Abstract
Background
Canine babesiosis is a severe disease caused by several Babesia spp. A number of names have been proposed for the canine-infecting piroplasmid pathogen initially named Theileria annae Zahler, Rinder, Schein & Gothe, 2000. It was shown to be a member of the Babesia (sensu lato) group infecting carnivores and is also closely related to the Babesia microti group. Subsequently, the same parasite species was reclassified as a member of the genus Babesia and the name Babesia vulpes Baneth, Florin-Christensen, Cardoso & Schnittger, 2015 was proposed for it. However, both names do not meet the requirements of the International Code of Zoological Nomenclature (no accompanying descriptions, no deposition of type-specimens) and cannot be recognized as available names from the nomenclatural point of view. The purpose of this study was to further characterize this parasite in order to confirm its validity, to provide its description and to introduce zoological nomenclature for it with the name Babesia vulpes n. sp.
Results
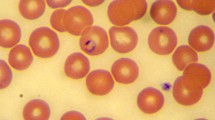
Morphological description of the parasite in canine erythrocytes demonstrated that it takes the shape of small (1.33 × 0.98 µm), round to oval forms reminiscent of the pyriform and ring shapes of other small canine Babesia spp., such as Babesia gibsoni Patton, 1910 and Babesia conradae Kjemtrup, Wainwright, Miller, Penzhorn & Carreno, 2006. However, these parasite forms were overall smaller than those measured for the latter two species and no tetrad (Maltese cross) form was reported. Furthermore, phylogenetic analysis using the cytochrome c oxidase subunit 1 (COX1) amino acid sequences substantiates the species identity of this parasite as previously demonstrated based on phylogenetic analysis of the 18S rRNA and β-tubulin genes. The holotype of the parasite species was designated and deposited in an accessible public collection.
Conclusions
This study ratifies the name Babesia vulpes n. sp. proposed for the parasite previously referred to as Theileria annae Zahler, Rinder, Schein & Gothe, 2000, Babesia annae (Zahler, Rinder, Schein & Gothe, 2000) or Babesia vulpes Baneth, Florin-Christensen, Cardoso & Schnittger, 2015, or mentioned as “Babesia microti-like piroplasm”, “Babesia Spanish dog isolate” and Babesia cf. microti.
Similar content being viewed by others
Background
Babesia Starcovici, 1893 and Theileria Bettencourt, França & Borges, 1907 are tick-borne protozoan genera classified in the phylum Apicomplexa, class Piroplasmea and order Piroplasmida, which infect domestic and wild animals, and humans, and may cause severe disease. Piroplasmids referred to as Theileria (sensu stricto) have been originally defined by the presence of a pre-erythrocytic life stage in leukocyte host cells and trans-stadial transmission in ticks. In contrast, schizont propagation is absent in species of Babesia (sensu stricto) and they display the characteristic of tick transovarial transmission [1, 2]. An additional group of piroplasmids are referred to as Babesia (sensu lato) since they cannot be assigned to either of the above groups [3].
Molecular phylogeny corroborates the taxonomic entities of Babesia (s.s.) and Theileria (s.s.) as each conforms to a monophyletic group referred to as Clade VI and Clade V, respectively [3]. In contrast, Babesia (s.l.) parasites can be clearly distinguished from the above entities and represent a complex of species that can be assigned to at least two other monophyletic groupings designated Clade I (“Babesia microti-like piroplasmids”) and Clade II (Western group) [3]. Domestic dogs and wild canines are infected by several piroplasmid species that can cause severe disease. During the past 30 years, several Babesia spp. that infect canines have been described in detail and genetically characterized [4]. As outlined in detail in Baneth et al. [5], equivocal phylogenetic placement has led to a subsequent erroneous taxonomic assignment of this parasite species within the genus Theileria as “Theileria annae Zahler, Rinder, Schein & Gothe, 2000”. To counter this inaccuracy, the parasite was addressed by a plethora of alternative designations and names such as “B. microti-like piroplasm” [6], “Babesia Spanish dog isolate” [7], “Babesia annae” [8], “Babesia (Theileria) annae” [9] and Babesia cf. microti [10]. The parasite infects red and gray foxes (Vulpes vulpes Linnaeus, 1758 and Urocyon cinereoargenteus Schreber, 1775) [9, 11,12,13,14], as well as golden jackals (Canis aureus Linnaeus, 1758), and domestic dogs (Canis lupus familiaris Linnaeus, 1758) [15, 16]; and it is associated with disease in dogs presenting with pale mucous membranes, anemia, anorexia and lethargy [17, 18].
