Abstract
Alternaria alternata that threatens pepper production and causes major economic harm is responsible for the leaf spot/blight disease. Chemical fungicides have been widely employed; unfortunately, fungicidal resistance is a current concern. Therefore, finding new environmentally friendly biocontrol agents is a future challenge. One of these friendly solutions is the use of bacterial endophytes that have been identified as a source of bioactive compounds. The current study investigates the in vivo and in vitro fungicidal potential of Bacillus amyloliquefaciens RaSh1 (MZ945930) against pathogenic A. alternata. In vitro, the results revealed that RaSh1 exhibited strong antagonistic activity against A. alternata. In addition to this, we inoculated pepper (Capsicum annuum L.) plants with B. amyloliquefaciens RaSh1 and infected them with A. alternata. As a result of A. alternata infection, which generated the highest leaf spot disease incidence (DI), the plant's growth indices and physio-biochemical characteristics significantly decreased, according to our findings. Our results also showed the abnormal and deformed cell structure using light and electron microscopy of A. alternata-infected leaves compared with other treatments. However, DI was greatly reduced with B. amyloliquefaciens RaSh1 application (40%) compared to pepper plants infected with A. alternata (80%), and this led to the largest increases in all identified physio-biochemical parameters, including the activity of the defense-related enzymes. Moreover, inoculation of pepper plants with B. amyloliquefaciens RaSh1 decreased electrolyte leakage by 19.53% and MDA content by 38.60% as compared to A. alternata infected ones. Our results show that the endophyte B. amyloliquefaciens RaSh1 has excellent potential as a biocontrol agent and positively affects pepper plant growth.
Similar content being viewed by others
Introduction
Plants are constantly threatened by a variety of pathogenic microorganisms present in their environments, such as bacteria, fungi, and viruses which cause diseases that significantly contribute to the overall loss in crop yield worldwide [1, 2]. Among these pathogenic microorganisms, fungi are the most dangerous biological stresses that cause harmful effects on crops quality and quantity leading to severe damage to agricultural crops in Egypt [3]. In response to fungal infection, there was a noticeable drop in plant physiological activity and chlorophyll concentration. In additions, this infection causes an imbalance in the movement of water and nutrients throughout the plant organs. As a result, this disrupts plant processes and leads to disease throughout the entire plant [4]. Alternaria is one of the most famous pathogens of fungal diseases causing a negative impact on vegetable crops [5]. Alternaria causes early blight and leads to a sharp decline in yield; the infection begins on the lower leaves of the plant first and then extends upwards, where small brown spots appear on the leaves [6, 7]. The most popular method of preventing fungal diseases is the application of chemical fungicides. However, these fungicides have several disadvantages, including high cost and potential impacts on the environment and human health [8, 9]. Additionally, the continued use of these pesticides has caused phytopathogens to become resistant [10, 11].
The use of microbial antagonists for biological control may offer a reliable, efficient, and environmentally friendly alternative method for the management of fungal diseases [12, 13]. Among these promising biocontrol agents are endophytes that have received increasing interest as the rhizosphere or phyllosphere acts as a source for numerous endophytes [14]. For a period of their life cycles, these endophytes live as symbionts in plant tissues asymptomatically [15]. Endophytes most likely use direct biocontrol strategies like antibiosis and competition as well as indirect strategies like inducing plant defense reactions against invasive diseases. Besides, they may stimulate plant development through phyto-stimulation and/or bio-fertilization [12, 16]. Endophytes are being evaluated for their capacity to produce new biologically active substances as well as their ability to maintain plant health due to their biological control and biological fertilizer qualities [16].
Additionally, endophytes have the ability to suppress disease, which may be introduced by the production of a variety of secondary metabolites, such as salicylic acid, siderophores, antibiotics, extracellular enzymes that break down the cell walls of pathogens, and the synthesis of volatile organic compounds, in addition to the induction of host plant-induced systemic resistance [17, 18]. Endophytes, according to Passari et al. [19], produce ammonia, phytohormones, hydrogen cyanide, siderophores, and solubilize phosphate, all of which are factors in encouraging plant growth.
Endophytic bacteria, including Bacillus sp., Burkholderia sp., Enterobacter sp., Pseudomonas sp., and Serratia sp., have been examined for their biocontrol potential against several plant diseases [20,21,22]. Kazerooni et al. [23] investigated B. amyloliquefaciens' capacity to promote Capsicum sp. growth and inhibit Botrytis grey mold and Alternaria leaf spot. Hazarika et al. [24] reported that B. subtilis SCB-1 screened for its antifungal potential against Alternaria sp., Cochliobolus sp., Curvularia sp., and Fusarium sp. Rashad et al. [16] observed that B. amyloliquefaciens GGA also had antagonistic action against Sclerotium cepivorum in vitro.
Due to their high nutritional value, peppers (Capsicum annuum L., family Solanaceae) are a well-known and significant economic vegetable crop [23, 25]. They are a rich source of vitamins, minerals, and antioxidants and assist to prevent inflammation, cancer, and cell damage. According to Attia et al. [26], fungi are one of the most dangerous pathogens of pepper plants. Pepper is vulnerable to a variety of fungal infections such as root rot, leaf spot, and wilt diseases [3, 27]; as a result, such stresses reduce yield and productivity, resulting in economic losses [28]. Previously, B. amyloliquefaciens RaSh1 (MZ945930) had been isolated from leaves of Brassica oleracea and it was reported for its ability to produce a number of bioactive compounds that have antifungal activities [10]. Consequently, the prime objective of our research is to assess antifungal capacity of B. amyloliquefaciens RaSh1 against A. alternata in vivo and in vitro as a biocontrol agent alternative to chemical products and further assess their growth-promoting effects on pepper plants in the greenhouse.
Materials and methods
Isolation, purification and morphological identification of the pathogenic Alternaria sp.
Leaves of diseased pepper plants (C. annuum L.) showing typical Alternaria leaf spot disease symptoms were collected in paper bags from several El-Sharkia governorate locations. Samples were surface sterilized for two min with 0.5% sodium hypochlorite solution before being cleaned with sterilized distilled water. After that, samples were dried between two sheets of sterile filter paper. Using a sterilized scalpel, the dried, sterilized spotted leaf tissues were chopped into small pieces along with the surrounding healthy tissues and put on the Potato Dextrose Agar (PDA) medium (Sigma-Aldrich, St. Louis, MO, USA) in 9 cm Petri dishes. Inoculated dishes were incubated at 25 °C for 4 days. Pure culture was obtained using a single spore culture technique according to Noman et al. [29]. The purified fungus was identified according to its morphological characteristics using the description of Ellis [30].
Molecular identification of pathogenic Alternaria sp.
Using the Gene Jet Plant genomic DNA purification Kit, genomic DNA was isolated (Thermo procedure). Sigma Scientific Services Company (Cairo, Egypt) performed the PCR in a DNA Engine Thermal Cycler with a hot start at 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s, followed by a final extension at 72 °C for 10 min. The sequencing was carried out by the GATC Company in Germany using an ABI 3730 × 1 DNA sequencer. The retrieved sequences were compared to the Gene Bank database using the NCBI BLAST programme. The 18S rRNA and ITS sequences in the Gene Bank database were compared to the sequences using BLASTN. The MEGA 6.0 programme was used to create a phylogenetic tree [31]. Finally, the sequence was uploaded to GenBank and assigned an accession number.
Fungal inoculum preparation
Alternaria alternata inoculum was prepared from cultures grown on PDA medium then incubated at 25 °C for 7 days. The developed cultures were flooded using 50 mL sterilized water. The growth (mycelial mates and spores) was carefully scraped from the medium surface. The mycelium and obtained spore suspension were filtered through sterilized cheesecloth to eliminate mycelial fragments. The obtained fungal suspensions were adjusted to 105 cfu/mL using the hemocytometer technique. Droplets of Tween 20 (0.5 mL/L) were finally added. Prepared suspension was used for spraying leaves in vivo.
Bacterial inoculum preparation
B. amyloliquefaciens RaSh1 (MZ945930) was previously isolated from B. oleracea leaves, identified, and reported for its ability to produce a variety of bioactive compounds with antifungal properties [32]. B. amyloliquefaciens RaSh1 was cultivated in 1000 mL Erlenmeyer flasks with 250 mL of nutrient medium and incubated for 48 h at 37 °C on an incubator shaker at 100 rpm.
Evaluation of the endophytic B. amyloliquefaciens RaSh1’s in vitro antagonistic activity
Using the dual culture plate method, B. amyloliquefaciens RaSh1’s antifungal activity was evaluated against A. alternata. Each PDA plate had a 6 mm-diameter disc of Alternaria sp. culture that had been grown for seven days in the center, with a loop of B. amyloliquefaciens RaSh1 streaking 1 cm from the plate's edge. Experimental controls included PDA plates that had only been infected with the fungal disc. The calculation of fungus growth inhibition using the following equation:
*R1 represents the control plate’s inward linear growth, and R2 represents the dual culture plate's inward linear growth.
Antifungal assay of Thiram was done using poisoned food technique according to Mohammad et al. [33] with slight modification. 20 mL of sterilized melted PDA with 0.2% Thiram were poured in sterilized petri plates and agitated gently to become homogenized. The same procedure was done without Thiram as control. All petri dishes were allowed to solidify. Each Petri dish was inoculated with 10 mm mycelial disc of pure isolate taken from actively growing culture of A. alternata. Each mycelial disc was placed aseptically at the center of each petri plate. All Petri dishes were incubated at 25 °C for 5 days, at the end of incubation period the growth of mycelium was noticed for each treatment. Three replicates were maintained for each treatment.
