Abstract
Dysregulation of the Notch signaling pathway, which is highly conserved across species, can drive aberrant epigenetic modification, transcription, and translation. Defective gene regulation caused by dysregulated Notch signaling often affects networks controlling oncogenesis and tumor progression. Meanwhile, Notch signaling can modulate immune cells involved in anti- or pro-tumor responses and tumor immunogenicity. A comprehensive understanding of these processes can help with designing new drugs that target Notch signaling, thereby enhancing the effects of cancer immunotherapy. Here, we provide an up-to-date and comprehensive overview of how Notch signaling intrinsically regulates immune cells and how alterations in Notch signaling in tumor cells or stromal cells extrinsically regulate immune responses in the tumor microenvironment (TME). We also discuss the potential role of Notch signaling in tumor immunity mediated by gut microbiota. Finally, we propose strategies for targeting Notch signaling in cancer immunotherapy. These include oncolytic virotherapy combined with inhibition of Notch signaling, nanoparticles (NPs) loaded with Notch signaling regulators to specifically target tumor-associated macrophages (TAMs) to repolarize their functions and remodel the TME, combining specific and efficient inhibitors or activators of Notch signaling with immune checkpoint blockers (ICBs) for synergistic anti-tumor therapy, and implementing a customized and effective synNotch circuit system to enhance safety of chimeric antigen receptor (CAR) immune cells. Collectively, this review aims to summarize how Notch signaling intrinsically and extrinsically shapes immune responses to improve immunotherapy.
Similar content being viewed by others
Introduction
The Notch signaling pathway, which is highly conserved across species, is implicated in numerous aspects of cancer biology, including the cancer stem cell phenotype, tumor angiogenesis, metastasis, and tumor immune evasion [1,2,3,4,5]. After decades of study, scientists have revealed that Notch signaling plays an essential regulatory role in immune cells and the tumor microenvironment (TME) [5, 6]. Its double-edged roles in anti-tumor or pro-tumor immune regulations involve modulating tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs), and other immune cells in the TME.
Previous studies have shown that Notch signaling regulates the activation, infiltration, and phenotypic switching of various immune cells (e.g., macrophages, T cells, among others). In addition, a synthetic Notch (synNotch) receptor can customize the anti-tumor response programs of T cells to kill tumor cells in a precise and localized manner. The synNotch system can deliver non-native therapeutic antibodies [e.g., programmed cell death protein 1 (PD-1) antibodies, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies] as well as derived customized cytokines [interleukin 2 (IL-2) and secreted interleukin 12 (IL-12)] [7]. Therefore, modulation of Notch signaling may be able to coordinate with immune responses to tumor cells. In this review, we provide an up-to-date overview of existing and emerging findings of Notch signaling in immune cells and of TME-related immune responses. We also discuss potential therapeutic strategies for reducing unwanted side effects of Notch signaling and examine how Notch signaling might be redirected to improve immunotherapy.
Components, basic function and inhibition of Notch signaling
Notch signaling is highly conserved through evolution as a determinant of cell fate by mediating direct contact between adjacent cells [5, 6]. Regulation of Notch signaling participates in numerous aspects of tumor biology, including tumor angiogenesis, maintenance of tumor stem cells, and the responses of immune cells (e.g., DCs, T cells, and macrophages) [1,2,3,4,5, 8,9,10]. Notch signaling is also regulated by a variety of mechanisms, including post-transcriptional regulation, glycosylation, transcriptional repression/activation, epigenetic modifications, as well as other mechanisms. Additionally, its activity can be modulated by different signaling pathways (e.g., AKT, RUNX1, SIRT6, and DEC1) [5, 6]. The Notch signaling pathway in mammals has three major components: (i) Ligands for binding the extracellular segments of Notch receptors (Jagged1, Jagged2, Dll1, Dll3, and Dll4); (ii) Notch receptors (Notch1, Notch2, Notch3, and Notch4); (iii) RBP-J-dependent canonical downstream effectors (e.g., Hes family proteins) and RBP-J-independent non-canonical downstream effectors of Notch signaling (e.g., Iκκ, NF-κB, and PI3K/AKT) [11, 12].
The Notch signaling pathway is assembled and triggered via complex mechanisms. (i) The Notch receptor protein, a type I transmembrane protein originally synthesized in the endoplasmic reticulum (ER), is transported into the Golgi apparatus, cleaved into two fragments by furin, and then transported to the cell surface to form a heterodimer [6, 13]; (ii) Binding of ligands from signal-sending cells to the extracellular domain of Notch receptor (NECD) of signal-receiving cells, or activation of ligand-independent Notch receptors, causes the receptors’ extracellular subunits to dissociate from its transmembrane subunits, thereby releasing the activated intracellular domain of Notch receptors (NICD) [12, 14]; (iii) Activated NICD enters the nucleus and complexes with other proteins [e.g., recombination signal binding protein for immunoglobulin kappa (κ) J region (RBP-J) and mastermind-like (MAML)], to form a transcription complex, thereby regulating gene transcription (e.g., of Hes1 and Hey1 genes) [11, 13]; (iv) Also, activated NICD can directly activate the expression of genes (e.g., PI3K/AKT) through non-canonical regulations. The structures of Notch ligands and receptors, their basic functions of Notch signaling are summarized in Fig. 1A–C.
The Notch signaling pathway. A-B Composition of five Notch ligands (Jagged1, Jagged2, Dll1, Dll3, and Dll4) and four Notch receptors (Notch1, Notch2, Notch3, and Notch4). Each domain of Notch ligands and receptors is shown. The structures of Notch1-4 are highly homologous but show differences to certain extents. All of the four receptors have the same or similar LNR, HD, TMD, RAM, NLS, ANK, and PEST. The protein structures of Notch1 and Notch2 are highly similar, both of which have 36 EGF repeats in their NECDs. Compared with Notch1/2, Notch3 has 34 EGF repeats and lacks TAD structure, while Notch4 has only 29 EGF repeats and also lacks the TAD structure. SP: Signal peptide; MNNL: Module at N-terminal domain of Notch ligand; DSL: Delta, Serrate, and LAG-2 domain; DOS: Delta and OSM-11-like proteins domain; CR: Cysteine-rich domain; TMD: Transmembrane domain; PDZ: PDZ domain; LNR: Lin 12-Notch repeats; HD: Heterodimerization domain; RAM: RBP-J association module; NLS: Nuclear localization sequences; ANK: Ankyrin repeats; TAD: Transcription activation domain. PEST: proline, glutamic acid, serine, threonine-rich domain. C. Activation of Notch signaling: ①: Notch receptors are synthesized and processed in the ER and Golgi apparatus and then transported to the cell membrane to form heterodimers; ② and ③: Notch ligands from signal-sending cells bind to the NECD of signal-receiving cells. The binding triggers cleavage by ADAM and then γ-secretase, which releases activated NICD; ④ and ⑤: Activated NICD enters the nucleus and binds to MAML, RBP-J, and other proteins to form transcription complexes that promote the transcription of a series of genes (e.g., Hes1, Hey1) through RBP-J-dependent canonical Notch signaling; ⑥: Activated NICD directly activates the expression of a series of genes (e.g., PI3K/AKT) through RBP-J-independent non-canonical signaling. The six yellow rectangular boxes (a-f) represent clinically or preclinically used inhibitors, blocking antibodies, or target gene’s antisense oligonucleotides (ASOs) that inhibit Notch signaling. They are mainly: (a) inhibitors that inhibit the formation of Notch receptors (e.g., SERCA inhibitor); (b-c) antibodies that inhibit Notch receptors or ligands [e.g., OMP-52M51 (anti-Notch1), OMP-59R5 (anti-Notch2/3), MEDI0639 (anti-Dll4), Demcizumab (anti-Dll4), and CTX014 (anti-Jagged1/2)], and receptor’s or ligand’s ASOs; (d-e) ADAM inhibitors or γ-secretase inhibitors that inhibit the cleavage of Notch receptor (e.g., INCB7839, ZLDI-8, AL101, and MK0752); (f) Inhibitors that inhibit transcriptional complexes (e.g., CB-103, SAHM1, and IMR-1) and target gene’s ASOs. mAbs: monoclonal antibodies. Asterisks (*) indicate drugs that are being assessed in clinical trials. Figure was created with BioRender.com
Previous studies have revealed that Notch signaling regulates the fate choice of various cells under physiological conditions [6], whereas dysregulated Notch signaling, especially abnormal activation, can promote the development of various malignancies. Therefore, in the past decades, drugs (mainly specific inhibitors or blocking antibodies) against Notch signaling are being tested in clinical trials or preclinical research for both solid and hematological malignancies [5, 12, 15–25]. The specific inhibitors and blocking antibodies of Notch signaling in clinical trials or preclinical studies are summarized below (Tables 1 and 2).
Inhibitors that inhibit the synthesis of Notch receptors
The precursors of Notch receptors (pre-Notch receptors) are originally synthesized, their S1 portion is cleaved in the ER and Golgi apparatus, and then the cleaved Notch receptors are transported into the cell surface to further integrate with their ligands [6]. Previous studies showed that the inhibition of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) or zinc transporter impaired pre-Notch receptors synthesis, rendering them to be potential therapeutic targets [24, 25].
Curcumin, a natural phenolic compound that binds and inhibits SERCA, has been tested in pancreatic cancer [26], colorectal cancer (CRC) [27, 28], and prostate cancer (PCa) clinical trials. These results showed that oral administration of curcumin is generally safe and tolerated in CRC patients [27, 28], and in pancreatic cancer, two out of 21 patients showed clinical biological activity and one of the two experienced transient but significant 73% tumor regression [26]. NVS-ZP7-4, an inhibitor that inhibits zinc transporter in ER, has been tested in T-ALL at the preclinical stage [16]. FLI-06, an inhibitor that inhibits the secretion of pre-Notch receptors before leaving ER, has been tested in esophageal squamous cell carcinoma (ESCC) at the preclinical stage [23]. In both in vitro and in vivo studies, these two inhibitors have shown inhibitory effects on the synthesis of pre-Notch receptors, which is worth future testing in the clinic. The results of each clinical trials and preclinical studies are shown in Tables 1 and 2.
Blocking antibodies of Notch receptors and Notch ligands
After Notch ligands binds to NECD of Notch receptors, the extracellular subunits of Notch receptors are dissociated from their transmembrane subunits, resulting in NICD release and activation [6]. Some blocking antibodies that block the function of Notch receptors or ligands have been developed.
Blocking antibodies of Notch receptors: (i) OMP-52M51 (also called brontictuzumab), an anti-Notch1 monoclonal antibody (mAb), was tested in the clinic to treat lymphoid malignancies [29], solid tumor [30], adenoid cystic carcinoma (ACC) [31], and metastatic CRC. Overall, OMP-52M51 was well tolerated, exhibiting moderate anti-tumor activity with one partial response (PR) and two stable disease (SD) in twenty-four lymphoid malignancies [29], two PR and four SD in thirty-six (17%) assessable patients with a solid tumor [30], and one PR of one patient with Notch1-mutant ACC [31]. Diarrhea is the main toxicity after MP-52M51 treatment. (ii) OMP-59R5, an anti-Notch2/3 mAb, has been tested in clinical trials in solid tumor [32], stage IV pancreatic cancer [33], and stage IV small cell lung cancer (SCLC). Overall, the therapeutic effect of OMP-59R5 is not impressive. Either as a single agent or in combination with the first-line chemotherapy drugs (e.g., gemcitabine), OMP-59R5 did not improve overall survival (OS), progression-free survival (PFS), or objective response rate (ORR) of patients.
Blocking antibodies of Notch ligands: (i)–(ii) Rovalpituzumab tesirine (also called Rova-T) and SC-002, two anti-Dll3 mAbs, were each tested in multiple clinical trials, especially in SCLC (e.g., NCT01901653, phase I/II) [34,35,36,37,38,39,40,41,42]. Rova-T has controllable associated toxicities. In the treatment of SCLC, Rova-T exhibited moderate clinical activity. The results of clinical trial NCT01901653 showed that eleven of sixty (18%) evaluable patients received confirmed objective responses, including ten of twenty-six (38%) patients with high Dll3 expression [36]. Thus, it seems that Rova-T exhibits encouraging single dose anti-tumor activity with controllable safety, especially in patients with high Dll3 expression. (iii) MEDI0639, an anti-Dll4 mAb, was tested on clinical trials for solid tumors [43], and (iv) demcizumab, an anti-Dll4 mAb, was clinically tested in non-small cell lung cancer (NSCLC) [44], pancreatic cancer, primary peritoneal carcinoma [45], and other solid tumors [46]. For MEDI0639, in twenty solid tumor patients, only one melanoma patient with PRs; seven patients had stable disease lasting more than 12 weeks [43]; for demcizumab, in forty-six treatment-naive patients with NSCLC, twenty of forty (50%) evaluable patients had objective tumor responses [44]. CTX014, an anti-Jagged 1/2 mAb, was tested in solid tumor at the preclinical stage, and results showed that CTX014 treatment overcame tumor-induced T cell tolerance, increased the infiltration of reactivated CD8+ T cells into tumors, and enhanced the efficacy of T cell–based immunotherapy [17]. The results of each of clinical trials and pre-clinical studies are summarized in Tables 1 and 2.
ADAM inhibitors and γ-secretase inhibitors
Two important cleaving enzymes, a disintegrin and metalloprotease (ADAM) and γ-secretase, catalyze the cleavage of Notch receptors [6]. Specifically, ADAM promotes the cleavage of NECD from the transmembrane (TM) NICD domain (S2 cleavage), while γ-secretase promotes the release of NICD from the TM domain (S3 cleavage), thereby achieving nuclear translocation [6, 12]. Therefore, ADAM and γ-secretase are important targets in blocking Notch signaling.
ADAM inhibitors: INCB7839 (also called Aderbasib), a small molecule drug targeting ADAM, has been proposed for a phase I clinical trial of high-grade gliomas (NCT04295759). This clinical trial is recruiting, and no results have been reported yet. The curative effect of other ADAM inhibitors (e.g., ZLDI-8) was tested in hepatocellular carcinoma (HCC)-bearing mice. Results showed that ZLDI-8 significantly inhibited tumor growth [18].