A study published by our group has demonstrated that within the B. microti-like piroplasmids (Clade I), a new species named Babesia vulpes Baneth, Florin-Christensen, Cardoso & Schnittger, 2015 is positioned in a monophyletic group of Babesia parasites that exclusively infect carnivores and is closely related to the monophyletic B. microti group. Furthermore, we demonstrated that within the Babesia-infecting carnivore group, this parasite can be unequivocally delineated as a distinct species [5]. In the latter study, the name B. vulpes had been proposed as a new species designation [5]; nevertheless, as pointed out in a Letter to the Editor of this journal by Harris [19], according to Article 16.4 of the International Code of Zoological Nomenclature (ICZN), the naming was not statutory. This is because in order to name a species, the naming publication must contain the fixation of a holotype deposited in a specified collection and a description of the species, preferably containing morphological details. As Harris [19] mentioned, these details, as well as the designation of the proposed name as “sp. nov.” were also missing in the naming of T. annae by Zahler et al. [6]; therefore, both these names are currently considered nomina nuda (plural for nomen nudum, Latin for “naked name”, a name not statutorily in force, but which can be made available in subsequent naming procedures) [19]. According to the glossary of ICZN, a nomen nudum is not an available name (in the meaning used in the zoological nomenclature) and therefore the same name may be made available later for the same or a different concept.
The purpose of this study is therefore to further characterize and provide the missing requirements (description, designation of a name-bearing type) in order to establish B. vulpes n. sp. as a valid species name.
Methods
Blood smears fixed with methanol and stained with Hemacolor® (Merck, Darmstadt, Germany) were obtained from the Inno Veterinary Laboratory in Braga, Portugal, and evaluated for parasite morphology by light microscopy. Piroplasm parasites from these smears prepared in 2009 from two Portuguese dogs infected with this parasite then termed B. microti-like were previously examined, described and molecularly characterized [17]. The smears were examined by oil immersion microscopy (Zeiss, Jena, Germany) at 1000× magnification. Sizes of parasites were measured using a micrometer. Measurements are in micrometers and are given as the range followed by the mean and standard deviation in parentheses. A stained blood smear from one of these dogs containing the holotype was deposited in the National Natural History Collection of the Hebrew University of Jerusalem, Israel, and the remaining slides containing the paratypes were deposited in the Parasite Collection of the University of Oporto, Portugal.
PCR to amplify the cox1 gene was carried out using blood samples from three Israeli red foxes (V. vulpes) collected for a hemoparasite survey. The samples had been shown to be infected with the new species by PCR of the 18S rRNA gene followed by sequencing (GenBank: KJ871347, KJ871348, KJ871349), and for which a nearly complete longer gene sequence (GenBank: KJ871351) derived from one fox had been used in the phylogenetic analysis of Baneth et al. [5]. To this end, a region of the cox1 gene was amplified using primers cox1F133 and cox1R11130 essentially as previously described [20]. Conventional PCR was performed in a total volume of 25 μl using the PCR-ready High Specificity mix (Syntezza Bioscience, Jerusalem, Israel) with 400 nM of each primers and sterile DNase/RNase-free water (Sigma, St. Louis, MO, USA). Amplification was carried out using a programmable, conventional thermocycler (Biometra, Göttingen, Germany). PCR products were electrophoresed on 1.5% agarose gels stained with ethidium bromide and evaluated under UV light for the size of amplified fragments by comparison to a 100 bp DNA molecular weight marker. Direct sequencing of PCR allowed determining the cox1 nucleotide sequences (GenBank: KX169167, KX169168 and KX169169) and corresponding COX1 amino acid sequences (GenBank: APX55184, APX55185 and APX55186) for subsequent inclusion into phylogenetic analyses.
