Abstract
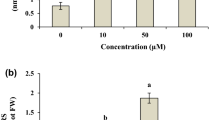
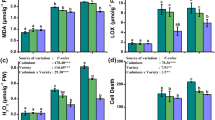
This study aims to evaluate the effect of chlorpyrifos on several metabolic and stress related parameters of Vigna radiata L. Twenty-day-old plants were exposed to several concentrations of chlorpyrifos, ranging from 0 to 1.5 mM through foliar spray in the field condition. Analyses were done at pre-flowering (Day 5), flowering (Day 10), and post-flowering (Day 20) stages after the treatment. Lipid peroxidation rate, proline, dehydroascorbate, oxidized and total glutathione were all ascended. Chlorpyrifos enhanced lipid peroxidation rate and proline content with 1.5 mM at Day 20 whereas dehydroascorbate, oxidized and total glutathione were increased in 1.5 mM at Day 10. However, dose dependence significantly declined in content of ascorbate and reduced glutathione levels were observed at all growth stages. Among the enzymatic antioxidants, activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase enhanced significantly in all the concentrations at Day 10. Maximum catalase activity was observed at Day 10 in control and it declined thereafter. The above results clearly depicted the provoked state of oxidative stress under chlorpyrifos exposure in Vigna radiata L. and therefore can be used to evaluate the degree of insecticide contamination to plant, which may be serving as biomarker in Vigna radiata L.

Similar content being viewed by others
References
Aditya CN, Banerjee H, Kole RK (1997) An appraisal of pesticide use in Indian agriculture with special reference to their consumption in West Bengal. Sci Cult 63:223–228
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:555–570
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplast and their functions. Plant Physiol 141:391–396
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bashir F, Siddiqi TO, Mahmooduzzafar, Iqbal M (2007a) Effect of different concentrations of mancozeb on the morphology and anatomy of Lens culinaris L. Indian J Environ Sci 11:71–74
Bashir F, Siddiqi TO, Mahmooduzzafar, Iqbal M (2007b) The anti oxidative response system in Glycine max (L.) Merr. Exposed to deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut 147:94–100
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Batish DR, Singh Hp, Setia N, Kaur S, Kohli K (2006) 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol Biochem 44:819–827
Bowler C, Anmontagu M, Inze D (1992) Superoxide dismutase and stress tolerance: annual review plant physiology. Plant Mol Biol 43:83–116
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–259
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase catalase and peroxidase activities in root tips of soybean (Glycine max L.). Physiol Plant 83:463–468
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Florence TM, Stauber JL (1986) Toxicity of copper complexes to the marine diatom Nitzschia closterium. Aquat Toxicol 8:11–26
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Lopez Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Planta 100:241–254
Gopi R, Jaleel CA, Sairam R, Lakshmanan GMA, Gomithinayagam M, Pannerselvem R (2007) Differentialn effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids Surf B Biointerfaces 60:180–186
Haddad R, Morris K, Buchanan-Wollaston V (2009) Molecular characterization of free radicals decomposing genes on plant developmental stages. In: Proceedings of world academy of science, engineering and technology, vol 37
Halliwell B, Gutteridge JMC (1985) Free radicals in biology and medicine. Clarendon Press, Oxford
Hazarika A, Sarkar SN, Hajare S, Kataria M, Malik JK (2003) Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology 185:1–8
Howard PH (ed) (1991) Handbook of environmental fate and exposure data for organic chemicals. In: Pesticides, vol III. Lewis Publishers, Chalsea, MI
Jaleel CA, Gopi R, Alagu Lal Shmanan GM, Panneerselvam R (2006) TDM induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus L. G. Don. Plant Sci 7:271–276
Jaleel CA, Jayakumar K, Xing ZC, Azooz MM (2009) Antioxidant potentials protect Vigna radiata (L.) Wilczek plants from soil cobalt stress and improve growth and pigment composition. Plant Omics J 2(3):120–126
Jan S, Parween T, Siddiqi TO, Mahmooduzzafar (2012a) Antioxidant modulation in response to gamma induced oxidative stress in developing seedlings of Psoralea corylifolia L. J Environ Radioact 113:142–149
Jan S, Parween T, Siddiqi TO, Mahmooduzzafar (2012b) Effect of gamma radiation on morphological, biochemical and physiological aspects of plants and plant products. Environ Rev 20:7–39
Jiang L, Yang H (2009) Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol Environ Safety 72:1687–1693
Jianga L, Maa L, Suia Y, Hna SQ, Wua ZY, Fenga YX, Yanga H (2010) Effect of manure compost on the herbicide prometryne bioavailability to wheat plants. J Hazard Mater 184:337–344