γ-secretase inhibitors (GSIs): since 2004, at least six types of γ-secretase inhibitors have been clinically tested in various cancer patients. The details are as follows: (i) MK0752 was tested in clinical trials designed for leukemia, advanced breast cancer (BC) [47], metastatic BC [48], pancreatic cancer [49], or other solid tumors [47]. Overall, the main toxic side effect of this drug was diarrhea. But some patients also showed positive treatment reactions. In high-grade gliomas patients, one patient completely response and additional ten patients remained stable for more than four months to MK0752 treatment in one hundred and three patients in total [47]. Among forty-four eligible pancreatic cancer patients, thirteen patients achieved stable disease after MK0752 was combined with first-line chemotherapy drug gemcitabine, and one patient achieved a confirmed PR, indicating that MK0752 has the potential to be used in combination with first-line chemotherapy drugs [49]. (ii) RO4929097 has conducted clinical trials in BC, sarcoma [50], melanoma [51], adult solid neoplasm, and other solid tumors [52]. Overall, only one of thirty-two metastatic melanoma patients treated with RO4929097 achieved PR. Although RO4929097 is well tolerated, but it has significant toxicity. (iii)–(v) LY900009 in advanced cancer [53], PF-03084014 in triple-negative breast cancer (TNBC), and LY303947 in solid tumors [54,55,56] have also been tested. Overall, the clinical treatment effects of these three drugs were not impressive, and participants showed limited clinical responses. (vi) AL101 has been studied in clinical trials of TNBC and in adenoid cystic cancer. These two clinical trials are recruiting, and no results have been reported yet. DAPT has been tested in head and neck squamous cell carcinoma (HNSCC) at the pre-clinical stage. Results showed that DAPT decreased tumor burden in a mouse model after prophylactic treatment [19]. The results of each clinical trials and preclinical studies are shown in Tables 1 and 2.
Notch transcription complex inhibitors
When activated NICD enters the nucleus, NICD binds with RBP-J and MAML to form a transcriptional complex, recruiting co-activators and triggering the transcription of Notch target genes [11, 13]. Therefore, targeted inhibition of the Notch transcription complex can also be an effective approach to block Notch signaling.
Notch transcription complex inhibitors: CB-103, the first drug to effectively control the Notch transcription complex, has been studied in advanced tumors and hematological malignancies (NCT03422679) in a phase I/II clinical trial. Results showed that CB-103 was well tolerated in cancer patients. The curative effect of the other two inhibitors, SAHM1 and IMR-1, has been tested in leukemic cells and an esophageal adenocarcinoma (EAC) patient-derived xenograft tumor model, respectively. Results showed that SAHM1 suppressed genome-wide suppression of Notch-activated genes in leukemic cells [20], and IMR-1 inhibited the growth of Notch-dependent EAC patient-derived xenograft tumors [21]. The results of each of clinical trials and pre-clinical studies are shown in Tables 1 and 2.
Collectively, among drugs that targeting Notch signaling, blocking antibodies of Notch ligands (e.g., anti-Dll3 mAb) and γ-secretase inhibitors (e.g., MK0752) have demonstrated encouraging therapeutic effects in clinical trials. Unfortunately, the therapeutic efficacy of other drugs does not seem to meet expectations, and further research is needed.
Regulation of Notch signaling in immune cells
Numerous studies have confirmed that Notch signaling regulates cell development, cancer stem cell differentiation and proliferation, and cancer cell fate by targeting various genes [5, 57,58,59,60]. In the TME, the regulation of immune cell properties by Notch signaling also plays important roles in tumor progression, as reviewed below.
Natural killer cells
Natural killer (NK) cells are crucial anti-viral and anti-tumor cells in the innate immune system [61, 62]. Early studies have found that Notch signaling plays an indispensable role in regulating their development and effector functions. For example, human umbilical cord blood (UCB) CD34+ precursors become committed to differentiate into NK cells in vitro after they are stimulated with Notch ligands (mainly Dll1, Dll4, and Jagged2) in the presence of cytokines [e.g., interleukin 7 (IL-7), Fms-like tyrosine kinase 3 (Flt3) ligand, and interleukin 15 (IL-15)]. These NK cells were able to lyse tumor cells because they upregulate their transcription and release of granzyme B (GZMB) and interferon-gamma (IFN-γ) [63,64,65]. As human NK cells mature, Dll1-mediated Notch signaling is activated to promote the expression of CD16 and killer Ig-like receptors (KIRs), resulting in cytotoxicity against tumor cells [66]. In human peripheral blood and decidual NK cells, activation of Notch1 and/or Notch2 by Dll1 and/or Dll4 promotes IFN-γ secretion [65]. Compared with normal human NK cells, Zakiryanova et al. found that the expression of Notch1 was significantly decreased in NK cells of patients with lung cancer or gastric cancer, while Notch2 was significantly reduced in patients with gastric cancer but not in those with lung cancer [67]. In murine NK cells, Kijima et al. found that DC-mediated NK cell activation was controlled by the interaction of Notch with Jagged2. Enforced expression of Jagged2 in DCs significantly enhanced the cytolytic effects of murine NK cells against YAC-1 cells by activating the NK cells’ Notch signaling [68]. Enforced expression of Jagged2 in A20 cells (a BALB/c-derived B cell lymphoma cell line with low expression of Jagged2) also enhanced the cytolytic efficacy of murine NK cells against A20 cells in vivo and in vitro [68]. Together, these observations suggest that Notch activation can significantly enhance the anti-tumor properties of NK cells. Therefore, targeted activation of Notch signaling in NK cells might be a promising strategy for enhancing NK cell therapy (Fig. 2A).
Mechanisms by which Notch signaling regulates anti- or pro-tumor functions of immune cells. A. I: After stimulation with Jagged2, Dll1, or Dll4, Notch signaling promotes the differentiation of CD34+ precursors into NK cells and enhances the anti-tumor properties of the NK cells by potentiating the secretion of IFN-γ and GZMB; II: Dll1 or other ligands that activate Notch1 or Notch2 increase the expression of CD16, KIRs, IFNG, enhance the maturation and cytotoxicity of NK cells. B. I: In IL22+ ILCs, AhR ligand enhances the transcription of Notch1 and Notch2 by activating AhR, sustains the NKp46+ ILC population, and, in part, also sustains the LTi-like cell population by activating Notch signaling; II: In LTi cells, T-bet promotes the transcription of Notch1 and Notch2 and also promotes the transition of LTi cells into NKp46+ ILCs; III: In NKp46− ILCs, Notch activation mediated by Notch2 signaling promote the transcription of T-bet, AhR, and RORγt through canonical Notch signaling, which is mediated by RBP-J. This promotes the transition of NKp46− ILCs into NKp46+ ILCs. C. I: Activation of canonical Notch signaling by Dll1 promotes the transcription of Socs3 and miR-125a but inhibits the transcription of SIRPα, promoting M1 macrophage polarization and inhibiting M2 macrophage polarization; II: In the liver TME, myeloid cell-mediated canonical Notch signaling positively regulates the differentiation of moTAMs, but negatively regulates the proliferation of kclTAMs by regulating WNT − β-CATENIN signaling transmission in kclTAMs. D. I: In the glioma TME, oHSVs induce Jagged1 expression on macrophages; Jagged1-presenting macrophages spread activation of Notch signaling on TAMs, promotes the release of CCL2 from TAMs, recruits MDSCs, and then inhibits the cytotoxicity of CD8+ T cells; II: In MDSCs, activated NICD inhibits MCT2 expression, reduces the uptake of lactate from the TME, inhibits Cox2 transcription, promotes MDSCs transition into M1(tumor-suppressive)-TAMs. E. Activation of RBP-J-mediated Notch signaling in DCs promotes the transcription of APC-related genes, migration-related genes, and CCR2; this activation enhances antigen presentation by DCs to T cells and accelerates tumor lysis. Dll4 of DCs activates Notch receptor of T cells, promotes the transcription of a series of genes (GATA3, T-bet, RORC, IL-4, IFNG and IL-17) that promote the differentiation of T cells or directly enhances the T cells’ cytotoxicity. F. Notch1/2 activated by Dll ligand promotes the transcription of Gzmb, Ifng, and Pdcd1, and enhances the anti-tumor ability of T cells by releasing IFN-γ and GZMB. However, Notch signaling activation also inhibits the anti-tumor property of T cells by increasing PD-1 expression. Transcriptional and immune response regulator (TCIM) inhibits Notch signaling, but enhancer of zeste homolog 2 (EZH2) activates Notch signaling by inhibiting the expression of Notch suppressors (Numb and Fbxw7). Figure was created with BioRender.com
Innate lymphoid cells
Innate lymphoid cells (ILCs) are newly discovered and defined lymphocytes involved in regulating innate and adaptive immune responses. They govern immune responses against viruses, intracellular pathogens, helminths, and tumors [69,70,71]. ILCs are widely distributed in various tissues and organs (e.g., liver, lymph nodes, small intestine lamina propria, and other mucosal tissues), and the various murine ILC lineages are distinguished by differences in transcription factor profiles and cytokine production. For example, T-bet [encoded by T-box transcription factor 21 (Tbx21)]-expressing group 1 ILCs (ILC1s) secrete mainly IFN-γ; GATA3-expressing group 2 ILCs (ILC2s) secrete mainly IL-5 and IL-13; and RORγt-expressing ILCs (ILC3s) secrete mainly IL-22 and IL-17. Murine ILC3s can be further divided into NKp46+ ILC3s, NKp46− ILC3s, and lymphoid tissue-inducer cells (LTi cells) [72, 73].
During the past decade, researchers have uncovered multifaceted roles for Notch signaling in ILC subsets. In 2011, Possot et al. cultured murine bone marrow (BM) common lymphoid progenitors (CLPs) on OP9-Dll4 stroma (to activate Notch signaling) or in the presence of DAPT (to inhibit γ-secretase and therefore Notch signaling) and found that the maturation of adult BM-derived RORγt+ ILCs (also defined as ILC3s) was Notch2-dependent manner [74]. A subsequent study of murine gut ILC22 cells (now known as ILC3s), which include NKp46+ILCs [CD3−NKp46+NK1.1lo–negRORγt+ cells (also defined as NKp46+ ILC3s)] and LTi cells, showed that Notch signaling was crucial for the downstream signaling of aryl hydrocarbon receptor (AhR) during the generation of murine NKp46+ ILCs (likely ILC3). In contrast, LTi-like cells were partly dependent on Notch signaling [75]. Compared with WT mice, RBP-Jκ-CD mice (conditional deletion of RBP-Jκ expression in the hematopoietic compartment) had considerably fewer NKp46+ ILCs in the lamina propria [75]. Mechanistically, the binding of AhR ligands on AhR promotes the translocation of AhR into the nucleus, where it binds to regulatory sites and promotes the expression of Notch receptors (mainly Notch1, Notch2), enhances IL-22 secretion, and ultimately sustains the NKp46+ ILC population and partly sustains LTi-like cells in small intestine (SI) lamina propria [75]. A subsequent study by Rankin et al. demonstrated that T-bet-mediated NKp46+ ILC development could also be achieved via Notch (mainly Notch1, Notch2) signaling [76]. Specifically, after being exposed to Dll1 for 9 days, murine SI lamina propria Rorc(γt)+/GFP Tbx21+/+ LTi cells generated NKp46+ ILCs, whereas Rorc(γt)+/GFPTbx21−/− LTi cells did not generate that subset in vitro. This suggests that Notch signaling plays an integral role in the T-bet-mediated transition of LTi cells into NKp46+ ILCs [76]. In addition, in murine SI lamina propria, RBP-J-mediated Notch2 signaling contributed to the transition of NCR− ILC3 precursors (NKp46− ILC3s) into NCR+ ILC3s (NKp46+ ILC3s) in a cell-autonomous manner. Mechanistically, activation of RBP-J-mediated Notch2 signaling mainly stimulates the expression of genes encoding transcription factors, such as T-bet, AhR, and RORγt [77]. These murine studies support the notion that Notch signaling regulates ILC3 plasticity by controlling the fate of NKp46+ cells. In human ILCs, researchers have also found that, in combination with IL-7, Notch signaling induces the differentiation of hematopoietic progenitor cell subpopulation one [HPC-1, CD45RA (RA)−Flt-3+c-Kithi cells] into NKp44+ ILC3s [78]. Thus, Notch signaling plays an essential regulatory role in the development and phenotypic transition of ILC subsets, mainly ILC3s. However, our understanding of the role of Notch signaling in ILC-mediated immune responses in cancer is still preliminary, as even ILCs’ role in cancer is not yet very clear. We recently started to elucidate the role of ILCs in cancer [79, 80]. Therefore, further studies that explore the role and regulatory mechanism of Notch signaling in immune responses mediated by ILCs are warranted (Fig. 2B).
Macrophages
Macrophages are specialized phagocytes in innate immunity. As one of the first responders to infection, they recognize and degrade tumor cells [81]. In the TME, macrophages are extremely plastic, and their interaction with tumor cells and/or the stromal microenvironment usually polarizes M1-like tumor-associated macrophages (M1-TAMs) into M2-like tumor-associated macrophages (M2-TAMs) [82]. Generally, M1-TAMs promote anti-tumor inflammatory responses and exert anti-tumor effects [82, 83]. In contrast, M2-TAMs are involved in neovascularization [84] and matrix deposition and remodeling [85], and they participate in immunosuppression, promoting tumor growth [84]. Therefore, promoting the polarization of macrophages into M1-TAMs or reversing M2-TAMs into M1-TAMs are key strategies for targeting macrophages in cancer immunotherapy [82, 86, 87].