Pot experiment and leaves inoculation under greenhouse conditions
In a completely randomized design, the pot experiment was conducted in the greenhouse of the Botany and Microbiology Department, Faculty of Science, Zagazig University, with temperatures ranging from 23 to 30 °C and relative humidity from 60 to 85% with 10 replicates for each particular treatment. Five treatments (T1–T5) were conducted as described in Table 1. Plastic bags (15 × 25 cm) were filled with sterile field clay soil (2 kg/bag). On seedlings that were 40 days old, experimental treatments were used, and 1 seedling was transplanted per bag. B. amyloliquefaciens RaSh1 inoculum suspension was initially used to immerse the pepper seedlings’ roots for 4 h before to transplanting, and then bacterial inoculum (50 mL/bag) was used in irrigation for bacterial treatments. A fungal pathogen (A. alternata) was inoculated on the surface of healthy leaves two days after transplantation by pipetting individual droplets of a fungal suspension (105 cfu/mL) onto the leaves. As a control, plants that were just sprayed and watered with tap water were employed. The infected plants were subjected to greenhouse conditions after being inoculated with the pathogen for 24 h to assure the infection process and maintain high humidity levels. The appearance of disease symptoms was noted four weeks following inoculation. Re-isolating A. alternata from diseased tissues and its identity was confirmed. The gathered samples were either used immediately or quickly frozen and preserved for further studies.
Measurements
Assessment of disease incidence
The following formula was used to calculate the disease’s incidence (DI) according to Rashad et al. 16:
Determination of morphological parameters
Pepper plants from the non-infected and A. alternata infected treatments were removed and rinsed with tap water 4 weeks after B. amyloliquefaciens RaSh1 application. We measured the pepper plant's shoot height and root length. The fresh weight (Fwt) was weighted and expressed by g. The samples were then heated to 70 °C for two days, during that time their dry weights (Dwt) were noted.
Measurement of the physiological parameters
Estimation of chlorophyll content
The development of disease in pepper plants was also evaluated by examining the chlorophyll content (Chl a, Chl b, and carotenoids) by the Metzner et al. [34] protocol. Chopped-up pepper leaves (0.1 g) from healthy and infected plants were extracted with 85% acetone. Using a spectrophotometer, the absorbance at 644, 663, and 452.5 nm was measured to determine the amount of chl (WP 0803006). Then, using the Lichtenthaler and Wellburn [35] formulas, Chl a, b, and carotenoids were further determined.
*OD refers to optical density, V to sample volume, and W to sample weight.
Measurement of the water status of pepper plant leaves
The pepper leaves were collected and divided into small pieces (5–10 cm2), and each piece's Fwt was measured. They were then submerged in dist. water for around 4 h. Following that, the turgid weight of leaves (Twt) was determined individually. The leaves were then dried entirely for 72 h in an oven at 65 °C, and their Dwt was calculated. Measurements were made on the water content (WC), relative water content (RWC), and water saturation deficit (WSD) [36].
Leakage of electrolytes (EL) and the membrane stability index (MSI)
The method of Shi et al. [37] was used to estimate the plasma membrane permeability or electrolyte leakage (EL) of both diseased and non-diseased pepper leaves. To get rid of the electrolytes generated during leaf disc excision, ten leaf discs (10 mm in diameter) from young, completely expanded leaves were put in 50 mL glass vials and rinsed with distilled water. Then, 30 mL of distilled water was added to the vials, and they were left at room temperature for 24 h in the dark. At the end of the incubation time, the bathing solution's initial conductivity (EC1) was measured. Vials were heated at 95 °C in a water bath for 15 min, cooled to room temperature, and the EC2 was then calculated. Farooq and Azam [38] calculated the membrane stability index (MSI). The following formula was used to get the relative EL and MSI:
*EC1 is a solution’s electrical conductivity before heating, and after heating, it is measured as EC2.
Measurement of the biochemical parameters
Thiobarbituric acid reactive substances [TBARS] content
Using a thiobarbituric acid (TBA) reaction, the TBARS content, a marker of lipid peroxidation, was assessed in the pepper leaves of healthy and infected pepper plants [39]. A 0.6 mL of 0.1% (w/v) trichloroacetic acid (TCA) was used to homogenize 0.25 g of fresh leaf, and the mixture was then centrifuged for 15 min at 6000 rpm. The supernatant was heated at 95 °C for 30 min while being combined with 4 mL of 20% (w/v) TCA containing 0.5% (w/v) thiobarbituric acid (TBA), and then it was immediately transferred to a cold bath. Following a 10-min centrifugation of the extracted samples, the absorbance of the utilized supernatants was measured at 532 and 600 nm.
Assay of the defense-related enzymes activities
According to Qiu et al. [40], fresh pepper plant leaves (1 g) were mixed with 10 mL of an extraction buffer containing 100 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, and 1% (w/v) polyvinyl pyrrolidone in order to assess the antioxidant enzyme activities in pepper plant leaves. The supernatant from centrifugation (12000 rpm for 15 min at 4 °C) was used to measure the enzyme activity. The consumption of H2O2 at 240 nm for two minutes was used to measure and assess the catalase (CAT; EC 1.11.1.6) activity [41]. A Lavid et al. technique [42] was used to find the activity of the enzyme polyphenol oxidase (PPO; EC.1.10.3.1). At 495 nm, the purpurogallin production was monitored.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
A known Fwt of leaf tissues (0.25 g) from healthy and diseased pepper samples was extracted with methanol and centrifuged for 10 min at 8000 rpm. The DPPH radical scavenging assay was used to determine the extracts' capacity to scavenge free radicals [43, 44]. A solution of 0.1 mM DPPH in methanol was prepared, and this solution was mixed with the methanolic extract at different concentrations (12.5–100 μg/mL). Methanol was utilized for the baseline correction, and the control was made in the same way as the above but without the sample extracts. After vigorous overtaxing, the reaction mixture was kept in the dark for 30 min. At 517 nm, the absorbance of the mixture was determined spectrophotometrically. Results were compared with ascorbic acid (ASA), a common antioxidant that was utilized as a reference. The following equation was used to compute the percentage of DPPH radical scavenging activity:
*The absorbance of the control is A0 and that of the sample extracts is A1.
Then, the percentage (%) of inhibition was plotted against concentration, and the IC50 was determined from the graph. The IC50 (the microgram of extract to scavenge 50% of the radicals) value was calculated using linear regression analysis.
Light and transmission electron microscopy (TEM)
Samples were collected from healthy (T1), infected (T2), and treated (T5) 1st and 3rd leaves and used for light and transmission electron microscopy examinations. The samples were first post-fixed in 2% osmium tetroxide for 2 h after being fixed in 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7) for 2 h [45]. The samples were then implanted in Epon-Spurr epoxy resin after being dehydrated in an escalating ethanol series and propylene oxide [46]. With the use of a Reichert Jung microtome, the semi-thin and ultra-thin sections were produced (Leica, Wetzlar, Germany). An analysis was performed using a compound microscope on the semi-thin sections. Uranyl acetate and lead citrate were used as the staining agents [47] for the ultra-thin sections and a Jeol 1010 TEM (Jeol, Tokyo, Japan) operating at 80 kV was used to investigate them.
Statistical analysis
Results from 10 replicates (n = 10) are represented as means ± standard errors in the graphs. Analysis of variance (ANOVA) was used to statistically confirm the findings. Duncan's multiple range test (p < 0.05) was used to determine the significant difference between the control and treatment groups. With SPSS® 18.0, the computations were carried out. Using SPSS, Pearson's correlation coefficients (r) were calculated to determine how growth indices and various biochemical markers interacted. Figures were assembled using OriginPro 8.5 for data analysis and graphing software.
Results and discussion
The establishment of disease-controlling crop management techniques that are environmentally benign is a critical challenge. A biological alternative to antibiotics is the use of bacterial antagonists [48]. One of the most appropriate management techniques in the integrated management program is to lessen pesticide use in the environment [49]. PGPRs have been studied as plant disease biocontrol agents and, in addition, as stimulators of disease resistance in plants [50].
Morphological characteristics of A. alternata
The morphological traits of the pure culture of Alternaria sp. isolated from diseased pepper plant leaves were used to identify it. The purified culture of Alternaria sp. formed aerial mycelium that was greyish black in color with a black reverse. On microscopic examination, septate brown hyphae were seen, along with septate and brown conidiophores containing conidia in chains. Conidiophores were pale brown, simple, and branching, with catenulate conidia at the apex and fertile sections of the apex and apical fertile regions. Conidia were prosperous, acropetally formed, dark brown, cylindrical or spindle-shaped, often with cylindrical beaks, and muriform with 3–4 transverse walls and 1–2 longitudinal walls (Fig. 1A, B).
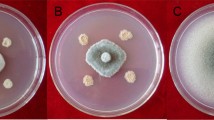
Morphological abnormalities in the mycelia of Alternaria sp upon interaction with B. amyloliquefaciens RaSh1 under the light microscope. Images A and B Untreated (control) Alternaria sp spores and mycelia. C Dual culture plate method showing inhibition of Alternaria sp. by B. amyloliquefaciens RaSh1. D Swelling and deformity of Alternaria sp mycelia treated with B. amyloliquefaciens RaSh1. E Untreated (control) Alternaria sp. cultivated on PDA media F Alternaria sp. cultivated on PDA media amended with 0.2% Thiram after 5 days of growth
Molecular identification of Alternaria sp.
The 18S rDNA gene sequence of an isolate of Alternaria sp. identified it as A. alternata RaSh3. As shown in Fig. 2, the acquired 18S rDNA gene partial sequence was entered into the GenBank database with the accession number OK053809.1.
