Abstract
This review article presents an in-depth analysis of the current state of research on receptor tyrosine kinase regulatory non-coding RNAs (RTK-RNAs) in solid tumors. RTK-RNAs belong to a class of non-coding RNAs (nc-RNAs) responsible for regulating the expression and activity of receptor tyrosine kinases (RTKs), which play a critical role in cancer development and progression. The article explores the molecular mechanisms through which RTK-RNAs modulate RTK signaling pathways and highlights recent advancements in the field. This include the identification of potential new RTK-RNAs and development of therapeutic strategies targeting RTK-RNAs. While the review discusses promising results from a variety of studies, encompassing in vitro, in vivo, and clinical investigations, it is important to acknowledge the challenges and limitations associated with targeting RTK-RNAs for therapeutic applications. Further studies involving various cancer cell lines, animal models, and ultimately, patients are necessary to validate the efficacy of targeting RTK-RNAs. The specificity of ncRNAs in targeting cellular pathways grants them tremendous potential, but careful consideration is required to minimize off-target effects, the article additionally discusses the potential clinical applications of RTK-RNAs as biomarkers for cancer diagnosis, prognosis, and treatment. In essence, by providing a comprehensive overview of the current understanding of RTK-RNAs in solid tumors, this review emphasizes their potential as therapeutic targets for cancer while acknowledging the associated challenges and limitations.
Similar content being viewed by others
Background
Receptor tyrosine kinases (RTKs) are a diverse family of cell surface receptors that are crucial for normal cellular processes such as cell growth, differentiation, and survival [1]. These receptors are activated by binding to extracellular ligands, which then triggers a series of molecular events of intracellular signaling pathways that regulate gene expression and cellular behavior [2]. The initiation of this process commonly involves the dimerization of adjacent RTKs and subsequent autophosphorylation of tyrosine residues within the resulting dimer. This autophosphorylation event is triggered by the binding of an extracellular signaling ligand. Subsequently, a phosphorylation cascade is initiated within the downstream signaling pathway. Under normal circumstances, the activation of RTKs and their downstream pathways, as well as the binding of extracellular ligands, are tightly regulated. However, in pathological conditions like cancer, receptor tyrosine kinases RTKs can become constitutively activated in a ligand-independent manner [3]. Several crucial pathways, including epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), stem cell tyrosine kinase receptor (c-Kit), c-Met, and insulin-like growth factor receptor (IGFR) signaling pathways, are particularly significant in this context [4,5,6,7,8,9,10]. These pathways are targeted by miRNAs, further emphasizing their importance in regulating aberrant RTK signaling.
Dysregulation of RTK signaling, which is commonly observed in various types of cancer, has been implicated in tumor growth, invasion, and metastasis [11, 12]. To tightly control the expression and activity of RTKs, multiple mechanisms come into play, including post-translational modifications, protein–protein interactions, and regulation by non-coding RNAs (ncRNAs) [13]. ncRNAs, which do not code for proteins but play crucial roles in gene regulation are particularly important in this context. They can be classified into two major groups: housekeeping and regulatory ncRNAs. While the former classification was based on size, the newer categorization focuses on their importance in gene regulation (Fig. 1).
Classification of RNAs. RNAs can be classified into two major groups, coding RNA and non-coding RNAs. Traditionally, ncRNAs were categorized based on their size as small ncRNAs and long ncRNAs. However, a more recent classification system has emerged, which distinguishes between two main groups: housekeeping ncRNAs and regulatory ncRNAs
RTK-RNAs are a class of ncRNAs that specifically regulate the expression and activity of RTKs. These ncRNAs, mostly including microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) as a subtype of lncRNAs, have been shown to play important roles in the development and progression of solid tumors by regulating the expression of RTKs [14]. Depending on the specific RTK and RNA involved, RTK-RNAs can either activate or inhibit RTK signaling pathways [15]. For example, some RTK-RNAs may bind to and stabilize RTK mRNA, leading to increased expression and activation of the receptors. On the other hand, other RTK-RNAs can bind to and degrade RTK mRNA, resulting in decreased expression and activity of the receptors. Recent studies have identified several new RTK-RNAs that play important roles in cancer development and progression [16, 17]. These RNAs hold potential as targets for the development of novel cancer therapies. Additionally, therapeutic strategies targeting RTK-RNAs have shown promise in preclinical studies [18]. Overall, the regulation of RTK expression and activity by ncRNAs, specifically RTK-RNAs, is an important mechanism in cancer development and progression. Understanding the molecular mechanisms underlying the modulation of RTK signaling pathways by RTK-RNAs could lead to the development of new cancer therapies that target specific RTK-RNA interactions. Furthermore, the identification of RTK-RNAs as potential biomarkers for cancer diagnosis, prognosis, and treatment response could enable more personalized and effective cancer therapies.
This review article aims to provide a comprehensive overview of the potential therapeutic targets of ncRNAs in RTK signaling for solid tumors. The review specifically focuses on the different classes of ncRNAs, including miRNAs, tsRNAs, piRNAs, lncRNAs, and circRNAs, exploring their roles in cancer processes. The article then delves into the future prospects of ncRNA-based therapies targeting RTK signaling in solid tumors, emphasizing the potential to enhance cancer patient care. The growing understanding of the "dark matter" of the genome highlights the significant potential of targeting ncRNA signaling to impact cancer patient care. Throughout the review, the discussion centers on the current status and future directions of ncRNA-based therapies for RTK signaling in solid tumors.
RTK-RNAs and their role in cancer
For a long time, health research primarily focused on studying the small portion of the genome responsible for coding proteins. It was believed that the remaining 98% had no significant function [19]. However, the ENCODE project revealed that this part of our genetic material is transcribed into numerous RNA molecules, known as non-coding RNAs (ncRNAs). These ncRNAs play a crucial role in regulating our body's functions and can be implicated in diseases, including cancer [20].
ncRNAs have emerged as key players in gene regulation and intercellular communication. They are involved in various processes such as coding, decoding, regulation, and expression of genes. Studies on ncRNA regulatory roles have demonstrated the existence of diverse ncRNA networks associated with different types of cancer. This breakthrough enables scientists to develop targeted strategies for cancer treatment and prevention by focusing on the specific ncRNAs encoded by our genes [21].
Deregulated expression of ncRNA has been directly linked to the development and progression of cancer, specifically affecting RTKs signaling pathways. RTKs are proteins on the cell surface that play a crucial role in cell proliferation and differentiation. Genetic alterations in ncRNA genes have also been associated with RTK-related cancers [22]. The different classes of ncRNAs can be broadly categorized based on their size including small ncRNAs such as miRNAs, siRNAs, and piRNAs along with lncRNAs, all of which contribute significantly to cancer biology.
While genetic variations in genes encoding ncRNAs have been linked to cancer, the number of identified cases is fewer compared to protein-coding genes. Additionally, the production or reduction of specific ncRNA molecules related to cancer can occur through different mechanisms, such as epigenetic, transcriptional, or post-transcriptional processes. Further research is needed to fully understand the precise control mechanisms of ncRNAs on genes and to explore ways to harness this knowledge for effective cancer treatment and prevention.
Small non-coding RTK-RNAs
The role of microRNAs in the RTK pathway in cancer
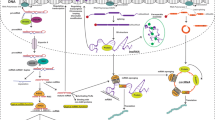
MicroRNAs (miRNAs) are a type of regulatory ncRNAs that primarily function to regulate gene expression by interacting with recognition sites in the 3'-UTR region of mRNA, thereby affecting mRNA stability [23]. The expression of miRNA involves several post-transcriptional cleavage steps. Initially, the transcription of the miRNA locus results in the production of primary miRNA (pri-miRNA). The pri-miRNA then undergoes two cleavage steps, first by the microprocessor enzyme in the nucleus and then by the dicer enzyme in the cytoplasm. The first cleavage step generates pre-miRNA, which is subsequently transported into the cytoplasm by exportin-5 for the final cleavage step, resulting in the formation of mature miRNA. The mature miRNA is a double-stranded polyribonucleotide consisting of a guide strand and a passenger strand. Following the production of the mature form, the miRNA is attached to the argonaute protein (AGO), the passenger strand is removed, and the miRNA-induced silencing complex (miRISC) is formed, consisting of AGO and the guide strand. Any alterations in miRNA expression can impact the expression of target genes and disrupt cellular homeostasis, potentially leading to the development of various diseases, including cancer [24]. These alterations can arise from changes in components of the miRNA processing pathways (e.g., mutations in the dicer gene), genetic variations in miRNA-encoding loci, epigenetic regulation of miRNA expression, and silencing of miRNA expression by long non-coding RNAs (Fig. 2) [25,26,27,28].
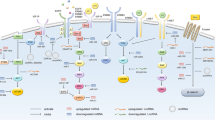
RTK-Mediated Signaling Pathways and Associated microRNAs in Solid Tumors This figure illustrates the signaling pathways mediated by receptor tyrosine kinases (RTKs) in solid tumors, along with the microRNAs that target different components of these pathways. The microRNAs have the ability to regulate the expression of both the RTK receptors and the downstream signaling cascades. The figure was created using the Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com, accessed on 10 July 2023). Please note that the figure is for illustrative purposes only and may not depict the exact molecular interactions or signaling pathways in every solid tumor
Among the various genes that are targeted by miRNAs, RTKs play a crucial role in conjunction with microRNAs, particularly in stress signaling. This association is especially significant in disease-related pathological conditions such as cancer. miRNAs have the ability to function as both oncogenes and tumor suppressors by participating in downstream RTK signaling pathways and regulating the expression of RTK genes [29, 30]. A variety of microRNAs have been associated with the regulation of the signaling pathways downstream of the activated RTKs. For instance, miR-216 modulates the EMT process by modulating the JAK2/STAT3 pathway [31].
The actions and effects of RTKs mentioned above have led to the observation of alterations in their expression in various types of malignancies. One of the key factors contributing to these alterations is the investigation of changes in the levels of miRNAs that target RTKs. This area of research has received significant attention and has been extensively studied.
miR-34a is a microRNA that targets various factors associated with cancer. It regulates cellular processes such as cellular survival, stemness and differentiation, cell cycle, invasion, epithelial-mesenchymal transition (EMT), metabolism, immunity, and epigenetics. RTKs including c-Met, EGFR, AXL, PDGFR-B, and ErbB2 are among the targets of miR-34a [32]. Down-regulation of miR-34a has been reported in several malignancies like breast cancer, colon cancer, gastric cancer, non-small cell lung cancer, and prostate cancer [33,34,35,36,37]. On the other hand, up-regulation of miR-34a in both tissue and serum levels has been observed in medullary thyroid cancer, making it a potential biomarker for this malignancy [38, 39]. In breast cancer, miR-34a negatively impacts processes like vasculogenic mimicry, migration, and invasion by affecting the AXL receptor tyrosine kinase axis [40]. Overexpression of miR-34a suppresses tumor growth, invasion, and drug resistance in breast cancer [41]. In a meta-analysis conducted in 2017 by Imani et al., the use of miR-34a as a biomarker for breast cancer risk was examined. The analysis indicated a pooled sensitivity of 85.5% (OR 83.8–87% (95% CI)) and specificity of 70% (OR 65.8–74.1% (95% CI)). The overall area under the curve (AUC) was calculated to be 0.8 [42]. These findings indicate that miR-34a shows promise as a potential biomarker for breast cancer. Furthermore, the tumor suppressive effects of miR-34a suggest that it could be utilized as a treatment approach for breast cancer. Several studies have already been conducted on this matter [43,44,45,46]. These studies have explored the possibility of using an appropriate drug delivery system to target and deliver miR-34a specifically to breast cancer cells, thereby enhancing its therapeutic effects. miR-34a is also a regulator of RTKs by affecting MET gene expression and plays a role in gastric cancer proliferation and metastasis [35, 47, 48]. Furthermore, miR-34a regulates downstream signaling pathways such as PI3K/Akt and Wnt/β-catenin [49, 50].
miR-7 is a well-studied regulatory microRNA that has been extensively researched. Its target genes such as EGFR and its downstream effectors, protein kinase B (Akt) and extracellular kinase regulator ½ (ERK ½), are associated with RTK [51]. Studies on breast cancer cell lines have demonstrated that miR-7 plays a role in regulating various cellular processes, including EMT, metastasis, tumor-associated angiogenesis, as well as increased radiosensitivity [52,53,54]. In gastric cancer, miR-7 directly inhibits the expression of IGF1R, EGFR, and mTOR, which are the downstream effectors of the PI3K/Akt pathway. This leads to increased sensitivity to cisplatin treatment, apoptosis, as well as negative regulation of metastasis and proliferation in gastric cancer tissues [55,56,57,58]. The under-expression of miR-7 is considered a poor prognostic indicator and therapeutic delivery of this microRNA has been shown to reduce vasculogenesis and inflammation in gastric cancer [59]. miR-7 influences a variety of RTK-associated signaling pathways including EGFR downstream pathways Ras/Raf/MEK/ERK1/2 and PI3K/Akt/mTOR, as well as IGF1R/IRS pathway. It is also implicated in gefitinib-mediated cytotoxicity by affecting the expression of these molecular components [60, 61]. Additionally, miR-7 acts as a mediator in regulating cellular proliferation, migration, and invasion in non-small cell lung cancer (NSCLC) through the ERK/MAPK signaling pathway [62].
