Abstract
Background
Delta-like ligand 4-Notch (DLL4-Notch) signaling contributes to the maintenance of chemotherapy-resistant cancer stem cells and tumor vasculature.
Objective
This phase IB trial of demcizumab, an IgG2 humanized monoclonal antibody directed against DLL4, was undertaken to determine its maximum tolerated dose, safety, immunogenicity, preliminary efficacy, pharmacokinetics, and pharmacodynamics, combined with standard chemotherapy.
Patients and Methods
Forty-six treatment-naive patients with metastatic non-squamous non-small cell lung cancer (NSCLC) were enrolled in this open-label, dose-escalation study using a standard 6 + 6 design. Demcizumab (2.5, 5.0, and 7.5 mg/kg) was given once every 3 weeks with standard doses of pemetrexed and carboplatin using a continuous (six cycles followed by demcizumab maintenance) or a truncated demcizumab regimen (four cycles followed by pemetrexed maintenance).
Results
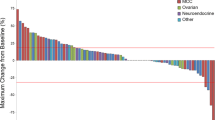
Initially, continuous demcizumab was given until progression but two patients developed grade 3 pulmonary hypertension and congestive heart failure after eight or more infusions. Thereafter, 23 patients were treated with a truncated regimen of demcizumab, which was not associated with any grade 3 or greater cardiopulmonary toxicity. Common adverse events were hypertension, raised brain natriuretic peptide, and those expected from carboplatin and pemetrexed alone. Twenty of 40 evaluable patients (50%) had objective tumor responses. In peripheral blood, demcizumab treatment modulated the expression of genes regulating Notch signaling and angiogenesis, and achieved concentrations exceeding those saturating DLL4 binding.
Conclusions
This study has identified a truncated dosing regimen and recommended phase II dose of demcizumab (5 mg/kg q3-weekly ×4) for subsequent clinical evaluation in combination with standard carboplatin and pemetrexed chemotherapy. NCT01189968.



Similar content being viewed by others
References
O’Flaherty JD, Barr M, Fennell D, Richard D, Reynolds J, O’Leary J, et al. The cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7(12):1880–90.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84.
Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, et al. Targeting notch to target cancer stem cells. Clin Cancer Res. 2010;16(12):3141–52.
Fischer M, Yen WC, Kapoun AM, Wang M, O'Young G, Lewicki J, et al. Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Res. 2011;71(5):1520–5.
Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5(2):168–77.
Yen WC, Fischer MM, Hynes M, Wu JJ, Kim E, Beviglia L, et al. Anti-DLL4 has broad Spectrum activity in pancreatic cancer dependent on targeting DLL4-notch Signaling in both tumor and vasculature cells. Clin Cancer Res. 2012;18(19):5374–86.
Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7(5):327–31.
Li JL, Sainson RCA, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67(23):11244–53.
Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–7.
Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444(7122):1083–7.
Smith DC, Eisenberg PD, Manikhas G, Chugh R, Gubens MA, Stagg RJ, et al. A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clin Cancer Res. 2014;20(24):6295–303.
Paller CJ, Bradbury PA, Ivy SP, Seymour L, LoRusso PM, Baker L, et al. Design of phase I combination trials: recommendations of the clinical trial design task force of the NCI investigational drug steering committee. Clin Cancer Res. 2014;20(16):4210–7.
Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. Non-small cell lung cancer, version 6.2015 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2015;13(5):515–24.
Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. NCCN guidelines (R) insights: non-small cell lung cancer, version 4.2016 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2016;14(3):255–64.
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–55.
Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–902.
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. 1996;11(3):189–228.
Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75.
Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudroit R, Irizarry R, Huber W, editors. Bioinformatics and computational biology solutions using R and bioconductor. Springer; 2005. p. 397-420.
Chiorean EG, LoRusso P, Strother RM, Diamond JR, Younger A, Messersmith WA, et al. A phase I first-in-human study of Enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin Cancer Res. 2015;21(12):2695–703.
Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R, Ish-Horowicz D. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69(2-3):135–44.
Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–80.
Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823–59.
Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:27–39.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67.
Mitsudomi T. Molecular epidemiology of lung cancer and geographic variations with special reference to EGFR mutations. Transl Lung Cancer Res. 2014;3(4):6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Oncomed Pharmaceuticals.
Conflict of Interest
AK, LX, and RS disclosed interests related to employment and stock ownership/options in Oncomed. MMcK, DK, BM, MH, MMil, MJ, DH, and BH had no interests to declare other than the funding of the study by Oncomed.
Electronic supplementary material
ESM 1
(DOCX 459 kb)
Rights and permissions
About this article
Cite this article
McKeage, M.J., Kotasek, D., Markman, B. et al. Phase IB Trial of the Anti-Cancer Stem Cell DLL4-Binding Agent Demcizumab with Pemetrexed and Carboplatin as First-Line Treatment of Metastatic Non-Squamous NSCLC. Targ Oncol 13, 89–98 (2018). https://doi.org/10.1007/s11523-017-0543-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0543-0