An alignment of COX1 amino acid sequences of piroplasmid species available on GenBank was done by MUSCLE [21, 22]. Aligned sequences comprised of 26 COX1 sequences including sequences of B. vulpes n. sp. that have been derived from three different canine species of distant geographical origin: red fox (V. vulpes) from Israel determined for this study as described above, Eurasian golden jackal (C. aureus) from Romania (GenBank amino acid sequence ARN62236 corresponding to nucleotide sequence KX712132), and a domestic dog (Canis lupus familiaris) from the USA (GenBank amino acid AGF95361 corresponding to nucleotide sequence KC207827). All positions containing gaps and missing data were eliminated, resulting in a final dataset of 293 positions. The JTT + G model with the shape parameter (G = 0.56) was selected based on Akaike information criterion (AIC) and a neighbor-joining tree inferred [23, 24].
Results
Family Babesiidae Poche, 1913
Genus Babesia Starcovici, 1893
Babesia vulpes n. sp.
Type-host: Domestic dog Canis lupus familiaris Linnaeus, 1758 (Mammalia: Canidae).
Other hosts: Red fox Vulpes vulpes (Linnaeus, 1758), gray fox (Urocyon cinereoargenteus Schreber, 1775), golden jackal (Canis aureus Linnaeus, 1758).
Type-locality: City of Braga (41°33′6″N, 8°25′22″W), Portugal.
Other localities: Austria [13], Bosnia and Herzegovina [25], Canada [9], Croatia [26], France [27], Germany [28], Great Britain [29], Hungary [10], Israel [14], Italy [30], Romania [16], Slovakia [31], Spain [15, 18], Turkey [32], USA [7, 12].
Type-material: A stained thin blood smear from a 4-year-old Portuguese female dog containing the holotype (Fig. 1b) was deposited in the National Natural History Collection of the Hebrew University of Jerusalem, Israel, under the accession number “HUJPROTOZ1001”. Blood smears containing paratypes were deposited at the Parasite Collection, Laboratory of Animal Pathology, CIIMAR-Centro Interdisciplinar de Investigação Marinha e Ambiental (Interdisciplinary Centre of Marine and Environmental Research), University of Oporto, Portugal under the accession number CIIMAR 2016.9. In addition, genomic DNA extracted from the blood of the Portuguese dog and three red foxes infected with the parasite from Israel (foxes nos. 910, 917 and 26217) has been deposited at the Koret School of Veterinary Medicine, Hebrew University of Jerusalem, Rehvot, Israel under the accession numbers 12019–32019.
Vector: Unknown. Ixodes hexagonus Leach, 1815, Ixodes ricinus Linnaeus, 1758, Ixodes canisuga Johnston, 1849, Dermacentor reticulatus Fabricius, 1794 and Rhipicephalus sanguineus Latreille, 1806 are suspected [28, 33,34,35].
Representative DNA sequences: Present study (GenBank: KX169167-KX169169; cox1); Baneth et al. [5] and Margalit-Levi et al. [14] (GenBank: KJ871346-KJ871352; 18S rRNA).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN) [36], details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:9A1011D2-063C-4E5A-B74D-DDD89EE0772F. The LSID for the new name Babesia vulpes is urn:lsid:zoobank.org:act:DF4C2543-0038-435B-AA52-01A2D9239DB7.
Etymology: The species is named after the red fox (V. vulpes) considered as the main wildlife host of this parasite. The species name “vulpes” is a noun in apposition (ICZN Article 31.1.2).
Description
Merozoites [Measurements based on 18 parasites; see Fig. 1.] Round to oval-shaped merozoites with an eccentric round nucleus presenting as single or two parasites in erythrocyte. Merozoites measuring 0.8–1.9 (1.33 ± 0.28) in length and 0.7–1.4 (0.98 ± 0.22) in width (n = 18), with nuclei measuring 0.4 in diameter (n = 4). No tetrad (Maltese cross) shapes observed.
