary progressive aphasia
- MAO:
-
Monoamine oxidase
- MAPT:
-
Microtubule-associated protein tau
- MCI:
-
Mild cognitive impairment
- MD:
-
Mean diffusivity
- MMSE:
-
Mini-mental state examination
- MoCA:
-
Montreal cognitive assessment
- MRE:
-
Magnetic resonance elastography
- MRI:
-
Magnetic resonance imaging
- MSA:
-
Multisystem atrophy
- MSA-C:
-
Multisystem atrophy with dominant cerebellar dysfunction
- MSA-P:
-
Multisystem atrophy with parkinsonian features
- MTI:
-
Magnetization transfer imaging
- MTR:
-
Magnetization transfer ratio
- NAA:
-
N-acetylaspartate
- NDD:
-
Neurodegenerative diseases
- nfPPA:
-
Nonfluent/agrammatic primary progressive aphasia
- NFTs:
-
Neurofibrillary tangles
- PCA:
-
Posterior cortical atrophy
- PCC:
-
Posterior cingulate cortex
- PD:
-
Parkinson’s disease
- PDD:
-
PD-related dementia
- PiB:
-
Pittsburgh compound B
- PSEN1:
-
Presenilin 1
- PSEN2:
-
Presenilin 2
- PSP:
-
Progressive supranuclear palsy
- ROI:
-
Region of interest
- rsfMRI:
-
Resting-state functional MRI
- SPECT:
-
Single-photon emission computed tomography
- ss-SPM:
-
Single-subject statistical parametric mapping
- SUVgluc:
-
Standard uptake value of glucose
- SUVr:
-
Standardized uptake value ratio
- svPPA:
-
Semantic primary progressive aphasia
- TDP-43:
-
TAR DNA-binding protein 43
- VaD:
-
Vascular dementia
- WMHs:
-
White matter abnormalities/hyperintensities
References
Cao Q, Tan CC, Xu W et al (2020) The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis 73:1157–1166. https://doi.org/10.3233/jad-191092
Beach TG, Monsell SE, Phillips LE, Kukull W (2012) Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol 71:266–273. https://doi.org/10.1097/NEN.0b013e31824b211b
Salloway S, Sperling R, Fox NC et al (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333. https://doi.org/10.1056/NEJMoa1304839
Vandenberghe R, Rinne JO, Boada M et al (2016) Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther 8:18. https://doi.org/10.1186/s13195-016-0189-7
Sevigny J, Chiao P, Bussière T et al (2016) The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537:50–56. https://doi.org/10.1038/nature19323
Honig LS, Vellas B, Woodward M et al (2018) Trial of Solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 378:321–330. https://doi.org/10.1056/NEJMoa1705971
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Park M, Moon WJ (2016) Structural MR imaging in the diagnosis of Alzheimer’s disease and other neurodegenerative dementia: current imaging approach and future perspectives. Korean J Radiol 17:827–845. https://doi.org/10.3348/kjr.2016.17.6.827
Zukotynski K, Kuo PH, Mikulis D et al (2018) PET/CT of dementia. AJR Am J Roentgenol 211:246–259. https://doi.org/10.2214/ajr.18.19822
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/bf00308809
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. https://doi.org/10.1007/s00401-006-0127-z
Laforce R Jr, Soucy JP, Sellami L et al (2018) Molecular imaging in dementia: past, present, and future. Alzheimers Dement 14:1522–1552. https://doi.org/10.1016/j.jalz.2018.06.2855
Duara R, Loewenstein DA, Potter E et al (2008) Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology 71:1986–1992. https://doi.org/10.1212/01.wnl.0000336925.79704.9f
Urs R, Potter E, Barker W et al (2009) Visual rating system for assessing magnetic resonance images: a tool in the diagnosis of mild cognitive impairment and Alzheimer disease. J Comput Assist Tomogr 33:73–78. https://doi.org/10.1097/RCT.0b013e31816373d8
Wolk DA, Das SR, Mueller SG, Weiner MW, Yushkevich PA (2017) Medial temporal lobe subregional morphometry using high resolution MRI in Alzheimer’s disease. Neurobiol Aging 49:204–213. https://doi.org/10.1016/j.neurobiolaging.2016.09.011
Xie L, Wisse LEM, Pluta J et al (2019) Automated segmentation of medial temporal lobe subregions on in vivo T1-weighted MRI in early stages of Alzheimer’s disease. Hum Brain Mapp 40:3431–3451. https://doi.org/10.1002/hbm.24607
Hill DLG, Schwarz AJ, Isaac M et al (2014) Coalition against major diseases/European medicines agency biomarker qualification of hippocampal volume for enrichment of clinical trials in predementia stages of Alzheimer’s disease. Alzheimers Dement 10:421–9.e3. https://doi.org/10.1016/j.jalz.2013.07.003
Tanpitukpongse TP, Mazurowski MA, Ikhena J, Petrella JR (2017) Predictive utility of marketed volumetric software tools in subjects at risk for Alzheimer disease: do regions outside the hippocampus matter? AJNR Am J Neuroradiol 38:546–552. https://doi.org/10.3174/ajnr.A5061
Salloway S, Chalkias S, Barkhof F et al (2022) Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients With early Alzheimer disease. JAMA Neurol 79:13–21. https://doi.org/10.1001/jamaneurol.2021.4161
Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP (2011) A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 32(2322):e5-18. https://doi.org/10.1016/j.neurobiolaging.2010.05.019
Nir TM, Jahanshad N, Villalon-Reina JE et al (2013) Effectiveness of regional DTI measures in distinguishing Alzheimer’s disease, MCI, and normal aging. Neuroimage Clin 3:180–195. https://doi.org/10.1016/j.nicl.2013.07.006
Ridha BH, Tozer DJ, Symms MR et al (2007) Quantitative magnetization transfer imaging in Alzheimer disease. Radiology 244:832–837. https://doi.org/10.1148/radiol.2443061128
Betts MJ, Cardenas-Blanco A, Kanowski M et al (2019) Locus coeruleus MRI contrast is reduced in Alzheimer’s disease dementia and correlates with CSF Aβ levels. Alzheimers Dement (Amst) 11:281–285. https://doi.org/10.1016/j.dadm.2019.02.001
Colonna I, Koini M, Pirpamer L et al (2021) Microstructural tissue changes in Alzheimer disease brains: insights from magnetization transfer imaging. AJNR Am J Neuroradiol 42:688–693. https://doi.org/10.3174/ajnr.A6975
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. https://doi.org/10.1097/NEN.0b013e318232a379
Chen Y, Chen T, Hou R (2022) Locus coeruleus in the pathogenesis of Alzheimer’s disease: a systematic review. Alzheimers Dement (N Y) 8:e12257. https://doi.org/10.1002/trc2.12257