In addition to miR-7, other microRNAs such as miR-146a, miR-375, and miR-495, target the EGFR-encoding mRNAs [48, 63, 64]. miR-146a is a regulator of cellular proliferation, apoptosis, and invasion. Its expression is down-regulated in gastric cancer tissue samples compared to controls [65,66,67,68]. The expression levels of miR-146a show a 98% sensitivity in distinguishing affected tissue from normal surroundings. In addition, higher expression of miR-146a indicates a better response to chemotherapy [69]. miR-375 targets ErbB2 and EGFR subtype, along with the receptor d’Origine Natais (RON) RTK, and the downstream signaling effector JAK2. It is involved in regulating cellular proliferation, migration, and invasion in gastric cancer. Higher levels of miR-375 are also an indicator of favorable treatment response [64, 70, 71]. Moreover, lower serum levels of this microRNA serve as a marker of poor prognosis with an AUC of 0.871 and are associated with lymph node metastasis and a higher tumor stage [72]. miR-375 is under-expressed in NSCLC tissue and plasma samples, and is associated with lymph node and brain metastasis, advanced disease stage, and shorter overall survival [73,74,75]. Such a pattern of alteration has also been implicated in lung squamous cell carcinoma [76]. miR-495 is also another regulator of cellular apoptosis and migration through modulation of the Akt/mTOR pathway and ErbB2 expression [47, 48]. On the other hand, miR-206 mediates the regulation of c-MET, IGF1R, and MAPK3’s expression in gastric cancer. It induces apoptosis and inhibits resistance to cisplatin, metastasis, and proliferation [77,78,79].
miR-145 is a microRNA that targets the PI3K/AKT pathway, which is a downstream signaling pathway of various RTKs. By inhibiting the expression of AKT3, miR-145 can enhance the response to treatment [80] and regulate the proliferation and migration of breast cancer cells [81]. Additionally, miR-145 also regulates the expression levels of IGF-1R, another RTK. Results of both in vitro and in vivo investigations on adenoviral transfection of the miR-145 encoding gene in breast cancer cells have shown promising results in inhibiting tumor growth [82].
On the other hand, there are microRNAs such as miR-21, miR-10b, miR-373, and miR-155 that are oncogenic and contribute to breast cancer progression. miR-10b targets HOXD10, which itself plays a role in cellular migration and angiogenic switch in malignant breast cancer. It is also highly expressed in the tumor vasculature. Upregulation of miR-10b expression is associated with breast cancer, as well as glioblastoma, colorectal cancer, and pancreatic adenocarcinoma [83]. miR-21 is also overexpressed in breast cancer tissues and induces metastasis and migration by targeting a variety of effectors like smad7 and PTEN [84, 85]. Both tissue and serum levels of miR-21 have been extensively investigated as potential biomarkers for breast cancer, showing significant associations with a variety of clinicopathological characteristics including tumor grade, risk of metastasis, and hormonal receptor expression profiles [86]. miR-21 has also been extensively studied in hepatocellular carcinoma (HCC) and lung cancer. In HCC, miR-21 targets the tumor suppressor protein PTEN and its overexpression has been observed in serum samples [87, 88]. A meta-analysis by Qu et al. investigating the use of serum miR-21 as a diagnostic biomarker for HCC reported a pooled sensitivity of 85.2%, specificity of 79.2%, and an AUC of 0.89, suggesting its potential for early disease detection [89]. In lung cancer, miR-21 is considered an oncogenic microRNA and has been found to be overexpressed in NSCLC tissues [90]. A recently conducted meta-analysis has estimated the pooled sensitivity and specificity of miR-21 in lung cancer diagnosis to be 77% and 86%, respectively, with an AUC of 0.87. In addition, this biomarker possesses prognostic values with a hazard ratio (HR) of 1.49 for overall survival [91]. Additionally, miR-155, which also regulates the expression of PTEN, is overexpressed in NSCLC tissues and is a predictor of poor outcome [92, 93]. A meta-analysis on the diagnostic and prognostic value of miR-155 reported a pooled sensitivity of 0.82, specificity of 0.78, AUC of 0.87, and a hazard ratio (HR) of 1.26 for poor overall survival [94].
The miR-155, which targets the SOCS1 gene, has shown heterogeneous results in various studies regarding its role in regulating and promoting breast cancer progression [95, 96]. Some studies have reported conflicting findings. However, higher levels of miR-155 have been detected in the serum of breast cancer patients compared to controls, suggesting its potential as a diagnostic biomarker. The validity of miR-155 as a biomarker was supported by an AUC of 0.89 and its elevated levels were significantly associated with tumor grade, stage, and size [97]. These discrepancies in study outcomes may be attributed to differences in study population and conditions. Therefore, it is crucial to conduct studies with controlled conditions and larger sample sizes to obtain more conclusive results.
miR-155 and miR-99a have also been indicated as potential biomarkers for HCC diagnosis. miR-99a, a tumor suppressor, is downregulated in HCC and targets IGF-1R and mTOR. Both microRNAs show promise as diagnostic and prognostic biomarkers, with AUC of 0.799 and 0.84, respectively, according to the findings of a recently conducted meta-analysis [98, 99]. Additionally, high levels of these microRNAs are significantly associated with poor survival time. Furthermore, miR-99a has been explored as a therapeutic approach in both in vitro and in vivo studies, utilizing nano-particle delivery systems [100].
MicroRNAs play a crucial role in regulating various metastasis-associated cellular processes in colorectal cancer (CRC). Among these processes, the EMT is regulated by miR-612, which targets AKT2, and miR-200b, miR-200c, and miR-141, which target ZEB1 and ZEB2. In metastatic CRC tissues, miR-612 is found to be under-expressed, while the miR-141, miR-200b, and miR-200c show patterns of over-expression compared to control tissues [101, 102]. Additionally, miR-1249, miR-590-5p, miR-206, and miR-126 are involved in regulating angiogenesis and hypoxia response by inhibiting the expression of VEGFA. These microRNAs are relatively under-expressed in metastatic CRC tissues. Furthermore, miR-25-3p and miR-143 targets the RTKs VEGFR2 and IGF1R, respectively, to regulate the aforementioned cellular processes [103,104,105,106,107,108]. miR-18a and Let-7c target KRAS mRNA and regulate cellular proliferation and invasion, respectively [109, 110]. Another important regulator, miR-124, targets STAT3 and is found to be downregulated in CRC tissue samples, which is a predictor of poor prognosis. In a study by Wang et al., the hazard ratio of the downregulated levels of miR-124 for overall survival and disease-free survival were estimated at 4.634 (p = 0.002) and 4.533 (p = 0.002), respectively [111].
miR-126 plays a significant role in the progression of lung cancer by targeting various genetic components. The Reduction in STAT3 expression levels results in induction of cellular proliferation and migration, as well as decreased susceptibility to apoptosis, as indicated by the under-expression of caspase 3 in NSCLC [112]. Furthermore, miR-126-mediated inactivation of the VEGFA/VEGFR2/ERK pathway is associated with apoptosis and inhibition of metastatic characteristics in NSCLC [113]. Apart from these processes, EMT and angiogenesis are also modulated by miR-126 via targeting PI3K/AKT/Snail and VEGF/FGF-mediated signaling pathways, respectively [114, 115].
The down-regulation of miR-125a-3p is a key factor in determining poor prognosis in NSCLC and is significantly correlated with tumor size and metastasis. Conversely, higher expression of this marker is associated with higher overall and disease-free survival rates in NSLC patients [116]. Similar to miR-126, miR-125a inhibits cellular proliferation and metastasis in NSCLC by targeting STAT3 [117]. It is also a modulator of the HER2 receptor in small cell lung cancer (SCLC), and its under-expression is associated with the anti-tumor effects of cytotoxic drugs in HER2-positive SCLC [118].
miRNA-1 is a tumor suppressor that is highly conserved and targets multiple pathways, including members of the tyrosine kinase receptor family such as c-met and EGFR [119]. The down-regulation of miRNA-1 expression has been associated with a variety of malignancies, including ovarian cancer, colorectal cancer, squamous cell carcinomas from different origins, osteosarcoma, prostate cancer, gastric cancer, lung cancer, and rhabdomyosarcoma [120,121,122,123,124,125,126,127,128,129]. These malignancies exhibit an up-regulation of the aforementioned tyrosine kinase receptors, contributing to the importance of miRNA-1 in their development and progression.
The significance of these alterations and their role in the development and advancement of malignancies has prompted the conduction of studies exploring the potential use of these non-coding RNAs as biomarkers and treatment approaches, yielding promising results. In addition to the studies discussed in this section, Table 1 provides a comprehensive summary of the most extensively investigated miRNA-based biomarkers. Moreover, Table 2 highlights some of the therapeutic applications of miRNAs that have been investigated in these studies.
The limited effectiveness of current therapeutic interventions contributes to the unfavorable prognosis of HCC [130]. Synthetic miRNA antagonists or mimics, when administered intravenously, tend to accumulate in the liver and kidney. This characteristic renders liver cancer an appropriate model for evaluating the efficacy of miRNA-based therapeutic strategies [131, 132]. Biodistribution studies of nanoparticles indicate that a significant proportion of administered nanomaterials tend to accumulate in the liver prior to undergoing hepatic clearance, rendering it a favorable target for various delivery systems. In cases of liver cancer, miR-122, which is known for its high abundance and liver-specific expression, has been observed to be downregulated. Restoring miR-122 through the use of a lentiviral expression vector has been shown to inhibit invasion, tumorigenesis, and metastasis in vivo. The inhibition of HCC metastasis is achieved through the modulation of ADAM17 and cyclin G1 by miR-122 [133, 134]. On the other hand, the expression of miR-21 is significantly upregulated in HCC. Inhibition of miR-21 in cultured HCC cells leads to increased expression of the PTEN tumor suppressor and decreased tumor cell proliferation, migration, and invasion [135, 136].
Lung cancer is a prominent contributor to cancer-associated mortality worldwide, with 5-year survival rates ranging from 4 to 17%. Liposomes derived from lung surfactants offer a practical option for delivering drugs to the lungs, making them suitable vehicles for miRNA-targeting agents. The downregulation of miR-34a, a miRNA known for its tumor suppressor function, has been observed in different types of solid tumors, including lung cancer. A study conducted by Wiggins et al. demonstrated that the administration of synthetic miR-34a encapsulated in liposomes effectively suppressed tumor growth in mice with NSCLC. Importantly, this treatment approach exhibited no signs of immunogenicity or toxicity. These findings align with prior in vitro studies conducted on genetic variations of NSCLC cell lines, which demonstrated that miR-34a decreased cell proliferation and the formation of cell colonies through the p53 pathways [137, 138]. Kasinski et al. introduced a novel approach involving the utilization of NOV340 liposomes for the concurrent delivery of tumor suppressors miR-34 and let-7b in NSCLC. The implementation of this method led to a notable 40% improvement in the survival rate of mutant mice [139]. Furthermore, the utilization of miR-200c loaded-NOV340 liposomes has been shown to augment the radiosensitivity of lung cancer cells through the upregulation of oxidative stress response mechanisms and inhibiting DNA double-strand breaks caused by radiation [140].