In vitro antifungal assay
The inhibition zone between A. alternata and B. amyloliquefaciens RaSh1was apparent in the dual culture test depicted in Fig. 1C. The obtained results indicated that B. amyloliquefaciens RaSh1 exhibited a strong antagonistic activity against A. alternata that proved its potent activity. Due to the release of antifungal metabolites, B. amyloliquefaciens RaSh1 has been examined for its fungitoxic action against a variety of soil-borne fungi [16, 32]. Among these metabolites that are produced by B. amyloliquefaciens are lipopeptides (e.g. bacillomycin, fengycin, and surfactin), hydrolytic enzymes, siderophores such as bacillibactin, and volatile compounds [8, 51, 52]. The feasible mechanisms of these metabolites’ actions include interference with cell membrane components, particularly sterol and phospholipid molecules, altering their structure and affecting membrane permeability [53]. Besides, inhibition of fungal DNA biosynthesis and cell lysis was also reported [16, 54].
Moreover, we observed a clear distortion and abnormality in the hyphal structure of A. alternata in the presence of B. amyloliquefaciens RaSh1 under light microscopy (Fig. 1D) as compared to the control one (Fig. 1B). Furthermore, as reported by Agarwal et al. [55] and Xu et al. [56], these outcomes suggest that antibiosis tends to be the biocontrol mechanism, employed by certain species of Bacillus against F. oxysporum, A. alternata, and Helminthosporium sp. Moreover, in this study, the antibiosis activity has been linked to the production of antifungal volatile compounds by B. amyloliquefaciens RaSh1 that are detected in its extract by GC–MS profiling, such as Bis (2-ethylhexyl) phthalate, Bis (2-ethylhexyl) ester, N,N-Dimethyldodecylamine, and Dibutyl phthalate [32]. These compounds are reported to have antimicrobial and antifungal activities against unicellular and filamentous fungi [57,58,59]. Antifungal assay of Thiram was done using poisoned food technique, results obtained in (Fig. 1 E, F) showed that Thiram has potent antifungal effect on the fungal linear growth.
B. amyloliquefaciens RaSh1 ameliorative response to pepper during disease incidence (DI)
A. alternata causes some of the most devastating destruction to crops by destroying plants, reducing growth and causing diseases. They also produce mycotoxins that are detrimental to human health and livestock [60, 61]. A. alternata causes widespread leaf spot/blight, which has become a hazard to pepper production [62, 63]. The typical disease symptoms appear as small, brown spots on leaves. As the disease progresses, these spots may take on an irregular shape and gradually cover a large surface, causing the leaves to wither, dry out, and fall off [64].
Synthetic chemical fungicides have been a mainstay in agriculture for controlling fungal diseases for decades; however, fungicides, like other pesticides, can have negative non-target effects on the environment, such as beneficial fungi to plant growth [65]. Wide fungicide use influences the mutualist fungi such as arbuscular mycorrhizal fungi [66, 67]. Our results showed that the inoculation of pepper plants with B. amyloliquefaciens RaSh1 bacterial endophyte significantly reduced the DI under A. alternata attack challenged conditions. Collected diseased samples were photographed and visible symptoms were also described as shown in Fig. 3(A), as the A. alternata strain was able to infect pepper plants, causing typical less spot symptoms. The highest DI (80%) was observed in A. alternata infected plants, while the lowest was recorded in plants infected with A. alternata and inoculated with B. amyloliquefaciens RaSh1 (40%) (Fig. 3B); the leaf spot disease symptoms were lessened by B. amyloliquefaciens RaSh1. B. amyloliquefaciens RaSh1 causes a great reduction in DI as compared to plants treated with A. alternata alone. Also, Thiram fungicide (0.2%) application decreased DI in A. alternata infected pepper plants (50%). However, in inoculated and non-inoculated B. amyloliquefaciens RaSh1 pepper plants grown under non-pathogenic conditions, certainly no disease symptoms were documented. Our finding was in agreement with previous reports which stated that endophytes are involved in controlling plant pathogens [22, 24, 68]. Additionally, Shahzad et al. [69] showed that B. amyloliquefaciens RWL-1 significantly reduced the growth of F. oxysporum in vitro when compared to the control.
B. amyloliquefaciens RaSh1 ameliorative response to pepper growth attributes after A. alternata infection
The capacity of B. amyloliquefaciens RaSh1 to act as biocontrol agents was confirmed via a greenhouse experiment. A. alternata inoculated to pepper plants either in the presence or absence of B. amyloliquefaciens RaSh1. Besides, the effects of B. amyloliquefaciens RaSh1 application on pepper plant growth attributes are recorded in Table 2. Generally, all the assessed growth parameters (Fwt, Dwt of shoot and roots, shoot height, and root length) were significantly reduced in the pepper plants affected by leaf spot/blight, compared with the healthy control ones. Ghanbary et al. [70] reported that under optimal circumstances, plants dedicate their energy to growth, cellular preservation, and reproduction, but under pathogen invasion, they need to balance energy production with plant defense to ensure survival [71]. However, most of these parameters were significantly increased in plants bacterized with B. amyloliquefaciens RaSh1 compared with the control ones, regardless of whether the plants were infected with A. alternata or not. Similarly, there was a significant difference among the treatments in terms of root lengths and plant heights of the pepper plant. Unlike plant heights and Fwt, there was no significant difference in Dwt. In A. alternata infected peppers, application of B. amyloliquefaciens RaSh1 significantly improved shoot height (16.5 cm), shoot Fwt (3.99 g) and shoot Dwt (0.5258 g) as compared to infected one (Table 2).
The results of our pot experiment on the enhancing capacity of B. amyloliquefaciens RaSh1 for pepper plant growth are consistent with the findings of Rashad et al. [16], Shahzad et al. [69] and Zhang et al. [72] who found that B. amyloliquefaciens GGA, RWL-1 and IBFCBF-1 inoculation significantly enhanced all the growth traits of pepper, tomato and garlic plants under both diseased as well as non-diseased conditions of Phytophthora blight, F. oxysporum and S. cepivorum white rot; respectively. Also, in this regard, Kazerooni et al. [23] reported that B. amyloliquefaciens resulted in increased growth, improved plant health, and suppressed Botrytis gray mold and Alternaria leaf spot diseases produced by B. pelargonii and A. alternata. Therefore, B. amyloliquefaciens RaSh1 displayed strong antagonism toward A. alternata infection and improved the growth of infected pepper plants.
The extensively distinguished mechanisms of plant growth promotion caused by PGPR are phytohormone production, providing the essential nutrients, N2 fixation, and phosphate solubilization [19, 23]. Rangjaroen et al. [73] reported that Klebsiella, Burkholderia, and Sphingomonas endophytic bacteria enhance Oryza growth by increasing the production of indole-3-acetic acid (IAA) and gibberellins, phosphate solubilization and siderophore formation. Moreover, endophytic bacteria may promote plant growth through phytostimulation and/or bio-fertilization [12, 16]. Shahzad et al [69] and Srivastava et al [74] discovered that endophytes’ gibberellins, organic acid, and secondary metabolite producing capabilities provide additional support to plants and increase plant development, thus increasing their resilience to biotic and abiotic challenges. These mechanisms seem to contribute to the plant growth-promoting potential of B. amyloliquefaciens RaSh1 on pepper plants.
Physiological parameters of pepper plants in response to A. alternata and B. amyloliquefaciens RaSh1
By taking over the plant’s physiology, endophytes can occasionally assist the host plant's defense mechanism against pathogenic microorganisms [75]. As the plant grows, it accumulates vigor and resilience to various abiotic and biotic challenges; this is one of the plant's defensive mechanisms against pathogens [76].
Photosynthetic pigments in pepper plants
Leaf pigment content, which includes Chl and carotenoids, is a key measure of plant physiological status that could be used to assess photosynthetic activity [77,78,79,80]. Amounts of Chl a, Chl b, and carotenoids in pepper leaves in response to the different treatments are summarized in Fig. 4A–C. Infection of pepper plants with A. alternata led to decreases in the Chl content compared to untreated plants. The maximum reduction in Chl a content was observed in A. alternata infected pepper plants, followed by those A. alternata infected and treated with Thiram fungicide, according to the formulae suggested by Lichtenthaler and Wellburn [35]. The Chl b and carotenoids content followed the same pattern and were found to be maximally reduced (0.371 and 0.593 mg g−1 Fwt) in pathogen treated samples; respectively. These observed reductions are consistent with the findings of Hossain et al. [81] and Ghanbary et al. [82].
Effect of B. amyloliquefaciens RaSh1 and A. alternata on different pigment fractions; A Chl a, B Chl b and C Carotenoids of pepper plants. *The values are the means of 10 replicates ± SE (n = 10). The same letter above each column indicates no significant difference between the treatments (p ≤ 0.05) as determined by Duncan’s multiple range test. Treatments: T1—control pepper (uninoculated), non-diseased; T2— infected with A. alternata, diseased; T3—infected with A. alternata and sprayed with Thiram (0.2%), diseased; T4—inoculated with B. amyloliquefaciens RaSh1), non-diseased; T5—inoculated with B. amyloliquefaciens RaSh1 and infected with A. alternata
Reduced chloroplast numbers, chloroplast collapse, altered leaf photochemistry, and inhibition of Rubisco and other photosynthetic enzymes are all common biotic and abiotic stress responses [70, 82, 83]. These are usually because of photo-oxidation, Chl degradation, or impaired Chl biosynthesis [84], and our data suggest that these processes have occurred in pepper plant leaves as a result of A. alternata infection. Besides, reductions in stomatal conductance may also occur under A. alternata attack because of hyphal development blocking the stomatal opening [85]. As well, toxins generated by fungal infections like A. alternata can also impair the host's defensive response and limit leaf photosynthetic area [70].