Waragai M, Moriya M, Nojo T (2017) Decreased N-Acetyl Aspartate/Myo-inositol ratio in the posterior cingulate cortex shown by magnetic resonance spectroscopy may be one of the risk markers of preclinical Alzheimer’s disease: A 7-year follow-up study. J Alzheimers Dis 60:1411–1427. https://doi.org/10.3233/jad-170450
Riederer I, Bohn KP, Preibisch C et al (2018) Alzheimer disease and mild cognitive impairment: integrated pulsed arterial spin-labeling MRI and (18)F-FDG PET. Radiology 288:198–206. https://doi.org/10.1148/radiol.2018170575
Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P (2017) Resting-state network dysfunction in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement (Amst) 8:73–85. https://doi.org/10.1016/j.dadm.2017.03.007
ElSheikh M, Arani A, Perry A et al (2017) MR elastography demonstrates unique regional brain stiffness patterns in dementias. AJR Am J Roentgenol 209:403–408. https://doi.org/10.2214/ajr.16.17455
Bloudek LM, Spackman DE, Blankenburg M, Sullivan SD (2011) Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis 26:627–645. https://doi.org/10.3233/jad-2011-110458
Silverman DH, Small GW, Chang CY et al (2001) Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 286:2120–2127. https://doi.org/10.1001/jama.286.17.2120
Smailagic N, Lafortune L, Kelly S, Hyde C, Brayne C (2018) 18F-FDG PET for prediction of conversion to Alzheimer’s disease dementia in people with mild cognitive impairment: an updated systematic review of test accuracy. J Alzheimers Dis 64:1175–1194. https://doi.org/10.3233/jad-171125
Drzezga A, Lautenschlager N, Siebner H et al (2003) Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 30:1104–1113. https://doi.org/10.1007/s00259-003-1194-1
Del Sole A, Clerici F, Chiti A et al (2008) Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: an FDG PET study. Eur J Nucl Med Mol Imaging 35:1357–1366. https://doi.org/10.1007/s00259-008-0773-6
Sanabria-Diaz G, Martínez-Montes E, Melie-Garcia L (2013) Glucose metabolism during resting state reveals abnormal brain networks organization in the Alzheimer’s disease and mild cognitive impairment. PLoS One 8:e68860. https://doi.org/10.1371/journal.pone.0068860
Herholz K, Carter SF, Jones M (2007) Positron emission tomography imaging in dementia. Br J Radiol 80:S160–S167. https://doi.org/10.1259/bjr/97295129
Sawyer DM, Kuo PH (2018) “Occipital Tunnel” sign on FDG PET for differentiating dementias. Clin Nucl Med 43:e59–e61. https://doi.org/10.1097/rlu.0000000000001925
Levin F, Ferreira D, Lange C et al (2021) Data-driven FDG-PET subtypes of Alzheimer’s disease-related neurodegeneration. Alzheimers Res Ther 13:49. https://doi.org/10.1186/s13195-021-00785-9
Gorno-Tempini ML, Hillis AE, Weintraub S et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
Bergeron D, Sellami L, Poulin S, Verret L, Bouchard RW, Laforce R Jr (2020) The behavioral/dysexecutive variant of Alzheimer’s disease: a case series with clinical, neuropsychological, and FDG-PET characterization. Dement Geriatr Cogn Disord 49:518–525. https://doi.org/10.1159/000511210
Herholz K (2014) The role of PET quantification in neurological imaging: FDG and amyloid imaging in dementia. Clin Transl Imaging 2:321–330. https://doi.org/10.1007/s40336-014-0073-z
Hutchins GD, Holden JE, Koeppe RA, Halama JR, Gatley SJ, Nickles RJ (1984) Alternative approach to single-scan estimation of cerebral glucose metabolic rate using glucose analogs, with particular application to ischemia. J Cereb Blood Flow Metab 4:35–40. https://doi.org/10.1038/jcbfm.1984.5
Huang SC (2000) Anatomy of SUV. Standardized uptake value. Nucl Med Biol 27:643–646. https://doi.org/10.1016/s0969-8051(00)00155-4
Langbaum JB, Chen K, Lee W et al (2009) Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer’s disease neuroimaging initiative (ADNI). Neuroimage 45:1107–1116. https://doi.org/10.1016/j.neuroimage.2008.12.072
Furst AJ, Rabinovici GD, Rostomian AH et al (2012) Cognition, glucose metabolism and amyloid burden in Alzheimer’s disease. Neurobiol Aging 33:215–225. https://doi.org/10.1016/j.neurobiolaging.2010.03.011
Della Rosa PA, Cerami C, Gallivanone F et al (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12:575–593. https://doi.org/10.1007/s12021-014-9235-4
Perani D, Della Rosa PA, Cerami C et al (2014) Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. Neuroimage Clin 6:445–454. https://doi.org/10.1016/j.nicl.2014.10.009
Ford JN, Sweeney EM, Skafida M et al (2021) Heuristic scoring method utilizing FDG-PET statistical parametric mapping in the evaluation of suspected Alzheimer disease and frontotemporal lobar degeneration. Am J Nucl Med Mol Imaging 11:313–326
Klunk WE, Engler H, Nordberg A et al (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol 55:306–319. https://doi.org/10.1002/ana.20009
Yeo JM, Waddell B, Khan Z, Pal S (2015) A systematic review and meta-analysis of (18)F-labeled amyloid imaging in Alzheimer’s disease. Alzheimers Dement (Amst) 1:5–13. https://doi.org/10.1016/j.dadm.2014.11.004
Hellwig S, Frings L, Bormann T, Vach W, Buchert R, Meyer PT (2019) Amyloid imaging for differential diagnosis of dementia: incremental value compared to clinical diagnosis and [(18)F]FDG PET. Eur J Nucl Med Mol Imaging 46:312–323. https://doi.org/10.1007/s00259-018-4111-3
Clark CM, Schneider JA, Bedell BJ et al (2011) Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 305:275–283. https://doi.org/10.1001/jama.2010.2008
Fodero-Tavoletti MT, Brockschnieder D, Villemagne VL et al (2012) In vitro characterization of [18F]-florbetaben, an Aβ imaging radiotracer. Nucl Med Biol 39:1042–1048. https://doi.org/10.1016/j.nucmedbio.2012.03.001
Buckley CJ, Sherwin PF, Smith AP, Wolber J, Weick SM, Brooks DJ (2017) Validation of an electronic image reader training programme for interpretation of [18F]flutemetamol β-amyloid PET brain images. Nucl Med Commun 38:234–241. https://doi.org/10.1097/mnm.0000000000000633
Lundeen TF, Seibyl JP, Covington MF, Eshghi N, Kuo PH (2018) Signs and artifacts in amyloid PET. Radiographics 38:2123–2133. https://doi.org/10.1148/rg.2018180160
Mormino EC, Brandel MG, Madison CM et al (2012) Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage 59:1152–1160. https://doi.org/10.1016/j.neuroimage.2011.07.098
Palmqvist S, Zetterberg H, Mattsson N et al (2015) Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 85:1240–1249. https://doi.org/10.1212/wnl.0000000000001991