In 2013, the initial phase of clinical testing involved the administration of MRX34, which consisted of miR-34 mimics enclosed within NOV340 liposomes. This therapeutic approach represented the first instance of utilizing miRNA for therapeutic purposes. Nevertheless, the study was terminated in 2016 as a result of the occurrence of significant unfavorable incidents among the participants [141, 142]. The research conducted by Wu et al. revealed that cationic lipoplexes based on DOTMA demonstrated a high level of efficacy in transporting miR-29b to both NSCLC cells and xenograft mouse models. Following a series of repeated administrations, the mice exhibited a notable decrease in tumor dimensions and a substantial increase in miR-29b expression, reaching a five-fold amplification specifically within the tumor tissue. This observation indicates that the release of miR-29b from DOTMA lipoplexes is highly effective [143]. In a phase I clinical trial, nonliving bacterial nano-cells were employed as vehicles for the administration of miR-16 to patients with NSCLC. The system specifically targeted cancer cells expressing EGFR, resulting in inhibited tumor growth. Nevertheless, there were documented toxicities that were dependent on the dosage, such as anaphylaxis, inflammation, and cardiac events. The study recorded varying response rates, with 5% of participants exhibiting partial response, 68% experiencing stable disease, and 27% demonstrating progressive disease [144].
Given that HER-2 positive breast cancers constitute approximately 30% of cases characterized by an unfavorable prognosis, there is an increasing focus on effectively targeting this overexpressed receptor. Within this particular framework, experiments conducted on live mice with breast cancer have shown that the introduction of the tumor suppressor miR-125a-5p through lentiviral delivery resulted in a decrease in tumor development, metastasis, and vasculature. This effect was achieved by specifically targeting histone deacetylase 4 (HDAC4). Hayward et al. demonstrated that introducing miR-125a-5p into hyaluronic acid (HA)-coated liposomes effectively suppressed the HER-2 proto-oncogene in 21MT-1 breast cancer cells through transfection. Consequently, the inactivation of the MAPK and PI3K/AKT signaling pathways led to decreased migratory and proliferative capabilities [145, 146]. The utilization of HA/miRNA nanoparticles has shown potential in the context of targeted clinical interventions for breast cancer. The study by Deng et al. employed HA-chitosan nanoparticles for the simultaneous encapsulation of doxorubicin and miR-34a, resulting in an improved response to chemotherapy and a reduction in cancer cells migration [147, 148]. The in vivo experiments demonstrated a significant 58% decrease in tumor volume upon the incorporation of miR-9, miR-21, and miR-145 sponges into magnetic particles within PEI particles [149, 150]. The study conducted by Panebianco et al. discovered that the utilization of silica nanoparticles facilitated the delivery of miR-34a into mammary tumors, resulting in reduced tumor growth in mice. The diminished expression of target genes, such as c-Myc, served as an indicator of the biological efficacy of the administered miR-34a [137].
tsRNAs and piRNAs role in RTK pathway in cancer
Transfer RNA-derived small RNAs (tsRNAs) are derived from tRNAs. The biogenesis of tsRNAs and their downstream mechanisms of action are still being studied, but it has been observed that they can be involved in regulating gene expression and translation, similar to miRNAs. tsRNAs have been found to be associated with Argonaute proteins and can mediate translational repression of mRNAs via binding to target 3' UTRs. Interestingly, a recent study has shown that a tsRNA derived from a leucine tRNA plays a role in global protein translation by regulating the expression of genes coding for ribosomal components [151]. Some tsRNAs have been found to be oncogenic, while others have been found to be tumor-suppressive. Therefore, tsRNAs have the potential to be a new target for anticancer therapy.
Emerging evidence suggests that piRNAs are involved in RTK signaling in solid tumors. piRNAs, a class of small ncRNA molecules typically 21–35 nucleotides in length, are primarily associated with the PIWI subfamily of Argonaute proteins and are involved in transposon silencing during germline development. In mammals, piRNAs are generated from long, single-stranded transcripts that are clustered throughout the genome, with approximately 20,000 piRNAs present in the human genome. While piRNAs were initially thought to function only in gonadal cells, recent research has shown that they are also expressed in somatic tissues, although at low levels, and misexpressed in cancers. Although the precise functional roles of piRNAs in RTK signaling in solid tumors are not fully understood, recent studies have suggested that these small ncRNAs may have therapeutic potential as targets in cancer treatment and may serve as useful biomarkers for diagnosis and prognosis [152, 153].
Long non-coding RTK-RNAs
Linear lncRNAs role in RTK pathway in cancer
lncRNAs are a class of regulatory molecules that are longer than 200 nucleotides with a fundamental role in gene expression through their interaction with chromatin, transcriptional machinery, and other cellular components. Their biogenesis begins with transcription, predominantly by RNA polymerase II, similar to messenger RNAs (mRNAs). The initial transcripts, known as primary lncRNAs (pri-lncRNAs), often undergo splicing, polyadenylation, and capping [154]. However, lncRNA transcripts can exhibit unique features, such as variable splicing and less efficient polyadenylation, which can impact their stability and cellular localization [155]. Following successful processing, mature lncRNAs participate in various cellular processes. For instance, nuclear lncRNAs can interact with chromatin and transcription factors, thereby affecting transcriptional regulation [156]. Cytoplasmic lncRNAs, on the other hand, can modulate mRNA stability or translation, interact with miRNAs, or influence signal transduction pathways [157] (Fig. 3).
Biogenesis and mechanism of action in Non-coding RNAs (TF = Transcription factor). This figure was created using the Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com, accessed on 11 July, 2023)
Regarding the interaction between lncRNA and microRNAs in promoting RTK expression, extensive research has been conducted in various types of cancer, such as colon, gastric, breast, osteosarcoma, HCC, and NSCLC [158,159,160,161]. For instance, lncRNA XIST has been found to upregulate RTK family member, AXL, via sponging miR-93-5p, resulting in the overexpression of hypoxia-inducible factor 1-alpha (HIF1A). Ultimately, this process participated in enhancing the tumorigenesis capacity in colon cancer cells [162]. On the other hand, lncRNA XIST expression can also be involved in the upregulation of RTK-like orphan receptor 1 (ROR1) by sponging miR-30a-5p, leading to promoting cellular growth [163]. Another study demonstrated that ERBB4, a member of the RTK family, is modulated by lncRNA, called Linc00152. Mechanistically, lncRNA Linc00152 regulates the expression of ERBB4 by targeting miR-193a-3p, which may hinder the sensitization of malignant cells toward oxaliplatin-based chemotherapy. Targeted downregulation of Linc00152 expression significantly reduces chemoresistance in malignant cells, suggesting promising implications for developing novel therapeutic strategies [164]. Another lncRNA, known as H19, acts on the ubiquitin ligase E3 family by modulating miR-675 to promote RTK stability, thereby contributing to breast cancer development [165].
Furthermore, in neuroblastoma, lncRNA MALAT1 has been discovered to promote cellular invasion and migration via upregulating AXL, an RTK member. Inhibition of AXL has emerged as a promising therapeutic approach [166]. Similarly, the upregulation of a novel lncRNA called CALIC has been identified to enhance colon cancer cell migration and metastasis through regulating AXL signaling [167]. Moreover, another investigation found that in anaplastic thyroid cancer, lncRNA MALAT1 overexpression can lead to the activation of the RTK signaling pathway via a cascade of intermediary cellular signaling pathways, offering a remarkable diagnostic advantage [168]. In breast cancer, lncRNA MAYA has been found to play a role in bone metastasis. Regarding the underlying mechanism, it has been demonstrated that lncRNA MAYA can stimulate the formation of a heterodimeric complex consisting of ROR1, HER3, and the other RTKs, which subsequently activate signaling cascades and contribute to cancer cell migration [169]. Another lncRNA, SPRY4-IT1, has been shown to regulate the expression of the RTK family member FGFR2 by interacting with chromatin and regulating the expression of nearby genes. Additionally, SPRY4-IT1 has a regulatory effect on EMT, which is considered an essential mechanism in cell migration [170].
In addition to lncRNAs, which act as activators of the RTK signaling pathway, there are several lncRNAs with inhibitory effects on RTK regulatory pathways, resulting in the suppression of tumor development and progression. For example, a lncRNA named LINC00526 could function as a tumor suppressor that establishes a negative feedback loop with AXL, indicating its potential therapeutic application in glioma treatment [171]. Conversely, RTK can interfere with the functionality of lncRNAs, thereby impacting cancer metastasis. In breast cancer, for example, EGF has been found to decrease the expression of a newly discovered lncRNA called LIMT, which subsequently promotes tumor invasion [172]. Tumor-suppressing lncRNAs also restrict the invasive potential of melanoma and HCC by inhibiting RTK-related pathways [173]. Interestingly, Eph tyrosine kinase receptor A4 functions as a suppressor of the EMT process, in which lncRNA TUSC acts as a sponge to sequester miR-10a and increase Eph expression, thereby restricting tumor growth. This pathway exhibits similarities to previous studies but with the opposite effect [174].
On the other hand, it has been demonstrated that RTK inhibitors (RTKIs) are effective therapeutic agents for treating cancer. In this context, researchers have identified 45 lncRNAs that are differentially expressed in NSCLC cells, which are resistant to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs). The downregulated LINC01128, acting as a specific miRNA sponge, decreases PTEN via sponging miR-25-3p. This activates the PI3K/Akt signaling pathway and promotes EGFR-TKI resistance. Another lncRNA, HIF1A-AS2, has been shown to be downregulated by RTKIs, resulting in reduced tumor growth in NSCLC [175]. HIF1A-AS2 also regulates the expression of specific genes involved in angiogenesis, such as VEGFA, by interacting with specific proteins. By inhibiting HIF1A-AS2 expression, RTKIs can also indirectly decrease the expression of these angiogenic factors, further inhibiting tumor growth [176]. Similarly, a study investigated the potential of a lncRNA called growth arrest-specific 5 (GAS5) in improving the efficacy of EGFR-TKIs in NSCLC therapy. The results showed that GAS5 is downregulated in lung adenocarcinoma tissues, and lower expression levels are associated with larger tumor sizes, poor differentiation, and advanced pathological stages. Furthermore, overexpression of GAS5 sensitizes resistant NSCLC cells to EGFR-TKIs and inhibits tumor growth in mice treated with gefitinib. These findings suggest that GAS5 may be a potential biomarker for diagnosing lung adenocarcinoma and a possible therapeutic target to reverse EGFR-TKI resistance [177]. Moreover, the lncRNA H19 has been shown to be involved in the regulation of the EGFR signaling pathway. H19 regulates EGFR signaling by binding to the EGFR protein and promoting its degradation. Therefore, lncRNA H19 may play a key role in the response of cancer cells, such as NSCLC, to EGFR-TKIs. Thereby, lncRNA H19 seems to be a key factor in regulating RTK signaling [178]. RTKIs with a similar effect on the mentioned pathways on UCA1 [179], CRNDE [180], and PCAT1 [181] genes lead to the development of other types of NSCLCs. In an alternative mechanism, LncRNA-SARCC functions as a suppressor of the androgen receptor (AR) protein, hindering the propagation of its downstream signals involving AKT, MMP-13, K-RAS, and P-ERK. This obstruction leads to the inhibition of invasiveness in RCC cells while also enhancing their sensitivity to Sunitinib therapy. The findings of this investigation underscore the potential effectiveness of targeting LncRNA-SARCC and its underlying pathway as a viable therapeutic approach for treating RCC. It should be noted, however, that certain RTKIs have been shown to induce the expression of a group of lncRNA molecules, including LncRNA-SARCC, which are associated with improved prognosis and contribute to the restoration of cancer suppression in RCC [182]. Therefore, this evidence could imply that lncRNAs may play an essential role in regulating RTKs.
Altogether, lncRNAs play a crucial role in modulating RTK signaling pathways and the development of cancer. However, comprehensive investigations are necessary to elucidate the intricate mechanisms underlying lncRNA-mediated regulation of RTK signaling and to recognize potential therapeutic targets for improving RTK-associated diseases. Nonetheless, gaining a deeper understanding of these pathways offers a new approach to managing different types of cancer, highlighting the need for more comprehensive investigation (Tables 3, 4).
Circular lncRNAs role in RTK pathway in cancer
CircRNAs are a novel class of endogenous lncRNAs that form covalently closed continuous loops and are generated from pre-mRNA back-splicing events, where a downstream splice donor is joined with an upstream splice acceptor [183]. The biogenesis of circRNAs is facilitated by several factors, including the presence of complementary Alu sequences and RNA-binding proteins like Quaking and Muscleblind [184]. The regulation of circRNA biogenesis, although not fully elucidated, is believed to be influenced by both cis-regulatory elements and trans-acting factors [185]. Importantly, circRNAs have been implicated in various biological processes, including acting as microRNA sponges and interacting with RNA-binding proteins [186] (Fig. 4).