However, these adverse effects on pigment synthesis in pepper plants were significantly mitigated by B. amyloliquefaciens RaSh1 inoculation. The Chl a and b quantities in the B. amyloliquefaciens RaSh1 treated pepper plants (1.254 and 0.537 mg g−1 Fwt) were greater than those of the non-B. amyloliquefaciens RaSh1 (0.636 and 0.371 mg g−1 Fwt) treated ones, whether the plants were pathogen-inoculated or not; so B. amyloliquefaciens RaSh1 application reduced the negative effects of the pathogen (A. alternata). Waqas et al. [86] showed that the Chl content of sunflower plant leaves was promoted in fungi-treated plants with or without the disease caused by S. rolfsii. Similar results were obtained by Shahzad et al. [69], who documented that B. amyloliquefaciens RWL-1 inoculation significantly improved the Chl content in tomato plants in comparison with F. oxysporum. Also, the inoculation of B. subtilis to diseased mung bean plants with Macrophomina phaseolina induced charcoal rot disease increased Chl content [87].
Moreover, Egamberdieva et al. [88] found that B. subtilis NUU4 inoculation causes 26% higher photosynthetic pigments in chickpea plant leaves compared to the un-inoculated ones. Photosynthetic pigments, particularly Chl, may be a good indicator of a plant's health and nitrogen status [89]. In previous studies, PGPR have been shown to boost the Chl content of plants by altering the amount of ethylene [90, 91]. Improved photosynthetic pigments in seedlings with endophytes may also be attributed to the plants' increased nutrient mobilization capabilities or by boosting the host nitrogen metabolism [91].
Correlation between plant growth and photosynthetic pigments in pepper plants upon A. alternata and B. amyloliquefaciens RaSh1 applications
The Pearson correlation between Chl a, Chl b, shoot height and root length, shoot and root Fwt, and shoot and root Dwt revealed that B amyliquefaciens RaSh1 had a beneficial effect on pepper plants infected with A. alternata (Table 3). Root Fwt (0.980), root length (0.966), shoot Fwt (0.967) and shoot height (0.920) showed significant positive correlations with Chl a. Chl b was found to have a significant positive correlation with root length, root Dwt, shoot Dwt and shoot length. Additionally, the correlation between shoot height, root length, and root Dwt was highly positive. Furthermore, there was a positive correlation between root length and root Dwt and shoot Dwt in plants.
Effect of B. amyloliquefaciens RaSh1 on RWC in A. alternata infected pepper plants
The change in the water potential of leaves is a key indicator of a plant's water status, and it is clearly linked to the moisture content of the soil [92]. Pathogens that damage the vascular system of plants have an impact on it. In this study, we observed that infection of pepper plants with A. alternata resulted in a decrease in WC and RWC as compared to those grown under non-diseased conditions. Our findings are in line with those of Burman and Lodha [93], who showed that cowpea plants treated to concurrent drought and Macrophomina phaseolina infection experienced a notable decrease in the shoot water potential.
Considerably increased chlorotic leaf spots in the pathogen treatments reflect the strongly reduced WC and RWC, which refer to impaired cuticle integrity and increased non-stomatal water loss [70]. Increased water loss is also due to pathogen-secreted toxins inhibiting stomatal closure and decreased stomatal resistance, all of which diminish plant leaf water potential [94]. Our findings on electrolyte leakage provide additional support for this hypothesis (see below). RWC-based signs of severe water deficiencies in plants frequently lead to metabolic alterations such as photosynthetic impairment (as previously mentioned) (Table 3) and increases in respiration [95].
Furthermore, A. alternata infection accelerated WSD in the diseased pepper leaves compared to control (Fig. 5). However, B. amyloliquefaciens RaSh1 inoculation decreased this parameter greatly. Also, we observed that B. amyloliquefaciens RaSh1 inoculated pepper plant leaves exhibited higher WC (85.87 and 82.10) and RWC (95.97 and 93.71) (p ≤ 0.05) under both control and A. alternata infection conditions (Fig. 5). This might be as a result of a decline in the pathogen’s inhibitory effect on pepper in the B. amyloliquefaciens RaSh1 inoculated plants. Our results are in accordance with Ghanbary et al. [70], who stated that the RWC of Q. libani seedling was significantly decreased by charcoal disease agents. According to Naveed et al. [20], the inoculation of B. phytofirmans with endophytic Enterobacter sp. greatly increased the RWC of maize under both normal and drought-stressed circumstances. Also, Dubey et al. [96] explored that there was a 35.3–48.41% rise in the leaf RWC in soybean seedlings inoculated with endophytes under drought stress conditions as compared to the control. These findings support investigations on PGPR-mediated reduction of osmotic stress [96].
Effect of B. amyloliquefaciens RaSh1 and A. alternata on water status (%); A relative water content (RWC), B water content (WC) and C water saturation deficit (WSD) of pepper plant leaves under different treatments. *The values are the means of 10 replicates ± SE (n = 10). The same letter above each column indicates no significant difference between the treatments (p ≤ 0.05) as determined by Duncan’s multiple range test. Treatments: T1— control pepper (uninoculated), non-diseased; T2—infected with A. alternata, diseased; T3—infected with A. alternata and sprayed with Thiram (0.2%), diseased; T4—inoculated with B. amyloliquefaciens RaSh1), non-diseased; T5—inoculated with B. amyloliquefaciens RaSh1 and infected with A. alternata
Effect of B. amyloliquefaciens RaSh1 on EL and MSI in A. alternata infected pepper plants
The cuticle and stomata are physically disrupted by higher fungi and oomycetes, which also impede stomatal closing in the dark and are linked to an impaired stomatal opening in the light. This is because of the production of several toxins by these fungi that impair stomatal function [97]. Figure 6 shows that B. amyloliquefaciens RaSh1 inoculation decreased EL and its value ranged from 8.50 to 19.53% in pepper plant leaves under normal and diseased conditions, respectively, compared to control under normal and diseased conditions. Its maximum increase (35.42%) was observed after A. alternata infection in pepper compared to control, as A. alternata pathogen infection accelerated relative membrane permeability in the diseased pepper leaves. In this study, we found that both inoculated and un-inoculated plants' MSI decreased as a result of pathogen infection. However, B. amyloliquefaciens RaSh1inoculation significantly increased the MSI compared to the un-inoculated controls. According to Ghanbary et al. [70], Q. libani and Q. infectoria seedlings exposed to a combination of charcoal disease pathogens and drought showed the highest rise in EL. The first observable symptoms of pathogen infestation that cause disease are changes in membrane permeability and integrity [98].
Effect of B. amyloliquefaciens RaSh1 and A. alternata on A electrolyte leakage (EL) and B membrane stability index (MSI) of pepper plant leaves under different treatments. *The values are the means of 10 replicates ± SE (n = 10). The same letter above each column indicates no significant difference between the treatments (p ≤ 0.05) as determined by Duncan’s multiple range test. Treatments: T1—control pepper (uninoculated), non-diseased; T2— infected with A. alternata, diseased; T3—infected with A. alternata and sprayed with Thiram (0.2%), diseased; T4—inoculated with B. amyloliquefaciens RaSh1), non-diseased; T5—inoculated with B. amyloliquefaciens RaSh1 and infected with A. alternata
Also, Naveed et al. [20] stated that B. phytofirmans besides Enterobacter sp. inoculation decreased the relative membrane permeability of maize under both normal and drought stress circumstances. Moreover, B. amyloliquefaciens RaSh1 inoculation resulted in an increase in MSI from 4.96% (without the pathogen) to 19.44% (with pathogen) compared to control or A. alternata-infected pepper plants, respectively (Fig. 6). In particular, B. amyloliquefaciens RaSh1 inoculation helped pepper leaves to maintain the relative membrane permeability besides reduced leaf damage compared to un-inoculated seedlings under A. alternata attack. Sandhya et al. [99] and Vardharajula et al. [100] found a positive association between stress sensitivity and membrane damage (EL). In addition, ROS that are produced during stress cause lipid peroxidation of membranes [20, 92, 101]. In our results, the B. amyloliquefaciens RaSh1 inoculation decreased the MDA content (as below) induced damage compared to control, fungicide application, and pathogen conditions (Fig. 7). Also, it is most feasible that RaSh1 inoculation colonization improved plant defense enzymes, to lessen the oxidative damage stimulated by A. alternata pathogen attack, suggesting that RaSh1 can enhance plant disease resistance. Similar to our results, Zhou et al. [102] found that Pinus tabulaeformis seedlings treated with PTD37 accumulated less MDA, broke down less Chl, and lost less tissue water than other treatments.
Effect of B. amyloliquefaciens RaSh1 on A lipid peroxidation (nmol/g Fwt) and the antioxidant activity of B CAT and C PPO in A. alternata infected pepper plants. *The values are the means of 10 replicates ± SE (n = 10). The same letter above each column indicates no significant difference between the treatments (p ≤ 0.05) as determined by Duncan’s multiple range test. Treatments: T1—control pepper (uninoculated), non-diseased; T2—infected with A. alternata, diseased; T3—infected with A. alternata and sprayed with Thiram (0.2%), diseased; T4—inoculated with B. amyloliquefaciens RaSh1), non-diseased; T5—inoculated with B. amyloliquefaciens RaSh1 and infected with A. alternata
Effect of B. amyloliquefaciens RaSh1 on lipid peroxidation in A. alternata infected pepper plants
The mobility of proteins, receptors, enzymes, and membrane ion channels is disturbed by the lipid peroxidation brought on by pathogenic stress [103]. As MDA is the marker for lipid peroxidation released from the cellular membranes of tissues and is formed by the reaction of ROS (H2O2 or/and O−2) with lipid molecules, the elevated concentration of ROS in the cellular system positively correlates with the oxidative changes affecting MDA content [92, 104,105,106,107]. According to our findings, A. alternata-induced leaf spot/blight resulted in a significant increase in the amount of lipid peroxidation produced in infected pepper plant leaves, followed by pepper plants infected with A. alternata and treated with Thiram fungicide and B. amyloliquefaciens RaSh1 treated samples over control plants (Fig. 7A). Even though inoculation of pepper plants with B. amyloliquefaciens RaSh1 decreased MDA content in their leaves by 38.60% as compared to A. alternata infected ones; this reflects the positive interaction of B. amyloliquefaciens RaSh1 in pepper plants.