Differential diagnosis
Intraerythrocytic parasites presented as round to oval-shaped, and an eccentric, basophilic-staining; round nucleus was conspicuous in some parasites (Fig. 1a, b). Of 18 parasites measured, 16 presented as single parasites, whereas the remaining two were located in the same erythrocyte. Parasites occupied only a small portion of the erythrocyte and were reminiscent of the pyriform and ring shapes described for other small-form Babesia species that infect dogs [37, 38]; however, no tetrad (Maltese cross) shapes were seen.
The morphological shape of B. vulpes n. sp. described here from dog erythrocytes is similar to the ring and pyriform shapes described for other small, canine-infecting Babesia spp. [38,39,40]. Nevertheless, the merozoites of B. vulpes n. sp. measuring on average 1.33 × 0.98 µm, are distinctly smaller than the merozoites of Babesia vogeli Reichenow, 1937, Babesia canis Pianna & Galli-Vallerio, 1895 and Babesia rossi (Nuttal, 1910) Wenyon, 1926, with size typically within the range of 4.5–5.0 × 2.0–2.5 µm (as summarized in [4]). They are also smaller than the ring forms described for Babesia conradae Kjemtrup, Wainwright, Miller, Penzhorn & Carreno, 2006, which measure 2.2 × 1.85 µm, and are closer in size to the pyriform shapes of B. conradae which measure 1.38 × 0.66 µm [38]. However, in contrast to B. conradae, no tetrad (Maltese cross) forms were observed in B. vulpes n. sp. Babesia gibsoni Patton, 1910, another small-form Babesia of dogs, which is also not reported to produce tetrads, is described as being considerably larger than B. vulpes n. sp. with the ring shape measuring 2.71 × 1.61 µm and the pyriform shape measuring 2.1 × 0.94 µm [40], or according to a different report, 1.9 × 1.2 µm, without a distinction between the shapes [39]. The above comparisons indicate that B. vulpes n. sp. is a distinct form consistent with the small-form piroplasms of canines. However, B. vulpes n. sp. tends to be smaller than B. conradae and B. gibsoni and has not been reported to form tetrads, thus further distinguishing it from B. conradae.
We consider that previous reports with morphological details on intraerythrocytic piroplasm forms seen by light microscopy in stained blood smears of synonyms of B. vulpes n. sp., e.g. “T. annae” [6, 18, 41, 42], “B. microti-like piroplasm” [6, 15, 17, 18, 43,44,45], and “Babesia (Theileria) annae” [9], from the domestic dog [6, 15, 17, 18, 43, 44] and the red fox [9, 45], actually represent B. vulpes n. sp. These reports describe intraerythrocytic ring-shaped or oval to round organisms morphologically compatible with small piroplasms [9, 18, 42, 45] which are 1–2 µm in diameter [6, 9, 42], as found for B. vulpes n. sp., and having a dark-staining dot-shaped nucleus [42]. The small piroplasms reported were present mostly as single parasites in erythrocytes and rarely as two intracellular organisms, and located centrally to paracentrally in their host erythrocytes [6, 9, 15]. PCR and sequencing of the parasites seen by microscopy in all of these reports indicated they have identical sequences to B. vulpes n. sp. and its synonyms [6, 9, 15, 17, 18, 41,42,43,44,45].