RTK-mediated signaling pathways and associated lncRNAs and circRNAs in solid tumors. lncRNAs and circRNAs target various components of these pathways, including both receptors and downstream signaling cascades. This figure was created using the Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com (accessed 10 July 2023))
Several studies have investigated the association between circular RNA and receptor tyrosine kinase in lung adenocarcinoma. One study demonstrated that hsa_circ_0070661 suppressed cancer progression by regulating the miR-556-5p/TEK axis [187]. Li et al. found that circ_0001058 inhibited lung adenocarcinoma development by modulating the miR-486-5p/TEK signaling pathway [188]. Moreover, Wang et al. discovered that overexpression of hsa_circ_0012673 promoted tumor proliferation by acting as a sponge for miR-22, which led to the upregulation of TEK expression [189]. Overall, these findings suggest that circRNAs regulate TEK signaling in lung adenocarcinoma and may serve as potential therapeutic targets for this disease. Studies have demonstrated a correlation between circular RNA and receptor tyrosine kinase in promoting glioma progression. For instance, circFAM53B promotes glioma proliferation and metastasis by activating the c-MET/PI3K/AKT pathway via sponging miR-532-3p [190]. Circ_0001588 upregulates ERBB4 to promote glioma malignant progression through sponging miR-128, [191] while circ_0001162 enhances cell proliferation and invasion of glioma through the miR-936/ERBB4 axis [192]. Furthermore, circRNA EPHB4 modulates stem properties and proliferation of gliomas via sponging miR-637 and upregulating SOX10, indicating that the RTK pathway may be involved in glioma development and progression mediated by circRNA-miRNA interactions [193]. These results highlight the potential of circRNA as a therapeutic target for glioma treatment and provide insights into the underlying mechanisms of glioma pathogenesis. Also, in other research, various types of cancer have been explored. For example, Circ_0044520 has been found to regulate the progression of laryngeal squamous cell carcinoma via the miR-338-3p/ROR2 axis [194]. Another study shows that circRNA_0006470 promotes gastric cancer cell proliferation and migration by functioning as a sponge of miR-27b-3p [195]. Additionally, tumor-derived exosomal circRNA_102481 has been shown to contribute to EGFR-TKIs resistance in non-small cell lung cancer via the miR-30a-5p/ROR1 axis [196]. NSD2 circular RNA has been found to promote colorectal cancer metastasis by targeting miR-199b-5p-mediated DDR1 and JAG1 signaling [197]. The study by Zheng et al. found that Circ_0079558 promotes papillary thyroid cancer progression by binding to miR-26b-5p, activating MET/AKT signaling [198]. Wang et al. found that the RNA-binding protein IGF2BP2 enhances the plenty of circ_0000745 and promotes the aggressiveness and stemness of ovarian cancer cells by activating the microRNA-3187-3p/ERBB4/PI3K/AKT signaling pathway [199]. Furthermore, circ_LAMP1 has been found to promote T-cell lymphoblastic lymphoma progression by acting as a competing endogenous RNA (ceRNA) for miR-615-5p to regulate DDR2 expression (200).
In developing resistance to specific cancer therapies, circular RNA correlates with receptor tyrosine kinase, as recent studies indicate. A study conducted on lung adenocarcinoma cells found that hsa_circ_0007312 promotes third-generation EGFR-TKI resistance through pyroptosis and apoptosis via the miR-764/MAPK1 axis [201]. Another study identified circ_0014235 as a factor responsible for conferring Gefitinib resistance and malignant behaviors in non-small cell lung cancer (NSCLC) by governing the miR-146b-5p/YAP/PD-L1 pathway [202]. Similarly, hsa_circ_0005576 promotes osimertinib resistance through the miR-512-5p/IGF1R axis in lung adenocarcinoma cells [203]. In contrast, Propofol was shown to suppress lung cancer tumorigenesis by modulating the circ-ERBB2/miR-7-5p/FOXM1 axis [204]. According to a study on non-small cell lung cancer patients receiving Gefitinib therapy, the circular RNA hsa_circ_0109320 showed significantly elevated expression levels in individuals who positively responded to EGFR-TKI treatment [205]. The study by Wang et al. revealed a novel protein encoded by circASK1 that plays a crucial role in overcoming gefitinib resistance in lung adenocarcinoma by competitively activating apoptosis signal-regulating kinase 1 (ASK1)-dependent apoptosis [206]. Finally, circRNA_001895 was found to promote sunitinib resistance to renal cell carcinoma through the regulation of apoptosis and DNA damage repair [207]. These findings have shown that circRNAs can play a crucial role in developing drug resistance in cancer cells, and further research is needed to explore their therapeutic potential (Tables 5, 6).
Future prospect
Recent advances in the field of ncRNA research have revealed the critical role of ncRNAs in the regulation of RTK signaling in solid tumors. Non-coding RNAs have shown great potential as therapeutic targets for the treatment of RTK-associated solid tumors. However, further research is needed to fully understand the intricate mechanisms underlying ncRNA-mediated regulation of RTK signaling and to identify promising therapeutic targets (Fig. 5).
An illustration of RTK-associated ncRNAs with therapeutic application in solid tumors. This figure was created using the Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com (accessed 10 July 2023))
One promising avenue for future research is the development of ncRNA-based therapies, such as miRNA mimics, miRNA inhibitors, and circRNA-based therapeutics. These therapies have shown promising results in preclinical studies and hold great potential for the treatment of solid tumors. Several ncRNA-based therapies have already been developed for the treatment of various diseases, including cancer. For example, the miRNA mimic, miR-34a, is currently being evaluated in clinical trials for the treatment of various solid tumors. Another miRNA mimic, miR-16, has been shown to inhibit the growth of solid tumors in preclinical studies. Additionally, the combination of ncRNA-based therapies with existing conventional therapies, such as chemotherapy and RTK inhibitors, may increase treatment efficacy and reduce resistance.
Another area of future research is the identification of novel ncRNAs involved in RTK signaling pathways. Recent advances in high-throughput sequencing technologies and bioinformatics have enabled the identification of previously unknown ncRNAs and their potential roles in disease pathogenesis. Targeting these novel ncRNAs may provide alternative therapeutic strategies for the treatment of RTK-associated solid tumors.
Furthermore, the development of reliable methods for the delivery of ncRNA-based therapeutics to target tissues remains a challenge. Various delivery methods, such as nanoparticles, liposomes, and exosomes, have been explored for the effective delivery of ncRNA-based therapeutics. However, further optimization is needed to improve their specificity, stability, and efficacy.
ncRNAs can also be used as biomarkers for the diagnosis and prognosis of solid tumors. Several studies have shown that the expression levels of certain ncRNAs are altered in solid tumors compared to normal tissues. For example, the expression of miR-21 is upregulated in various solid tumors and is associated with poor prognosis. The expression of miR-195 is downregulated in various solid tumors and is associated with a better prognosis.
Several methods are currently available to experimentally identify ncRNAs from samples, including enzymatic/chemical RNA sequencing, use of cDNA libraries, microarray analysis, and genomic SELEX. The RNA sequencing methods though not impeded by secondary/tertiary RNA structures (in contrast to cDNA-based methods), have some limitations including the inability to discriminate between RNAs with similar size, and limited size of identified RNAs, and it is limited to highly abundant RNAs. On the other hand, microarray analysis can simultaneously detect multiple RNAs and have great potential. Genomic SELEX methods are based on the generation of RNAs from genomic DNAs in vitro and can be used to identify RNAs that are not necessarily expressed [208].
Furthermore, the development of new technologies, such as single-cell sequencing and CRISPR/Cas9 genome editing, will allow for a more comprehensive understanding of the role of ncRNAs in the regulation of RTK signaling in solid tumors. Single-cell sequencing will enable the identification of ncRNAs that are specifically expressed in tumor cells, which can be targeted for therapy. CRISPR/Cas9 genome editing will allow for the precise manipulation of ncRNA expression levels, which can be used to study the function of ncRNAs in solid tumors.
Conclusion
Non-coding RNAs have emerged as promising therapeutic targets for the treatment of RTK-associated solid tumors. MicroRNAs, circular RNAs, and long ncRNAs have been extensively studied in the context of RTK signaling, and their dysregulation has been implicated in the development and progression of various solid tumors. Future research aims to develop ncRNA-based therapies, identify novel ncRNAs involved in RTK signaling pathways, use of ncRNAs as biomarkers for diagnosis and prognosis, and optimize delivery methods for these therapeutics. These efforts will hold great potential and advance our understanding of the role of ncRNAs in solid tumors, as well as lead to the development of more effective and personalized treatments for patients with solid tumors.
Availability of data and materials
As this is a review article, the data and material analyzed were obtained from previously published sources, and as such, all data analyzed are publicly available. Details of the sources of information used in this review are provided in the reference list. Additionally, any specific materials referenced in this review are obtainable through their respective sources, as cited in the text.
Abbreviations
- pri-miRNA:
-
Primary miRNA
- miRISC:
-
MiRNA-induced silencing complex
- AGO:
-
Argonaute
- EMT:
-
Epithelial mesenchymal transition
- AUC:
-
Area under the curve
- RON:
-
Receptor d’Origine Natais
- Pri-lncRNA:
-
Primary long non-coding RNA
- ADAM17:
-
A Disintegrin and Metalloproteinase 17
- PTEN:
-
Phosphatase and Tensin Homolog
- P53:
-
Tumor Protein P53
- RAS:
-
Rat sarcoma
- BCL2:
-
B-Cell Lymphoma 2
- MET:
-
Mesenchymal-Epithelial Transition Factor
- PRDX2:
-
Peroxiredoxin 2
- DNMT3B:
-
DNA (Cytosine-5)-Methyltransferase 3 Beta
- CDK6:
-
Cyclin-Dependent Kinase 6
- MCL1:
-
Myeloid Cell Leukemia 1
- EGFR:
-
Epidermal Growth Factor Receptor
- HDAC4:
-
Histone Deacetylase 4
- Notch:
-
Neurogenic locus notch homolog protein
- c-MYC:
-
Myelocytomatosis Proto-Oncogene Protein
- MAPK:
-
Mitogen-Activated Protein Kinase
- PI3K/AKT:
-
Phosphoinositide 3-Kinase/Protein Kinase B
- NSCLC:
-
Non-Small Cell Lung Cancer
- NOV340:
-
A type of liposome
- let-7b:
-
MicroRNA let-7b
- MRX34:
-
A therapeutic approach with miR-34 mimics in liposomes
- DOTMA:
-
N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium
- SiO2NPs:
-
Silica Nanoparticles
- HER-2:
-
Human Epidermal Growth Factor Receptor 2
- HDAC4:
-
Histone Deacetylase 4
- HA:
-
Hyaluronic acid
- PEI:
-
Polyethyleneimine
- GAPB/NRF2:
-
Glyceraldehyde-3-Phosphate Dehydrogenase Binding Protein/Nuclear factor erythroid 2-related factor 2
- SESN1:
-
Sestrin 1
- CDK4:
-
Cyclin-dependent kinase 4
- lncRNAs:
-
Long noncoding RNAs
- HCC:
-
Hepatocellular carcinoma
- SCLC:
-
Small cell lung cancer
- RTK:
-
Receptor tyrosine kinase
- miR:
-
MicroRNA
- RCC:
-
Renal cell carcinoma
- circRNA:
-
Circular RNA
- HIF1A:
-
Hypoxia-inducible factor 1-alpha
- ROR1:
-
RTK-like orphan receptor 1
- GAS5:
-
Growth arrest-specific 5
- EIciRNAs:
-
Exon–intron circRNAs
- TF:
-
Transcription factor
- XIST:
-
X-inactive specific transcript
- AXL:
-
AXL receptor tyrosine kinase
- Linc00152:
-
Long intergenic non-protein coding RNA 152
- CALIC:
-
Colon cancer-associated lncRNA in CTCs
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- MAYA:
-
Metastasis-associated Y-chromosome lncRNA
- SPRY4-IT1:
-
SPRY4 intronic transcript 1
- FGFR2:
-
Fibroblast growth factor receptor 2
- GAS5:
-
Growth arrest-specific 5
- HIF1A-AS2:
-
HIF1A antisense RNA 2
- VEGFA:
-
Vascular endothelial growth factor A
- AR:
-
Androgen receptor
- MMP-13:
-
Matrix metalloproteinase-13
- K-RAS:
-
Kirsten rat sarcoma viral oncogene homolog
- P-ERK:
-
Phosphorylated extracellular signal-regulated kinase
- UCA1:
-
Urothelial carcinoma-associated 1
- CRNDE:
-
Colorectal neoplasia differentially expressed
- PCAT1:
-
Prostate cancer-associated transcript 1
- TEK:
-
Tyrosine Kinase
- ERBB4:
-
Erythroblastic Leukemia Viral Oncogene Homolog 4
- EPHB4:
-
Ephrin Type-B Receptor 4
- SOX10:
-
SRY (Sex Determining Region Y)-Box 10
- DDR1:
-
Discoidin Domain Receptor Tyrosine Kinase 1
- JAG1:
-
Jagged 1
- IGF2BP2:
-
Insulin-like growth factor 2 mRNA binding protein 2
- EGFR-TKIs:
-
Epidermal growth factor receptor tyrosine kinase inhibitors
- NSD2:
-
Nuclear Receptor Binding SET Domain Protein 2
- MET:
-
Mesenchymal-Epithelial Transition
- AKT:
-
Protein Kinase B
- MAPK1:
-
Mitogen-Activated Protein Kinase 1
- PD-L1:
-
Programmed Death-Ligand 1
- Gefitinib:
-
Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
- YAP:
-
Yes-Associated Protein
- circ-ERBB2:
-
Circular RNA derived from ERBB2 gene
- FOXM1:
-
Forkhead Box Protein M1
- ASK1:
-
Apoptosis signal-regulating kinase 1
- circASK1:
-
Circular RNA derived from ASK1 gene
- tsRNA:
-
Transfer RNA-derived small RNA
- piRNA:
-
PIWI-interacting RNA
References
Schlessinger JJC. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25.