van Berckel BN, Ossenkoppele R, Tolboom N et al (2013) Longitudinal amyloid imaging using 11C-PiB: methodologic considerations. J Nucl Med 54:1570–1576. https://doi.org/10.2967/jnumed.112.113654
Thurfjell L, Lilja J, Lundqvist R et al (2014) Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med 55:1623–1628. https://doi.org/10.2967/jnumed.114.142109
Ng S, Villemagne VL, Berlangieri S et al (2007) Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J Nucl Med 48:547–552. https://doi.org/10.2967/jnumed.106.037762
Camus V, Payoux P, Barré L et al (2012) Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur J Nucl Med Mol Imaging 39:621–631. https://doi.org/10.1007/s00259-011-2021-8
Akamatsu G, Ikari Y, Ohnishi A et al (2019) Voxel-based statistical analysis and quantification of amyloid PET in the Japanese Alzheimer’s disease neuroimaging initiative (J-ADNI) multi-center study. EJNMMI Res 9:91. https://doi.org/10.1186/s13550-019-0561-2
Raman F, Fang YD, Grandhi S et al (2022) Dynamic amyloid PET: relationships to (18)F-Flortaucipir tau PET measures. J Nucl Med 63:287–293. https://doi.org/10.2967/jnumed.120.254490
Chien DT, Bahri S, Szardenings AK et al (2013) Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis 34:457–468. https://doi.org/10.3233/jad-122059
Baker SL, Harrison TM, Maass A, La Joie R, Jagust WJ (2019) Effect of off-target binding on (18)F-Flortaucipir variability in healthy controls across the life span. J Nucl Med 60:1444–1451. https://doi.org/10.2967/jnumed.118.224113
Walji AM, Hostetler ED, Selnick H et al (2016) Discovery of 6-(Fluoro-18F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([18F]-MK-6240): a positron emission tomography (PET) imaging agent for quantification of neurofibrillary tangles (NFTs). J Med Chem 59:4778–4789. https://doi.org/10.1021/acs.jmedchem.6b00166
Honer M, Gobbi L, Knust H et al (2018) Preclinical evaluation of 8F-RO6958948, 11C-RO6931643, and 11C-RO6924963 as Novel PET radiotracers for imaging tau aggregates in Alzheimer disease. J Nucl Med 59:675–681. https://doi.org/10.2967/jnumed.117.196741
Leuzy A, Chiotis K, Lemoine L et al (2019) Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry 24:1112–1134. https://doi.org/10.1038/s41380-018-0342-8
Sanabria Bohórquez S, Marik J, Ogasawara A et al (2019) [18F]GTP1 (Genentech Tau Probe 1), a radioligand for detecting neurofibrillary tangle tau pathology in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 46:2077–2089. https://doi.org/10.1007/s00259-019-04399-0
Kroth H, Oden F, Molette J et al (2019) Discovery and preclinical characterization of [(18)F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur J Nucl Med Mol Imaging 46:2178–2189. https://doi.org/10.1007/s00259-019-04397-2
Gobbi LC, Knust H, Körner M et al (2017) Identification of three novel radiotracers for imaging aggregated tau in Alzheimer’s disease with positron emission tomography. J Med Chem 60:7350–7370. https://doi.org/10.1021/acs.jmedchem.7b00632
Hostetler ED, Walji AM, Zeng Z et al (2016) Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J Nucl Med 57:1599–1606. https://doi.org/10.2967/jnumed.115.171678
Schöll M, Lockhart SN, Schonhaut DR et al (2016) PET imaging of tau deposition in the aging human brain. Neuron 89:971–982. https://doi.org/10.1016/j.neuron.2016.01.028
Biel D, Brendel M, Rubinski A et al (2021) Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res Ther 13:137. https://doi.org/10.1186/s13195-021-00880-x
Fleisher AS, Pontecorvo MJ, Devous MD Sr et al (2020) Positron emission tomography imaging With [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol 77:829–839. https://doi.org/10.1001/jamaneurol.2020.0528
Ossenkoppele R, Schonhaut DR, Schöll M et al (2016) Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139:1551–1567. https://doi.org/10.1093/brain/aww027
Xia C, Makaretz SJ, Caso C et al (2017) Association of In vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol 74:427–436. https://doi.org/10.1001/jamaneurol.2016.5755
Chiotis K, Saint-Aubert L, Rodriguez-Vieitez E et al (2018) Longitudinal changes of tau PET imaging in relation to hypometabolism in prodromal and Alzheimer’s disease dementia. Mol Psychiatry 23:1666–1673. https://doi.org/10.1038/mp.2017.108
Vemuri P, Lowe VJ, Knopman DS et al (2017) Tau-PET uptake: regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement (Amst) 6:21–30. https://doi.org/10.1016/j.dadm.2016.12.010
Varrone A, Oikonen V, Forsberg A et al (2015) Positron emission tomography imaging of the 18-kDa translocator protein (TSPO) with [18F]FEMPA in Alzheimer’s disease patients and control subjects. Eur J Nucl Med Mol Imaging 42:438–446. https://doi.org/10.1007/s00259-014-2955-8
Santillo AF, Gambini JP, Lannfelt L et al (2011) In vivo imaging of astrocytosis in Alzheimer’s disease: an 11C-L-deuteriodeprenyl and PIB PET study. Eur J Nucl Med Mol Imaging 38:2202–2208. https://doi.org/10.1007/s00259-011-1895-9
Edison P, Archer HA, Gerhard A et al (2008) Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis 32:412–419. https://doi.org/10.1016/j.nbd.2008.08.001
Wiley CA, Lopresti BJ, Venneti S et al (2009) Carbon 11-labeled Pittsburgh compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol 66:60–67. https://doi.org/10.1001/archneurol.2008.511
Yokokura M, Mori N, Yagi S et al (2011) In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 38:343–351. https://doi.org/10.1007/s00259-010-1612-0
Schuitemaker A, Kropholler MA, Boellaard R et al (2013) Microglial activation in Alzheimer’s disease: an (R)-[11C]PK11195 positron emission tomography study. Neurobiol Aging 34:128–136. https://doi.org/10.1016/j.neurobiolaging.2012.04.021
Carter SF, Schöll M, Almkvist O et al (2012) Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med 53:37–46. https://doi.org/10.2967/jnumed.110.087031
Rodriguez-Vieitez E, Saint-Aubert L, Carter SF et al (2016) Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain 139:922–936. https://doi.org/10.1093/brain/awv404
Carter SF, Chiotis K, Nordberg A, Rodriguez-Vieitez E (2019) Longitudinal association between astrocyte function and glucose metabolism in autosomal dominant Alzheimer’s disease. Eur J Nucl Med Mol Imaging 46:348–356. https://doi.org/10.1007/s00259-018-4217-7