In recent years, our research has suggested that Notch signaling plays a crucial regulatory role in switching macrophage phenotypes and thus in remodeling the TME [88,89,90,91]. Specifically, some Notch signaling molecules (e.g., Notch1, Notch2, Hes1) were expressed higher in M1-TAMs than in M2-TAMs in a B16F10 melanoma in vivo model. Forced activation of Notch signaling by co-culture with OP9-Dll4 cells promoted anti-tumor activity by polarizing macrophages into IL-12-producing M1-macrophages but not into M2-macrophages. Mechanistically, knockout of RBP-J-mediated Notch signaling inhibited M1 polarization by inhibiting LPS-induced suppressor of cytokine 3 (Socs3) expression [88]. Using NIC transgenic mice controlled by Lyz2-Cre (NICCA), we subsequently showed that forced activation of Notch signaling in macrophages in vivo repressed tumor growth while diminishing TAM phenotypes. Mechanistically, miR-125a has been identified as a key downstream miRNA of RBP-J-mediated Notch signaling activation. Overexpression of miR-125a promoted M1 polarization and suppressed M2 polarization, boosting anti-tumor activity [89]. In addition, signal regulatory protein α (SIRPα), a key inhibitor of macrophages, was identified as the key downstream molecule of RBP-J-mediated Notch signaling. Notch activation repressed SIRPα expression through the Hes family co-repressors and then enhanced tumor cell lysis partly by promoting polarization into the M1 phenotype. Soluble mSIRPαext polypeptides, which possess the extracellular domains of mouse SIRPα, promoted M1 polarization and increased phagocytosis of tumor cells by macrophages [90]. This study indicates that specifically activating Notch signaling to inhibit the SIRPα-CD47 axis might be a promising strategy for releasing macrophages from phagocytic inhibition. We recently generated a type 1 herpes simplex virus-based oncolytic virus (oHSV) that expresses a full-length anti-CD47 antibody (αCD47) to block the CD47 ‘don’t eat me’ signal. This engineered virus suppressed tumor growth in both glioblastoma and metastatic ovarian cancer models, partly by promoting the M1 polarization of macrophages [92, 93]. In the TME of murine orthotopic HCC, myeloid-specific RBP-J knockout significantly promoted the growth of orthotopic tumors [91]. Compared with control mice, the infiltration of CCR2-independent TAMs—mainly Kupffer cell-like TAMs (kclTAMs) but not monocyte-derived TAMs (moTAMs)—in the liver was significantly higher in RBP-J knockout mice [91]. Mechanistically, RBP-J deficiency in myeloid cells impeded the differentiation of moTAMs, but promoted the proliferation and pro-tumor cytokine [e.g., interleukin 10 (IL-10)] production of kclTAMs by upregulating WNT-β-CATENIN signaling, and then accelerating the progression of murine orthotopic HCC [91]. Together, these findings suggest that intrinsic activation of Notch signaling promotes the M1 polarization and suppressed M2 polarization of macrophage to boost anti-tumor activity, while intrinsic inhibition of Notch signaling promotes the proliferation of kclTAMs to boost pro-tumor activity (Fig. 2C).
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are another major immune response modifier in cancer, as they interfere with immune responses against tumors and facilitate tumor metastasis and angiogenesis [94]. According to the differences in cell surface markers, MDSCs can be divided into different subtypes. Granulocytic-MDSCs [G-MDSCs or polymorphonuclear (PMN)-MDSCs] and mononuclear MDSCs (M-MDSCs) are two important immunosuppressive subsets [95, 96].
Recently, Wang et al. showed that Notch signaling was significantly inhibited in PMN-MDSCs of tumor-bearing mice [97]. Compared with MDSCs of control mice, the MDSCs (mainly PMN-MDSCs) of mice with specific knockout of RBP-J in myeloid cells were significantly less immunosuppressive. Mechanistically, knockout of RBP-J inhibits the signal transducer and activator of transcription 3 (STAT3) signaling and reduces the inhibition capability of PMN-MDSCs on the proliferation and activation of allogenic T cells, while the deficiency of the Notch signaling has not much effect on M-MDSC [97]. Therefore, blocking RBP-J-mediated canonical Notch signaling, specifically in PMN-MDSCs, might be an ideal strategy for inhibiting tumor progression [97]. Using Cybersort and Gene Set Enrichment Analysis (GSEA), Otani et al. analyzed TCGA database and revealed that a higher Notch score positively correlated with M-MDSC recruitment in glioma patients [98]. Mechanistically, treating mice bearing intracranial glioma with oHSV induced Jagged1 expression on macrophages. These Jagged1-presenting macrophages spread Notch activation in the TME, especially in TAMs. TAMs with Notch activation induce the secretion of CCL2, further amplifying M-MDSCs recruitment and attenuating anti-tumor immune response of T cells [98]. Blockading Notch signaling with GSI (γ-secretase inhibitor) significantly reduced the M-MDSC-mediated immunosuppressive TME and activated CD8+ T cell-dependent anti-tumor memory response [98]. A study from our group showed that activating Notch signaling in murine myeloid cells significantly inhibited tumor progression. Activated NICD inhibited lactate import 2 (MCT2) expression via Hes1, thus reducing lactate intake in myeloid cells. Activated NICD also promoted the differentiation of M-MDSCs into M1-type TAM but not into PMN-MDSCs in the TME [99].
Together, these studies highlight the complex roles of Notch signaling in the differentiation of different MDSC subtypes, suggesting that targeting activation or inhibition of Notch signaling in MDSCs for cancer treatment must be context-dependent. However, oncolytic virotherapy combined with Notch blockade may be a promising strategy for synergistically inhibiting the immunosuppressive function of M-MDSCs and thereby enhancing therapeutic benefits in glioma or other tumors that respond positively to inhibition of Notch signaling (Fig. 2D).
Dendritic cells
Dendritic cells are professional antigen-presenting cells (APCs) that can efficiently intake and process antigens, and then present them to T cells, leading to activation of adaptive immune responses against pathogens and tumors [100]. Meng et al. identified a new human DC subset that highly expresses the Notch ligand Dll4 (Dll4+ DCs) [101]. Compared with Dll4− DCs, these Dll4+ DCs can better promote the differentiation and expansion of T helper (Th) cells (e.g., Th1 and Th17 cells) and effector CD8+ T cells because they upregulate the transcription of differentiation-related transcription factors (e.g., GATA3, T-bet, RORC) and the production of anti-tumor effector cytokines (e.g., IL-4, IFN-γ, and IL-17). This suggests that high levels of Dll4 in DCs indicate the high anti-tumor potential because of upregulated antigen presentation and adaptive immune responses [101, 102].
In addition to Notch ligands, we found that Notch receptors and their downstream effectors are essential for DCs’ effector function. Thus, activation of RBP-J-mediated Notch signaling was critical in DC-dependent anti-tumor immune responses [9]. Compared with murine RBP-J+/− DCs, RBP-J−/− DCs (specific knockout of RBP-J in DCs) lost inhibition of tumors (e.g., B16F10 melanoma, H22 hepatoma, and Lewis lung carcinoma) in vivo because DC migration and antigen presentation to T cells were inhibited [9]. During the progression of colitis-associated CRC, mice whose DCs were deficient in Notch signaling were more susceptible to the disease than mice with normal DCs [103]. In contrast, adoptive transfer of Notch-primed DCs in mice restrained the progression of inflammation-associated CRC [103]. Mechanistically, chemokine receptors [mainly CC-chemokine receptor 7 (CCR7)] of DCs were identified as a critical downstream component of RBP-J-mediated Notch2 signaling, and upregulation of CCR7 mediated by activated Notch2 signaling facilitated DC migration and cross-presentation of antigens to CD8+ T cells [103]. Kirkling et al. found that Notch signaling facilitated the differentiation and CCR7-dependent migration of conventional DCs (cDCs) and then promoted their cross-presentation of antigens to T cells [104]. In addition, Notch signaling can be activated in DCs by a polysaccharide [Lycium barbarum polysaccharide (LBP)] and can then induce the phenotypic and functional maturation of DCs to promote DC-mediated cytotoxicity of T lymphocytes (CTLs) [105]. In general, Notch signaling is a positive regulator of DC maturation, antigen presentation, and adaptive immune responses, but the specific regulation mechanisms remain to be explored (Fig. 2E).
T cells
T cells, especially CD8+ T cells, are well known for their cytolytic effects that require prior sensitization during adaptive immune responses [106]. Previous reports indicated that activation of Dll1-mediated Notch signaling (mainly Notch2 signaling) promoted the differentiation and cytolytic function of murine T cells both in vitro and in vivo [107]. By using a Notch2f/fE8I-Cre+ mouse model (lacking Notch2 expression in peripheral CD8+ T cells but not in CD4+ T cells), the authors found that the knocking out of Notch2 inhibited (compared to the control) the differentiation of naive CD8+ T cells into CTLs and could not control the growth of OVA-expressing EG7 thymoma cells and EG7 cells in vivo. These observations indicate that Notch2 is crucial for the anti-tumor response of CTL cells [107, 108]. Using a ChIP assay to explore the mechanism, the authors found that a complex of activated NICD, phosphorylated-CREB1, and transcriptional coactivator p300 bound to the promoter of the Gzmb gene, enhancing its transcription [107, 108]. Of note, in vitro and in vivo tumor models (e.g., breast adenocarcinoma, lung cancer, thymoma) and three follow-up studies also demonstrated that activation of Notch signaling significantly enhanced the anti-tumor and/or anti-tumor memory capacity of CD8+ T cells by promoting the expression of IFN-γ and GZMB. In these studies, Notch signaling was activated by (i) using mice whose CD8+ T cells contained a specifically activated NIC (Notch1 intracellular domain) [109], (ii) treating CD8+ T cells with the proteasome inhibitor bortezomib [110], or (iii) treating CD8+ T cells with the Notch ligand Dll1 [111]. However, in a different TME (e.g., in murine HCC or ovarian cancer), Notch signaling was regulated by a series of genes [e.g., transcriptional and immune response regulator (1810011O10 Rik, also known as TCIM) and enhancer of zeste homolog 2 (EZH2)] to modulate the anti-tumor immune response of T cells. Specifically, high expression of TCIM in T cells inhibited the nuclear translocation of activated NICD of the Notch2 receptor and thus suppressed the activation of downstream effector molecules, thereby reducing the cytotoxicity of CD8+ T cells [112]. Restricting glucose uptake of T cells from TME inhibited EZH2 expression, which indirectly inhibited the Notch signaling through suppressing the two Notch repressors, Numb and Fbxw7, leading to dampening anti-tumor activity of T cells [113]. Together, these studies indicate that Notch signaling plays a positive regulatory role in the anti-tumor properties of T cells. Therefore, activating Notch signaling in T cells, especially CD8+ T cells, might be a good strategy for enhancing anti-tumor responses.
As well as boosting T cells’ anti-tumor effects, Notch signaling might accelerate T cell exhaustion. The transcriptional activation complex of canonical Notch signaling directly binds to the promoter of Pdcd1 (encoding PD-1, a marker gene that promotes T cell exhaustion) to promote Pdcd1 transcription in CD8+ T cells [114]. Compared with colorectal T cells from healthy individuals, the expression of PD-1 and Notch signaling molecules (NOTCH1, NOTCH2, HES1, and HES5) was elevated in tumor-infiltrating CD8+ T cells from CRC patients [115]. Inhibition of Notch signaling not only promoted the cytotoxicity of tumor-infiltrating CD8+ T cells, but also enhanced CD8+ T cells’ production of proinflammatory cytokines [including IFN-γ, tumor necrosis factor alpha-like (TNF-α), interleukin-1 beta (IL-1β), IL-6, and IL-8] in those patients. This process was accompanied by decreased PD-1 expression in CD8+ T cells but did not affect cell proliferation [115]. This result suggests that Notch signaling has potential immunosuppressive properties that might inhibit the cytolytic and non-cytolytic functions of CD8+ T cells by inducing PD-1 in colorectal cancer patients [115]. In addition, the single-cell RNA sequencing of T cells in the human TME (e.g., lung cancer, pan-cancer) demonstrated that RBP-J expression was also related to the cytotoxicity or exhaustion of T cells [116, 117]. Together, the above evidence suggests that Notch activation can significantly enhance anti-tumor properties but may also potentially promote T cell exhaustion. However, targeted activation of Notch signaling in T cells combined with immune checkpoint blockers (ICBs), such as αPD-1, might be a promising strategy for enhancing T cell therapy (Fig. 2F).
In summary, Notch signaling plays a “double-edged sword” role in regulating immune responses, as Notch signaling can modulate the functions of anti- or pro-tumor immune cells. Specifically, for innate immune cells (e.g., NK cells, DCs, and macrophages), activation of Notch signaling mainly: (1) enhances the anti-tumor property of NK cells directly; (2) promotes the maturation and antigen presentation of DCs; and (3) facilitates the transition of macrophages into an M1 type. All of these can inhibit tumor progression. However, in the different TME, Notch signaling plays different roles in MDSCs-mediated tumor immunity. In the TME of murine lung carcinoma model, activating Notch signaling in myeloid cells promotes the differentiation of M-MDSCs into M1-TAMs and thus inhibits tumor progression. However, in the glioma TME, activating Notch signaling promotes M-MDSC-mediated immunosuppression and thus facilitates tumor progression. For adaptive T immune cells (e.g., T cells), activation of Notch signaling enhances their anti-tumor property, but Notch signaling also potentially enhances the exhaustion of T cells by upregulating PD-1 expression.
SynNotch can be used as a tool to increase T cell cytotoxicity and specificity
Adoptive T cell therapy, especially with CAR-T cells, has achieved unprecedented success against hematological malignancies [e.g., chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and lymphoma] but has shown only modest progress with solid tumors [118]. Historically, adoptive T cell therapy (especially with CAR-T cells) has been facing many challenges, inducing expected and unexpected toxicities (e.g., cytokine release syndrome, ‘on-target/off-tumor’ recognition), and is prone to exhaustion in the TME [119, 120]. Thus, many researchers are looking for strategies to overcome these obstacles [121]. In 2016, the Lim group took advantage of the Notch receptor’s unique structure to replace and customize its extracellular and intracellular domains, transcription factor domains, and downstream effectors. This led to the development of a synthetic Notch (synNotch) system, which allows engineered T cells to respond to tumor antigens in a very precise and localized way. The structure of synNotch system is shown in Fig. 3A [7]. The synNotch platform was used to engineer T cells that produce a customized therapeutic response after they encounter a tumor antigen. For example: (i) synNotch T cells can produce selective cytokines (e.g., IL-2 and IL-12) to precisely regulate immune responses; (ii) synNotch T cells can increase the expression of differentiation-related molecules (e.g., T-bet) to promote T cells differentiation into anti-tumor Th1 cells, thus controlling the fate choice of T cells; (iii) synNotch T cells can bind to specific receptors, trigger self-destruction of cancer cells and further promote their demise by producing TNF-related apoptosis-inducing ligand (TRAIL); (iv) upon contact with cancer cells, synNotch T cells prompt T cells to produce antibodies (e.g., αPD-1, αCTLA-4, or αCD3/CD19 BiTE) against specific immune checkpoints (ICs) or antigens, enhancing the efficacy of immunotherapy [7]. SynNotch T cells have shown good therapeutic effects against a variety of solid tumors.