Pawson T. Regulation and targets of receptor tyrosine kinases. Eur J Cancer. 2002;38:S3–10.
Aveic S, Tonini GP. Resistance to receptor tyrosine kinase inhibitors in solid tumors: can we improve the cancer fighting strategy by blocking autophagy? Cancer Cell Int. 2016;16:62.
Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2(87): re6.
Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets. 2012;12(3):197–209.
Mabeta P, Steenkamp V. The VEGF/VEGFR axis revisited: implications for cancer therapy. Int J Mol Sci. 2022;23(24):14485.
Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97–107.
Zou X, Tang XY, Qu ZY, Sun ZW, Ji CF, Li YJ, et al. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: a review. Int J Biol Macromol. 2022;202:539–57.
Sheikh E, Tran T, Vranic S, Levy A, Bonfil RD. Role and significance of c-KIT receptor tyrosine kinase in cancer: a review. Bosn J Basic Med Sci. 2022;22(5):683–98.
Giglio S, Vecchione A. c-Met and miRs in cancer. Biomedicines. 2015;3(1):32–44.
Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17:1–13.
Ebrahimi N, Fardi E, Ghaderi H, Palizdar S, Khorram R, Vafadar R, et al. Receptor tyrosine kinase inhibitors in cancer. Cell Mol Life Sci. 2023;80(4):104.
Hopkins BD, Hodakoski C, Barrows D, Mense SM, Parsons RE. PTEN function: the long and the short of it. Trends Biochem Sci. 2014;39(4):183–90.
Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179(5):1033–55.
Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–21.
Mirzaei S, Gholami MH, Hushmandi K, Hashemi F, Zabolian A, Canadas I, et al. The long and short non-coding RNAs modulating EZH2 signaling in cancer. J Hematol Oncol. 2022;15(1):18.
Zeng X, Xiao J, Bai X, Liu Y, Zhang M, Liu J, et al. Research progress on the circRNA/lncRNA–miRNA–mRNA axis in gastric cancer. Pathol Res Pract. 2022;238: 154030.
Chen B, Dragomir MP, Yang C, Li Q, Horst D, Calin GA, et al. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7(1):121.
Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(supp_1):R17–29.
Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–8.
Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev. 2018;18(1):5–18.
Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(2):321–37.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54.
Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14(4): dmm047662.
Menigatti M, Staiano T, Manser CN, Bauerfeind P, Komljenovic A, Robinson M, et al. Epigenetic silencing of monoallelically methylated miRNA loci in precancerous colorectal lesions. Oncogenesis. 2013;2(7): e56.
Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R, et al. Circulating microRNAs in cancer: potential and challenge. Front Genet. 2019;10:626.
de Kock L, Rivera B, Revil T, Thorner P, Goudie C, Bouron-Dal Soglio D, et al. Sequencing of DICER1 in sarcomas identifies biallelic somatic DICER1 mutations in an adult-onset embryonal rhabdomyosarcoma. Br J Cancer. 2017;116(12):1621–6.
Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227.
Donzelli S, Cioce M, Muti P, Strano S, Yarden Y, Blandino G. MicroRNAs: non-coding fine tuners of receptor tyrosine kinase signalling in cancer. Semin Cell Dev Biol. 2016;50:133–42.
Jiang Z, Jiang C, Yu C, Fang J. MicroRNA-208b inhibits human osteosarcoma progression by targeting ROR2. Tumour Biol. 2017;39(6):1010428317705751.
Tao Y, Yang S, Wu Y, Fang X, Wang Y, Song Y, et al. MicroRNA-216a inhibits the metastasis of gastric cancer cells by targeting JAK2/STAT3-mediated EMT process. Oncotarget. 2017;8(51):88870–81.
Li WJ, Wang Y, Liu R, Kasinski AL, Shen H, Slack FJ, et al. MicroRNA-34a: potent tumor suppressor, cancer stem cell inhibitor, and potential anticancer therapeutic. Front Cell Dev Biol. 2021;9: 640587.
Li C, Wang Y, Lu S, Zhang Z, Meng H, Liang L, et al. MiR-34a inhibits colon cancer proliferation and metastasis by inhibiting platelet-derived growth factor receptor α. Mol Med Rep. 2015;12(5):7072–8.
Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH, Ambs S, Caplen NJ. Identification of the receptor tyrosine kinase AXL in breast cancer as a target for the human miR-34a microRNA. Breast Cancer Res Treat. 2011;130(2):663–79.
Wei B, Huang QY, Huang SR, Mai W, Zhong XG. MicroRNA-34a attenuates the proliferation, invasion and metastasis of gastric cancer cells via downregulation of MET. Mol Med Rep. 2015;12(4):5255–61.
Xiong R, Sun XX, Wu HR, Xu GW, Wang GX, Sun XH, et al. Mechanism research of miR-34a regulates Axl in non-small-cell lung cancer with gefitinib-acquired resistance. Thorac Cancer. 2020;11(1):156–65.
Yamamura S, Saini S, Majid S, Hirata H, Ueno K, Deng G, et al. MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS ONE. 2012;7(1): e29722.
Shabani N, Razaviyan J, Paryan M, Tavangar SM, Azizi F, Mohammadi-Yeganeh S, et al. Evaluation of miRNAs expression in medullary thyroid carcinoma tissue samples: miR-34a and miR-144 as promising overexpressed markers in MTC. Hum Pathol. 2018;79:212–21.
Shabani N, Sheikholeslami S, Paryan M, Zarif Yeganeh M, Tavangar SM, Azizi F, et al. An investigation on the expression of miRNAs including miR-144 and miR-34a in plasma samples of RET-positive and RET-negative medullar thyroid carcinoma patients. J Cell Physiol. 2020;235(2):1366–73.
Lim D, Cho JG, Yun E, Lee A, Ryu HY, Lee YJ, et al. MicroRNA 34a-AXL axis regulates vasculogenic mimicry formation in breast cancer Cells. Genes. 2020;12(1):9.
Mohammady M, Ghetmiri SI, Baharizade M, Morowvat MH, Torabi S. Expanding the biotherapeutics realm via miR-34a: “potent clever little” agent in breast cancer therapy. Curr Pharm Biotechnol. 2019;20(8):665–73.
Imani S, Zhang X, Hosseinifard H, Fu S, Fu J. The diagnostic role of microRNA-34a in breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(14):23177–87.
Han TY, Hou LS, Li JX, Huan ML, Zhou SY, Zhang BL. Bone targeted miRNA delivery system for miR-34a with enhanced anti-tumor efficacy to bone-associated metastatic breast cancer. Int J Pharm. 2023;635: 122755.
Xie X, Chen Y, Chen Z, Feng Y, Wang J, Li T, et al. Polymeric hybrid nanomicelles for cancer theranostics: an efficient and precise anticancer strategy for the codelivery of doxorubicin/miR-34a and magnetic resonance Imaging. ACS Appl Mater Interfaces. 2019;11(47):43865–78.
Wang S, Cao M, Deng X, Xiao X, Yin Z, Hu Q, et al. Degradable hyaluronic acid/protamine sulfate interpolyelectrolyte complexes as miRNA-delivery nanocapsules for triple-negative breast cancer therapy. Adv Healthc Mater. 2015;4(2):281–90.
Zhang H, Li Y, Rao F, Liufu C, Wang Y, Chen Z. A novel UTMD system facilitating nucleic acid delivery into MDA-MB-231 cells. Biosci Rep. 2020;40(2): BSR20192573.
Wang J, Feng W, Dong Y, Mao X, Guo F, Luo F. MicroRNA-495 regulates human gastric cancer cell apoptosis and migration through Akt and mTOR signaling. Oncol Rep. 2018;40(6):3654–62.
Li N, Han M, Zhou N, Tang Y, Tang XS. MicroRNA-495 confers increased sensitivity to chemotherapeutic agents in gastric cancer via the mammalian target of rapamycin (mTOR) signaling pathway by interacting with human epidermal growth factor receptor 2 (ERBB2). Med Sci Monit. 2018;24:5960–72.
Cheng C, Qin Y, Zhi Q, Wang J, Qin C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107(Pt B):2620–9.
Cao W, Yang W, Fan R, Li H, Jiang J, Geng M, et al. miR-34a regulates cisplatin-induce gastric cancer cell death by modulating PI3K/AKT/survivin pathway. Tumour Biol. 2014;35(2):1287–95.
Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284(9):5731–41.
Kong X, Li G, Yuan Y, He Y, Wu X, Zhang W, et al. MicroRNA-7 inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via targeting FAK expression. PLoS ONE. 2012;7(8): e41523.
Cui YX, Bradbury R, Flamini V, Wu B, Jordan N, Jiang WG. MicroRNA-7 suppresses the homing and migration potential of human endothelial cells to highly metastatic human breast cancer cells. Br J Cancer. 2017;117(1):89–101.
Lee KM, Choi EJ, Kim IA. microRNA-7 increases radiosensitivity of human cancer cells with activated EGFR-associated signaling. Radiother Oncol. 2011;101(1):171–6.
Xu N, Lian YJ, Dai X, Wang YJ. miR-7 increases cisplatin sensitivity of gastric cancer cells through suppressing mTOR. Technol Cancer Res Treat. 2017;16(6):1022–30.
Zhao X, Dou W, He L, Liang S, Tie J, Liu C, et al. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32(11):1363–72.
Lin J, Liu Z, Liao S, Li E, Wu X, Zeng W. Elevated microRNA-7 inhibits proliferation and tumor angiogenesis and promotes apoptosis of gastric cancer cells via repression of Raf-1. Cell Cycle. 2020;19(19):2496–508.
Expression of Concern. Elevated microRNA-7 inhibits proliferation and tumor angiogenesis and promotes apoptosis of gastric cancer cells via repression of Raf-1. Cell Cycle. 2021;20(24):2672.
Ye T, Yang M, Huang D, Wang X, Xue B, Tian N, et al. MicroRNA-7 as a potential therapeutic target for aberrant NF-κB-driven distant metastasis of gastric cancer. J Exp Clin Cancer Res. 2019;38(1):55.
Gu DN, Huang Q, Tian L. The molecular mechanisms and therapeutic potential of microRNA-7 in cancer. Expert Opin Ther Targets. 2015;19(3):415–26.
Zhao JG, Men WF, Tang J. MicroRNA-7 enhances cytotoxicity induced by gefitinib in non-small cell lung cancer via inhibiting the EGFR and IGF1R signalling pathways. Contemp Oncol (Pozn). 2015;19(3):201–6.