Therefore, B. amyloliquefaciens RaSh1 inoculation alleviated the oxidative stress in A. alternata-infected pepper plants. The biocontrol agents such as B. amyloliquefaciens RaSh1 can inhibit lipid peroxidation, which is what generates the oxidative burst caused by A. alternata in infected plants [87, 108]. Our findings support Lubaina and Murugan [109] finding that A. sesame infection increases the level of lipid peroxidation in the pathogen-inoculated leaf samples. Hashem et al. [87] reported that inoculation of B. subtilis to diseased mung bean plants with M. phaseolina inhibited MDA content while enhancing plant growth. These findings imply that the biochemical defense pathway is more in favor of the pathogen. The impact of B. amyloliquefaciens RaSh1 reduced the peroxidation of membrane lipids caused by A. alternata activation of excessive ROS generation in pepper plants. Thiram (2%) fungicide causes an increase in MDA content in diseased pepper plant leaves relative to control ones. This result was compatible with results of Metwally and Abdelhameed [67] in cucumber leaves treated with Ridomil. Thiram (2%) caused oxidative stress in pepper by producing ROS, which resulted in peroxidation of membranous lipids and the formation of MDA [110].
Role of B. amyloliquefaciens RaSh1 and A. alternata on defense-related enzymes activities in pepper plants
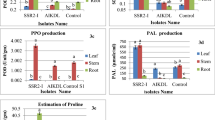
When the antioxidant system fails to manage the intracellular ROS levels, oxidative stress and damage ensue; the generation of ROS as a result of this process can damage proteins and cell membranes, resulting in EL and ultimately cell death [103, 111,112,113]. The detoxification of ROS is necessary to safeguard critical cellular processes and the potential recovery of plants from oxidative damage [114]. The biocontrol strategy of B. amyloliquefaciens has also been shown to involve the induction of plant systemic resistance to invading pathogens. The effects of the applications of B. amyloliquefaciens RaSh1 on the activities of defense-related enzyme activities of pepper plants infected with leaf spot disease are summarized in Fig. 7. The antioxidant enzyme activities in pepper plants were significantly increased by the biotic interaction of B. amyloliquefaciens RaSh1 and A. alternata. The activity of CAT and PPO was considerably (Fig. 7B, C) increased to 60.31 and 7.14%, respectively, in A. alternata-infected pepper plants than in their control to scavenge the H2O2 produced due to pathogen attack.
In addition, further stimulation of CAT (46.55%) and PPO (35.65%) was observed in pepper plant leaves owing to B. amyloliquefaciens RaSh1 inoculation to mitigate the stress effects. Meanwhile, the greatest amounts of enzyme activities were for the A. alternata inoculated plants treated with B. amyloliquefaciens RaSh1, when compared with the control plants. The upregulation of antioxidant enzymes due to B. amyloliquefaciens RaSh1 inoculation protected the A. alternata-infected pepper plant’s metabolism by imparting fast removal of ROS. Our results are in accordance with Vellosillo et al. [115], who reported that pathogens such as fungi, bacteria, and viruses attack plants and produce hazardous ROS, which causes severe cell damage by triggering a sequence of destructive reactions.
Moreover, Li et al. [116] reported that cucurbit seedlings treated with B. amyloliquefaciens LJ02 reduced the Sphaerotheca fuliginea infection by triggering the defense-related enzymes biosynthesis. As a result, B. amyloliquefaciens RaSh1's effectiveness showed that pepper plants were more resistant to the leaf spot/blight disease because it prevented the initiation of the Haber–Weiss reaction, the formation of hydroxyl (OH–) radicals, and membrane dysfunction. Also, B. amyloliquefaciens RaSh1 can mediate the pepper plant’s growth by eliminating free radicals and maintaining photosynthetic rate, cellular redox potential, membrane integrity, and induced disease resistance in infected plants [108]. This suggests that the biocontrol activity of B. amyloliquefaciens RaSh1 against the pepper leaf spot pathogen was influenced by the induction of the host systemic resistance. It is apparent that the application of Thiram (2%) stimulates the antioxidant enzymes activities (PPO; 35.71%) in the diseased pepper plant leaves more than in control ones, as shown in Fig. 7, to mitigate the stress effects. According to Shakir et al. [117], the application of pesticides can quickly produce many types of physiological responses and oxidative damage in plants. It can also accelerate the formation of free radicals and ROS, which in turn causes oxidative damage [118].
DPPH free radical scavenging activity
Plants and pathogens both suffer significant oxidative damage as a result of plant-pathogen interactions that lead to a buildup of ROS in the cells. Both the host and the pathogen have created antioxidant systems to quench excessive ROS and keep ROS generation and scavenging mechanisms under control in order to repair this damage. Radical scavenging activities are very important to prevent the deleterious role of these radicals in different diseases. So, in our study, the DPPH free radical scavenging technique was used as an accepted mechanism for screening the antioxidant activity of methanolic extracts of pepper plants under different treatments (Fig. 8A, B). Figure 8A shows the free radical scavenging activity of the methanolic extracts of different treatments and standard ASA. B. amyloliquefaciens RaSh1 inoculated and infected with A. alternata pepper plant leaves possessed the highest free radical scavenging activity. At a concentration of 100 μg/mL, the scavenging activity in A. alternata infected peppers and treated either with B. amyloliquefaciens RaSh1 or Thiram (0.2%) was 83.19 and 81.08, respectively, compared to diseased (62.13%) or healthy (60.21%) ones (Fig. 8A).
Effect of B. amyloliquefaciens RaSh1 and A. alternata on A DPPH radical scavenging activity and B IC50 of methanolic extracts of the different treatments: ASA Ascorbic acid; T1— control pepper (uninoculated), non-diseased; T2—infected with A. alternata, diseased; T3—infected with A. alternata and sprayed with Thiram (0.2%), diseased; T4—inoculated with B. amyloliquefaciens RaSh1), non-diseased; T5—inoculated with B. amyloliquefaciens RaSh1 and infected with A. alternata
The IC50 (half maximal inhibitory concentration) value was calculated to determine the concentration of the sample required to inhibit 50% of the radical [107]. The observed IC50 value of A. alternata infected plants and treated either with B. amyloliquefaciens RaSh1 or Thiram (0.2%) was 21.74 and 27.01 μg/mL, respectively, compared with A. alternata infected (63.45 μg/mL) and control (80.87 μg/mL) (Fig. 8B). The lower IC50 value indicates greater antioxidant activity as this value is inversely proportional to the free radical scavenging activity/antioxidant property of the sample. Our obtained results suggest that the combined treatment of B. amyloliquefaciens RaSh1 and A. alternata showed the highest radical scavenging activity (the lowest IC50), which indicates their electron transfer or hydrogen donating ability, and this was confirmed by the significant role of B. amyloliquefaciens RaSh1 in increasing the antioxidant enzymes (CAT and PPO) as previously mentioned in the results (Fig. 7). The effect of antioxidants on DPPH is thought to be due to their hydrogen donating ability, keeping in mind the role of polyphenols and tocopherols in scavenging the DPPH radicals by their hydrogen donating ability [119, 120].
Correlation between biochemical and oxidative stress parameters
Inoculation of pepper plants with B. amyloliquefaciens RaSh1 had a positive effect on the plants subjected to Alternaria leaf spot disease conditions. This premise is supported by conducting a Pearson correlation analysis of the different biochemical and oxidative stress parameters that were measured (Table 3). The results indicated that Chl a content in pepper plant leaves was positively correlated with MSI (0.937), RWC (0.762), WC (0.685), and Chl b content (0.979) and negatively correlated with MDA (-0.925), EL (-0.899) and WSD (-0.869). The Pearson correlation coefficients (r) were < 0.01 and < 0.05 for all of the compared attributes (Table 3).
Light and transmission electron microscopy (TEM)
The movement of the pathogen toward the host, attachment to the plant’s surface, penetration of the host by the pathogen, and rapid proliferation of the pathogen once inside the host are necessary for a pathogen to successfully infect a host plant. Leaves collected from healthy pepper plants (T1), A. alternata-infected (T2), and those infected and inoculated with B. amyloliquefaciens RaSh1 (T5) (MZ945930) as biocontrol agent were examined at the structural and ultrastructural level using light and transmission electron microscopy. Structural changes of pepper leaf sections as detected by light microscope Fig. 9A–D) showed anatomical changes in the leaf tissues between different treatments.
Light microscopy images showing the anatomy of pepper leaves A and B Necrotized inoculation sites of pepper leaves infected with A. alternata C Uninfected control leaves and D Pepper leaves infected with A. alternata treated with B. amyloliquefaciens RaSh1 (MZ945930) as biocontrol agent. E, epidermis; N, necrosis; P, palisade mesophyll; S, spongy mesophyll; V.B, vascular bundle
The ultrastructure of cells from healthy pepper leaves revealed that the mesophyll cells had a normal ultrastructure, with a large central vacuole and a narrow layer of cytoplasm, with normal classic chloroplasts characterized by a regularly arranged thylakoid, grana, well defined envelope, starch grains, and few plastoglobuli (Fig. 10). The same results have been previously described by Macioszek et al. [121]. Moreover, electron microscopic analyses of pepper leaves infected with A. alternata spores showed anatomical changes with severe damage in chloroplasts, abnormal enlarged plastoglobuli and starch grains and thylakoids degradation compared to the control leaves (Fig. 11A–C). However, chloroplast with preserved thylakoid, grana system and large starch grains were observed in leaves treated with B. amyloliquefaciens RaSh1(Fig. 11D, F). Similarly, disorganization of the membrane system of the chloroplasts in the infected mesophyll cells, breakdown of the envelope, damage of the cell wall, disappearance of starch grains or the appearance of large starch grains with an increase of plastoglobuli are indicative of infection [121,122,123]. Our results were also in agreement with Gabara et al. [124] and Kozieł et al. [125].