Molecular phylogeny
Phylogenetic analysis of amino acid COX1 sequences for Theileria spp. and Babesia spp. resulted in a tree that recovers Clades I [Babesia (s.l.), Babesia microti-like group], II [Babesia (s.l.) of the Western Clade], IV [Theileria equi (Laveran, 1901) Melhorn & Schein, 1998], V [Theileria (s.s.)], and VI [Babesia (s.s.)] as previously reported by Schreeg et al. [46] and based on 18S rRNA gene sequences by Schnittger et al. [3] (Fig. 2). Canine-infecting Babesia sp. Coco, B. vogeli, B. rossi, B. canis and B. gibsoni were clustered with strong support into the Babesia (s.s.) Clade VI (bootstrap support, bs = 100), whereas canine-infecting B. conradae, segregated into the well-supported Clade II [Babesia (s.l.) of the Western Clade, bs = 81]. Importantly, the strongly supported joint placement of COX1 sequences of isolates from geographically distant locations and diverse canine hosts (V. vulpes from Israel, C. aureus from Romania and C. l. familiaris from the USA) attests the distinct species status of B. vulpes n. sp. (bs = 100). The clade to which B. vulpes n. sp. is most closely related, yet can be clearly distinguished from, is the strongly supported B. microti group (bs = 86). Furthermore, Babesia rodhaini Van den Berghe, Vincke, Chardome & Van den Bulcke, 1950 represented a strongly supported sister species to the clade comprising of B. vulpes n. sp. and the B. microti group. The results based on the COX1 amino acid sequences coincide and support the previously presented results on the species identity of B. vulpes n. sp. by phylogenetic analysis of 18S RNA and β-tubulin gene sequences [5]. In addition, a neighbor-joining tree based on 25 cox1 nucleotide sequences with a final dataset of 879 positions of B. vulpes n. sp. and other piroplasmid species was inferred and corroborated results obtained by COX1 amino acid sequences. Specifically, the same topology and an identical bootstrap support were determined for the corresponding relevant clades of the trees inferred by amino acid and nucleotide sequences [B. microti group/B. vulpes n. sp. clade (bs = 100) and (B. vulpes n. sp. clade (bs = 100)] (Additional file 1: Figure S1).
Neighbor-joining tree of COX1 amino acid sequences of Babesia vulpes n. sp. and other piroplasmid species. Sequences analyzed in the context of this study are designated by bold accession numbers of taxon labels. Clade designations are presented as defined previously [3, 50]. The percentage of replicate trees as determined by 1000 replicates of a bootstrap test are shown next to the branches. A Plasmodium falciparum COX1 sequence has been included as the outgroup. The scale-bar represents the evolutionary distance in the units of the number of amino acid substitutions per site. Gray dots designate Babesia species that infect domestic dogs [51]
Discussion
This study establishes B. vulpes n. sp. as a new taxon fulfilling the requirements of the ICZN guidelines. A morphological description with measurements of the parasite forms in canine erythrocytes and the deposition of the holotype and paratypes in suitable collections have been made in compliance with the ICZN guidelines [36]. The generic placement of B. vulpes n. sp. is derived from the molecular phylogenetic analysis of the 18S RNA and β-tubulin genes, and COX1 protein sequences, whereas the species name has been chosen because the red fox (V. vulpes) is considered the main natural host of this piroplasmid (see also [5]). As mentioned above, according to the ICZN regulations, “T. annae” [6] is considered a non-available name (nomen nudum), which has never been valid in terms of the Code, and thus the principle of priority does not apply in this case. Accordingly, as previously stated [19], the species name “annae” does not need to be carried into the proposed species designation. The renaming of “T. annae” as B. vulpes n. sp. should now replace the use of all synonyms for this species, such as “B. microti-like piroplasm”, Babesia cf. microti, “B. annae” and “Babesia Spanish dog isolate”, thus ending the confusion when referring to this parasite species. Furthermore, in accordance with recent findings on the molecular phylogeny of this and other piroplasmid species, the proposed name clearly distinguishes this parasite from species of the genus Theileria Bettencourt, França & Borges, 1907.
The COX1 has been increasingly applied in molecular phylogenetic studies of piroplasmids [20, 46, 47]. The phylogenetic analysis using COX1 demonstrated that B. vulpes n. sp. does not segregate into Theileria (s.s.) (Clade V) nor into Babesia (s.s.) (Clade VI), but into a group of Babesia (s.l.) species that is placed into Clade I (B. microti-like parasites or Archaeopiroplasmida; see [11]). Within Clade I, B. vulpes n. sp. is strongly supported as a distinct species of a subclade of Babesia (s.l.) species that has so far been found to exclusively infect carnivores of the families Mustelidae and Canidae. The subclade including B. vulpes n. sp. can be clearly distinguished from the subclades of the B. microti group and B. rodhaini together forming Clade I of B. microti-like piroplasmids (Fig. 2; [3]). As previously outlined in detail, the 18S rRNA and β-tubulin gene phylogenetic analyzes are in accordance with this result [5]. Babesia vulpes n. sp. is the first species defined within its own subclade group and it is expected that additional species in this group will be described in the future (see also [5]).