Warnock GI, Aerts J, Bahri MA et al (2014) Evaluation of 18F-UCB-H as a novel PET tracer for synaptic vesicle protein 2A in the brain. J Nucl Med 55:1336–1341. https://doi.org/10.2967/jnumed.113.136143
Nabulsi NB, Mercier J, Holden D et al (2016) Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med 57:777–784. https://doi.org/10.2967/jnumed.115.168179
Chen MK, Mecca AP, Naganawa M et al (2018) Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol 75:1215–1224. https://doi.org/10.1001/jamaneurol.2018.1836
Bastin C, Bahri MA, Meyer F et al (2020) In vivo imaging of synaptic loss in Alzheimer’s disease with [18F]UCB-H positron emission tomography. Eur J Nucl Med Mol Imaging 47:390–402. https://doi.org/10.1007/s00259-019-04461-x
Hogan DB, Fiest KM, Roberts JI et al (2016) The prevalence and incidence of dementia with lewy bodies: a systematic review. Can J Neurol Sci 43(Suppl 1):S83-95. https://doi.org/10.1017/cjn.2016.2
McKeith IG, Boeve BF, Dickson DW et al (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89:88–100. https://doi.org/10.1212/wnl.0000000000004058
Tsuboi Y, Dickson DW (2005) Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinsonism Relat Disord 11(Suppl 1):S47-51. https://doi.org/10.1016/j.parkreldis.2004.10.014
Tsuang D, Leverenz JB, Lopez OL et al (2013) APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 70:223–228. https://doi.org/10.1001/jamaneurol.2013.600
Elahi FM, Miller BL (2017) A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol 13:457–476. https://doi.org/10.1038/nrneurol.2017.96
Burton EJ, Karas G, Paling SM et al (2002) Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage 17:618–630
Burton EJ, Barber R, Mukaetova-Ladinska EB et al (2009) Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132:195–203. https://doi.org/10.1093/brain/awn298
Lebedev AV, Westman E, Beyer MK et al (2013) Multivariate classification of patients with Alzheimer’s and dementia with Lewy bodies using high-dimensional cortical thickness measurements: an MRI surface-based morphometric study. J Neurol 260:1104–1115. https://doi.org/10.1007/s00415-012-6768-z
Whitwell JL, Weigand SD, Shiung MM et al (2007) Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain 130:708–719. https://doi.org/10.1093/brain/awl388
Bozzali M, Falini A, Cercignani M et al (2005) Brain tissue damage in dementia with Lewy bodies: an in vivo diffusion tensor MRI study. Brain 128:1595–1604. https://doi.org/10.1093/brain/awh493
Firbank MJ, Blamire AM, Krishnan MS et al (2007) Atrophy is associated with posterior cingulate white matter disruption in dementia with Lewy bodies and Alzheimer’s disease. Neuroimage 36:1–7. https://doi.org/10.1016/j.neuroimage.2007.02.027
Kantarci K, Avula R, Senjem ML et al (2010) Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology 74:1814–1821. https://doi.org/10.1212/WNL.0b013e3181e0f7cf
Watson R, Blamire AM, Colloby SJ et al (2012) Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 79:906–914. https://doi.org/10.1212/WNL.0b013e318266fc51
Firbank MJ, Watson R, Mak E et al (2016) Longitudinal diffusion tensor imaging in dementia with Lewy bodies and Alzheimer’s disease. Parkinsonism Relat Disord 24:76–80. https://doi.org/10.1016/j.parkreldis.2016.01.003
Shams S, Fällmar D, Schwarz S et al (2017) MRI of the swallow tail sign: a useful marker in the diagnosis of lewy body dementia? AJNR Am J Neuroradiol 38:1737–1741. https://doi.org/10.3174/ajnr.A5274
Kamagata K, Nakatsuka T, Sakakibara R et al (2017) Diagnostic imaging of dementia with Lewy bodies by susceptibility-weighted imaging of nigrosomes versus striatal dopamine transporter single-photon emission computed tomography: a retrospective observational study. Neuroradiology 59:89–98. https://doi.org/10.1007/s00234-016-1773-z
Kitao S, Matsusue E, Fujii S et al (2013) Correlation between pathology and neuromelanin MR imaging in Parkinson’s disease and dementia with Lewy bodies. Neuroradiology 55:947–953. https://doi.org/10.1007/s00234-013-1199-9
Madelung CF, Meder D, Fuglsang SA et al (2022) Locus coeruleus shows a spatial pattern of structural disintegration in Parkinson’s disease. Mov Disord 37:479–489. https://doi.org/10.1002/mds.28945
Lowther ER, O’Brien JT, Firbank MJ, Blamire AM (2014) Lewy body compared with Alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry Res 223:192–201. https://doi.org/10.1016/j.pscychresns.2014.06.004
Sourty M, Thoraval L, Roquet D, Armspach JP, Foucher J, Blanc F (2016) Identifying dynamic functional connectivity changes in dementia with lewy bodies based on product hidden markov models. Front Comput Neurosci 10:60. https://doi.org/10.3389/fncom.2016.00060
Marshall VL, Reininger CB, Marquardt M et al (2009) Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord 24:500–508. https://doi.org/10.1002/mds.22108
O’Brien JT, Oertel WH, McKeith IG et al (2014) Is ioflupane I123 injection diagnostically effective in patients with movement disorders and dementia? Pooled analysis of four clinical trials. BMJ Open 4:e005122. https://doi.org/10.1136/bmjopen-2014-005122
Thomas AJ, Attems J, Colloby SJ et al (2017) Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 88:276–283. https://doi.org/10.1212/wnl.0000000000003512
King AE, Mintz J, Royall DR (2011) Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord 26:1218–1224. https://doi.org/10.1002/mds.23659
Yoshita M, Taki J, Yamada M (2001) A clinical role for [<sup>123</sup>I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer’s-type and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 71:583. https://doi.org/10.1136/jnnp.71.5.583
Klein JC, Eggers C, Kalbe E et al (2010) Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 74:885–892. https://doi.org/10.1212/WNL.0b013e3181d55f61
Lim SM, Katsifis A, Villemagne VL et al (2009) The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med 50:1638–1645. https://doi.org/10.2967/jnumed.109.065870
Rowe CC, Ng S, Ackermann U et al (2007) Imaging beta-amyloid burden in aging and dementia. Neurology 68:1718–1725. https://doi.org/10.1212/01.wnl.0000261919.22630.ea