Cao Q, Mao ZD, Shi YJ, Chen Y, Sun Y, Zhang Q, et al. MicroRNA-7 inhibits cell proliferation, migration and invasion in human non-small cell lung cancer cells by targeting FAK through ERK/MAPK signaling pathway. Oncotarget. 2016;7(47):77468–81.
Kogo R, Mimori K, Tanaka F, Komune S, Mori M. Clinical significance of miR-146a in gastric cancer cases. Clin Cancer Res. 2011;17(13):4277–84.
Zhou N, Qu Y, Xu C, Tang Y. Upregulation of microRNA-375 increases the cisplatin-sensitivity of human gastric cancer cells by regulating ERBB2. Exp Ther Med. 2016;11(2):625–30.
Chen Y, Zhou B, Xu L, Fan H, Xie J, Wang D. MicroRNA-146a promotes gastric cancer cell apoptosis by targeting transforming growth factor β-activated kinase 1. Mol Med Rep. 2017;16(1):755–63.
Jiang P, Liang B, Zhang Z, Fan B, Zeng L, Zhou Z, et al. MicroRNA-146a-5p induces cell cycle arrest and enhances apoptosis in gastric cancer via targeting CDC14A. Front Cell Dev Biol. 2023;11:1181628.
Hou Z, Xie L, Yu L, Qian X, Liu B. MicroRNA-146a is down-regulated in gastric cancer and regulates cell proliferation and apoptosis. Med Oncol. 2012;29(2):886–92.
Yao Q, Tu C, Lu D, Zou Y, Liu H, Zhang S. Clinicopathological significance of the microRNA-146a/WASP-family verprolin-homologous protein-2 axis in gastric cancer. Cancer Sci. 2017;108(7):1285–92.
Luo Z, Li X, Zhao Z, Yang X, Xiao S, Zhou Y. MicroRNA-146a affects the chemotherapeutic sensitivity and prognosis of advanced gastric cancer through the regulation of LIN52. Oncol Lett. 2017;13(3):1386–92.
Lian S, Park JS, Xia Y, Nguyen TT, Joo YE, Kim KK, et al. MicroRNA-375 functions as a tumor-suppressor gene in gastric cancer by targeting recepteur d’Origine Nantais. Int J Mol Sci. 2016;17(10):1633.
Yan XL, Luo QY, Zhou SN, Pan WT, Zhang L, Yang DJ, et al. MicroRNA-375 reverses the expression of PD-L1 by inactivating the JAK2/STAT3 signaling pathways in gastric cancer. Clin Res Hepatol Gastroenterol. 2021;45(5): 101574.
Chen B, Guo S, Yu Z, Feng Y, Hui L. Downregulation of microRNA-375, combined with upregulation of its target gene Janus kinase 2, predicts unfavorable prognosis in patients with gastric cancer. Int J Clin Exp Pathol. 2017;10(11):11106–13.
Li Y, Jiang Q, Xia N, Yang H, Hu C. Decreased expression of microRNA-375 in nonsmall cell lung cancer and its clinical significance. J Int Med Res. 2012;40(5):1662–9.
Chen LJ, Li XY, Zhao YQ, Liu WJ, Wu HJ, Liu J, et al. Down-regulated microRNA-375 expression as a predictive biomarker in non-small cell lung cancer brain metastasis and its prognostic significance. Pathol Res Pract. 2017;213(8):882–8.
Yu H, Jiang L, Sun C, Li Guo L, Lin M, Huang J, et al. Decreased circulating miR-375: a potential biomarker for patients with non-small-cell lung cancer. Gene. 2014;534(1):60–5.
Chen WJ, Gan TQ, Qin H, Huang SN, Yang LH, Fang YY, et al. Implication of downregulation and prospective pathway signaling of microRNA-375 in lung squamous cell carcinoma. Pathol Res Pract. 2017;213(4):364–72.
Ren J, Huang HJ, Gong Y, Yue S, Tang LM, Cheng SY. MicroRNA-206 suppresses gastric cancer cell growth and metastasis. Cell Biosci. 2014;4:26.
Chen Z, Gao YJ, Hou RZ, Ding DY, Song DF, Wang DY, et al. MicroRNA-206 facilitates gastric cancer cell apoptosis and suppresses cisplatin resistance by targeting MAPK2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):171–80.
Zheng Z, Yan D, Chen X, Huang H, Chen K, Li G, et al. MicroRNA-206: effective inhibition of gastric cancer progression through the c-Met pathway. PLoS ONE. 2015;10(7): e0128751.
Zhou Y, Cai W, Lu H. Overexpression of microRNA-145 enhanced docetaxel sensitivity in breast cancer cells via inactivation of protein kinase B gamma-mediated phosphoinositide 3-kinase -protein kinase B pathway. Bioengineered. 2022;13(4):11310–20.
Zheng M, Sun X, Li Y, Zuo W. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol. 2016;37(6):8189–96.
Kim SJ, Oh JS, Shin JY, Lee KD, Sung KW, Nam SJ, et al. Development of microRNA-145 for therapeutic application in breast cancer. J Control Release. 2011;155(3):427–34.
Biagioni F, Bossel Ben-Moshe N, Fontemaggi G, Yarden Y, Domany E, Blandino G. The locus of microRNA-10b: a critical target for breast cancer insurgence and dissemination. Cell Cycle. 2013;12(15):2371–5.
Han M, Wang F, Gu Y, Pei X, Guo G, Yu C, et al. MicroRNA-21 induces breast cancer cell invasion and migration by suppressing smad7 via EGF and TGF-β pathways. Oncol Rep. 2016;35(1):73–80.
Fang H, Xie J, Zhang M, Zhao Z, Wan Y, Yao Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am J Transl Res. 2017;9(3):953–61.
Chen J, Wang X. MicroRNA-21 in breast cancer: diagnostic and prognostic potential. Clin Transl Oncol. 2014;16(3):225–33.
Lai CY, Yeh KY, Lin CY, Hsieh YW, Lai HH, Chen JR, et al. MicroRNA-21 plays multiple oncometabolic roles in the process of NAFLD-related hepatocellular carcinoma via PI3K/AKT, TGF-β, and STAT3 signaling. Cancers. 2021;13(5):940.
Bharali D, Banerjee BD, Bharadwaj M, Husain SA, Kar P. Expression analysis of microRNA-21 and microRNA-122 in hepatocellular carcinoma. J Clin Exp Hepatol. 2019;9(3):294–301.
Qu J, Yang J, Chen M, Cui L, Wang T, Gao W, et al. MicroRNA-21 as a diagnostic marker for hepatocellular carcinoma: a systematic review and meta-analysis. Pak J Med Sci. 2019;35(5):1466–71.
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta. 2010;411(11–12):846–52.
Wang W, Li X, Liu C, Zhang X, Wu Y, Diao M, et al. MicroRNA-21 as a diagnostic and prognostic biomarker of lung cancer: a systematic review and meta-analysis. Biosci Rep. 2022;42(5):BSR0211653.
Xue X, Liu Y, Wang Y, Meng M, Wang K, Zang X, et al. MiR-21 and MiR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget. 2016;7(51):84508–19.
Xu S, Shi L. High expression of miR-155 and miR-21 in the recurrence or metastasis of non-small cell lung cancer. Oncol Lett. 2019;18(1):758–63.
Shao C, Yang F, Qin Z, Jing X, Shu Y, Shen H. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer: a systematic review with meta-analysis. BMC Cancer. 2019;19(1):1103.
Wang J, Wang Q, Guan Y, Sun Y, Wang X, Lively K, et al. Breast cancer cell-derived microRNA-155 suppresses tumor progression via enhancing immune cell recruitment and antitumor function. J Clin Investag. 2022;132(19): e157248.
Zhang G, Zhong L, Luo H, Wang S. MicroRNA-155-3p promotes breast cancer progression through down-regulating CADM1. Onco Targets Ther. 2019;12:7993–8002.
Hosseini Mojahed F, Aalami AH, Pouresmaeil V, Amirabadi A, Qasemi Rad M, Sahebkar A. Clinical evaluation of the diagnostic role of microRNA-155 in breast cancer. Int J Genomics. 2020;2020:9514831.
Li D, Liu X, Lin L, Hou J, Li N, Wang C, et al. MicroRNA-99a inhibits hepatocellular carcinoma growth and correlates with prognosis of patients with hepatocellular carcinoma. J Biol Chem. 2011;286(42):36677–85.
Ning S, Liu H, Gao B, Wei W, Yang A, Li J, et al. miR-155, miR-96 and miR-99a as potential diagnostic and prognostic tools for the clinical management of hepatocellular carcinoma. Oncol Lett. 2019;18(3):3381–7.
Cai C, Xie Y, Wu L, Chen X, Liu H, Zhou Y, et al. PLGA-based dual targeted nanoparticles enhance miRNA transfection efficiency in hepatic carcinoma. Sci Rep. 2017;7:46250.
Sheng L, He P, Yang X, Zhou M, Feng Q. miR-612 negatively regulates colorectal cancer growth and metastasis by targeting AKT2. Cell Death Dis. 2015;6(7): e1808.
Zhu SH, He XC, Wang L. Correlation analysis of miR-200b, miR-200c, and miR-141 with liver metastases in colorectal cancer patients. Eur Rev Med Pharmacol Sci. 2017;21(10):2357–63.
Chen X, Zeng K, Xu M, Liu X, Hu X, Xu T, et al. P53-induced miR-1249 inhibits tumor growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2. Cell Death Dis. 2019;10(2):131.
Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui H, et al. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7(10): e2413.
Xu Z, Zhu C, Chen C, Zong Y, Feng H, Liu D, et al. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018;9(10):974.
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395.
Qian X, Yu J, Yin Y, He J, Wang L, Li Q, et al. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12(9):1385–94.
Zhang Y, Wang X, Xu B, Wang B, Wang Z, Liang Y, et al. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol Rep. 2013;30(4):1976–84.
Tsang WP, Kwok TT. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30(6):953–9.
Han HB, Gu J, Zuo HJ, Chen ZG, Zhao W, Li M, et al. Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J Pathol. 2012;226(3):544–55.
Wang MJ, Li Y, Wang R, Wang C, Yu YY, Yang L, et al. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int J Colorectal Dis. 2013;28(2):183–9.
Zhang Z, Wang J, Cheng J, Yu X. Effects of miR-126 on the STAT3 signaling pathway and the regulation of malignant behavior in lung cancer cells. Oncol Lett. 2018;15(6):8412–6.
Shi H, Bi H, Sun X, Dong H, Jiang Y, Mu H, et al. Antitumor effects of tubeimoside-1 in NCI-H1299 cells are mediated by microRNA-126-5p-induced inactivation of VEGF-A/VEGFR-2/ERK signaling pathway. Mol Med Rep. 2018;17(3):4327–36.
Jia Z, Zhang Y, Xu Q, Guo W, Guo A. miR-126 suppresses epithelial-to-mesenchymal transition and metastasis by targeting PI3K/AKT/Snail signaling of lung cancer cells. Oncol Lett. 2018;15(5):7369–75.
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71.
Hou L, Luo P, Ma Y, Jia C, Yu F, Lv Z, et al. MicroRNA-125a-3p downregulation correlates with tumorigenesis and poor prognosis in patients with non-small cell lung cancer. Oncol Lett. 2017;14(4):4441–8.
Huang H, Huang J, Yao J, Li N, Yang Z. miR-125a regulates HAS1 and inhibits the proliferation, invasion and metastasis by targeting STAT3 in non-small cell lung cancer cells. J Cell Biochem. 2020;121(5–6):3197–207.
Yagishita S, Fujita Y, Kitazono S, Ko R, Nakadate Y, Sawada T, et al. Chemotherapy-regulated microRNA-125-HER2 pathway as a novel therapeutic target for trastuzumab-mediated cellular cytotoxicity in small cell lung cancer. Mol Cancer Ther. 2015;14(6):1414–23.
Khan P, Ebenezer NS, Siddiqui JA, Maurya SK, Lakshmanan I, Salgia R, et al. MicroRNA-1: diverse role of a small player in multiple cancers. Semin Cell Dev Biol. 2022;124:114–26.
Qu W, Chen X, Wang J, Lv J, Yan D. MicroRNA-1 inhibits ovarian cancer cell proliferation and migration through c-Met pathway. Clin Chim Acta. 2017;473:237–44.