The synNotch system increases T cell specificity. A: Top, customized recognition domain of synNotch receptors (e.g., αCD19 scFV) detects a signal molecule (e.g., CD19) on target tumor cells. Middle, the core regulatory region of the Notch receptor that governs proteolysis or cleavage is activated by the interaction between the receptor and the tumor target signal (top), and a cytoplasmic orthogonal transcription factor is released; Bottom, the orthogonal transcription factor enters the nucleus of engineered T cells and controls the function-related transcriptional programs. B. I: αCD19-synNotch T cells recognize CD19+ tumor cells and release customized cytokines (e.g., IL-2, IL-12, and IL-10) that destroy CD19+ tumor cells; II: αCD19-synNotch T cells recognize CD19+ tumor cells, promote the transcription of differentiation-related genes (e.g., T-bet), and then promote T cells differentiation into Th1 cells; III: αGFP-synNotch T cells recognize GFP+ tumor cells, promote the transcription of TRAIL, and accelerate the lysis of tumor cells; IV: αGFP-synNotch T cells recognize GFP+ tumor cells, promote the production of antibodies (e.g., αPD-1, αCTLA-4 and αCD19/αCD3 BiTE), and accelerate tumor lysis. C. I: EpCAM/B7-H3-synNotch-αROR1-CAR-T cells recognize EpCAM+/B7-H3+ tumor cells, promote the expression of αROR1-CAR on T cells, and enable T cells to recognize and lyse EpCAM+/B7-H3+ROR1+ tumor cells; II: MET-synNotch-αMART1-T cells recognize MET+ tumor cells, and then enable T cells to lyse MET+MART1+ melanocytes; III: GFP-synNotch1-HER2-synNotch2-αCD19-CAR-T cells recognize GFP+ tumor cells, promote the expression of αCD19-CAR on T cells, and recognize and lyse GFP+CD19+ tumor cells. However, if GFP-synNotch1-HER2-synNotch2-αCD19-CAR-T cells recognize HER2+ normal cells, the proapoptotic factor tBID (truncated BH3-interacting domain death agonist) will be expressed by T cells to casue T cell apoptosis; IV: EGFR-synNotch1-MET-synNotch2-αHER2-CAR-T cells recognize two antigens from tumor cells (first one is EGFR and second one is MET). This promotes the expression of αHER2-CAR on T cells, and enable T cells to lyse EGFR+MET+HER2+ tumor cells; V: Both MET-synNotch-αHER2/αEGFR-CAR-T cells and EGFR/HER2-synNotch-αMET-CAR-T cells recognize and lyse EGFR+MET+/HER2+MET+ tumor cells; VI: GD2-synNotch-αB7-H3-CAR-T cells, APPL2-synNotch-αMCAM/αMSLN/αHER2-CAR-T cells, or EGFRvIII-synNotch-αEphA2/αIL13Rα2-CAR-T cells recognize the first antigen (GD2, APPL2, or EGFRvIII) on tumor cells, promote the expression of αB7-H3-CAR, αMCAM/αMSLN/αHER2-CAR, or αEphA2/αIL13Rα2-CAR on T cells, and enable T cells to lyse specific tumor cells (GD2+B7-H3+ tumor cells, APPL2+MCAM+/MSLN+/HER2+ tumor cells, or EGFRvIII+EphA2+/IL13Rα2+ tumor cells). Figure was created with BioRender.com
In a series of subsequent studies, synNotch was combined with CAR-T cells to give the cells more specificity. In 2020, the Lim group deployed multiple synNotch in the same T cell to generate a complex combined sensing circuit [122]. Specifically, the authors designed a diverse library of multi-receptor cell recognition circuits by using synNotch to transcriptionally interconnect multiple molecular recognition events. These synthetic circuits allow engineered CAR-T cells to integrate extracellular and intracellular antigen recognition, and they achieve precise recognition by integrating up to three antigens with positive or negative logic, providing a powerful and precise recognition tool for CAR-T cells [122]. At the same time, various groups conducted therapeutic studies on solid tumors (e.g., glioma, mesothelioma, ovarian cancer), demonstrating that synNotch CAR-T cells produce a stronger anti-tumor effect and have greater specificity than conventional CAR-T cells. Conventional receptor tyrosine kinase-like orphan receptor 1 (ROR1)-targeted CAR-T cells not only lyse ROR1+ tumor cells but also attack ROR1+ normal stromal cells, which may cause therapeutic iatrogenic toxicity [123, 124]. Srivastava et al. developed ROR1-targeted CAR T cells expressiong synNotch receptors for epithelial cell adhesion molecules (EpCAM) or B7-H3, which are expressed on tumor cells but not on normal stromal cells [125]. In mouse and human solid tumor models, these synNotch CAR-T cells selectively killed EpCAM+ ROR1+ or B7-H3+ ROR1+ tumors cells but not killed EpCAM−ROR1+ cells or B7-H3−ROR1+ cells in normal tissues, resulting in tumor regression without toxicity [125]. Thus, this strategy safely targets tumors while sparing normal stromal cells, greatly reducing the extratumoral toxic effects of conventional CAR-T cell therapy [125]. In 2021, Choe et al. developed a synNotch CAR-T cell system whose synNotch receptor recognizes a specific priming antigen, such as the heterogeneous but tumor-specific glioblastoma neoantigen epidermal growth factor receptor splice variant III (EGFRvIII). After it is primed, the CAR-T cells are locally induced to express a second chimeric receptor targeting two more homogeneous tumor-specific antigens [EPH receptor A2 (EphA2) antigen or IL13Rα2 antigen] so as to switch on their highly specific killing program [126]. These synNotch CAR-T cells specifically recognized and killed EGFRvIII+EphA2+/IL13Rα2+glioblastoma cells while sparing healthy tissues [126].
Alkaline phosphatase placental-like 2 (ALPPL2), a tumor-specific antigen, is highly expressed in a spectrum of solid tumors (e.g., mesothelioma, ovarian cancer). Hyrenius-Wittsten et al. designed a synNotch CAR-T cell that targets ALPPL2 and another tumor-associated antigen [e.g., melanoma cell adhesion molecule (MCAM), mesothelin, or human epidermal growth factor receptor 2 (HER2)]. In mouse models of human mesothelioma and ovarian cancer, the synNotch CAR-T cells exerted superior control over tumor burden compared with traditional CAR-T cells, and they maintained long memory and a non-exhausted phenotype [127]. In neuroblastoma, Moghimi et al. engineered a specific synNotch protein on the surface of T cells to recognize the disialoganglioside (GD2) antigen [128]. When T cells recognized GD2, the synNotch protein instructed them to activate their CAR-T properties, allowing them to recognize a second antigen, B7-H3 [128]. These T cells followed these specific instructions to kill neuroblasts that carry both GD2 and B7-H3 [128].
In general, the above studies confirm that T cells, especially CAR-T cells, that contain engineered synNotch are better able to control solid tumors than conventional T cells. Thus, the synNotch system is a advantageous tumor recognition strategy that may navigate the concurrent challenges of specificity and heterogeneity to increase the therapeutic benefits of T cells against tumors, especially solid tumors (Fig. 3).
Dysregulated Notch signaling in the tumor microenvironment and targeting it for cancer immunotherapy
The TME strongly affects responsiveness to immunotherapy, indicating that it plays a crucial role in accelerating or inhibiting cancer progression [129, 130]. Numerous studies have shown that tumor cells, stromal cells (e.g., cancer-associated fibroblasts, pericytes, mesenchymal stromal cells), as well as extracellular matrix (ECM) and secreted molecules in the TME (e.g., growth factors, cytokines, chemokines, and extracellular vesicles) can affect the infiltration and effector functions of immune cells, thus regulating tumor progression [131]. Compelling evidence indicates that alterations (activation or inhibition) in Notch signaling of tumor cells or stromal cells influence the effector functions of immune cells that infiltrate the TME, making Notch signaling a promising target in cancer immunotherapy.
Dysregulated Notch signaling in tumor cells affects immune cell function in the TME
In BC patients, Jagged1 expression correlated with tumor progression. High Jagged1 expression correlated positively with infiltration of stromal M2-TAMs, which predicts poor patient survival and resistance to aromatase inhibitor therapy. However, BC cells pretreated with GSI and co-cultured with macrophages significantly inhibited the polarization of macrophages into M2-TAMs [132]. In BC, high expression of a long noncoding RNA, Linc00514, also promoted the expression of Jagged1, which in turn activated Notch signaling to promote the secretion of IL-4 and IL-6 from BC cells; these events then induced M2 polarization of macrophages. This suggests that activation of Notch signaling mediated by Jagged1 positively promotes M2-TAM polarization [133]. In multiple spontaneous BC models (e.g., 4T1 BC, PyMT-A BC), overexpression of tumor-derived Jagged1 promoted tumorigenesis [134]. By utilizing genetically engineered murine models of mammary-gland-specific Jagged1 overexpression or knockout mice, the researcher found that Notch activation by tumor-derived Jagged1 promoted the secretion of multiple cytokines (e.g., IL-6, WISP1) and TAM recruitment; the proliferation and tumoricidal activity of T cells were then inhibited, partially through upregulation of the T cells’ PD-1 [134]. Also, the combination of Notch inhibitor (GSI) with ICBs (αPD-1) significantly inhibited tumor growth in TNBC [134]. In pancreatic ductal adenocarcinoma (PDAC) patients, high Jagged1 expression in PDAC cells was associated positively with CD68+ macrophage infiltration and decreased patient survival [135]. Zhang et al. have shown that the upregulated expression of Dll1 in BC cells induces long-term normalization of tumor vascular and promotes the accumulation of CD8+ T cells and the polarization of M1-TAMs [136]. By recruiting 130 patients with invasive BC for bioinformatics and statistical analysis, the researcher found that high expression of Dll3 was associated with poor survival and with high levels of Treg cell infiltration [137]. High infiltration of tumor-associated neutrophils (TANs) was associated with immune tolerance and dismal prognosis in epithelial ovarian cancer (EOC) [138]. High expression of Jagged2 in tumor cells enhanced TAN infiltration, in turn inhibiting CD8+ T cell cytotoxicity. Blockade of Notch signaling [anti-Jagged2 antibody or LY3039478 (γ-secretase inhibitor)] reactivated CD8+ T cell-mediated anti-tumor properties, inhibiting tumor progression [138]. The above studies show that, in BC, PDAC, and EOC, high expression of Notch ligands (mainly Jagged1, Jagged2, and Dll3) of tumor cells promotes an immunosuppressive microenvironment in the TME, eventually allowing tumors to tolerate immunotherapy.
In addition to Notch ligands, abnormal expression of tumor-derived Notch receptors and downstream signaling genes affects the infiltration of immune cells and therefore tumor progression. By analyzing tumor samples from 152 patients with hormone receptor-positive and -negative phenotypes (luminal and triple-negative/basal-like) of BC, the author found that low mRNA levels of Notch receptors (mainly Notch1, Notch2, and Notch4) mainly in tumor cells were associated with higher infiltration of Treg cells into the tumors, predicting poor prognosis and poor survival [139]. However, in murine B16F10 melanoma models with subcutaneous and lung metastases, ectopic over-expression of Notch1 in B16F10 cells accelerated tumor progression and promoted tumor immunosuppression by upregulating TGF-β1. Specifically, forced high expression of Notch1 in B16F10 cells reduced the release of IFN-γ into the TME and inhibited the infiltration of CD8+ T cells and NK cells, while enhancing Treg cell and MDSC infiltration in vivo. PD-1 of CD4+ cells and CD8+ T cells were upregulated, accelerating T cell exhaustion [140]. In a mouse TNBC model, loss of function of ubiquitin-specific peptidase 9x-linked (USP9x) in tumor cells abolished NICD activation reduced the production of proinflammatory cytokines (e.g., CCL2, IL-1β), which further reduced the tumor inflammation through inhibiting the infiltration of CD206+ TAMs and Treg cells, augmenting the anti-tumor immune response through increase the infiltration of CD8+ T cells, suppressing BC tumor cell growth in vivo [141]. In a Tgfbr1/Pten knockout mouse model of HNSCC, Notch1 − Hes1 signaling was activated [19]. A γ-secretase inhibitor-DAPT, which inhibited Notch signaling, significantly decreased the burden of HNSCC tumors in that model [19]. Flow cytometry analysis demonstrated that the γ-secretase inhibitor also reduced the infiltration of MDSCs, TAMs, and Tregs into the spleen, draining lymph nodes and the TME as well as decreasing the expression of ICs (e.g., PD-1, CTLA-4, TIM-3, and LAG-3) in T cells in the circulation and tumor TME [19]. This study suggests that blocking Notch1 − Hes1 signaling in HNSCC might be an effective way to reduce immunosuppression and enhance therapeutic efficacy [19] (Table 2).