Gomperts SN, Rentz DM, Moran E et al (2008) Imaging amyloid deposition in Lewy body diseases. Neurology 71:903–910. https://doi.org/10.1212/01.wnl.0000326146.60732.d6
Edison P, Rowe CC, Rinne JO et al (2008) Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 79:1331–1338. https://doi.org/10.1136/jnnp.2007.127878
Gomperts SN, Locascio JJ, Marquie M et al (2012) Brain amyloid and cognition in Lewy body diseases. Mov Disord 27:965–973. https://doi.org/10.1002/mds.25048
Albin RL, Fisher-Hubbard A, Shanmugasundaram K et al (2015) Post-Mortem evaluation of amyloid-dopamine terminal positron emission tomography dementia classifications. Ann Neurol 78:824–830. https://doi.org/10.1002/ana.24481
Gomperts SN, Locascio JJ, Makaretz SJ et al (2016) Tau positron emission tomographic imaging in the lewy body diseases. JAMA Neurol 73:1334–1341. https://doi.org/10.1001/jamaneurol.2016.3338
Kantarci K, Lowe VJ, Boeve BF et al (2017) AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 81:58–67. https://doi.org/10.1002/ana.24825
Fodero-Tavoletti MT, Mulligan RS, Okamura N et al (2009) In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur J Pharmacol 617:54–58. https://doi.org/10.1016/j.ejphar.2009.06.042
Zhang X, Jin H, Padakanti PK et al (2014) Radiosynthesis and in vivo evaluation of two PET radioligands for imaging α-synuclein. Appl Sci (Basel) 4:66–78. https://doi.org/10.3390/app4010066
Chu W, Zhou D, Gaba V et al (2015) Design, synthesis, and characterization of 3-(Benzylidene)indolin-2-one derivatives as ligands for α-synuclein fibrils. J Med Chem 58:6002–6017. https://doi.org/10.1021/acs.jmedchem.5b00571
Plassman BL, Langa KM, Fisher GG et al (2007) Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29:125–132. https://doi.org/10.1159/000109998
Sachdev PS, Brodaty H, Valenzuela MJ et al (2004) The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 62:912–919. https://doi.org/10.1212/01.wnl.0000115108.65264.4b
de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM (2000) Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry 57:1071–1076. https://doi.org/10.1001/archpsyc.57.11.1071
Román GC, Tatemichi TK, Erkinjuntti T et al (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43:250–260. https://doi.org/10.1212/wnl.43.2.250
Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226:75–80. https://doi.org/10.1016/j.jns.2004.09.019
van Straaten EC, Scheltens P, Knol DL et al (2003) Operational definitions for the NINDS-AIREN criteria for vascular dementia: an interobserver study. Stroke 34:1907–1912. https://doi.org/10.1161/01.Str.0000083050.44441.10
Price CC, Jefferson AL, Merino JG, Heilman KM, Libon DJ (2005) Subcortical vascular dementia: integrating neuropsychological and neuroradiologic data. Neurology 65:376–382. https://doi.org/10.1212/01.wnl.0000168877.06011.15
Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H (2010) Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 41:2483–2490. https://doi.org/10.1161/strokeaha.110.591586
O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS (2001) Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57:2307–2310. https://doi.org/10.1212/wnl.57.12.2307
van Norden AG, de Laat KF, van Dijk EJ et al (2012) Diffusion tensor imaging and cognition in cerebral small vessel disease: the RUN DMC study. Biochim Biophys Acta 1822:401–407. https://doi.org/10.1016/j.bbadis.2011.04.008
Fu JL, Zhang T, Chang C, Zhang YZ, Li WB (2012) The value of diffusion tensor imaging in the differential diagnosis of subcortical ischemic vascular dementia and Alzheimer’s disease in patients with only mild white matter alterations on T2-weighted images. Acta Radiol 53:312–317. https://doi.org/10.1258/ar.2011.110272
Wang R, Liu N, Tao YY et al (2020) The application of rs-fMRI in vascular cognitive impairment. Front Neurol 11:951. https://doi.org/10.3389/fneur.2020.00951
Kerrouche N, Herholz K, Mielke R, Holthoff V, Baron JC (2006) 18FDG PET in vascular dementia: differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab 26:1213–1221. https://doi.org/10.1038/sj.jcbfm.9600296
Pascual B, Prieto E, Arbizu J, Marti-Climent J, Olier J, Masdeu JC (2010) Brain glucose metabolism in vascular white matter disease with dementia: differentiation from Alzheimer disease. Stroke 41:2889–2893. https://doi.org/10.1161/strokeaha.110.591552
Villemagne VL, Ong K, Mulligan RS et al (2011) Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med 52:1210–1217. https://doi.org/10.2967/jnumed.111.089730
Lee JH, Kim SH, Kim GH et al (2011) Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology 77:18–25. https://doi.org/10.1212/WNL.0b013e318221acee
Yun HJ, Moon SH, Kim HJ et al (2017) Centiloid method evaluation for amyloid PET of subcortical vascular dementia. Sci Rep 7:16322. https://doi.org/10.1038/s41598-017-16236-1
Ye BS, Seo SW, Kim JH et al (2015) Effects of amyloid and vascular markers on cognitive decline in subcortical vascular dementia. Neurology 85:1687–1693. https://doi.org/10.1212/wnl.0000000000002097
Pluta R, Ułamek-Kozioł M, Januszewski S, Czuczwar SJ (2018) Tau protein dysfunction after brain ischemia. J Alzheimers Dis 66:429–437. https://doi.org/10.3233/jad-180772
Olney NT, Spina S, Miller BL (2017) Frontotemporal dementia. Neurol Clin 35:339–374. https://doi.org/10.1016/j.ncl.2017.01.008
Coyle-Gilchrist IT, Dick KM, Patterson K et al (2016) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86:1736–1743. https://doi.org/10.1212/wnl.0000000000002638
Knopman DS, Roberts RO (2011) Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci 45:330–335. https://doi.org/10.1007/s12031-011-9538-y
Pick A (1892) Uber die beziehungen der senilen hirnatrophie zur aphasie. Prager Medizinische Wochenschrift 17:165–167
Rohrer JD, Lashley T, Schott JM et al (2011) Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134:2565–2581. https://doi.org/10.1093/brain/awr198
Goldman JS, Farmer JM, Wood EM et al (2005) Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65:1817–1819. https://doi.org/10.1212/01.wnl.0000187068.92184.63
Lu PH, Mendez MF, Lee GJ et al (2013) Patterns of brain atrophy in clinical variants of frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 35:34–50. https://doi.org/10.1159/000345523