Migliore C, Martin V, Leoni VP, Restivo A, Atzori L, Petrelli A, et al. MiR-1 downregulation cooperates with MACC1 in promoting MET overexpression in human colon cancer. Clin Cancer Res. 2012;18(3):737–47.
Reid JF, Sokolova V, Zoni E, Lampis A, Pizzamiglio S, Bertan C, et al. miRNA profiling in colorectal cancer highlights miR-1 involvement in MET-dependent proliferation. Mol Cancer Res. 2012;10(4):504–15.
Jiang S, Zhao C, Yang X, Li X, Pan Q, Huang H, et al. miR-1 suppresses the growth of esophageal squamous cell carcinoma in vivo and in vitro through the downregulation of MET, cyclin D1 and CDK4 expression. Int J Mol Med. 2016;38(1):113–22.
Koshizuka K, Hanazawa T, Fukumoto I, Kikkawa N, Matsushita R, Mataki H, et al. Dual-receptor (EGFR and c-MET) inhibition by tumor-suppressive miR-1 and miR-206 in head and neck squamous cell carcinoma. J Hum Genet. 2017;62(1):113–21.
Novello C, Pazzaglia L, Cingolani C, Conti A, Quattrini I, Manara MC, et al. miRNA expression profile in human osteosarcoma: role of miR-1 and miR-133b in proliferation and cell cycle control. Int J Oncol. 2013;42(2):667–75.
Chang YS, Chen WY, Yin JJ, Sheppard-Tillman H, Huang J, Liu YN. EGF receptor promotes prostate cancer bone metastasis by downregulating miR-1 and activating TWIST1. Cancer Res. 2015;75(15):3077–86.
Chiu KL, Lin YS, Kuo TT, Lo CC, Huang YK, Chang HF, et al. ADAM9 enhances CDCP1 by inhibiting miR-1 through EGFR signaling activation in lung cancer metastasis. Oncotarget. 2017;8(29):47365–78.
Han C, Zhou Y, An Q, Li F, Li D, Zhang X, et al. MicroRNA-1 (miR-1) inhibits gastric cancer cell proliferation and migration by targeting MET. Tumour Biol. 2015;36(9):6715–23.
Yan D, Dong Xda E, Chen X, Wang L, Lu C, Wang J, et al. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem. 2009;284(43):29596–604.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62.
Baboci L, Capolla S, Di Cintio F, Colombo F, Mauro P, Dal Bo M, et al. The dual role of the liver in nanomedicine as an actor in the elimination of nanostructures or a therapeutic target. J Oncol. 2020;2020:4638192.
Alehossein P, Taheri M, Tayefeh Ghahremani P, Dakhlallah D, Brown CM, Ishrat T, et al. Transplantation of exercise-induced extracellular vesicles as a promising therapeutic approach in ischemic stroke. Transl Stroke Res. 2023;14(2):211–37.
Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu C-G, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Can Res. 2007;67(13):6092–9.
Tsai W-C, Hsu PW-C, Lai T-C, Chau G-Y, Lin C-W, Chen C-M, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49(5):1571–82.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58.
Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, et al. Association of microRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14(2):419–27.
Panebianco F, Climent M, Malvindi MA, Pompa PP, Bonetti P, Nicassio F. Delivery of biologically active miR-34a in normal and cancer mammary epithelial cells by synthetic nanoparticles. Nanomed Nanotechnol Biol Med. 2019;19:95–105.
Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Can Res. 2010;70(14):5923–30.
Kasinski AL, Kelnar K, Stahlhut C, Orellana E, Zhao J, Shimer E, et al. A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene. 2015;34(27):3547–55.
Cortez MA, Valdecanas D, Zhang X, Zhan Y, Bhardwaj V, Calin GA, et al. Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Mol Ther. 2014;22(8):1494–503.
Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y-K, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35:180–8.
Hong DS, Kang Y-K, Borad M, Sachdev J, Ejadi S, Lim HY, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122(11):1630–7.
Wu Y, Crawford M, Mao Y, Lee RJ, Davis IC, Elton TS, et al. Therapeutic delivery of MicroRNA-29b by cationic lipoplexes for lung cancer. Mol Ther Nucleic Acids. 2013;2(4): e84.
van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18(10):1386–96.
Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai CY, Hou MF, et al. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2015;6(1):494–509.
Hayward SL, Francis DM, Kholmatov P, Kidambi S. Targeted delivery of microRNA125a-5p by engineered lipid nanoparticles for the treatment of HER2 positive metastatic breast cancer. J Biomed Nanotechnol. 2016;12(3):554–68.
Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35(14):4333–44.
Imani S, Wu R-C, Fu J. MicroRNA-34 family in breast cancer: from research to therapeutic potential. J Cancer. 2018;9(20):3765.
Zhi F, Dong H, Jia X, Guo W, Lu H, Yang Y, et al. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro. PLoS ONE. 2013;8(3): e60034.
Yu Y, Yao Y, Yan H, Wang R, Zhang Z, Sun X, et al. A tumor-specific microRNA recognition system facilitates the accurate targeting to tumor cells by magnetic nanoparticles. Mol Ther Nucleic Acids. 2016;5(5): e318.
Kim HK, Fuchs G, Wang S, Wei W, Zhang Y, Park H, et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature. 2017;552(7683):57–62.
Iwasaki YW, Siomi MC, Siomi H. PIWI-interacting RNA: its biogenesis and functions. Annu Rev Biochem. 2015;84:405–33.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66.
Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62.
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–7.
Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46.
Hong Q, Li O, Zheng W, Xiao W-Z, Zhang L, Wu D, et al. LncRNA HOTAIR regulates HIF-1α/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis. 2017;8(5): e2772.
Li Q, Wang X, Jiang N, Xie X, Liu N, Liu J, et al. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med. 2020;9(17):6354–66.
Yu X, Lin Q, Liu F, Yang F, Mao J, Chen X. LncRNA TMPO-AS1 facilitates the proliferation and metastasis of NSCLC cells by up-regulating ERBB2 via sponging miR-204-3p. Int J Immunopathol Pharmacol. 2020;34:2058738420958947.
Zhang H, Liao Z, Liu F, Su C, Zhu H, Li Y, et al. Long noncoding RNA HULC promotes hepatocellular carcinoma progression. Aging (Albany NY). 2019;11(20):9111.
Yang L, Cao M, Zhang J, Li X, Sun Q. LncRNA XIST modulates HIF-1A/AXL signaling pathway by inhibiting miR-93-5p in colorectal cancer. Mol Genet Genomic Med. 2020;8(4): e1112.
Zhang R, Wang Z, Yu Q, Shen J, He W, Zhou D, et al. Atractylenolide II reverses the influence of lncRNA XIST/miR-30a-5p/ROR1 axis on chemo-resistance of colorectal cancer cells. J Cell Mol Med. 2019;23(5):3151–65.
Yue B, Cai D, Liu C, Fang C, Yan D. Linc00152 functions as a competing endogenous RNA to confer oxaliplatin resistance and holds prognostic values in colon cancer. Mol Ther. 2016;24(12):2064–77.
Vennin C, Spruyt N, Dahmani F, Julien S, Bertucci F, Finetti P, et al. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget. 2015;6(30):29209.
Bi S, Wang C, Li Y, Zhang W, Zhang J, Lv Z, et al. LncRNA-MALAT1-mediated Axl promotes cell invasion and migration in human neuroblastoma. Tumor Biol. 2017;39(5):1010428317699796.
Kawasaki Y, Miyamoto M, Oda T, Matsumura K, Negishi L, Nakato R, et al. The novel lnc RNA CALIC upregulates AXL to promote colon cancer metastasis. EMBO Rep. 2019;20(8): e47052.
Samimi H, Haghpanah V, Irani S, Arefian E, Sohi AN, Fallah P, et al. Transcript-level regulation of MALAT1-mediated cell cycle and apoptosis genes using dual MEK/Aurora kinase inhibitor “BI-847325” on anaplastic thyroid carcinoma. DARU J Pharm Sci. 2019;27:1–7.
Li C, Wang S, Xing Z, Lin A, Liang K, Song J, et al. A ROR1–HER3–lncRNA signalling axis modulates the Hippo–YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19(2):106–19.
Drak Alsibai K, Meseure D. Tumor microenvironment and noncoding RNAs as co-drivers of epithelial–mesenchymal transition and cancer metastasis. Dev Dyn. 2018;247(3):405–31.
Yan J, Xu C, Li Y, Tang B, Xie S, Hong T, et al. Long non-coding RNA LINC00526 represses glioma progression via forming a double negative feedback loop with AXL. J Cell Mol Med. 2019;23(8):5518–31.
Sas-Chen A, Aure MR, Leibovich L, Carvalho S, Enuka Y, Körner C, et al. LIMT is a novel metastasis inhibiting lnc RNA suppressed by EGF and downregulated in aggressive breast cancer. EMBO Mol Med. 2016;8(9):1052–64.
Huang Z, Zhou JK, Peng Y, He W, Huang C. The role of long noncoding RNAs in hepatocellular carcinoma. Molecular cancer. 2020;19:1–8. https://doi.org/10.1186/s12943-020-01188-4.
Wang Y, Liu Z, Yao B, Dou C, Xu M, Xue Y, et al. Long non-coding RNA TUSC7 acts a molecular sponge for miR-10a and suppresses EMT in hepatocellular carcinoma. Tumor Biol. 2016;37:11429–41.
Ding D, Zhang J, Luo Z, Wu H, Lin Z, Liang W, et al. Analysis of the lncRNA–miRNA–mRNA network reveals a potential regulatory mechanism of EGFR-TKI resistance in NSCLC. Front Genet. 2022;13: 851391.
Si J, Ma Y, Lv C, Hong Y, Tan H, Yang Y. HIF1A-AS2 induces osimertinib resistance in lung adenocarcinoma patients by regulating the miR-146b-5p/IL-6/STAT3 axis. Mol Ther Nucleic Acids. 2021;26:613–24.
Dong S, Qu X, Li W, Zhong X, Li P, Yang S, et al. The long non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J Hematol Oncol. 2015;8(1):1–13.
Lv P, Yang S, Liu W, Qin H, Tang X, Wu F, et al. Circulating plasma lncRNAs as novel markers of EGFR mutation status and monitors of epidermal growth factor receptor-tyrosine kinase inhibitor therapy. Thorac Cancer. 2020;11(1):29–40.
Cheng N, Cai W, Ren S, Li X, Wang Q, Pan H, et al. Long non-coding RNA UCA1 induces non-T790M acquired resistance to EGFR-TKIs by activating the AKT/mTOR pathway in EGFR-mutant non-small cell lung cancer. Oncotarget. 2015;6(27):23582.
Takahashi S, Noro R, Seike M, Zeng C, Matsumoto M, Yoshikawa A, et al. Long non-coding RNA CRNDE is involved in resistance to EGFR tyrosine kinase inhibitor in EGFR-mutant lung cancer via eIF4A3/MUC1/EGFR signaling. Int J Mol Sci. 2021;22(8):4005.
Wang S, Liu C, Lei Q, Wu Z, Miao X, Zhu D, et al. Relationship between long non-coding RNA PCAT-1 expression and gefitinib resistance in non-small-cell lung cancer cells. Respir Res. 2021;22(1):1–10.
Zhai W, Sun Y, Guo C, Hu G, Wang M, Zheng J, et al. LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor (AR)/miRNA-143-3p signals. Cell Death Differ. 2017;24(9):1502–17.
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141–57.
Li X, Liu C-X, Xue W, Zhang Y, Jiang S, Yin Q-F, et al. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell. 2017;67(2):214–27.
Chen L-L, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–8.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8.
Chen Y, Wu Y. Hsa_circ_0070661 inhibits cancer progression through miR-556–5p/TEK axis in lung adenocarcinoma. Cancer Biomark. 2023;37:1–13.
Li W, Wang H, Zheng Y. Circ_0001058 represses the progression of lung adenocarcinoma through governing of the miR-486-5p/TEK signaling axis. Anticancer Drugs. 2022;33(8):710–9.
Wang X, Zhu X, Zhang H, Wei S, Chen Y, Chen Y, et al. Increased circular RNA hsa_circ_0012673 acts as a sponge of miR-22 to promote lung adenocarcinoma proliferation. Biochem Biophys Res Commun. 2018;496(4):1069–75.