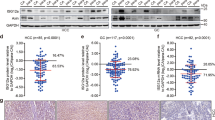
In the glioma TME, tumor cells escape immune surveillance and increase invasiveness by reducing Notch signaling. Specifically, loss of Notch signaling (mainly Notch1, Notch2, RBP-J, and Hey1) in glioma cells suppressed the expression of MHC-I and cytokines [e.g., C-X-C motif chemokine ligand 9 (CXCL9) and IL-15], reduced the recruitment of anti-tumor immune cells (e.g., CD8+ T cells), but favored the infiltration of microglia and pro-tumor TAMs [142]. In gastric cancer (GC) patients, both tumor tissue and peripheral blood showed significantly higher expression of Notch receptor (NOTCH1, NOTCH2) mRNA than normal human gastric tissue, and they also had higher proportions of Treg cells and Th17 cells [143]. Inhibiting Notch signaling with DAPT significantly suppressed Treg cell function in GC patients [143]. Another group also found that high Notch receptor (NOTCH3) expression was a poor prognostic factor when compared with 395 other genes in GC patients [144]. Specifically, high expression of Notch3 was associated with lower infiltration of anti-tumor immune cells (e.g., activated CD8+ T cells) and higher infiltration of immunosuppressive cells (e.g., Treg cells, M2-TAMs). In addition, high expression of Notch3 was accompanied by increased expression of a series of ICs [e.g., CD276, adenosine Aa2a receptor (ADORA2A)], resulting in a dampened anti-tumor immune response [144]. In addition to solid tumors, abnormal expression of Notch signaling in tumor cells of hematologic malignancies can also affect the infiltration of immune cells. For example, in diffuse large B cell lymphoma (DLBCL), mutation or knockdown of lysine methyltransferase 2D (KMT2D) in tumor cells indirectly activated Notch signaling (increased NICD protein), boosted the expression of downstream molecules (e.g., MYC and TGF-β1), and accelerated tumor progression by recruiting Treg cells [145]. Also in DLBCL, another group found that mutations in histone acetylation-related molecules [CREB binding protein (CREBBP) or E1A binding protein p300 (EP300)] in tumor cells contributed to tumor progression through indirectly upregulate Notch signaling [146]. Mechanistically, CREBBP or EP300 mutations indirectly activate Notch signaling (increased NICD protein, HEY1,and HEY2 mRNA) and downstream CCL2 − colony-stimulating factor 1(CSF1) in tumor cells, altering macrophage polarization into M2-TAMs and accelerating tumor progression[146] (Table 2). Based on these findings, we conclude that the abnormally expression of Notch receptors and their downstream signaling molecules in tumor cells affects tumor progression partially by regulating immune cells infiltration. Meanwhile, the specific regulatory mechanism of Notch signaling in tumor cell is complex and context-dependent (Table 3).
Dysregulated Notch signaling in stromal cells affects immune cell function in the TME
As well as tumor cells, stromal cells in the TME can also regulate immune cell infiltration and function through Notch signaling. In the TME of KRASG12D-driven CRC, Jackstadt et al. found that epithelial Notch1 signaling was critical in disease subtypes with the poorest prognoses and liver metastasis of CRC [147]. Mechanistically, activation of Notch1 in epithelial cells promoted the secretion of TGF-β into the TME, increased the recruitment of TGF-β-dependent neutrophils, and inhibited the anti-tumor function of CD8+ T cells [147]. In contrast, recruitment of neutrophils was significantly inhibited by 1D11 (a ligand-trapping antibody targeting TGF-β1/2/3) and then suppressed CRC tumor liver metastasis [147] (Table 2). As the author demonstrated that epithelial Notch1 signaling was critical for the secretion of TGF-β [147], we speculate that blocking Notch1 signaling of intestinal epithelial cells might be a potential strategy for inhibiting CRC metastasis. It could therefore suggest a clinical treatment for liver metastasis in CRC.
Potential role of Notch signaling in tumor immunity mediated by gut microbiota
Gut microbiota (GM) (e.g., bacteriophages, viruses, bacteria, helminths, and fungi) are microorganisms in the gastrointestinal tract of humans or mammals, with bacteria accounting for more than 99% of the species [148]. Gut microbiota can directly or indirectly regulate immune cells to affect tumor progression [149,150,151]. For example, gut microbes or their metabolites can modulate the responses of immune cells (e.g., ILC3s, Th1 cells, and CD8+ T cells) to control CRC progression [152,153,154]. In an MHCIIΔILC3 murine CRC model, it was demonstrated that MHC II+ ILC3s supported the colonization of gut microbiota that boosted the anti-tumor properties of Th1 and T-bet+ CD8+ T cells [152]. The colonized microbes also regulated the differentiation and activation of Treg cells, Th1 cells, and Th17 cells to control intestinal disease (e.g., cancer, autoimmune diseases) [153, 155,156,157]. Metabolites of gut microbiota (e.g., butyric acid, pentanoate, and butyrate) also induced Treg cell differentiation [158], increased the secretion of anti-tumor cytokines (e.g., IFN-γ and TNF-α) by CD8+ T cells, and enhanced the anti-tumor responses of antigen-specific CTLs and CAR-T cells [159]. In collaboration with the Wang lab, we found that feeding black raspberries, a natural product, significantly induced distinct changes in murine gut microbiota, increased the abundance of anti-inflammatory microbial species (e.g., Akkermansia and Desulfovibrio), activated anti-tumor immune cells (e.g., NK cells), and enhanced those cells’ anti-tumor immune responses [160,161,162,163]. Conversely, dysbiosis of gut microbiota in mice increased susceptibility to colon tumors because it overstimulated CD8+ T cells, which in turn promoted chronic inflammation and early T cell exhaustion, thereby reducing the cells’ anti-tumor immune response [154].
Gut microbiota have profound effects on host physiology through classical signaling (e.g., Notch signaling, WNT signaling, and PI3K − Akt signaling) [164]. In recent years, interactions between host microbiota and Notch signaling have also been revealed. Roy et al. [165] found that controlling the hyperactivation of Notch signaling was important for preventing intestinal inflammation mediated by Citrobacter rodentium in humans and mice. The activity of microbiota, determined by innate immune signaling, correlated with activation of Notch signaling in the intestinal epithelium, suggesting that Notch signaling played a role in maintaining gut homeostasis and that its dysregulation would lead to chronic inflammation or cancer [166]. Inhibition of Notch1 activation by indoleamine 2,3-dioxygenase-1 (IDO1) in mice significantly increased both the thickness of the intestinal mucus layer and the proportion of intestinal Akkermansia muciniphila and Mucispirillum schaedleri. Additionally, mice that received IDO1 to inhibit Notch1 activation had 85% fewer ileal bacteria after a challenge with enteropathogenic E. coli compared with control mice [167]. In other studies, NKp46+ ILC3s played a positive role in controlling tumor progression [168, 169], and Notch signaling activation proved crucial for sustaining AhR-mediated production of NKp46+ ILC3s in the lamina propria [75]. From the above study, we speculate that Notch signaling plays a key role in mediating the anti-tumor effects of gut microbiota on NKp46+ ILC3s, but the specific regulatory mechanism requires further in-depth study. In general, we speculate that Notch signaling could have a regulatory role in tumor immunity mediated by gut microbiota, such as by boosting NKp46+ ILC3 numbers. Therefore, a deeper understanding of the potential functional interactions of Notch signaling-mediated immune cells with gut microbes may provide new strategies for developing innovative immunotherapies against cancer.
Strategies for targeting Notch signaling in cancer immunotherapy
In the above discussion, we concluded that Notch signaling, including ligands (e.g., Jagged1, Dll1, and Dll4), receptors (e.g., Notch1, Notch2), and downstream utility molecules (e.g., RBP-J, Hes1), is directly involved in the regulation of immune cells’ anti- or pro-tumor immune responses in various ways. At the same time, abnormal expression of Notch signaling in tumor cells or stromal cells can regulate immune cell infiltration, resulting in an immunosuppressive TME and accelerated tumor progression.
Targeting Notch signaling for cancer immunotherapy could be achieved by: (1) combining oncolytic virotherapy with inhibition of Notch signaling to efficiently inhibit the proliferation and other properties of immunosuppressive cells (e.g., MDSCs); (2) customizing the delivery of Notch activators into TAMs via nanoparticles to promote the M1 polarization of TAMs and activate CD8+ T cells and ultimately remodel the TME; (3) combining Notch drugs with ICBs to synergistically enhance anti-tumor immunotherapy; (4) customizing the synNotch circuit into CAR cells (e.g., CAR-T cells, CAR-NK cells, or CAR-Macrophages) to enhance the precision of CAR immune cell therapy. Below, we discuss these potential strategies in more detail.
Oncolytic virotherapy combined with inhibition of Notch signaling
Oncolytic virotherapy, an emerging cancer immunotherapy, has received extensive attention in recent years [170]. One of the most widely investigated oncolytic viruses is oHSV, as it efficiently lyses tumor cells while leaving normal cells unscathed. In 2015, the US Food and Drug Administration (FDA) approved the first oncolytic HSV—oHSV-talimogene laherparepvec (T-VEC)—for treating melanoma patients (Clinical Trial.gov identifier: NCT02173171) [171]. Now, oHSV is used to treat glioblastoma (GBM) [93], melanoma [171], breast cancer [172], and ovarian cancer [92]. One study from our group showed that customized oHSV had significant efficacy against GBM in pre-clinical mouse models [173]. Our customized OV-CDH1 oncolytic virus was able to spread into tumors and lyse tumor cells more effectively than control oHSV. It also selectively prevented KLRG1+ NK cells from lysing OV-CDH1-infected tumor cells, improving the efficacy of cancer virotherapy [173]. In two recent studies, we customized an oHSV to express a full-length anti-CD47-IgG1 antibody [92, 93]. After that OV-αCD47 infected murine GBM or ovarian tumor in vivo, it lysed tumor cells, released αCD47 into the TME, and induced antibody-dependent cellular cytotoxicity (ADCC) of NK cells and antibody-dependent cellular phagocytosis (ADCP) of macrophages, thus cooperatively enhancing the therapeutic efficacy of cancer virotherapy [93].
In the TME of GBM, oHSV infection abnormally activates Notch signaling, causing TAMs to secrete large amounts of cytokines (e.g., CCL2, IL-10). It then recruits MDSCs to inhibit the therapeutic effect of oncolytic virotherapy [98]. Adding GSI, a pharmacological blocker of Notch signaling, rescued the oHSV-induced immunosuppressive TME and activated CD8+ T cell-dependent anti-tumor memory responses, resulting in therapeutic benefits [98]. Therefore, by combining our previously developed oncolytic viruses (e.g., OV-αCD47) with gene sequences encoding antibodies (e.g., αNotch1, αDll1) that block Notch signaling or by combining DAPT, GSI, or other inhibitors of Notch signaling with OVs, we can inhibit cytokine (e.g., CCL2) secretion of TAMs and the recruitment of MDSCs, reactivating CD8+ T cells for cancer immunotherapy. These combination therapies have important implications for the clinical treatment of solid tumors (e.g., glioma) that respond positively to inhibition of Notch signaling (Fig. 4A).
Strategies for targeting Notch signaling in cancer immunotherapy. A: Oncolytic virotherapy combined with Notch signaling inhibition. αCD47 can be incorporated into OVs along with gene sequences of antibodies that inhibition of Notch signaling (e.g., αNotch1, αDll1). Also, combination of DAPT, GSI, or other inhibitors of Notch signaling with OVs inhibits the CCL2 secretion of TAMs and the recruitment of MDSCs, and reactivation of CD8+ T cells; B: Specific delivery of Notch signaling activators into TAMs. Dll1 ligand and Notch1 overexpression plasmid are packaged into mannose-NPs to specifically target TAMs, promoting the polarization of TAMs to M1-TAMs and activating CD8+ T cells; C: ICBs combined with drugs targeting Notch signaling. D: The synNotch circuit system can be incorporated into CAR immune cells for immunotherapy. Several types of CAR cells can be used to increase anti-tumor capacity. They include synNotch CAR-T cells, synNotch CAR-NK cells, or synNotch CAR-M cells that recognize multiple antigens. These synNotch strategies can enhance the specificity of CAR cells and improve their anti-tumor functions. Figure was created with BioRender.com
Encapsulating drugs that target Notch signaling into nanoparticles and specifically delivering them to TAMs in the TME
Targeted delivery of drugs into specific immune cells or the TME to transform “cold tumor” into “hot tumor” is an emerging and promising strategy for cancer immunotherapy [174]. In recent years, nanoparticles (NPs) have shown great clinical potential in drug delivery systems, as they can accurately and effectively deliver many types of drugs (e.g., oligonucleotides, siRNAs, or protein-based drugs) into TAMs of the TME. For example, siRNAs that modulate NF-κB signaling [175] and VEGF signaling [85] can be payloaded into polymeric NPs; anti-CSF-1R siRNA can be incorporated into lipid-based NPs [176]; and cytosine-phosphate-guanine (CpG) [Toll-like receptor 9 (TLR9) agonist] can be payloaded into carbon NPs [177]. These NPs have been successfully delivered into TAMs of the TME, affecting the cells’ functionality.
Our group and others have found that activating Notch signaling in TAMs of the TME (e.g., in murine lung cancer) promotes TAM polarization into proinflammatory M1-TAMs, thereby increasing the infiltration of CD8+ T cells, further inhibiting tumor progression [88, 91]. By integrating Notch-activating ligands (e.g., Dll1) and/or Notch1 overexpression plasmid into mannose-NPs, Notch signaling can be activated in TAMs but not in other cells. Thus, TAMs can be polarized into M1-TAMs, fulfilling the goal of remodeling the TME to improve tumor immunotherapy (Fig. 4B).
Combining ICBs with drugs that target Notch signaling
ICs are key regulators of immune system suppression [178]. ICBs (e.g., αCTLA-4, αPD-1/αPDL-1) can block inhibitory checkpoints, thereby unleashing suppressed anti-tumor immune responses [179]. In recent years, ICB-based immunotherapy, including αPD-1/αPD-L1 and αCTLA-4, has significantly improved the survival rates of patients with metastatic solid tumors, especially melanoma and lung cancer [180, 181]. Also, ICBs have correlated significantly with Notch signaling changes (activation or inhibition) in various tumors. Activation of Notch signaling in human neuroendocrine (NE) SCLC cell lines induced low NE differentiation and increased intrinsic tumor immunity [182]. Activation of Notch signaling was found to be an important predictor of the clinical benefit of ICB used in two relapsed SCLC cohorts [182]. In CRC, Notch signaling mutations in tumor cells were associated with the enrichment of cytotoxicity-related molecules (e.g., GZMB and PRF1) but also exhaustion-related molecules (e.g., PD-1) [183]. We found that, in some tumors (e.g., TNBC), ICBs combined with GSI inhibited tumor progression [134]. In other tumors (e.g., HNSCC, GC), high Notch expression promoted the expression of ICs, indicating that ICBs may have better therapeutic effects in these cancer patients with high Notch expression compared to those with low expression [19, 140, 146].
