Abstract
Purpose
The aim of this study was to investigate the regional differences between the morphologic and functional changes in the same patients with frontotemporal dementia (FTD) using statistical parametric mapping and voxel-based morphometry (VBM).
Methods
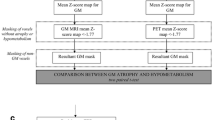
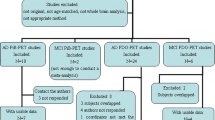
Thirteen FTD patients (mean age, 64.9 years old; mean MMSE score, 17.7), 20 sex-matched Alzheimer’s disease (AD) patients (mean age, 65.0 years old; mean MMSE score, 17.5), and 20 normal volunteers (mean age, 65.2 years old; mean MMSE score, 29.0) underwent both [18F]FDG positron emission tomography and three-dimensional spoiled gradient echo MRI. Statistical parametric mapping was used to conduct a VBM analysis of the morphologic data, which were compared voxel by voxel with the results of a similar analysis of glucose metabolic data.
Results
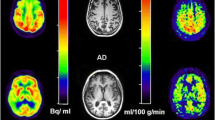
FTD patients showed decreased grey matter volume and decreased glucose metabolism in the frontal lobe and anterior temporal lobe. In addition, there was a clear asymmetry in grey matter volume in FTD patients by the VBM analysis while the glucose metabolic data showed little asymmetry. In AD patients, glucose metabolic reduction occurred in the bilateral posterior cingulate gyri and parietal lobules while grey matter density decreased the least in the same patients.
Conclusion
In FTD, metabolic and morphologic changes occur in the bilateral frontal lobe and temporal lobe with a limited asymmetry whereas there was considerable discordance in the AD group.



Similar content being viewed by others
References
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54.
Pasquier F, Grymonprez L, Lebert F, Van der Linden M. Memory impairment differs in frontotemporal dementia and Alzheimer’s disease. Neurocase 2001;7:161–71.
Lindau M, Almkvist O, Kushi J, Boone K, Johansson SE, Wahlund LO, et al. First symptoms: frontotemporal dementia versus Alzheimer’s disease. Dement Geriatr Cogn Disord 2000;11:286–93.
Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, Kang E, et al. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nucl Med 2005;46:233–9.
Diehl J, Grimmer T, Drzezga A, Riemenschneider M, Förstl H, Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia A PET study. Neurobiol Aging 2004;25:1051–6.
Grimmer T, Diehl J, Drzezga A, Förstl H, Kurz A. Region-specific decline of cerebral glucose metabolism in patients with frontotemporal dementia: a prospective 18F-FDG-PET study. Dement Geriatr Cogn Disord 2004;18:32–6.
Ishii K, Sakamoto S, Sasaki M, Kitagaki H, Yamaji S, Hashimoto M, et al. Cerebral glucose metabolism in patients with frontotemporal dementia. J Nucl Med 1998;39:1875–8.
Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain 2004;127:628–49.
Gee J, Ding L, Xie Z, Lin M, DeVita C, Grossman M. Alzheimer’s disease and frontotemporal dementia exhibit distinct atrophy-behavior correlates a computer-assisted imaging study. Acad Radiol 2003;10:1392–401.
Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol 2005;62:1402–8.
Mendez MF, Shapira JS, McMurtray A, Licht E, Miller BL. Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol 2007;64:830–5.
Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988;24:637–9.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44.
Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med 1997;38:925–8.
Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp 1995;3:165–89.
Ashburner J, Neelin P, Collins DL, Evans AC, Friston KJ. Incorporating prior knowledge into image registration. Neuroimage 1997;6:344–52.
Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp 1999;7:254–66.
Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001;14:685–700.
Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain 2007;130:708–19.
Jeong Y, Song YM, Chung PW, Kim EJ, Kang SJ, Kim JM, et al. Correlation of ventricular asymmetry with metabolic asymmetry in frontotemporal dementia. J Neuroradiol. 2005;32:247–54.
Santens P, De Bleecker J, Goethals P, Strigckmans K, Lemahieu I, Slegers G, et al. Differential regional cerebral uptake of 18F-fluoro-2-deoxy-D-glucose in Alzheimer’s disease and frontotemporal dementia at initial diagnosis. Eur Neurol 2001;45:19–27.
Salmon E, Garraux G, Delbeuck X, Collette F, Kalbe E, Zuendorf G, et al. Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. Neuroimage 2003;20:435–40.
Shinagawa S, Ikeda M, Fukuhara R, Tanabe H. Initial symptoms in frontotemporal dementia and semantic dementia compared with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:74–80.
Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL et al. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005;65:75–80.
Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002;58:198–208.
Whitwell JL, Jack CR Jr, Baker M, Rademakers R, Adamson J, Boeve BF, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol 2007;64:371–6.
Whitwell JL, Jack CR Jr. Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Top Magn Reson Imaging 2005;16:409–25.
Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, et al. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 2007;130:1159–66.
Hooten WM, Lyketsos CG. Frontotemporal dementia: a clinicopathological review of four postmortem studies. J Neuropsychiatry Clin Neurosci 1996;8:10–9.
Boccardi M, Laakso MP, Bresciani L, Galluzzi S, Geraldi C, Beltramello A, et al. The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging 2003;24:95–103.
Fukui T, Kertesz A. Volumetric study of lobar atrophy in Pick complex and Alzheimer’s disease. J Neurol Sci 2000;174:111–21.
Ishii K, Sasaki H, Kono AK, Miyamoto N, Fukuda T, Mori E. Comparison of gray matter and metabolic reduction in mild Alzheimer’s disease using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging 2005;32:959–63.
Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer’s disease and semantic dementia. Neuroimage 2006;30:1010–20.
Mosconi L, Pupi A, De Cristofaro MT, Fayyaz M, Sorbi S, Herholz K. Functional interactions of the entorhinal cortex: an 18F-FDG PET study on normal aging and Alzheimer’s disease. J Nucl Med 2004;45:382–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanda, T., Ishii, K., Uemura, T. et al. Comparison of grey matter and metabolic reductions in frontotemporal dementia using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging 35, 2227–2234 (2008). https://doi.org/10.1007/s00259-008-0871-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0871-5