Zhou J, Greicius MD, Gennatas ED et al (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133:1352–1367. https://doi.org/10.1093/brain/awq075
Agosta F, Sala S, Valsasina P et al (2013) Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 81:134–143. https://doi.org/10.1212/WNL.0b013e31829a33f8
Dopper EG, Rombouts SA, Jiskoot LC et al (2014) Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology 83:e19-26. https://doi.org/10.1212/wnl.0000000000000583
Steketee RM, Bron EE, Meijboom R et al (2016) Early-stage differentiation between presenile Alzheimer’s disease and frontotemporal dementia using arterial spin labeling MRI. Eur Radiol 26:244–253. https://doi.org/10.1007/s00330-015-3789-x
Salmon E, Garraux G, Delbeuck X et al (2003) Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. Neuroimage 20:435–440. https://doi.org/10.1016/s1053-8119(03)00346-x
Kanda T, Ishii K, Uemura T et al (2008) Comparison of grey matter and metabolic reductions in frontotemporal dementia using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging 35:2227–2234. https://doi.org/10.1007/s00259-008-0871-5
Cerami C, Dodich A, Greco L et al (2017) The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J Alzheimers Dis 55:183–197. https://doi.org/10.3233/jad-160682
Smith R, Puschmann A, Schöll M et al (2016) 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain 139:2372–2379. https://doi.org/10.1093/brain/aww163
Spina S, Schonhaut DR, Boeve BF et al (2017) Frontotemporal dementia with the V337M MAPT mutation: Tau-PET and pathology correlations. Neurology 88:758–766. https://doi.org/10.1212/wnl.0000000000003636
Mak E, Nicastro N, Malpetti M et al (2021) Imaging tau burden in dementia with Lewy bodies using [(18)F]-AV1451 positron emission tomography. Neurobiol Aging 101:172–180. https://doi.org/10.1016/j.neurobiolaging.2020.11.006
Tan RH, Kril JJ, Yang Y et al (2017) Assessment of amyloid β in pathologically confirmed frontotemporal dementia syndromes. Alzheimers Dement (Amst) 9:10–20. https://doi.org/10.1016/j.dadm.2017.05.005
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29:1583–1590. https://doi.org/10.1002/mds.25945
Wenning GK, Colosimo C, Geser F, Poewe W (2004) Multiple system atrophy. Lancet Neurol 3:93–103. https://doi.org/10.1016/s1474-4422(03)00662-8
Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676. https://doi.org/10.1212/01.wnl.0000324625.00404.15
Kraft E, Schwarz J, Trenkwalder C, Vogl T, Pfluger T, Oertel WH (1999) The combination of hypointense and hyperintense signal changes on T2-weighted magnetic resonance imaging sequences: a specific marker of multiple system atrophy? Arch Neurol 56:225–228. https://doi.org/10.1001/archneur.56.2.225
Lee WH, Lee CC, Shyu WC, Chong PN, Lin SZ (2005) Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR Am J Neuroradiol 26:2238–2242
Watanabe H, Saito Y, Terao S et al (2002) Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125:1070–1083. https://doi.org/10.1093/brain/awf117
Savoiardo M, Strada L, Girotti F et al (1990) Olivopontocerebellar atrophy: MR diagnosis and relationship to multisystem atrophy. Radiology 174:693–696. https://doi.org/10.1148/radiology.174.3.2305051
Mascalchi M, Vella A, Ceravolo R (2012) Movement disorders: role of imaging in diagnosis. J Magn Reson Imaging 35:239–256. https://doi.org/10.1002/jmri.22825
Rulseh AM, Keller J, Rusz J et al (2016) Diffusion tensor imaging in the characterization of multiple system atrophy. Neuropsychiatr Dis Treat 12:2181–2187. https://doi.org/10.2147/ndt.S109094
Eckert T, Sailer M, Kaufmann J et al (2004) Differentiation of idiopathic Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 21:229–235
Zheng W, Ren S, Zhang H et al (2019) Spatial patterns of decreased cerebral blood flow and functional connectivity in multiple system atrophy (cerebellar-type): a combined arterial spin labeling perfusion and resting state functional magnetic resonance imaging study. Front Neurosci 13:777. https://doi.org/10.3389/fnins.2019.00777
Eckert T, Barnes A, Dhawan V et al (2005) FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 26:912–921. https://doi.org/10.1016/j.neuroimage.2005.03.012
Nishimori M, Murata Y, Iwasa H et al (2018) Comparison of MRI and (123)I-FP-CIT SPECT for the evaluation of MSA-P clinical severity. Biomed Rep 8:523–528. https://doi.org/10.3892/br.2018.1086
Litvan I, Agid Y, Jankovic J et al (1996) Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 46:922–930. https://doi.org/10.1212/wnl.46.4.922
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17:74–82. https://doi.org/10.1111/j.1750-3639.2007.00054.x
Kato N, Arai K, Hattori T (2003) Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 210:57–60. https://doi.org/10.1016/s0022-510x(03)00014-5
Seppi K, Poewe W (2010) Brain magnetic resonance imaging techniques in the diagnosis of parkinsonian syndromes. Neuroimaging Clin N Am 20:29–55. https://doi.org/10.1016/j.nic.2009.08.016
Spotorno N, Hall S, Irwin DJ et al (2019) Diffusion tensor MRI to distinguish progressive supranuclear palsy from α-synucleinopathies. Radiology 293:646–653. https://doi.org/10.1148/radiol.2019190406
Bharti K, Bologna M, Upadhyay N et al (2017) Abnormal resting-state functional connectivity in progressive supranuclear palsy and corticobasal syndrome. Front Neurol 8:248. https://doi.org/10.3389/fneur.2017.00248
Brendel M, Barthel H, van Eimeren T et al (2020) Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol 77:1408–1419. https://doi.org/10.1001/jamaneurol.2020.2526
Vlaar AM, de Nijs T, Kessels AG et al (2008) Diagnostic value of 123I-ioflupane and 123I-iodobenzamide SPECT scans in 248 patients with parkinsonian syndromes. Eur Neurol 59:258–266. https://doi.org/10.1159/000115640
Brooks DJ (2002) Diagnosis and management of atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 72(Suppl 1):I10–I16. https://doi.org/10.1136/jnnp.72.suppl_1.i10
Lee SE, Rabinovici GD, Mayo MC et al (2011) Clinicopathological correlations in corticobasal degeneration. Ann Neurol 70:327–340. https://doi.org/10.1002/ana.22424
Dickson DW, Bergeron C, Chin SS et al (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946. https://doi.org/10.1093/jnen/61.11.935