In summary, activation or inhibition of Notch signaling in tumor cells can affect the expression of ICs, thus likely modulating the therapeutic effect of ICBs. However, a previous study found that, in anti-tumor T cells, activation of Notch signaling enhanced the cells’ cytotoxicity but could also promote the expression of PD-1, potentially promoting T cell exhaustion. Therefore, we should adopt different synergistic therapeutic strategies in different contexts: (i) For a TME in which inhibition of Notch signaling enhances IC expression (e.g., PD-1), we could combine clinically used inhibitors that target Notch signaling (e.g., γ-secretase inhibitors, ADAM inhibitors) with ICBs (e.g., αPD-1/αPDL-1) to obtain synergistic anti-tumor effects; (ii) For a TME in which activation of Notch signaling enhances the expression of ICs (e.g., PD-1), we could develop NPs loaded with a Notch signaling activator [e.g., NICD, Dll1, Dll3, Dll4, or Notch homolog 1-translocation-associated (Notch1 TFA)] specifically into target cells (e.g., T cells). Combining these NPs with ICBs (e.g., αPD-1/αPDL-1) would produce additive or synergistic anti-tumor activity (Fig. 4C).
Developing a synNotch circuit for CAR immune cell therapy
CAR (chimeric antigen receptor) protein is a synthetic cell surface receptor that confers immune cells (e.g., T cells, NK cells, and macrophages) with specific anti-tumor properties that can target corresponding antigenic proteins [184]. CAR-T cells have achieved unprecedented success with some hematological malignancies, and a couple of products have already been approved by the US FDA [185, 186]. Other CAR immune cells [including CAR-NK cells, CAR-NKT, CAR-macrophage (CAR-M), and CAR-γδT] have been approved for or are in clinical trials, as documented in our recent review of CAR immune cells’ great potential for improving cancer immunotherapy [184]. Because allogenic CAR-NK cells are efficacious against tumor cells but do not produce cytokine storms or graft-versus-host disease (GVHD) [187], they are being developed into ‘off the-shelf’ drugs for immunotherapy. Using animal models, we obtained a significant anti-tumor effect when we recently used ‘off-the-shelf’ human EGFR-CAR-NK cells and human PSCA-CAR-s15NK cells to treat solid tumors (e.g., glioma and pancreatic cancer) [188, 189]. Also, CAR-M cells have attracted great interest as potential immunotherapies in recent years. Researchers have found that modifying human macrophages with specific CARs can improve the presentation of tumor antigens (especially those on solid tumors) and increase macrophages’ phagocytic activity. These CAR immune cell-mediated tumor therapies have produced good results or shown great potential in the majority of hematological tumors and some non-homogeneous solid tumors. However, very few antigens are truly tumor-specific, and thus, conventional CAR-T cell therapies often cause lethal toxicities such as on-target, off-tumor cross-reaction of CAR cells with normal tissues; they also have poor specificity [126, 190,191,192]. In fact, the majority of tumor antigens are often expressed heterogeneously, and treatment with conventional CAR cells allows antigen-negative tumor cells to escape immune surveillance [193]. Therefore, there is an urgent need to develop a new tumor recognition system of CAR cells—one that can recognize tumor cells carrying multiple antigens—to deal with tumor heterogeneity and thereby increase the therapeutic effectiveness of CAR immune cells against solid cancers.
Recently, the synNotch system, developed by the Lim group at the new frontier of cancer research, was launched. This system can accurately teach T cells (especially CAR-T cells) to recognize two or three antigens of solid tumors (e.g., mesothelioma, ovarian tumor, or glioblastoma) [122]. In the section—“SynNotch can be used as a tool to increase T cell cytotoxicity and specificity”, we explained how the synNotch system enhances anti-tumor specificity mediated by T cells (mainly CAR-T cells), especially in solid tumors. SynNotch circuits allow CAR-T cells to integrate extracellular and intracellular antigen recognition signals and accurately identify and kill tumor cells, as they use positive or negative logic to combine two or multiple different antigens [122, 126,127,128]. Important future developments will likely include: (i) synNotch circuits that can simultaneously recognize multiple different antigens on tumor cells by CAR-T cells to precisely kill highly heterogeneous solid tumors; (ii) synNotch circuits that can simultaneously recognize multiple antigens on tumor cells by CAR-NK or CAR-M cells; (iii) CAR immune cells can persist in vivo and kill solid tumors by more accurately dissolving or swallowing tumor cells (Fig. 4D).
Conclusions and perspectives
In this review, we summarized recent advances in understanding the mechanism of Notch signaling in immune cells and its roles in immune responses. It is important to acknowledge, however, that this area of investigation is complex, and much is still to be learned. More comprehensive understanding of the biological function of Notch signaling in immune responses should facilitate the development of Notch targets for more precise tumor immunotherapy.
We also proposed rational strategies for ameliorating cancer immunotherapy based on targeting Notch signaling, including the development of: (i) Notch inhibitors packaged into oncolytic viruses and released into the TME, where they effectively inhibit the recruitment of immunosuppressive cells such as MDSCs; (ii) targeted delivery of Notch activators into TAMs via NPs to re-educate TAMs and reprogram them to the M1 phenotype to ultimately remodel the TME; (iii) combinations of Notch drugs with ICBs to synergistically enhance the effects of anti-tumor immunotherapy, as ICB therapy has been a breakthrough in cancer treatment. As noted above, Notch signaling not only alters IC expression patterns in multiple cancers but also modulates ICB efficacy in some preclinical animal models. At present, drugs targeting Notch signaling are in clinical trials for several solid tumors, but there are few studies on combining ICBs with drugs that affect Notch signaling. Based on previous investigations, we speculate that such combinations could be tested in clinical trials for their synergistic anti-tumor effects in humans; (iv) transformation of CAR immune cells with the synNotch circuit to enhance synergistic therapeutic effects and CAR cell safety.
However, the therapeutic interventions of Notch signaling are challenging because the undesired “on-target, off-tumor” activity may potentially lead to significant toxicity. Also, non-specific intervention of Notch signaling can have the opposite effect on controlling tumor development because of targeting both tumor cells and immune cells. For example, the intervention to inhibit tumor cells may also suppress immune responses to tumor cells. Thus, a deeper understanding of Notch signaling in different cell types and the interactions between the Notch signaling pathway and other pathways may contribute to the development of more innovative and precise targeted therapeutics that will provide better clinical outcomes in cancer patients.
Availability of data and materials
This is a review article that does not have original data.
Abbreviations
- TME:
-
Tumor microenvironment
- NPs:
-
Nanoparticles
- TAMs:
-
Tumor-associated macrophages
- ICBs:
-
Immune checkpoint blockers
- CAR:
-
Chimeric antigen receptor
- MDSCs:
-
Myeloid-derived suppressor cells
- DCs:
-
Dendritic cells
- synNotch:
-
Synthetic Notch
- PD-1:
-
Programmed cell death protein 1
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen-4
- IL-2:
-
Interleukin 2
- IL-12:
-
Interleukin 12
- ER:
-
Endoplasmic reticulum
- NECD:
-
Extracellular domain of Notch receptors
- NICD:
-
Intracellular domain of Notch receptors
- RBP-J:
-
Recombination signal binding protein for immunoglobulin kappa (к) J region
- MAML:
-
Mastermind-like
- pre-Notch receptors:
-
precursors of Notch receptors
- SERCA:
-
Sarcoendoplasmic reticulum Ca2+-ATPase
- CRC:
-
Colorectal cancer
- PCa:
-
Prostate cancer
- ESCC:
-
Esophageal squamous cell carcinoma
- mAb:
-
Monoclonal antibody
- ACC:
-
Adenoid cystic carcinoma
- PR:
-
Partial response
- SCLC:
-
Small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- ORR:
-
Objective response rate
- NSCLC:
-
Non-small cell lung cancer
- ADAM:
-
A disintegrin and metalloprotease
- TM:
-
Transmembrane
- HCC:
-
Hepatocellular carcinoma
- GSIs:
-
γ-Secretase inhibitors
- BC:
-
Breast cancer
- TNBC:
-
Triple-negative breast cancer
- HNSCC:
-
Head and neck squamous cell carcinoma
- EAC:
-
Esophageal adenocarcinoma
- NK cells:
-
Natural killer cells
- UCB:
-
Umbilical cord blood
- IL-7:
-
Interleukin 7
- Flt3:
-
Fms-like tyrosine kinase 3
- IL-15:
-
Interleukin 15
- GZMB:
-
Granzyme B
- IFN-γ:
-
Interferon-gamma
- KIRs:
-
Killer Ig-like receptors
- ILCs:
-
Innate lymphoid cells
- Tbx21:
-
T-box transcription factor 21
- ILC1s:
-
Group 1 ILCs
- ILC2s:
-
Group 2 ILCs
- ILC3s:
-
RORγt-expressing ILCs
- LTi cells:
-
Lymphoid tissue-inducer cells
- BM:
-
Bone marrow
- CLPs:
-
Common lymphoid progenitors
- AhR:
-
Aryl hydrocarbon receptor
- SI:
-
Small intestine
- HPC-1:
-
Hematopoietic progenitor cell subpopulation one
- M1-TAMs:
-
M1-like tumor-associated macrophages
- M2-TAMs:
-
M2-like tumor-associated macrophages
- Socs3:
-
Suppressor of cytokine 3
- SIRPα:
-
Signal regulatory protein α
- oHSV:
-
Type 1 herpes simplex virus-based oncolytic virus
- αCD47:
-
Anti-CD47 antibody
- kclTAMs:
-
Kupffer cell-like TAMs
- moTAMs:
-
Monocyte-derived TAMs
- IL-10:
-
Interleukin 10
- MDSCs:
-
Myeloid-derived suppressor cells
- PMN-MDSCs:
-
Polymorphonuclear-MDSCs
- M-MDSCs:
-
Mononuclear MDSCs
- STAT3:
-
Signal transducer and activator of transcription 3
- GSEA:
-
Cybersort and Gene Set Enrichment Analysis
- MCT2:
-
Lactate import 2
- APCs:
-
Antigen-presenting cells
- CCR7:
-
CC-chemokine receptor 7
- cDCs:
-
Conventional DCs
- LBP:
-
Lycium barbarum polysaccharide
- CTLs:
-
Cytotoxic T lymphocytes
- TCIM:
-
Transcriptional and immune response regulator
- EZH2:
-
Enhancer of zeste homolog 2
- TNF-α:
-
Tumor necrosis factor alpha-like
- IL-1β:
-
Interleukin-1 beta
- CLL:
-
Chronic lymphocytic leukemia
- ALL:
-
Acute lymphoblastic leukemia
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- ICs:
-
Immune checkpoints
- ROR1:
-
Receptor tyrosine kinase-like orphan receptor 1
- EpCAM:
-
Epithelial cell adhesion molecule
- EGFRvIII:
-
Epidermal growth factor receptor splice variant III
- EphA2:
-
EPH receptor A2
- ALPPL2:
-
Alkaline phosphatase placental-like 2
- MCAM:
-
Melanoma cell adhesion molecule
- HER2:
-
Epidermal growth factor receptor 2
- GD2:
-
Disialoganglioside
- ECM:
-
Extracellular matrix
- PDAC:
-
Pancreatic ductal adenocarcinoma
- TANs:
-
Tumor-associated neutrophils
- EOC:
-
Epithelial ovarian cancer
- USP9x:
-
Ubiquitin-specific peptidase 9x-linked
- IL-1β:
-
Interleukin-1 beta
- CXCL9:
-
C-X-C motif chemokine ligand 9
- GC:
-
Gastric cancer
- ADORA2A:
-
Adenosine A2a receptor
- DLBCL:
-
Diffuse large B cell lymphoma
- KMT2D:
-
Lysine methyltransferase 2D
- CREBBP:
-
CREB binding protein
- EP300:
-
E1A binding protein p300
- CSF1:
-
Colony-stimulating factor 1
- GM:
-
Gut microbiota
- IDO1:
-
Indoleamine 2,3-dioxygenase-1
- FDA:
-
Food and drug administration
- T-VEC:
-
OHSV-talimogene laherparepvec
- GBM:
-
Glioblastoma
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- ADCP:
-
Antibody-dependent cellular phagocytosis
- CpG:
-
Cytosine-phosphate-guanine
- TLR9:
-
Toll-like receptor 9
- NE:
-
Neuroendocrine
- CAR-M:
-
CAR-macrophages
- GVHD:
-
Graft-versus-host disease
References
Bolós V, Blanco M, Medina V, Aparicio G, Díaz-Prado S, Grande E. Notch signalling in cancer stem cells. Clin Transl Oncol. 2009;11(1):11–9.
Rehman AO, Wang CY. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 2006;16(6):293–300.
Hu YY, Zheng Mh, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Notch Signaling in Embryology and Cancer. 2012; pp186–198.
Li L, Tang P, Li S, Qin X, Yang H, Wu C, et al. Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy. Med Oncol. 2017;34(10):1–10.
Meurette O, Mehlen P. Notch signaling in the tumor microenvironment. Cancer Cell. 2018;34(4):536–48.
Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, et al. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7(1):95.
Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell. 2016; 167(2):419–32. e416.
Tsukumo Si, Yasutomo K. Regulation of CD8+ T cells and antitumor immunity by Notch signaling. Front Immunol. 2018; 9:101.
Feng F, Wang YC, Hu XB, Liu XW, Ji G, Chen YR, et al. The transcription factor RBP-J-mediated signaling is essential for dendritic cells to evoke efficient anti-tumor immune responses in mice. Mol Cancer. 2010;9(1):90.
Palaga T, Wongchana W, Kueanjinda P. Notch signaling in macrophages in the context of cancer immunity. Front Immunol. 2018;9:652.
Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–33.
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369(1):20–7.
Kovall RA, Gebelein B, Sprinzak D, Kopan R. The canonical Notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell. 2017;41(3):228–41.
Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34(7):1420–30.
Majumder S, Crabtree JS, Golde TE, Minter LM, Osborne BA, Miele L. Targeting Notch in oncology: the path forward. Nat Rev Drug Discov. 2021;20(2):125–44.
Nolin E, Gans S, Llamas L, Bandyopadhyay S, Brittain SM, Bernasconi-Elias P, et al. Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat Chem Biol. 2019;15(2):179–88.
Sierra RA, Trillo-Tinoco J, Mohamed E, Yu L, Achyut BR, Arbab A, et al. Anti-jagged immunotherapy inhibits MDSCs and overcomes tumor-induced tolerance. Cancer Res. 2017;77(20):5628–38.