Pei J, Dou H, Deng X. CircFAM53B promotes the proliferation and metastasis of glioma through activating the c-MET/PI3K/AKT pathway via sponging miR-532-3p. Cell Cycle. 2022;21(5):462–76.
Wang J, Li J, Duan P, Dang Y, Shi T. Circ_0001588 upregulates erbb4 to promote glioma malignant progression through sponging mir-1281. Neurotox Res. 2022;89:1–14.
Zhou D, Lin X, Wang P, Yang Y, Zheng J, Zhou D. Circular RNA circ_0001162 promotes cell proliferation and invasion of glioma via the miR-936/ERBB4 axis. Bioengineered. 2021;12(1):2106–18.
Jin C, Zhao J, Zhang ZP, Wu M, Li J, Liu B, et al. CircRNA EPHB4 modulates stem properties and proliferation of gliomas via sponging miR-637 and up-regulating SOX10. Mol Oncol. 2021;15(2):596–622.
Yang H, Yu G, Wang Y, Guo X. Circ_0044520 regulates the progression of laryngeal squamous cell carcinoma via the miR-338–3p/ROR2 axis. Histol Histopathol. 2022;37(6):513–26.
Yejia C, Jin C, Huang S, Jinjun Y, Huang H, Dan L, et al. circRNA_0006470 promotes the proliferation and migration of gastric cancer cells by functioning as a sponge of miR-27b-3p. Neoplasma. 2021;68(6):1245.
Yang B, Teng F, Chang L, Wang J, Liu D-L, Cui Y-S, et al. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging (Albany NY). 2021;13(9):13264.
Chen LY, Zhi Z, Wang L, Zhao YY, Deng M, Liu YH, et al. NSD2 circular RNA promotes metastasis of colorectal cancer by targeting miR-199b-5p-mediated DDR1 and JAG1 signalling. J Pathol. 2019;248(1):103–15.
Zheng H, Fu Q, Ma K, Shi S, Fu Y. Circ_0079558 promotes papillary thyroid cancer progression by binding to miR-26b-5p to activate MET/AKT signaling. Endocr J. 2021;68(11):1247–66.
Wang S, Li Z, Zhu G, Hong L, Hu C, Wang K, et al. RNA-binding protein IGF2BP2 enhances circ_0000745 abundancy and promotes aggressiveness and stemness of ovarian cancer cells via the microRNA-3187-3p/ERBB4/PI3K/AKT axis. J Ovarian Res. 2021;14:1–17.
Deng L, Liu G, Zheng C, Zhang L, Kang Y, Yang F. Circ-LAMP1 promotes T-cell lymphoblastic lymphoma progression via acting as a ceRNA for miR-615-5p to regulate DDR2 expression. Gene. 2019;701:146–51.
Dai C, Ma Z, Si J, An G, Zhang W, Li S, et al. Hsa_circ_0007312 promotes third-generation epidermal growth factor receptor-tyrosine kinase inhibitor resistance through pyroptosis and apoptosis via the MiR-764/MAPK1 axis in lung adenocarcinoma cells. J Cancer. 2022;13(9):2798.
Niu R, Li D, Chen J, Zhao W. Circ_0014235 confers Gefitinib resistance and malignant behaviors in non-small cell lung cancer resistant to Gefitinib by governing the miR-146b-5p/YAP/PD-L1 pathway. Cell Cycle. 2022;21(1):86–100.
Liu S, Jiang Z, Xiao P, Li X, Chen Y, Tang H, et al. Hsa_circ_0005576 promotes osimertinib resistance through the miR-512-5p/IGF1R axis in lung adenocarcinoma cells. Cancer Sci. 2022;113(1):79–90.
Gao J, Ding C, Zhou J, Wu G, Han Z, Li J, et al. Propofol suppresses lung cancer tumorigenesis by modulating the circ-ERBB2/miR-7-5p/FOXM1 axis. Thorac Cancer. 2021;12(6):824–34.
Liu Y-T, Han X-H, Xing P-Y, Hu X-S, Hao X-Z, Wang Y, et al. Circular RNA profiling identified as a biomarker for predicting the efficacy of Gefitinib therapy for non-small cell lung cancer. J Thorac Dis. 2019;11(5):1779.
Wang T, Liu Z, She Y, Deng J, Zhong Y, Zhao M, et al. A novel protein encoded by circASK1 ameliorates gefitinib resistance in lung adenocarcinoma by competitively activating ASK1-dependent apoptosis. Cancer Lett. 2021;520:321–31.
Tan L, Huang Z, Chen Z, Chen S, Ye Y, Chen T, et al. CircRNA_001895 promotes sunitinib resistance of renal cell carcinoma through regulation of apoptosis and DNA damage repair. J Chemother. 2023;35(1):11–8.
Hüttenhofer A, Vogel J. Experimental approaches to identify non-coding RNAs. Nucleic Acids Res. 2006;34(2):635–46.
Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91.
Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, et al. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep. 2013;30(2):877–89.
Zhao FL, Dou YC, Wang XF, Han DC, Lv ZG, Ge SL, et al. Serum microRNA-195 is down-regulated in breast cancer: a potential marker for the diagnosis of breast cancer. Mol Biol Rep. 2014;41(9):5913–22.
Sun Y, Wang M, Lin G, Sun S, Li X, Qi J, et al. Serum microRNA-155 as a potential biomarker to track disease in breast cancer. PLoS ONE. 2012;7(10): e47003.
Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B, Li Y, et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol. 2013;34(4):2175–81.
Jiang M, Li X, Quan X, Li X, Zhou B. MiR-92a family: a novel diagnostic biomarker and potential therapeutic target in human cancers. Front Mol Biosci. 2019;6:98.
Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR, et al. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann Surg. 2014;259(4):735–43.
Zuo Z, Li Y, Zeng C, Xi Y, Tao H, Guo Y. Integrated analyses identify key molecules and reveal the potential mechanism of miR-182-5p/FOXO1 axis in alcoholic liver disease. Front Med. 2021;8: 767584.
Soliman SE, Elabd NS, El-Kousy SM, Awad MF. Down regulation of miR-30a-5p and miR-182-5p in gastric cancer: clinical impact and survival analysis. Biochem Biophys Rep. 2021;27: 101079.
Wu J, Li G, Wang Z, Yao Y, Chen R, Pu X, et al. Circulating microRNA-21 is a potential diagnostic biomarker in gastric cancer. Dis Markers. 2015;2015: 435656.
Peng Q, Shen Y, Lin K, Zou L, Shen Y, Zhu Y. Comprehensive and integrative analysis identifies microRNA-106 as a novel non-invasive biomarker for detection of gastric cancer. J Transl Med. 2018;16(1):127.
Cai H, Yuan Y, Hao YF, Guo TK, Wei X, Zhang YM. Plasma microRNAs serve as novel potential biomarkers for early detection of gastric cancer. Med Oncol. 2013;30(1):452.
Farasati Far B, Vakili K, Fathi M, Yaghoobpoor S, Bhia M, Naimi-Jamal MR. The role of microRNA-21 (miR-21) in pathogenesis, diagnosis, and prognosis of gastrointestinal cancers: a review. Life Sci. 2023;316: 121340.
Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila). 2009;2(9):807–13.
Xia J, Cao T, Ma C, Shi Y, Sun Y, Wang ZP, et al. miR-7 suppresses tumor progression by directly targeting MAP3K9 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:121–32.
Ye ZQ, Zou CL, Chen HB, Jiang MJ, Mei Z, Gu DN. MicroRNA-7 as a potential biomarker for prognosis in pancreatic cancer. Dis Markers. 2020;2020:2782101.
Paik WH, Song BJ, Kim HW, Kim HR, Hwang JH. MicroRNA-200c as a prognostic biomarker for pancreatic cancer. Korean J Gastroenterol. 2015;66(4):215–20.
Dong L, Hou X, Liu F, Tao H, Zhang Y, Zhao H, et al. Regulation of insulin resistance by targeting the insulin-like growth factor 1 receptor with microRNA-122-5p in hepatic cells. Cell Biol Int. 2019;43(5):553–64.
Dai M, Li L, Qin X. Clinical value of miRNA-122 in the diagnosis and prognosis of various types of cancer. Oncol Lett. 2019;17(4):3919–29.
Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56(1):167–75.
Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu RX, et al. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36(4):3035–42.
Grimolizzi F, Monaco F, Leoni F, Bracci M, Staffolani S, Bersaglieri C, et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep. 2017;7(1):15277.
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H, et al. Diagnostic value of serum miR-182, miR-183, miR-210, and miR-126 levels in patients with early-stage non-small cell lung cancer. PLoS ONE. 2016;11(4): e0153046.
Hou B, Ishinaga H, Midorikawa K, Nakamura S, Hiraku Y, Oikawa S, et al. Let-7c inhibits migration and epithelial-mesenchymal transition in head and neck squamous cell carcinoma by targeting IGF1R and HMGA2. Oncotarget. 2018;9(10):8927–40.
Dou H, Wang Y, Su G, Zhao S. Decreased plasma let-7c and miR-152 as noninvasive biomarker for non-small-cell lung cancer. Int J Clin Exp Med. 2015;8(6):9291–8.
Huang Y-F, Zhang Y, Fu X. Long non-coding RNA DANCR promoted non-small cell lung cancer cells metastasis via modulating of miR-1225–3p/ErbB2 signal. Eur Rev Med Pharm Sci. 2021;25(2):758–69.
Du Y, Chen Y, Wu T, Fan X, Lin W, Jiang Z. miR-2682-3p antagonizes its host lncRNA-MIR137HG by interacting with the same target FUS to regulate the progression of gastric cancer. BMC Cancer. 2022;22(1):1–15.
Han X, Jia Y, Chen X, Sun C, Sun J. lncRNA TINCR attenuates the proliferation and invasion, and enhances the apoptosis of cutaneous malignant melanoma cells by regulating the miR-424-5p/LATS1 axis. Oncol Rep. 2021;46(5):1–11.
Wen L, Zheng Y, Wen X, Zhang Y, Zeng W. Increased expression of long noncoding RNA GAS6-AS2 promotes proliferation and inhibits apoptosis of melanoma cells via upregulating GAS6 expression. IUBMB Life. 2019;71(10):1503–14.
Dai C, Liu B, Li S, Hong Y, Si J, Xiong Y, et al. Construction of a circRNA-miRNA-mRNA regulated pathway involved in EGFR-TKI lung adenocarcinoma resistance. Technol Cancer Res Treat. 2021;20:15330338211056808.
Acknowledgements
We would like to express our gratitude to all the researchers and authors whose work has been cited in this review article. Their contributions have been invaluable in shaping this review and providing a comprehensive understanding of the topic. We also extend our thanks to the editors and reviewers who provided valuable feedback and suggestions for improving the quality of this manuscript. Finally, we acknowledge the support of our respective institutions in providing the necessary resources for the completion of this work. We would like to express our sincere gratitude to Dr. Reza Mirfakhraie for his valuable contributions and time dedicated to this article, despite his decision to not participate as a coauthor. His insights and expertise were instrumental in shaping the initial stages of this research. We acknowledge his efforts and appreciate his support.
Funding
As this is a review article, it did not receive any specific funding. All authors have contributed their time and effort voluntarily towards the completion of this manuscript.
Author information
Authors and Affiliations
Contributions
AM was responsible for the miRNA section and the creation of figures. MN, NS, and AB were responsible for the lncRNA and circRNA sections. MMa contributed to the miRNA section. STF, HS, and MMi provided revisions to the manuscript. M-RG contributed to the introduction, future prospects, conclusion, and the finalization of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study obtained approval from the Research Ethics Committee of the Faculty of Medicine at Shahid Beheshti University of Medical Sciences, in adherence to the principles outlined in the Declaration of Helsinki. It should be noted that although this review acknowledges the ethical approval obtained and adherence to the Declaration of Helsinki, it does not pertain to consent to participate, as it is not applicable to the nature of this review.
Consent for publication
The authors who have made a contribution to this manuscript have provided their collective agreement for its publication in this journal.
Competing interests
The authors declare that they have no competing interests relevant to this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moeinafshar, A., Nouri, M., Shokrollahi, N. et al. Non-coding RNAs as potential therapeutic targets for receptor tyrosine kinase signaling in solid tumors: current status and future directions. Cancer Cell Int 24, 26 (2024). https://doi.org/10.1186/s12935-023-03203-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-03203-2