Transmission electron micrographs; A–C showing disorganization of chloroplast structure in the mesophyll cells of pepper leaves infected with A. alternata showing large starch grains and numerous plastoglobules, destroyed outer and inner membranes and collapsed grana and thylakoids. D–F showing chloroplast with preserved thylakoid and grana system and large starch grains in infected pepper leaves and inoculated with B. amyloliquefaciens RaSh1 (MZ945930) as biocontrol agent. Ch chloroplast, CW cell wall, G collapsed granum, P plastoglobule, S starch grain, St stroma
Successful infection of a susceptible host by a necrotrophic fungus depends on many environmental factors including host plant age and conidial concentration [126, 127]. Toxins and secondary metabolites may cause changes in the ultrastructure of organelles [128]. A. alternata pathotypes produce different HSTs (host-specific and non-host-specific toxins) which are responsible for the degradation of different organelles within an infected cell in over 200 plant species. Toxins can affect cell division, protein synthesis, membrane permeabilization and chloroplast photo-phosphorylation [129,130,131].
Conclusion
Our results clearly demonstrate the significance of B. amyloliquefaciens RaSh1 for pepper development and performance, both in healthy and diseased conditions of A. alternata leaf spot/blight. This bacterial strain has the ability to release antioxidant enzymes and solubilize nutrients, which promote host development, physiology, and RWC while mitigating the disease in pepper plants. The B. amyloliquefaciens RaSh1 bacteria not only promotes pepper growth but also increases resistance to A. alternata infections. According to our findings, B. amyloliquefaciens RaSh1 application was effective and advantageous for C. annum under biotic stress (Alternaria leaf spot) and may be a candidate for the management of crop diseases. Given the increased disease tolerance against A. alternata, this suggests that the devastating effects of the leaf spot/blight disease caused by A. alternata in the C. annum are likely to get worse in the future.
Availability of data and materials
All datasets generated for this study are included in the manuscript. The relevant datasets supporting the results of this article are included within the article and the [GenBank NCBI] at https://www.ncbi.nlm.nih.gov/nuccore/OK053809.1
Abbreviations
- CAT:
-
Catalase
- DI:
-
Disease incidence
- Dwt:
-
Dry weight
- EL:
-
Electrolyte leakage
- Fwt:
-
Fresh weight
- MSI:
-
Membrane stability index
- PGPR:
-
Plant growth promoting rhizobacteria
- PPO:
-
Polyphenol oxidase
- WC:
-
Water content
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- WSD:
-
Water saturation deficit
References
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evolut. 2019;3(3):1.
Peng Y, Li SJ, Yan J, Tang Y, Cheng JP, Gao AJ, Yao X, Ruan JJ, Xu BL. Research progress on phytopathogenic fungi and their role as biocontrol agents. Front Microbiol. 2021;12:670135.
Abdelaziz AM, Dacrory S, Hashem AH, Attia MS, Hasanin M, Fouda HM, Kamel, ElSaied SH. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal Agric Biotechnol. 2021;35:102083. https://doi.org/10.1016/j.bcab.2021.102083.
Abdelaziz AM, El-Wakil DA, Attia MS, Ali OM, AbdElgawad H, Hashem AH. Inhibition of Aspergillus flavus growth and aflatoxin production in Zea mays L. Using Endophytic Aspergillus fumigatus. J Fungi. 2022;8(5):482.
Mancini V, Romanazzi G. Seed treatments to control seed borne fungal pathogens of vegetable crops. Pest Manag Sci. 2014;70(6):860–8.
Attia MS, Sharaf A, Zayed AS. Protective action of some bio-pesticides against early blight disease caused by Alternaria solani in tomato plant. JISET Int J Innov Sci Eng Tech. 2017;4:67–94.
Attia MS, Hashem AH, Badawy AA. Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: improving plant immunological, physiological and antifungal activities. Bot Stud. 2022;63:26. https://doi.org/10.1186/s40529-022-00357-6.
Duan Y, Chen R, Zhang R, Jiang W, Chen X, Yin C, Mao Z. Isolation, Identification, and antibacterial mechanisms of Bacillus amyloliquefaciens QSB-6 and its effect on plant roots. Front Microbiol. 2021;12:746799.
Metwally RA, Abdelhameed RE, Soliman SA, Al-Badwy AH. Potential use of beneficial fungal microorganisms and C-phycocyanin extract for enhancing seed germination, seedling growth and biochemical traits of Solanum lycopersicum L. BMC Microbiol. 2022;22:108.
Cheng X, Man X, Wang Z, Liang L, Zhang F, Wang Z. Fungicide SYP-14288 inducing multidrug resistance in Rhizoctonia solani. Plant Dis. 2020;104:2563–70.
Metwally RA, Soliman SA, Abdel Latef AA, Abdelhameed RE. The individual and interactive role of arbuscular mycorrhizal fungi and Trichoderma viride on growth, protein content, amino acids fractionation, and phosphatases enzyme activities of onion plants amended with fish waste. Ecotoxicol Environ Saf. 2021;214:112072.
Hassan SELD, Salem SS, Fouda A, Awad MA, El-Gamal MS, Abdo AM. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nano particles using endophytic actinomycetes. J Radiat Res Appl Sci. 2018;11:262–70.
Chowdhury SK, Majumdar S, Mandal V. Application of Bacillus sp. LBF-01 in Capsicum annuum plant reduces the fungicide use against Fusarium oxysporum. Biocatal Agric Biotechnol. 2020;27:101714.
Truyens S, Weyens N, Cuypers A, Vangronsveld J. Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environ Microbiol Rep. 2014;7:40–50.
Strobel G. The emergence of endophytic microbes and their biological promise. J Fungi. 2018;4:57.
Rashad YM, Abbas MA, Soliman HM, Abdel-Fattah GG, Abdel-Fattah GM. Synergy between endophytic Bacillus amyloliquefaciens GGA and arbuscular mycorrhizal fungi induces plant defense responses against white rot of garlic and improves host plant growth. Phytopathol Mediterr. 2020;59(1):169–86.
Saied EM, Banhart S, Bürkle SE, Heuer D, Arenz C. A series of ceramide analogs modified at the 1-position with potent activity against the intracellular growth of chlamydia trachomatis. Future Med Chem. 2015;7:1971–80.
Zhao L, Xu Y. Lai X (2018) Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz J Microbiol. 2018;49:269–78.
Passari AK, Mishra VK, Leo VV, Gupta VK, Singh BP. Phytohormone production endowed with antagonistic potential and plant growth promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol Res. 2016;193:57–73.
Naveed M, Mitter B, Reichenauer T, Wieczorek K, Sessitsch A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot. 2014;97:30–9.
Zhang X, Gao J, Zhao F, Zhao Y, Li Z. Characterization of a salt-tolerant bacterium Bacillus sp. from a membrane bioreactor for saline wastewater treatment. J Environ Sci. 2014;26:1369–74.
de Almeida Lopes KB, Carpentieri- Pipolo V, Fira D, Balatti PA, López SMY, Oro TH, Stefani Pagliosa E, Degrassi G. Screening of bacterial endophytes as potential biocontrol agents against soybean diseases. J Appl Microbiol. 2018;125(5):1466–81.
Kazerooni EA, Maharachchikumbura SSN, Al-Sadi AM, Kang S-M, Yun B-W, Lee I-J. Biocontrol potential of Bacillus amyloliquefaciens against Botrytis pelargonii and Alternaria alternata on Capsicum annuum. J Fungi. 2021;7:472.
Hazarika DJ, Goswami G, Gautom T, Parveen A, Das P, Barooah M. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019;19:71.
Padmanabhan P, Cheema A, Paliyath G. Solanaceous fruits including tomato, eggplant, and peppers. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of food and health. Oxford: Academic Press; 2016.
Attia MS, Elsayed SM, Abdelaziz AM, Ali MM. Potential impacts of Ascophyllum nodosum, Arthrospira platensis extracts and calcium phosphite as therapeutic nutrients for enhancing immune response in pepper plant against Fusarium wilt disease. Biomass Conv Bioref. 2023. https://doi.org/10.1007/s13399-023-03949-9.
El-kazzaz MK, Ghoneim KE, Agha MKM, Helmy A, Behiry SI, Abdelkhalek A, Saleem MH, Al-Askar AA, Arishi AA, Elsharkawy MM. Suppression of pepper root rot and wilt diseases caused by Rhizoctonia solani and Fusarium oxysporum. Life. 2022;12(4):587.
Parisi M, Alioto D, Tripodi P. Overview of biotic stresses in pepper (Capsicum spp): sources of genetic resistance, molecular breeding and genomics. Int J Mol Sci. 2020;21:2587.
Noman E, Al-Gheethi AA, Rahman NK, Talip B, Mohamed RNH, Kadir OA. Single spore isolation as a simple and efficient technique to obtain fungal pure culture. IOP Conf Ser: Earth Environ Sci. 2018;140:012055.
Ellis MB. Dematiaceus hyphomycetes. Kew: Commonwealth Mycological Institute; 1971.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
Soliman SA, Khaleil MM, Metwally RA. Evaluation of the antifungal activity of Bacillus amyloliquefaciens and B. velezensis and characterization of the bioactive secondary metabolites produced against plant pathogenic fungi. Biology. 2022;11:1390.