Zhang Y, Li D, Jiang Q, Cao S, Sun H, Chai Y, et al. Novel ADAM-17 inhibitor ZLDI-8 enhances the in vitro and in vivo chemotherapeutic effects of Sorafenib on hepatocellular carcinoma cells. Cell Death Dis. 2018;9(7):743.
Mao L, Zhao ZL, Yu GT, Wu L, Deng WW, Li YC, et al. γ-Secretase inhibitor reduces immunosuppressive cells and enhances tumour immunity in head and neck squamous cell carcinoma. Int J Cancer. 2018;142(5):999–1009.
Moellering RE, Cornejo M, Davis TN, Bianco CD, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462(7270):182–8.
Astudillo L, Da Silva TG, Wang Z, Han X, Jin K, VanWye J, et al. The small molecule IMR-1 inhibits the notch transcriptional activation complex to suppress tumorigenesis. Cancer Res. 2016;76(12):3593–603.
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, et al. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7(1):87.
Lu Z, Ren Y, Zhang M, Fan T, Wang Y, Zhao Q, et al. FLI-06 suppresses proliferation, induces apoptosis and cell cycle arrest by targeting LSD1 and Notch pathway in esophageal squamous cell carcinoma cells. Biomed Pharmacother. 2018;107:1370–6.
Roti G, Carlton A, Ross KN, Markstein M, Pajcini K, Su AH, et al. Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell. 2013;23(3):390–405.
Hara T, Yoshigai E, Ohashi T, Fukada T. Zinc transporters as potential therapeutic targets: an updated review. J Pharmacol Sci. 2022;148(2):221–8.
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–9.
Howells LM, Iwuji CO, Irving GR, Barber S, Walter H, Sidat Z, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149(7):1133–9.
Irving GR, Iwuji CO, Morgan B, Berry DP, Steward WP, Thomas A, et al. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): study protocol for a randomised control trial. Trials. 2015;16:110.
Casulo C, Ruan J, Dang NH, Gore L, Diefenbach C, Beaven AW, et al. Safety and preliminary efficacy results of a phase I first-in-human study of the novel Notch-1 targeting antibody brontictuzumab (OMP-52M51) administered intravenously to patients with hematologic malignancies. Blood. 2016;128(22):5108.
Ferrarotto R, Eckhardt G, Patnaik A, LoRusso P, Faoro L, Heymach J, et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. 2018;29(7):1561–8.
Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol. 2017;35(3):352–60.
Smith DC, Chugh R, Patnaik A, Papadopoulos KP, Wang M, Kapoun AM, et al. A phase 1 dose escalation and expansion study of Tarextumab (OMP-59R5) in patients with solid tumors. Invest New Drugs. 2019;37(4):722–30.
Hu ZI, Bendell JC, Bullock A, LoConte NK, Hatoum H, Ritch P, et al. A randomized phase II trial of nab‐paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019;8(11):5148–57.
Goldman JW, Barve M, Patel JD, Wozniak A, Dowlati A, Starodub A, et al. Effects of rovalpituzumab tesirine on ventricular repolarization in patients with small-cell lung cancer. Clin Transl Sci. 2021;14(2):664–70.
Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25(23):6958–66.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51.
Hann CL, Burns TF, Dowlati A, Morgensztern D, Ward PJ, Koch MM, et al. A phase 1 study evaluating rovalpituzumab tesirine in frontline treatment of patients with extensive-stage SCLC. J Thorac Oncol. 2021;16(9):1582–8.
Malhotra J, Nikolinakos P, Leal T, Lehman J, Morgensztern D, Patel JD, et al. A phase 1–2 study of rovalpituzumab tesirine in combination with nivolumab plus or minus ipilimumab in patients with previously treated extensive-stage SCLC. J Thorac Oncol. 2021;16(9):1559–69.
Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16(9):1547–58.
Xie H, Kaye FJ, Isse K, Sun Y, Ramoth J, French DM, et al. Delta-like protein 3 expression and targeting in Merkel cell carcinoma. Oncologist. 2020;25(9):810–7.
Morgensztern D, Johnson M, Rudin CM, Rossi M, Lazarov M, Brickman D, et al. SC-002 in patients with relapsed or refractory small cell lung cancer and large cell neuroendocrine carcinoma: phase 1 study. Lung Cancer. 2020;145:126–31.
Udagawa H, Akamatsu H, Tanaka K, Takeda M, Kanda S, Kirita K, et al. Phase I safety and pharmacokinetics study of rovalpituzumab tesirine in Japanese patients with advanced, recurrent small cell lung cancer. Lung Cancer. 2019;135:145–50.
Falchook GS, Dowlati A, Naing A, Gribbin MJ, Jenkins DW, Chang LL, et al. Phase I study of MEDI0639 in patients with advanced solid tumors. Proc Am Soc Clin Oncol. 2015;33(15):1.
McKeage MJ, Kotasek D, Markman B, Hidalgo M, Millward MJ, Jameson MB, et al. Phase IB trial of the anti-cancer stem cell DLL4-binding agent demcizumab with pemetrexed and carboplatin as first-line treatment of metastatic non-squamous NSCLC. Targeted Oncol. 2018;13(1):89–98.
Coleman RL, Handley KF, Burger R, Dal Molin GZ, Stagg R, Sood AK, et al. Demcizumab combined with paclitaxel for platinum-resistant ovarian, primary peritoneal, and fallopian tube cancer: The SIERRA open-label phase Ib trial. Gynecol Oncol. 2020;157(2):386–91.
Johnson M, Rasco D, Schneider B, Shu C, Jotte R, Parmer H, et al. A phase 1b, open-label, dose escalation and expansion study of demcizumab plus pembrolizumab in patients with locally advanced or metastatic solid tumors. Mol Cancer Ther. 2018;17:A081.
Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30(19):2307–13.
Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19(6):1512–24.
Cook N, Basu B, Smith DM, Gopinathan A, Evans J, Steward WP, et al. A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br J Cancer. 2018;118(6):793–801.
Gounder MM, Rosenbaum E, Wu N, Dickson MA, Sheikh TN, D’Angelo SP, et al. A phase Ib/II randomized study of RO4929097, a gamma-secretase or Notch inhibitor with or without vismodegib, a hedgehog inhibitor, in advanced sarcoma. Clin Cancer Res. 2022;28(8):1586–94.
Lee SM, Moon J, Redman BG, Chidiac T, Flaherty LE, Zha Y, et al. Phase 2 study of RO 4929097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer. 2015;121(3):432–40.
Diaz-Padilla I, Hirte H, Oza AM, Clarke BA, Cohen B, Reedjik M, et al. A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest New drugs. 2013;31(5):1182–91.
Pant S, Jones SF, Kurkjian CD, Infante JR, Moore KN, Burris HA, et al. A first-in-human phase I study of the oral Notch inhibitor, LY900009, in patients with advanced cancer. Eur J Cancer. 2016;56:1–9.
Massard C, Cassier P, Azaro A, Anderson B, Yuen E, Yu D, et al. A phase 1b study of crenigacestat (LY3039478) in combination with gemcitabine and cisplatin or gemcitabine and carboplatin in patients with advanced or metastatic solid tumors. Cancer Chemother Pharmacol. 2022;90(4):335–44.
Azaro A, Massard C, Tap WD, Cassier PA, Merchan J, Italiano A, et al. A phase 1b study of the Notch inhibitor crenigacestat (LY3039478) in combination with other anticancer target agents (taladegib, LY3023414, or abemaciclib) in patients with advanced or metastatic solid tumors. Invest New Drugs. 2021;39(4):1089–98.
Doi T, Tajimi M, Mori J, Asou H, Inoue K, Benhadji KA, et al. A phase 1 study of crenigacestat (LY3039478), the Notch inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2021;39(2):469–76.
Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, et al. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–6.
Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, et al. RBPjκ-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137(9):1461–71.
Keerthivasan S, Suleiman R, Lawlor R, Roderick J, Bates T, Minter L, et al. Notch signaling regulates mouse and human Th17 differentiation. J Immunol. 2011;187(2):692–701.
Miyamoto S, Rosenberg DW. Role of Notch signaling in colon homeostasis and carcinogenesis. Cancer Sci. 2011;102(11):1938–42.
Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–18.
Li X, Ma S, Deng Y, Yi P, Yu J. Targeting the RNA m6A modification for cancer immunotherapy. Mol Cancer. 2022;21(1):76.
Beck RC, Padival M, Yeh D, Ralston J, Cooke KR, Lowe JB. The Notch ligands Jagged2, Delta1, and Delta4 induce differentiation and expansion of functional human NK cells from CD34+ cord blood hematopoietic progenitor cells. Biol Blood Marrow Transplant. 2009;15(9):1026–37.
DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105(9):3521–7.
Manaster I, Gazit R, Goldman-Wohl D, Stern-Ginossar N, Mizrahi S, Yagel S, et al. Notch activation enhances IFNγ secretion by human peripheral blood and decidual NK cells. J Reprod Immunol. 2010;84(1):1–7.
Felices M, Ankarlo DE, Lenvik TR, Nelson HH, Blazar BR, Verneris MR, et al. Notch signaling at later stages of NK cell development enhances KIR expression and functional maturation. J Immunol. 2014;193(7):3344–54.
Zakiryanova GK, Kustova E, Urazalieva NT, Baimukhametov ET, Makarov VA, Turaly GM, et al. Notch signaling defects in NK cells in patients with cancer. Cancer Immunol Immunother. 2021;70(4):981–8.
Kijima M, Yamaguchi T, Ishifune C, Maekawa Y, Koyanagi A, Yagita H, et al. Dendritic cell-mediated NK cell activation is controlled by Jagged2–Notch interaction. Proc Natl Acad Sci USA. 2008;105(19):7010–5.
Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301.
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–66.
Vallentin B, Barlogis V, Piperoglou C, Cypowyj S, Zucchini N, Chéné M, et al. Innate lymphoid cells in cancer. Cancer Immunol Res. 2015;3(10):1109–14.
Kim CH, Hashimoto-Hill S, Kim M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol. 2016;37(1):68–79.
Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20(9):552–65.
Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, et al. Notch signaling is necessary for adult, but not fetal, development of RORγt+ innate lymphoid cells. Nat Immunol. 2011;12(10):949–58.
Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144–51.
Rankin LC, Groom JR, Chopin M, Herold MJ, Walker JA, Mielke LA, et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat Immunol. 2013;14(4):389–95.
Chea S, Perchet T, Petit M, Verrier T, Guy-Grand D, Banchi EG, et al. Notch signaling in group 3 innate lymphoid cells modulates their plasticity. Sci Signal. 2016;9(426):ra45.
Kyoizumi S, Kubo Y, Kajimura J, Yoshida K, Hayashi T, Nakachi K, et al. Fate decision between group 3 innate lymphoid and conventional NK cell lineages by notch signaling in human circulating hematopoietic progenitors. J Immunol. 2017;199(8):2777–93.
Li Z, Ma R, Ma S, Tian L, Lu T, Zhang J, et al. ILC1s control leukemia stem cell fate and limit development of AML. Nat Immunol. 2022;23(5):718–30.
Caligiuri M, Li Z, Ma R, Tang H, Zhang J, Marcucci G, et al. Human ILC1s target leukemia stem cells and control development of AML. 2023.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61.
Caux C, Ramos RN, Prendergast GC, Bendriss-Vermare N, Ménétrier-Caux C. A milestone review on how macrophages affect tumor growth. Cancer Res. 2016;76(22):6439–42.
Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10(11):2156–70.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):1–13.
Song Y, Tang C, Yin C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials. 2018;185:117–32.
Shrivastava R, Asif M, Singh V, Dubey P, Malik SA, Tewari BN, et al. M2 polarization of macrophages by Oncostatin M in hypoxic tumor microenvironment is mediated by mTORC2 and promotes tumor growth and metastasis. Cytokine. 2019;118:130–43.
Ma S, Sun B, Duan S, Han J, Barr T, Zhang J, et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nat Immunol. 2023;23(2):255–66.
Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70(12):4840–9.
Zhao JL, Huang F, He F, Gao CC, Liang SQ, Ma PF, et al. Forced activation of notch in macrophages represses tumor growth by upregulating miR-125a and disabling tumor-associated macrophages. Cancer Res. 2016;76(6):1403–15.
Lin Y, Zhao JL, Zheng QJ, Jiang X, Tian J, Liang SQ, et al. Notch signaling modulates macrophage polarization and phagocytosis through direct suppression of signal regulatory protein α expression. Front Immunol. 2018; 9:1744.
Ye YC, Zhao JL, Lu YT, Gao CC, Yang Y, Liang SQ, et al. NOTCH signaling via WNT regulates the proliferation of alternative, CCR2-independent tumor-associated macrophages in hepatocellular carcinoma. Cancer Res. 2019;79(16):4160–72.
Tian L, Xu B, Teng KY, Song M, Zhu Z, Chen Y, et al. Targeting Fc receptor-mediated effects and the “Don’t Eat Me” signal with an oncolytic virus expressing an anti-CD47 antibody to treat metastatic ovarian cancer. Clin Cancer Res. 2022;28(1):201–14.
Xu B, Tian L, Chen J, Wang J, Ma R, Dong W, et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma. Nat Commun. 2021;12(1):5908.
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362.
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7(1):12150.
Kramer ED, Abrams SI. Granulocytic myeloid-derived suppressor cells as negative regulators of anticancer immunity. Front Immunol. 2020;11:1963.
Wang SH, Lu QY, Guo YH, Song YY, Liu PJ, Wang YC. The blockage of Notch signalling promoted the generation of polymorphonuclear myeloid-derived suppressor cells with lower immunosuppression. Eur J Cancer. 2016;68:90–105.
Otani Y, Yoo JY, Lewis CT, Chao S, Swanner J, Shimizu T, et al. NOTCH-induced MDSC recruitment after oHSV virotherapy in CNS cancer models modulates antitumor immunotherapy. Clin Cancer Res. 2022;28(7):1460–73.
Zhao JL, Ye YC, Gao CC, Wang L, Ren KX, Jiang R, et al. Notch-mediated lactate metabolism regulates MDSC development through the Hes1/MCT2/c-Jun axis. Cell Rep. 2022;38(10): 110451.
Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14(11):719–30.
Meng L, Bai Z, He S, Mochizuki K, Liu Y, Purushe J, et al. The Notch ligand DLL4 defines a capability of human dendritic cells in regulating Th1 and Th17 differentiation. J Immunol. 2016;196(3):1070–80.