Overall, the congruent phylogenetic analysis of the 18S and β-tubulin genes and the COXI protein-sequence encoded by the mitochondrial genome, and the fact that B. vulpes n. sp. has not been shown to infect rodents and humans, distinguishes it as a species from the zoonotic B. microti located in the B. microti group (Fig. 2). Furthermore, B. microti from mice belonging to the zoonotic B. microti group was not found to be infectious to dogs, pigs, chicken and goats in an experimental transmission study, while it was infectious to rats [48].
The mode of transmission and tick vectors of B. vulpes n. sp. have not been determined yet. Although the DNA of this parasite has been detected in several tick species (reviewed in [5]), including I. hexagonus, which was proposed as a vector [49], and D. reticulatus [35], no study to date has provided sufficient proof for the vectorial capacity of any particular tick species, and further research is needed to elucidate this issue.
Conclusions
The fixation of holotype and the morphological description and differentiation of the new species provided here establish the species name B. vulpes n. sp. by fulfilling the ICZN requirements for description of a new species. The name B. vulpes n. sp. should replace all the synonyms that have been used for this parasite including “Theileria annae”, “Babesia annae”, “B. microti-like piroplasm”, Babesia cf. microti and “Babesia Spanish dog isolate”.
Abbreviations
- COX1:
-
cytochrome c oxidase 1
- ICZN:
-
International Code of Zoological Nomenclature
- PCR:
-
polymerase chain reaction
References
Mehlhorn H, Schein E. The piroplasms: life cycle and sexual stages. Adv Parasitol. 1984;23:37–103.
O’Donoghue P. Haemoprotozoa: making biological sense of molecular phylogenies. Int J Parasitol Parasites Wildl. 2017;6:241–56.
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012;12:1788–809.
Solano-Gallego L, Baneth G. Babesiosis in dogs and cats - expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48–60.
Baneth G, Florin-Christensen M, Cardoso L, Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors. 2015;8:207.
Zahler M, Rinder H, Schein E. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–8.
Yeagley TJ, Reichard MV, Hempstead JE, Allen KE, Parsons LM, White MA, et al. Detection of Babesia gibsoni and the canine small Babesia ‛Spanish isolateʼ in blood samples obtained from dogs confiscated from dogfighting operations. J Am Vet Med Assoc. 2009;235:535–9.
Camacho AT, Guitian FJ, Pallas E, Gestal JJ, Olmeda S, Goethert H, et al. Serum protein response and renal failure in canine Babesia annae infection. Vet Res. 2005;36:713–22.
Clancey N, Horney B, Burton S, Birkenheuer A, McBurney S, Tefft K. Babesia (Theileria) annae in a red fox (Vulpes vulpes) from Prince Edward Island, Canada. J Wildl Dis. 2010;46:615–21.
Farkas R, Takács N, Hornyák Á, Nachum-Biala Y, Hornok S, Baneth G. First report on Babesia cf. microti infection of red foxes (Vulpes vulpes) from Hungary. Parasit Vectors. 2015;8:55.
Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part I. Epizootiological aspects. Vet Parasitol. 2003;113:189–201.
Birkenheuer AJ, Horney B, Bailey M, Scott M, Sherbert B, Catto V, et al. Babesia microti-like infections are prevalent in North American foxes. Vet Parasitol. 2010;172:179–82.
Duscher GG, Fuehrer HP, Kübber-Heiss A. Fox on the run - molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasit Vectors. 2014;7:521.
Margalit-Levi M, Nachum-Biala Y, King R, Baneth G. A survey of Babesia spp. and Hepatozoon spp. in wild canids in Israel. Parasit Vectors. 2018;11:150.
Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS, Goethert HK, et al. Infection of dogs in north-west Spain with a Babesia microti-like agent. Vet Rec. 2001;149:552–5.
Mitková B, Hrazdilová K, D’Amico G, Duscher GG, Suchentrunk F, Forejtek P, et al. Eurasian golden jackal as host of canine vector-borne protists. Parasit Vectors. 2017;10:183.