Mohammad Q, Muhammad IK, Muhammad S. Comparison of poison food technique and drench method for In Vitro control of Alternaria Sp, the cause of leaf spot of bitter gourd. J Agric Sci Soil Sci. 2016;4:126–30.
Metzner H, Rau H, Senger H. Untersuchungen Zur synchronisierbarkeit einzelner pigment-mangel mutanten von chlorella. Planta. 1965;65(2):186–94.
Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and Chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–2.
Barr HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci. 1962;15:413–28.
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006;48:127–35.
Farooq S, Azam F. The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J Plant Physiol. 2006;163(6):629–37. https://doi.org/10.1016/j.jplph.2005.06.006.
Heath R, Packer L. Photoperoxidation in isolated chloroplasts of fatty acid peroxidation chlorophyll. Arch Biochem biophisics. 1968;126:189–98.
Qiu RL, Zhao X, Tang XZ, Yu FM, Hu PJ. Antioxidative response to Cd in a newly discovered cadmium hyperaccumulator, Arabis paniculata F. Chemosphere. 2008;74:6–12.
Aebi H. Catalase in vitro. Methods in enzymol, vol. 105. Cambridge: Academic Press; 1984. p. 121–6.
Lavid N, Schwartz A, Yarden O. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta. 2001;212:323–31. https://doi.org/10.1007/s004250000400.
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200.
Desmarchelier C, Bermudez MJN, Coussio J, Ciccia G, Boveris A. Antioxidant and prooxidant activities in aqueous extract of Argentine plants. Int J Pharmacogn. 1997;35:116–20.
Montasser MS, Dashti NJ, Ali NY, Bhardwaj RG, Alhamar B. Occurrence of three strains of cucumber mosaic virus affecting tomato in Kuwait. Plant Pathol J. 2006;22:51–62.
Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26(1):31–43.
Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–12.
Baysal Ő, Lai D, Xu H-H, Siragusa M, C¸alıs¸kan M. A Proteomic approach provides new insights into the control of soil-borne plant pathogens by Bacillus species. PLoS ONE. 2013;8(1):e53182.
Prabha S, Yadav A, Kumar A, Yadav. Biopesticides—an alternative and eco-friendly source for the control of pests in agricultural crops. Plant Archives. 2016;16:902–6.
El-Sayed WS, Akhkha A, El-Naggar MY, ElBadry M. In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front Microbiol. 2014;5:651.
Fan H, Zhang Z, Li Y, Zhang X, Duan Y, Wang Q. Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization. Front Microbiol. 2017;8:1973.
Zhang QX, Zhang Y, He LL, Ji ZL, Tong YH. Identification of a small antimycotic peptide produced by Bacillus amyloliquefaciens 6256. PesticideBiochem Physiol. 2018;150:78–82.
Sur S, Romo TD, Grossfield A. Selectivity and mechanism of fengycin, an antimicrobial lipopeptide, from molecular dynamics. J Phys Chem B. 2018;122(8):2219–26.
Liu J, Zhou T, He D, Li X-Z, Wu H, Gao X. Functions of lipopeptides bacillomycin D and fengycin in antagonism of Bacillus amyloliquefaciens C06 towards Monilinia fructicola. J Mol Microbiol Biotechnol. 2011;20:43–52.
Agarwal M, Dheeman S, Dubey RC, Kumar P, Maheshwari DK, Bajpai VK. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol Res. 2017;205:40–7. https://doi.org/10.1016/j.micres.2017.08.012.
Xu W, Wang K, Wang H, Liu Z, Shi Y, Gao Z, et al. Evaluation of the biocontrol potential of Bacillus sp. WB against Fusarium oxysporum f. sp. niveum. Biol Control. 2020;147:104288. https://doi.org/10.1016/j.biocontrol.2020.104288.
Massawe VC, Hanif A, Farzand A, Mburu DK, Ochola SO, Wu L, et al. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology. 2018;108:1373–85.
Mohamad O, Ma JB, Liu YH, Zhang D, Hua S, Bhute S, Hedlund BP, Li WJ, Li L. Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front Plant Sci. 2020;11:47.
Huang L, Zhu X, Zhou S, Cheng Z, Shi K, Zhang C, Shao H. Phthalic acid esters: natural sources and biological activities. Toxins. 2021;13:495.
Mousa WK, Raizada MN. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front Microbiol. 2013;4:65.
Grabka R, d’Entremont TW, Adams SJ, Walker AK, Tanney JB, Abbasi PA, Ali S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants. 2022;11:384.
Sarkar D, Barhate BG, Joshi VR. Studies on leaf spot of chili. Int J Plant Prot. 2017;10:369–74.
Soomro HB, Khaskheli MI, Hyder M, Khan RAA, Bukero A, Panhwar S, Bukero AA, Larik AQ. Disease intensity and eco-friendly management of Alternaria alternata in chili (Capsicum annuum L.). Pure Appl Biol. 2019;8:2333–42.
Shoaib A, Akhtar M, Javaid A, Ali H, Nisar Z, Javed S. Antifungal potential of zinc against leaf spot disease in chili pepper caused by Alternaria alternata. Physiol Mol Biol Plants. 2021;27(6):1361–76.
Vázquez MB, Moreno MV, Amodeo MR, Bianchinotti MV. Effects of glyphosate on soil fungal communities: a field study. Rev Argent Microbiol. 2021;53:349–58.
Metwally RA, Azab HS, Al-Shannaf HM, Rabie GH. Prospective of mycorrhiza and Beauvaria bassiana silica nanoparticles on Gossypium hirsutum L. plants as biocontrol agent against cotton leafworm Spodoptera littoralis. BMC Plant Biol. 2022;22:409.
Metwally RA, Abdelhameed RE. Impact of ridomil, bavistin and agrothoate on arbuscular mycorrhizal fungal colonization, biochemical changes and potassium content of cucumber plants. Ecotoxicology. 2019;28:487–98.
Kumar V, Jain L, Jain SK, Chaturvedi S, Kaushal P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. S Afr J Bot. 2020;134:50–63.
Shahzad R, Khan AL, Bilal S, Asaf S, Lee I. Plant growth-promoting endophytic bacteria versus pathogenic infections: an example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. Peer J. 2017;5:e3107.
Ghanbary E, Fathizadeh O, Pazhouhan I, Zarafshar M, Tabari M, Jafarnia S, Parad GA, Bader MK-F. Drought and pathogen effects on survival, leaf physiology, oxidative damage, and defense in two middle eastern oak species. Forests. 2021;12:247.
Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot. 2012;63:3523–43.
Zhang M, Li J, Shen A, Tan S, Yan Z, Yu Y, et al. Isolation and Identification of Bacillus amyloliquefaciens IBFCBF-1 with potential for biological control of phytophthora blight and growth promotion of pepper. J Phytopathol. 2016;164:1012–21.
Rangjaroen C, Rekarsem B, Teaumroong N, Noisangiam R, Lumyong S (2014). Promoting plant growth in a commercial rice cultivar by endophytic diazotrophic bacteria isolated from rice landraces. Ann Microbiol. 1–14.
Srivastava S, Bist V, Srivastava S, Singh PC, Trivedi PK, Asif MH, Chauhan PS, Nautiyal CS. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front Plant Sci. 2016;7:587. https://doi.org/10.3389/fpls.2016.00587.
Gimenez-Ibanez S, Solano R. Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens. Front Plant Sci. 2013;4:72. https://doi.org/10.3389/fpls.2013.00072.
Fadiji AE, Babalola OO. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol. 2020;8:467.
Abdelhameed RM, Abdelhameed RA, Kamel HA. Iron-based metal-organic-frameworks as fertilizers for hydroponically grown Phaseolus vulgaris. Mater Lett. 2019;237:72–9.
Metwally RA, Al-Amri SM. Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett Appl Microbiol. 2019;70:79–86.
Abdelhameed RE, Abu-Elsaad NI, Abdel Latef AAH, Metwally RA. Tracking of zinc ferrite nanoparticle effects on pea (Pisum sativum L.) plant growth, pigments, mineral content and arbuscular mycorrhizal colonization. Plants. 2021;10:583.
Abdelhameed RE, Metwally RA. Assessment of beneficial fungal microorganism’s bio-efficacy in stimulating morphological and physiological parameters of Allium cepa plants grown in soil amended with fish wastes. BMC Plant Biol. 2022;22:617.
Hossain M, Veneklaas EJ, Hardy GESJ, Poot P. Tree host–pathogen interactions as influenced by drought timing: linking physiological performance, biochemical defense and disease severity. Tree Physiol. 2019;39:6–18.
Ghanbary E, Tabari Kouchaksaraei M, Zarafshar M, Bader MK-F, Mirabolfathy M, Ziaei M. Differential physiological and biochemical responses of Quercus infectoria and Q. libani to drought and charcoal disease. Physiol Plant. 2020;168:876–92.
Ranjbar A. Comparative study on the effect of water stress and rootstock on photosynthetic function in pistachio (Pistacia vera L). Trees. 2017;8:151–9.
Heuser T, Zimmer W. Quantitative analysis of phytopathogenic ascomycota on leaves of pedunculate oaks (Quercus robur L.) by real-time PCR. FEMS Microbiol Let. 2002;209:295–9.
Gómez-Gallego M, Bader MK-F, Scott PM, Leuzinger S, Williams NM. Phytophthora pluvialis studies on douglas-fir require swiss needle cast suppression. Plant Dis. 2017;101:1259–62.
Waqas M, Khan A, Hamayun M, Shahzad R, Kang S, Kim J, Lee I. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J Plant Int. 2015;10(1):280–7.