Meng L, Hu S, Wang J, He S, Zhang Y. DLL4+ dendritic cells: key regulators of Notch Signaling in effector T cell responses. Pharmacol Res. 2016;113:449–57.
Wang L, Yu S, Chan ER, Chen KY, Liu C, Che D, et al. Notch-regulated dendritic cells restrain inflammation-associated colorectal carcinogenesis. Cancer Immunol Res. 2021;9(3):348–61.
Kirkling ME, Cytlak U, Lau CM, Lewis KL, Resteu A, Khodadadi-Jamayran A, et al. Notch signaling facilitates in vitro generation of cross-presenting classical dendritic cells. Cell Rep. 2018;23(12):3658–72.e6.
Wang W, Liu M, Wang Y, Yang T, Li D, Ding F, et al. Lycium barbarum polysaccharide promotes maturation of dendritic cell via notch signaling and strengthens dendritic cell mediated T lymphocyte cytotoxicity on colon cancer cell CT26-WT. Evid Based Complement Alternat Med. 2018;2018:2305683.
den Haan JM, Arens R, van Zelm MC. The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunol Lett. 2014;162(2):103–12.
Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9(10):1140–7.
Sugimoto K, Maekawa Y, Kitamura A, Nishida J, Koyanagi A, Yagita H, et al. Notch2 signaling is required for potent antitumor immunity in vivo. J Immunol. 2010;184(9):4673–8.
Sierra RA, Thevenot P, Raber PL, Cui Y, Parsons C, Ochoa AC, et al. Rescue of Notch-1 signaling in antigen-specific CD8+ T cells overcomes tumor-induced T-cell suppression and enhances immunotherapy in cancer. Cancer Immunol Res. 2014;2(8):800–11.
Thounaojam MC, Dudimah DF, Pellom ST Jr, Uzhachenko RV, Carbone DP, Dikov MM, et al. Bortezomib enhances expression of effector molecules in anti-tumor CD8+ T lymphocytes by promoting Notch-nuclear factor-κB crosstalk. Oncotarget. 2015;6(32):32439–55.
Biktasova AK, Dudimah DF, Uzhachenko RV, Park K, Akhter A, Arasada RR, et al. Multivalent forms of the notch ligand DLL-1 enhance antitumor T-cell immunity in lung cancer and improve efficacy of EGFR-targeted therapy. Cancer Res. 2015;75(22):4728–41.
Dai K, Huang L, Huang Y, Chen Z, Yang L, Jiang Y. 1810011o10 Rik inhibits the antitumor effect of intratumoral CD8+ T cells through suppression of Notch2 pathway in a murine hepatocellular carcinoma model. Front Immunol. 2017; 8:320.
Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17(1):95–103.
Mathieu M, Cotta-Grand N, Daudelin JF, Thébault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8+ T-cell activation. Immunol Cell Biol. 2013;91(1):82–8.
Yu W, Wang Y, Guo P. Notch signaling pathway dampens tumor-infiltrating CD8+ T cells activity in patients with colorectal carcinoma. Biomed Pharmacother. 2018;97:535–42.
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277–89.
Zheng L, Qin S, Si W, Wang A, Xing B, Gao R, et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. 2021; 374(6574):abe6474.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5.
Schubert ML, Schmitt M, Wang L, Ramos C, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34–48.
Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011.
Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021;20(7):531–50.
Williams JZ, Allen GM, Shah D, Sterin IS, Kim KH, Garcia VP, et al. Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science. 2020;370(6520):1099–104.
Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, et al. The B-cell tumor–associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116(22):4532–41.
Balakrishnan A, Goodpaster T, Randolph-Habecker J, Hoffstrom BG, Jalikis FG, Koch LK, et al. Analysis of ROR1 protein expression in human cancer and normal tissues. Clin Cancer Res. 2017;23(12):3061–71.
Srivastava S, Salter AI, Liggitt D, Yechan-Gunja S, Sarvothama M, Cooper K, et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer cell. 2019; 35(3):489–503. e8.
Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. 2021; 13(591):eabe7378.
Hyrenius-Wittsten A, Su Y, Park M, Garcia JM, Alavi J, Perry N, et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci Transl Med. 2021; 13(591):eabd8836.
Moghimi B, Muthugounder S, Jambon S, Tibbetts R, Hung L, Bassiri H, et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun. 2021;12(1):511.
Frankel T, Lanfranca MP, Zou W. The role of tumor microenvironment in cancer immunotherapy. Adv Exp Med Biol. 2017;1036:51–64.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–66.
Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–82.
Liu H, Wang J, Zhang M, Xuan Q, Wang Z, Lian X, et al. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res Treat. 2017;166(1):95–107.
Tao S, Chen Q, Lin C, Dong H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J Exp Clin Cancer Res. 2020;39(1):191.
Meng J, Jiang Yz, Zhao S, Tao Y, Zhang T, Wang X, et al. Tumor-derived Jagged1 promotes cancer progression through immune evasion. Cell Rep. 2022; 38(10):110492.
Geng Y, Fan J, Chen L, Zhang C, Qu C, Qian L, et al. A Notch-dependent inflammatory feedback circuit between macrophages and cancer cells regulates pancreatic cancer metastasis. Cancer Res. 2021;81(1):64–76.
Zhang N, Yin R, Zhou P, Liu X, Fan P, Qian L, et al. DLL1 orchestrates CD8+ T cells to induce long-term vascular normalization and tumor regression. Proc Natl Acad Sci USA. 2021;118(22): e2020057118.
Yuan C, Chang K, Xu C, Li Q, Du Z. High expression of DLL3 is associated with a poor prognosis and immune infiltration in invasive breast cancer patients. Transl Oncol. 2021;14(7): 101080.
Yang M, Zhang G, Wang Y, He M, Xu Q, Lu J, et al. Tumour-associated neutrophils orchestrate intratumoural IL-8-driven immune evasion through Jagged2 activation in ovarian cancer. Br J Cancer. 2020;123(9):1404–16.
Ortiz-Martinez F, Gutierrez-Avino FJ, Sanmartin E, Pomares-Navarro E, Villalba-Riquelme C, Garcia-Martinez A, et al. Association of Notch pathway down-regulation with Triple Negative/Basal-like breast carcinomas and high tumor-infiltrating FOXP3+ Tregs. Exp Mol Pathol. 2016;100(3):460–8.
Yang Z, Qi Y, Lai N, Zhang J, Chen Z, Liu M, et al. Notch1 signaling in melanoma cells promoted tumor-induced immunosuppression via upregulation of TGF-β1. J Exp Clin Cancer Res. 2018;37(1):1–13.
Jaiswal A, Murakami K, Elia A, Shibahara Y, Done SJ, Wood SA, et al. Therapeutic inhibition of USP9x-mediated Notch signaling in triple-negative breast cancer. Proc Natl Acad Sci USA. 2021;118(38): e2101592118.
Parmigiani E, Ivanek R, Rolando C, Hafen K, Turchinovich G, Lehmann FM, et al. Interferon-γ resistance and immune evasion in glioma develop via Notch-regulated co-evolution of malignant and immune cells. Dev Cell. 2022;57(15):1847–65.e9.
Yang L, Zhao KL, Qin L, Ji DX, Zhang B, Zheng PF, et al. Notch signaling pathway regulates CD4+CD25+CD127dim/− regulatory T cells and T helper 17 cells function in gastric cancer patients. Biosci Rep. 2019; 39(5): BSR20182044.
Cui Y, Li Q, Li W, Wang Y, Lv F, Shi X, et al. NOTCH3 is a prognostic factor and is correlated with immune tolerance in gastric cancer. Front Oncol. 2021;10:574937
Liu QX, Zhu Y, Yi HM, Shen YG, Wang L, Cheng S, et al. KMT2D mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-induced regulatory T cell trafficking via FBXW7-NOTCH-MYC/TGF-β1 axis. 2022. https://doi.org/10.21203/rs.3.rs-1520534/v1.
Huang YH, Cai K, Xu PP, Wang L, Huang CX, Fang Y, et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-associated macrophage polarization via FBXW7-NOTCH-CCL2/CSF1 axis. Signal Transduct Target Ther. 2021;6(1):10.
Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36(3):319–36.e7.
Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506.
Sharma P, Jain T, Sethi V, Iyer S, Dudeja V. Gut microbiome: the third musketeer in the cancer-immune system cross-talk. J Pancreatol. 2020;3(4):181–7.
Pope JL, Tomkovich S, Yang Y, Jobin C. Microbiota as a mediator of cancer progression and therapy. Transl Res. 2017;179:139–54.
Goc J, Lv M, Bessman NJ, Flamar AL, Sahota S, Suzuki H, et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell. 2021;184(19):5015–30.e16.
Cheng H, Guan X, Chen D, Ma W. The Th17/Treg cell balance: a gut microbiota-modulated story. Microorganisms. 2019;7(12):583.
Amy IY, Zhao L, Eaton KA, Ho S, Chen J, Poe S, et al. Gut microbiota modulate CD8 T cell responses to influence colitis-associated tumorigenesis. Cell Rep. 2020;31(1): 107471.
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6.
Ivanov II, de Llanos FR, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–49.
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41.
Qiao S, Lian X, Yue M, Zhang Q, Wei Z, Chen L, et al. Regulation of gut microbiota substantially contributes to the induction of intestinal Treg cells and consequent anti-arthritis effect of madecassoside. Int Immunopharmacol. 2020;89: 107047.
Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077.
Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, et al. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr Cancer. 2017;69(6):943–51.
Pan P, Oshima K, Huang YW, Yearsley M, Zhang J, Arnold M, et al. Gut bacteria are required for the benefits of black raspberries in ApcMin/+ mice. J Berry Res. 2018;8(4):239–49.
Huang YW, Lin CW, Pan P, Shan T, Echeveste CE, Mo YY, et al. Black raspberries suppress colorectal cancer by enhancing Smad4 expression in colonic epithelium and natural killer cells. Front Immunol. 2020;11: 570683.
Huang YW, Pan P, Echeveste CE, Wang HT, Oshima K, Lin CW, et al. Transplanting fecal material from wild-type mice fed black raspberries alters the immune system of recipient mice. Food Front. 2020;1(3):253–9.
Kipanyula MJ, Etet PFS, Vecchio L, Farahna M, Nukenine EN, Kamdje AHN. Signaling pathways bridging microbial-triggered inflammation and cancer. Cell Signal. 2013;25(2):403–16.
Roy BC, Ahmed I, Stubbs J, Zhang J, Attard T, Septer S, et al. DCLK1 isoforms and aberrant Notch signaling in the regulation of human and murine colitis. Cell Death Discov. 2021;7(1):169.
Troll JV, Hamilton MK, Abel ML, Ganz J, Bates JM, Stephens WZ, et al. Microbiota promote secretory cell determination in the intestinal epithelium by modulating host Notch signaling. Development. 2018;145(4):dev155317.
Alvarado DM, Chen B, Iticovici M, Thaker AI, Dai N, VanDussen KL, et al. Epithelial indoleamine 2, 3-dioxygenase 1 modulates aryl hydrocarbon receptor and notch signaling to increase differentiation of secretory cells and alter mucus-associated microbiota. Gastroenterology. 2019;157(4):1093-108.e11.
Eisenring M, Vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue–inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11(11):1030–8.
Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, et al. NCR+ ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun. 2015;6(1):8280.
Ma R, Li Z, Chiocca EA, Caligiuri MA, Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. 2023;9(2):122–39.
Greig SL. Talimogene laherparepvec: first global approval. Drugs. 2016;76(1):147–54.
Chen X, Han J, Chu J, Zhang L, Zhang J, Chen C, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7(19):27764–77.
Xu B, Ma R, Russell L, Yoo JY, Han J, Cui H, et al. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Nat Biotechnol. 2019;37(1):45–54.
Rodallec A, Sicard G, Fanciullino R, Benzekry S, Lacarelle B, Milano G, et al. Turning cold tumors into hot tumors: Harnessing the potential of tumor immunity using nanoparticles. Expert Opin Drug Metab Toxicol. 2018;14(11):1139–47.
Ortega RA, Barham W, Sharman K, Tikhomirov O, Giorgio TD, Yull FE. Manipulating the NF-κB pathway in macrophages using mannosylated, siRNA-delivering nanoparticles can induce immunostimulatory and tumor cytotoxic functions. Int J Nanomedicine. 2016;11:2163–77.
Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–49.
Tao Y, Ju E, Ren J, Qu X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials. 2014;35(37):9963–71.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Patel SA, Minn AJ. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48(3):417–33.
Hodi FS, O'day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311.
Roper N, Velez MJ, Chiappori A, Kim YS, Wei JS, Sindiri S, et al. Notch signaling and efficacy of PD-1/PD-L1 blockade in relapsed small cell lung cancer. Nat Commun. 2021;12(1):1–13.
Wang F, Long J, Li L, Zhao Zb, Wei F, Yao Y, et al. Mutations in the notch signalling pathway are associated with enhanced anti‐tumour immunity in colorectal cancer. J Cell Mol Med. 2020;24(20):12176–87.
Ran GH, Lin YQ, Tian L, Zhang T, Yan DM,Yu J, et al. Natural killer cell homing and trafficking in tissues and tumors: from biology to application. Signal Transduct Target Ther. 2022;7(1):205.
RajeN, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380(18):1726–37.
Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–31.
Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13(1):168.
Ma R, Lu T, Li Z, Teng KY, Mansour AG, Yu M, et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 2021;81(13):3635–48.
Teng KY, Mansour AG, Zhu Z, Li Z, Tian L, Ma S, et al. Off-the-shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology. 2022;162(4):1319–33.
Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–46.
Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–26.
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–51.
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJ, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017; 9(399):eaaa0984.
Acknowledgements
Figures 1, 2, 3 and 4 are made using www.BioRender.com.
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2020A1515110094).
Author information
Authors and Affiliations
Contributions
XL, JY, BK, and HH conceived the review. XL, XY, YW, and JY wrote the manuscript. All the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This is not applicable to this review.
Consent for publication
This is not applicable to this review.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Yan, X., Wang, Y. et al. The Notch signaling pathway: a potential target for cancer immunotherapy. J Hematol Oncol 16, 45 (2023). https://doi.org/10.1186/s13045-023-01439-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-023-01439-z