Koyama M, Yagishita A, Nakata Y, Hayashi M, Bandoh M, Mizutani T (2007) Imaging of corticobasal degeneration syndrome. Neuroradiology 49:905–912. https://doi.org/10.1007/s00234-007-0265-6
Erbetta A, Mandelli ML, Savoiardo M et al (2009) Diffusion tensor imaging shows different topographic involvement of the thalamus in progressive supranuclear palsy and corticobasal degeneration. AJNR Am J Neuroradiol 30:1482–1487. https://doi.org/10.3174/ajnr.A1615
Teune LK, Bartels AL, de Jong BM et al (2010) Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord 25:2395–2404. https://doi.org/10.1002/mds.23291
Mille E, Levin J, Brendel M et al (2017) Cerebral glucose metabolism and dopaminergic function in patients with corticobasal syndrome. J Neuroimaging 27:255–261. https://doi.org/10.1111/jon.12391
Josephs KA, Whitwell JL, Tacik P et al (2016) [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132:931–933. https://doi.org/10.1007/s00401-016-1618-1
Lowe VJ, Curran G, Fang P et al (2016) An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4:58. https://doi.org/10.1186/s40478-016-0315-6
Palleis C, Brendel M, Finze A et al (2021) Cortical [(18) F]PI-2620 binding differentiates corticobasal syndrome subtypes. Mov Disord 36:2104–2115. https://doi.org/10.1002/mds.28624
Pirker W, Asenbaum S, Bencsits G et al (2000) [123I]beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord 15:1158–1167. https://doi.org/10.1002/1531-8257(200011)15:6%3c1158::aid-mds1015%3e3.0.co;2-0
Badoud S, Van De Ville D, Nicastro N, Garibotto V, Burkhard PR, Haller S (2016) Discriminating among degenerative parkinsonisms using advanced (123)I-ioflupane SPECT analyses. Neuroimage Clin 12:234–240. https://doi.org/10.1016/j.nicl.2016.07.004
Pantelakis S (1954) A particular type of senile angiopathy of the central nervous system: congophilic angiopathy, topography and frequency. Monatsschr Psychiatr Neurol 128:219–256
Biffi A, Greenberg SM (2011) Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 7:1–9. https://doi.org/10.3988/jcn.2011.7.1.1
Jellinger KA (2002) Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm (Vienna) 109:813–836. https://doi.org/10.1007/s007020200068
Charidimou A, Boulouis G, Frosch MP et al (2022) The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol 21:714–725. https://doi.org/10.1016/s1474-4422(22)00208-3
Miller-Thomas MM, Sipe AL, Benzinger TL, McConathy J, Connolly S, Schwetye KE (2016) Multimodality review of amyloid-related diseases of the central nervous system. Radiographics 36:1147–1163. https://doi.org/10.1148/rg.2016150172
Baron JC, Farid K, Dolan E et al (2014) Diagnostic utility of amyloid PET in cerebral amyloid angiopathy-related symptomatic intracerebral hemorrhage. J Cereb Blood Flow Metab 34:753–758. https://doi.org/10.1038/jcbfm.2014.43
Farid K, Hong YT, Aigbirhio FI et al (2015) Early-phase 11C-PiB PET in amyloid angiopathy-related symptomatic cerebral hemorrhage: potential diagnostic value? PLoS One 10:e0139926. https://doi.org/10.1371/journal.pone.0139926
Charidimou A, Farid K, Tsai HH, Tsai LK, Yen RF, Baron JC (2018) Amyloid-PET burden and regional distribution in cerebral amyloid angiopathy: a systematic review and meta-analysis of biomarker performance. J Neurol Neurosurg Psychiatry 89:410–417. https://doi.org/10.1136/jnnp-2017-316851
Bergeret S, Queneau M, Rodallec M et al (2021) [(18) F]FDG PET may differentiate cerebral amyloid angiopathy from Alzheimer’s disease. Eur J Neurol 28:1511–1519. https://doi.org/10.1111/ene.14743
Schoemaker D, Charidimou A, Zanon Zotin MC et al (2021) Association of memory impairment with concomitant tau pathology in patients with cerebral amyloid angiopathy. Neurology 96:e1975–e1986. https://doi.org/10.1212/wnl.0000000000011745
Roberts GW, Allsop D, Bruton C (1990) The occult aftermath of boxing. J Neurol Neurosurg Psychiatry 53:373–378. https://doi.org/10.1136/jnnp.53.5.373
McKee AC, Cantu RC, Nowinski CJ et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735. https://doi.org/10.1097/NEN.0b013e3181a9d503
McKee AC, Stern RA, Nowinski CJ et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. https://doi.org/10.1093/brain/aws307
Koerte IK, Hufschmidt J, Muehlmann M et al (2016) Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma 33:346–353. https://doi.org/10.1089/neu.2015.3880
Bang SA, Song YS, Moon BS et al (2016) Neuropsychological, metabolic, and GABAA receptor studies in subjects with repetitive traumatic brain injury. J Neurotrauma 33:1005–1014. https://doi.org/10.1089/neu.2015.4051
Multani N, Goswami R, Khodadadi M et al (2016) The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol 263:1332–1341. https://doi.org/10.1007/s00415-016-8141-0
Wilde EA, Hunter JV, Li X et al (2016) Chronic effects of boxing: diffusion tensor imaging and cognitive findings. J Neurotrauma 33:672–680. https://doi.org/10.1089/neu.2015.4035
Mayer AR, Ling JM, Dodd AB, Meier TB, Hanlon FM, Klimaj SD (2017) A prospective microstructure imaging study in mixed-martial artists using geometric measures and diffusion tensor imaging: methods and findings. Brain Imaging Behav 11:698–711. https://doi.org/10.1007/s11682-016-9546-1
Stern RA, Adler CH, Chen K et al (2019) Tau positron-emission tomography in former national football league players. N Engl J Med 380:1716–1725. https://doi.org/10.1056/NEJMoa1900757
Lesman-Segev OH, La Joie R, Stephens ML et al (2019) Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. Neuroimage Clin 24:102025. https://doi.org/10.1016/j.nicl.2019.102025
Masrori P, Van Damme P (2020) Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol 27:1918–1929. https://doi.org/10.1111/ene.14393
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. https://doi.org/10.1126/science.1134108
Comi G, Rovaris M, Leocani L (1999) Review neuroimaging in amyotrophic lateral sclerosis. Eur J Neurol 6:629–637. https://doi.org/10.1046/j.1468-1331.1999.660629.x
Hecht MJ, Fellner F, Fellner C, Hilz MJ, Heuss D, Neundörfer B (2001) MRI-FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2-, T1- and proton-density-weighted images. J Neurol Sci 186:37–44. https://doi.org/10.1016/s0022-510x(01)00503-2
Jin J, Hu F, Zhang Q, Jia R, Dang J (2016) Hyperintensity of the corticospinal tract on FLAIR: a simple and sensitive objective upper motor neuron degeneration marker in clinically verified amyotrophic lateral sclerosis. J Neurol Sci 367:177–183. https://doi.org/10.1016/j.jns.2016.06.005