Hashem A, AbdAllah EF, Alqarawi AA, Radhakrishnan R, Kumar A. Plant defense approach of Bacillus subtilis (BERA 71) against Macrophomina phaseolina (Tassi) Goid in mung bean. J Plant Interact. 2017;12(1):390–401.
Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, AbdAllah EF. Endophytic Bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and Induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol. 2017;8:1887.
Feng W, He L, Zhang HY, Guo BB, Zhu YJ, Wang CY, Guo TC. Assessment of plant nitrogen status using chlorophyll fluorescence parameters of the upper leaves in winter wheat. Eur J Agron. 2015;64:78–87.
Habib S, Kausar H, Saud H. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in Okra 468 through ROS-scavenging enzymes. BioMed Res Int. 2016. https://doi.org/10.1155/2016/6284547.
Verma S, White J. Indigenous endophytic seed bacteria promote seedling development and defend against fungal disease in browntop millet (Urochloa ramosa L.). J Appl Microbiol. 2018;124:764–78. https://doi.org/10.1111/jam.13673.
Metwally RA, Abdelhameed RE. Synergistic effect of arbuscular mycorrhizal fungi in growth and physiology of salt-stressed Trigonella foenum-graecum plants. Biocatal Agric Biotechnol. 2018;16:538–44.
Burman U, Lodha S. Macrophomina phaseolina induced changes in plant water relations of resistant and susceptible cowpea genotypes. Indian Phytopathol. 1996;49:254–9.
Duniway JM, Durbin RD. Detrimental effect of rust infection on the water relations of bean. Plant Physiol. 1971;48:69–72.
González L, González-Vilar M. Determination of relative water content. In: Reigosa MJ, editor. Handbook of plant eco-physiology techniques. Dordrecht, The Netherlands: Springer; 2001. p. 207–12.
Dubey A, Saiyam D, Kumar A, Hashem A, Abd Allah EF, Khan ML. Bacterial root endophytes: characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. Int J Environ Res Public Health. 2021;18(3):931.
Grimmer M, Foulkes M, Paveley N. Foliar pathogenesis and plant water relations: a review. J Exp Bot. 2012;63(12):4321–31.
Geat N, Singh D, Khirbat S. Effect of non-conventional chemicals and synthetic fungicide on biochemical characteristics of chilli against fruit rot pathogen Colletotrichum capsici. J Plant Pathol Microbiol. 2016;7:605–13.
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62:21–30. https://doi.org/10.1007/s10725-010-9479-4.
Vardharajula S, Shaik ZA, Grover M, Reddy G, Venkateswarlu B. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact. 2011;6:1–14. https://doi.org/10.1080/17429145.2010.535178.
Abdelhameed RE, Abdel Latef AA, Shehata RS. Physiological responses of salinized fenugreek (Trigonella foenum-graecum L.) plants to foliar application of salicylic acid. Plants. 2021. https://doi.org/10.3390/plants10040657.
Zhou X, Dai L, Xu G, Wang H. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Scientifc Reports. 2021;11:7637.
Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30.
Abdelhameed RE, Metwally RA. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int J Phytoremediation. 2019;21:663–71.
Cui-Juan W, Ying-Zi W, Zhao-Hui C, Pei-Song W, Bao-You L, Bao-Yan L, Xiao-Li Y, Bing-Hui L. Endophytic Bacillus amyloliquefaciens YTB1407 elicits resistance against two fungal pathogens in sweet potato (Ipomoea batatas (L.) Lam.). Journal of Plant Physiology. 2020;253:153260.
Metwally RA, Soliman SA (2023) Alleviation of the adverse effects of NaCl stress on tomato seedlings (Solanum lycopersicum L.) by Trichoderma viride through the antioxidative defense system c8232cb7–7e69–4caf-88d4–001bc699ac5b” Botanical Studies, in press.
Abdalla H, Adarosy MH, Hegazy HS, Abdelhameed RE. Potential of green synthesized titanium dioxide nanoparticles for enhancing seedling emergence, vigor and tolerance indices and DPPH free radical scavenging in two varieties of soybean under salinity stress. BMC Plant Biol. 2022;22:560. https://doi.org/10.1186/s12870-022-03945-7.
Radhakrishnan R, Shim KB, Lee BW, Hwang CD, Pae SB, Park CH, Kim SU, Lee CK, Baek IY. IAA producing Penicillium sp NICS01 triggers plant growth and suppresses Fusarium induced oxidative stress in sesame (Sesamum indicum L.). J Microbiol Biotechnol. 2013;23(6):856–63.
Lubaina AS, Murugan K. Ultrastructural changes and oxidative stress markers in wild and cultivar Sesamum orientale L. following Alternaria sesame (Kawamura) Mohanty and Behera. inoculation. Indian J Exp Biol. 2013;51:670–80.
Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482(3):419–25.
Shukla N, Awasthi R, Rawat L, Kumar J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem. 2012;54:78–88.
Abu-Elsaad NI, Abdelhameed RE. Copper ferrite nanoparticles as nutritive supplement for cucumber plants grown under hydroponic system. J Plant Nutr. 2019. https://doi.org/10.1080/01904167.2019.1630428.
Nasrallah DA, Morsi MA, El-Sayed F, Metwally RA. Structural, optical and electrical properties of copper chloride filled polyvinyl chloride/polystyrene blend and its antifungal properties against Aspergillus avenaceus and Aspergillus terreus. Compos Commun. 2020;22:100451.
Hasanuzzaman M, Raihan MRH, Masud AAC, Rahman K, Nowroz F, Rahman M, Nahar K, Fujita M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int J Mol Sci. 2021;22(17):9326.
Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C. Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiol. 2010;154:444–8.
Li Y, Gu Y, Li J, Xu M, Wei Q, Wang Y. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front Microbiol. 2015;28(6):883.
Shakir SK, Irfan S, Akhtar B, Rehman S, Daud MK, Taimur N, Azizullah A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology. 2018;27:919–35.
Parween T, Jan S, Mahmooduzzafar FT. Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int J Environ Sci Technol. 2012;9:605–12.
Baumann J, Wurn G, Bruchlausen FV. Prostaglandin synthetase inhibiting O2 radical scavenging properties of some flavonoids and related phenolic compounds. Deutsche Pharmakologische Gesellschaft abstracts of the 20th spring meeting, Naunyn-Schmiedebergs abstract no: R27 cited. Arc Pharmacol. 1979;307:1–77.
Huang D, Ou B, Prior RI. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56.
Macioszek VK, Sobczak M, Skoczowski A, Oliwa J, Michlewska S, Gapin´ska M, Ciereszko I, Kononowicz AK. The effect of photoperiod on necrosis development, photosynthetic efficiency and ‘Green Islands’ formation in Brassica juncea infected with Alternaria brassicicola. Int J Mol Sci. 2021;22:8435.
Macioszek VK, Gapińska M, Zmienko A, Sobczak M, Skoczowski A, Oliwa J, Kononowicz AK. Complexity of Brassica oleracea–Alternaria brassicicola Susceptible Interaction Reveals downregulation of photosynthesis at ultrastructural, transcriptional, and physiological levels. Cells. 2020;9:2329.
Montasser MS, Al-Own FD, Haneif AM, Afzal M. Effect of tomato yellow leaf curl bigeminivirus (TYLCV) infection on tomato cell ultrastructure and physiology. Can J Plant Path. 2012;34(1):114–25.
Gabara B, Kuz´niak E, Skłodowska M, Surówka E, Miszalski Z. Ultrastructural and metabolic modifications at the plant-pathogen interface in Mesembryanthemum crystallinum leaves infected by Botrytis cinerea. Environ Exp Bot. 2012;77:33–43.
Kozieł E, Otulak-Kozieł K, Bujarski JJ. Modifications in tissue and cell ultrastructure as elements of immunity- like reaction in Chenopodium quinoa against prune dwarf virus (PDV). Cells. 2020;9:148.
Deep S, Sharma P. Host age as predisposing factor for incidence of black leaf spot of cauliflower caused by Alternaria brassicae and Alternaria brassicicola. Indian Phytopathol. 2012;65:71–5.
Nowakowska M, Wrzesińska M, Kamiński P, Szczechura W, Lichocka M, Tartanus M, Kozik EU, Nowicki M. Alternaria brassicicola – Brassicaceae pathosystem: insight into the infection process and resistance mechanism under optimized artificial bio-assay. Eur J Plant Pathol. 2019;153:131–51.
Cho Y. How the necrotrophic fungus Alternaria brassicicola kills plant cells remains an enigma. Eukaryot Cell. 2015;14:335–44.
Tsuge T, Harimoto Y, Akimitsu K, Ohtani K, Kodama M, Akagi Y, Egusa M, Yamamoto M, Otani H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol Rev. 2013;37:44–66.
Akimitsu K, Tsuge T, Kodama M, Yamamoto M, Otani H. Alternaria host-selective toxins: determinant factors of plant disease. J Gen Plant Pathol. 2014;80:109–22.
Meena M, Samal S. Alternaria host-specific (HSTs) toxins: an overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol Rep. 2019;6:745–58.
Acknowledgements
We appreciate the financial support from the Academy of Scientific Research and Technology (ASRT-2022), Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization: RAM, REA and SAS; Methodology: REA, SAS, and RAM; Formal analysis and investigation: RAM and REA; Writing: RAM, SAS and REA; Review and editing: RAM, SAS and REA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Soliman, S.A., Abdelhameed, R.E. & Metwally, R.A. In vivo and In vitro evaluation of the antifungal activity of the PGPR Bacillus amyloliquefaciens RaSh1 (MZ945930) against Alternaria alternata with growth promotion influences on Capsicum annuum L. plants. Microb Cell Fact 22, 70 (2023). https://doi.org/10.1186/s12934-023-02080-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-023-02080-8