Simões PB, Cardoso L, Araújo M, Yisaschar-Mekuzas Y, Baneth G. Babesiosis due to the canine Babesia microti-like small piroplasm in dogs - first report from Portugal and possible vertical transmission. Parasit Vectors. 2011;4:50.
Miró G, Checa R, Paparini A, Ortega N, González-Fraga JL, Gofton A, et al. Theileria annae (syn. Babesia microti-like) infection in dogs in NW Spain detected using direct and indirect diagnostic techniques: clinical report of 75 cases. Parasit Vectors. 2015;8:217.
Harris DJ. Naming no names: Comments on the taxonomy of small piroplasmids in canids. Parasit Vectors. 2016;9:289.
He L, Zhang Y, Zhang QL, Zhang WJ, Feng HH, Khan MK, et al. Mitochondrial genome of Babesia orientalis, apicomplexan parasite of water buffalo (Bubalus babalis Linnaeus, 1758) endemic in China. Parasit Vectors. 2014;7:82.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.
Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–82.
Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015;8:88.
Beck R, Vojta L, Mrljak V, Marinculić A, Beck A, Zivicnjak T, et al. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int J Parasitol. 2009;39:843–8.
René-Martellet M, Moro CV, Chêne J, Bourdoiseau G, Chabanne L, Mavingui P. Update on epidemiology of canine babesiosis in southern France. BMC Vet Res. 2015;11:223.
Najm NA, Meyer-Kayser E, Hoffmann L, Herb I, Fensterer V, Pfister K, et al. A molecular survey of Babesia spp. and Theileria spp. in red foxes (Vulpes vulpes) and their ticks from Thuringia. Germany. Ticks Tick Borne Dis. 2014;5:386–91.
Bartley PM, Hamilton C, Wilson C, Innes EA, Katzer F. Detection of Babesia annae DNA in lung exudate samples from red foxes (Vulpes vulpes) in Great Britain. Parasit Vectors. 2016;9:84.
Zanet S, Trisciuoglio A, Bottero E, de Mera IG, Gortazar C, Carpignano MG, et al. Piroplasmosis in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasit Vectors. 2014;7:70.
Koneval M, Miterpáková M, Hurníková Z, Blaňarová L, Víchová B. Neglected intravascular pathogens, Babesia vulpes and haemotropic Mycoplasma spp. in European red fox (Vulpes vulpes) population. Vet Parasitol. 2017;243:176–82.
Orkun Ö, Karaer Z. Molecular characterization of Babesia species in wild animals and their ticks in Turkey. Infect Genet Evol. 2017;55:8–13.
Lledó L, Giménez-Pardo C, Domínguez-Peñafiel G, Sousa R, Gegúndez MI, Casado N, et al. Molecular detection of hemoprotozoa and Rickettsia species in arthropods collected from wild animals in the Burgos Province, Spain. Vector Borne Zoonotic Dis. 2010;10:735–8.
Iori A, Gabrielli S, Calderini P, Moretti A, Pietrobelli M, Tampieri MP, et al. Tick reservoirs for piroplasms in central and northern Italy. Vet Parasitol. 2010;170:291–6.
Hodžić A, Zörer J, Duscher GG. Dermacentor reticulatus, a putative vector of Babesia cf. microti (syn. Theileria annae) piroplasm. Parasitol Res. 2017;116:1075–7.
ICZN. International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull Zool Nomencl. 2012;69:161–9.
Thomford JW, Conrad PA, Boyce WM, Holman PJ, Jessup DA. Isolation and in vitro cultivation of Babesia parasites from free-ranging desert bighorn sheep (Ovis canadensis nelsoni) and mule deer (Odocoileus hemionus) in California. J Parasitol. 1993;79:77–84.
Kjemtrup AM, Wainwright K, Miller M, Penzhorn BL, Carreno RA. Babesia conradae sp. nov., a small canine Babesia identified in California. Vet Parasitol. 2006;138:103–11.
Anderson JF, Magnarelli LA, Donner CS, Spielman A, Piesman J. Canine Babesia new to North America. Science. 1979;204:1431–2.