Foerster BR, Carlos RC, Dwamena BA et al (2014) Multimodal MRI as a diagnostic biomarker for amyotrophic lateral sclerosis. Ann Clin Transl Neurol 1:107–114. https://doi.org/10.1002/acn3.30
Cheong I, Marjańska M, Deelchand DK, Eberly LE, Walk D, Öz G (2017) Ultra-high field proton MR spectroscopy in early-stage amyotrophic lateral sclerosis. Neurochem Res 42:1833–1844. https://doi.org/10.1007/s11064-017-2248-2
Costagli M, Donatelli G, Biagi L et al (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin 12:965–969. https://doi.org/10.1016/j.nicl.2016.04.011
Zhou F, Gong H, Li F et al (2013) Altered motor network functional connectivity in amyotrophic lateral sclerosis: a resting-state functional magnetic resonance imaging study. NeuroReport 24:657–662. https://doi.org/10.1097/WNR.0b013e328363148c
Van Laere K, Vanhee A, Verschueren J et al (2014) Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol 71:553–561. https://doi.org/10.1001/jamaneurol.2014.62
Pagani M, Chiò A, Valentini MC et al (2014) Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 83:1067–1074. https://doi.org/10.1212/wnl.0000000000000792
Shinotoh H, Shimada H, Kokubo Y et al (2019) Tau imaging detects distinctive distribution of tau pathology in ALS/PDC on the Kii Peninsula. Neurology 92:e136–e147. https://doi.org/10.1212/wnl.0000000000006736
McColgan P, Tabrizi SJ (2018) Huntington’s disease: a clinical review. Eur J Neurol 25:24–34. https://doi.org/10.1111/ene.13413
Tabrizi SJ, Scahill RI, Owen G et al (2013) Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol 12:637–649. https://doi.org/10.1016/s1474-4422(13)70088-7
Estevez-Fraga C, Scahill R, Rees G, Tabrizi SJ, Gregory S (2020) Diffusion imaging in Huntington’s disease: comprehensive review. J Neurol Neurosurg Psychiatry 92:62–69. https://doi.org/10.1136/jnnp-2020-324377
Ciarmiello A, Cannella M, Lastoria S et al (2006) Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med 47:215–222
Andrews TC, Weeks RA, Turjanski N et al (1999) Huntington’s disease progression. PET and clinical observations. Brain 122(Pt 12):2353–2363. https://doi.org/10.1093/brain/122.12.2353
Cybulska K, Perk L, Booij J, Laverman P, Rijpkema M (2020) Huntington’s disease: a review of the known PET imaging biomarkers and targeting radiotracers. Molecules. https://doi.org/10.3390/molecules25030482
Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M (2020) Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect Dis 20:e2–e10. https://doi.org/10.1016/s1473-3099(19)30615-2
Fragoso DC, Gonçalves Filho AL, Pacheco FT et al (2017) Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics 37:234–257. https://doi.org/10.1148/rg.2017160075
Tschampa HJ, Kallenberg K, Urbach H et al (2005) MRI in the diagnosis of sporadic Creutzfeldt-Jakob disease: a study on inter-observer agreement. Brain 128:2026–2033. https://doi.org/10.1093/brain/awh575
Galanaud D, Haik S, Linguraru MG et al (2010) Combined diffusion imaging and MR spectroscopy in the diagnosis of human prion diseases. AJNR Am J Neuroradiol 31:1311–1318. https://doi.org/10.3174/ajnr.A2069
Kim EJ, Cho SS, Jeong BH et al (2012) Glucose metabolism in sporadic Creutzfeldt-Jakob disease: a statistical parametric mapping analysis of (18) F-FDG PET. Eur J Neurol 19:488–493. https://doi.org/10.1111/j.1468-1331.2011.03570.x
Renard D, Castelnovo G, Collombier L, Thouvenot E, Boudousq V (2017) FDG-PET in Creutzfeldt-Jakob disease: analysis of clinical-PET correlation. Prion 11:440–453. https://doi.org/10.1080/19336896.2017.1387348
Villemagne VL, McLean CA, Reardon K et al (2009) 11C-PiB PET studies in typical sporadic Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 80:998–1001. https://doi.org/10.1136/jnnp.2008.171496
Matías-Guiu JA, Guerrero-Márquez C, Cabrera-Martín MN et al (2017) Amyloid- and FDG-PET in sporadic Creutzfeldt-Jakob disease: correlation with pathological prion protein in neuropathology. Prion 11:205–213. https://doi.org/10.1080/19336896.2017.1314427
Day GS, Gordon BA, Perrin RJ et al (2018) In vivo [(18)F]-AV-1451 tau-PET imaging in sporadic Creutzfeldt-Jakob disease. Neurology 90:e896–e906. https://doi.org/10.1212/wnl.0000000000005064
Kim HJ, Cho H, Park S et al (2019) THK5351 and flortaucipir PET with pathological correlation in a Creutzfeldt-Jakob disease patient: a case report. BMC Neurol 19:211. https://doi.org/10.1186/s12883-019-1434-z
Kiloh LG (1961) Pseudo-dementia. Acta Psychiatr Scand 37:336–351. https://doi.org/10.1111/j.1600-0447.1961.tb07367.x
Small GW, Liston EH, Jarvik LF (1981) Diagnosis and treatment of dementia in the aged. West J Med 135:469–481
Yesavage JA, Brink TL, Rose TL et al (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49. https://doi.org/10.1016/0022-3956(82)90033-4
Rasgon NL, Kenna HA, Geist C, Small G, Silverman D (2008) Cerebral metabolic patterns in untreated postmenopausal women with major depressive disorder. Psychiatry Res 164:77–80. https://doi.org/10.1016/j.pscychresns.2007.12.006
Loreto F, Gunning S, Golemme M et al (2021) Evaluating cognitive profiles of patients undergoing clinical amyloid-PET imaging. Brain Commun 3:fcab035. https://doi.org/10.1093/braincomms/fcab035
Acknowledgements
We thank Sarah Klingenberger, Radiology Graphics Imaging Specialist at the University of Rochester Medical Center, and Kevin Hopkins, CNMT, for their assistance with preparing the figures for print.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JL was the major contributor in writing the manuscript. SP and SM contributed to the radiologic images and critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As the images contain no PHI, ethics approval is waived by the research subjects review board at the University of Rochester.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loftus, J.R., Puri, S. & Meyers, S.P. Multimodality imaging of neurodegenerative disorders with a focus on multiparametric magnetic resonance and molecular imaging. Insights Imaging 14, 8 (2023). https://doi.org/10.1186/s13244-022-01358-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-022-01358-6