Namikawa K, Sunaga F, Kanno Y. Morphology of Babesia gibsoni in canine erythrocytes. Nihon Juigaku Zasshi. 1988;50:936–8.
García AT. Piroplasma infection in dogs in northern Spain. Vet Parasitol. 2006;138:97–102.
Falkenö U, Tasker S, Osterman-Lind E, Tvedten HW. Theileria annae in a young Swedish dog. Acta Vet Scand. 2013;55:50.
Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS. Natural infection by a Babesia microti-like piroplasm in a splenectomised dog. Vet Rec. 2002;150:381–2.
Camacho AT, Guitian EJ, Pallas E, Gestal JJ, Olmeda AS, Goethert HK, et al. Azotemia and mortality among Babesia microti-like infected dogs. J Vet Intern Med. 2004;18:141–6.
Checa R, López-Beceiro AM, Montoya A, Barrera JP, Ortega N, Gálvez R, et al. Babesia microti-like piroplasm (syn. Babesia vulpes) infection in red foxes (Vulpes vulpes) in NW Spain (Galicia) and its relationship with Ixodes hexagonus. Vet Parasitol. 2018;252:22–8.
Schreeg ME, Marr HS, Tarigo JL, Cohn LA, Bird DM, Scholl EH, et al. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS One. 2016;11:e0165702.
Tuvshintulga B, Sivakumar T, Battsetseg B, Narantsatsaral SO, Enkhtaivan B, Battur B, et al. The PCR detection and phylogenetic characterization of Babesia microti in questing ticks in Mongolia. Parasitol Int. 2015;64:527–32.
Wu J, Cao J, Zhou Y, Zhang H, Gong H, Zhou J. Evaluation on infectivity of Babesia microti to domestic animals and ticks outside the Ixodes genus. Front Microbiol. 2017;8:1915.
Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS, Telford SR, et al. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet Parasitol. 2003;112:157–63.
Lack JB, Reichard MV, Van Den Bussche RA. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int J Parasitol. 2012;42:353–63.
Baneth G. Babesia of domestic dogs. In: Florin-Christensen M, Schnittger L, editors. Parasitic protozoa of farm animals and pets. Basel: Springer International Publishing; 2018. p. 241–58.
Acknowledgements
Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th CVBD World Forum Symposium. The authors thank Drs Yaarit Nachum-Biala and Maayan Margalit-Levi for their assistance in analyzing the animal samples.
Funding
The study was funded by the author’s internal resources with no external funding.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Analyzed cox1 nucleotide sequences used for tree construction were submitted to the GenBank database under the accession numbers KX169167-KX169169 and correspond to COX1 amino acid sequences submitted under the accession numbers APX55184-APX55186 that have likewise been used for tree construction in this study. The holotype was deposited in the National Natural History Collection of the Hebrew University of Jerusalem, Israel, under the accession number “HUJPROTOZ1001” and paratypes were deposited in the Parasite Collection, Laboratory of Animal Pathology, CIIMAR, University of Oporto, Portugal.
Authors’ contributions
GB, LC and LS planned and conceived the study and wrote the manuscript. PBS participated in detecting and characterizing the parasites. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable. The study included material from previously published studies with no additional sampling of animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
A neighbor-joining tree of 25 cox1 nucleotide sequences of B. vulpes n. sp. and other piroplasmid species. Clade designations are presented as defined previously [3, 50]. After alignment of nucleotide sequences, all positions containing gaps and missing data were eliminated, resulting in a final dataset of 879 positions. The T92 + G model with the shape parameter (G = 0.42) was selected based on Akaike information criterion (AIC) and the neighbor-joining tree inferred [23, 24]. The percentages of replicate trees as determined by 1000 replicates of a bootstrap test are shown next to the branches. A Plasmodium falciparum cox1 sequence has been included as the outgroup. The scale-bar represents the evolutionary distance in the units of the number of nucleotide substitutions per site. Gray dots designate Babesia spp. that infect domestic dogs [51].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Baneth, G., Cardoso, L., Brilhante-Simões, P. et al. Establishment of Babesia vulpes n. sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Parasites Vectors 12, 129 (2019). https://doi.org/10.1186/s13071-019-3385-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3385-z