Jiménez A, Hernández JA, del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Khan SM, Kour G (2007) Sub acute oral toxicity of chlorpyriphos and protective effect of green tea extract. Pest Biochem Physiol 89:118–123
Khan H, Zeb A, Ali Z, Shah SM (2009) Impact of five insecticides on chickpea (Cicer arietinum L.) nodulation, yield and nitrogen fixing rhizospheric bacteria. Soil Environ 28:56–59
Kok De L, Stulen I (1993) Role of glutathione in plants under oxidative stress. In: De Kok LJ, Stuten I, Rennenberg H, Brunold C, Rauser WE (eds) Sulfur nutrition and assimilation in higher plants: regulatory agricultural and environmental aspects. SPB Acedamic Publishing, The Hague, pp 125–138
Kovacik J, Klejdus B, Hedbavny J, Backor M (2009) Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18:44–554
Kumar NG, Nirmala P, Jayappa AH (2010) Effect of various methods of application of insecticides on the incidence of serpentine leaf miner, Liriomyza trifolii (burgess) and other pests in soybean. Karnataka J Agric Sci 23:130–132
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplast. Biochem J 210:99–903
MacRae EA, Ferguson IB (1985) Changes in catalase activity and hydrogen peroxide concentration in plants in response to low temperature. Physiol Plant 65:51–56
Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabi Y (2011) Effect of drought stress and subsequent recovery on protein, carbohydrate contents, catalase and peroxidase activities in three chickpea (Cicer arietinum) cultivars. AJCS 5(10):1255–1260
Mishra V, Srivastava G, Prasad SM, Abraham G (2008) Growth, photosynthetic pigment and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata L.) in response to UV-B and dimethoate. Pestic Biochem Physiol 92:30–37
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trend Plant Sci 7:05–410
Morimura T, Ohya T, Ikawa T (1996) Presence of ascorbate-peroxidizing enzymes in roots of Brassica campestris L. cv Komatsuna. Plant Sci 117:55–63
Nagesh BR, Devaraj VR (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Aus J Crop Sci 2:40–48
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Panduranga MG, Mahadeva PG, Sudarshana MS (2005) Toxicity of different imbibitions periods of dimethoate on germination, chlorophyll a/b, and dry matter of Glycine max (L) Merrill. Cv. 548 KHSB-2, during early seedlings growth. J Physiol Res 18:199–201
Parween T, Jan S, Mahmooduzzafar, Fatma T (2011) Alteration in nitrogen metabolism and plant growth during different developmental stages of green gram (Vigna radiata L.) in response to chlorpyrifos. Acta physiol plant 33:2321–2328
Peixoto F, Alves-Fernandes D, Santos D, Fontaınhas-Fernandes A (2006) Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pesticide Biochem Physiol 85:91–96
Pinto E, Sigaud-Kutner TCS, Leitao MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:008–1018
Prasad SM, Kumar D, Zeeshan M (2005) Growth, Photosynthesis, active oxygen species and antioxidants responses of paddy field cyano bacterium Plectonema boryanum to endosulfan stress. J Geb Appl Microbiol 51:115–123
Qureshi MI, Abdin MZ, Qadir S, Iqbal M (2007) Lead-induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol Plant 51:121–128
Rao MV (1992) Cellular detoxifying mechanisms determine age dependent injury in tropical plants exposed 410 to SO2. J Plant Physiol 140:733–740
Rastgool L, Alemzadeh A (2011) Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aus J Crop Sci 5(4):375–383
Rusyniak DE, Nanagas KA (2004) Organophosphate poisoning. Semen Neurol 24:197–204
Saraf M, Sood N (2002) Influence of monocrotophos on growth, oxygen uptake and exopolysaccharide production of rhizobium NCIM 2271 on chickpea. J Indian Bot Soc 82:157–164
Song NH, Yin X, Chen GF, Yang H (2007) Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 69:1779–1787
Stevens M, Reined RF, Combs NE, Helliwell S, Jianhua Mo (2008) Influence of imidacloprid seed treatments on rice germination and early seedling growth. Pest Manag Sci 64:215–222
Stolt JP, Sneller FEC, Bryngelsson T, Lundborg T, Schat H (2003) Phytochelatin and cadmium accumulation in wheat. Environ Exp Bot 49:21–28
Venkateswara Rao J, Parvati K, Kavitha P, Jakka NM, Pallela R (2005) Effect of chlorpyrifos and monocrotophos on loco motor behavior and acetyl cholinesterase activity of subterranean termites, Odontotermes obesus. Pest Manage Sci 61:417–421
Wang L, Jiang X, Yan D, Wu J, Bian Y, Wang F (2007) Behaviour and fate of chlorpyrifos introduced into soil crop systems by irrigation. Chemosphere 66:391–396
Wingate VPM, Lawton MA, Lamb CJ (1988) Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol 87:06–210
Winglse G, Karpinski S (1996) Differential redox regulation by glutathione and glutathione reductase and Cu–Zn SOD gene expression in Pinus sylvestris L. needles. Planta 198:151–157
Wu J, Laird DA (2003) Abiotic transformation of chlorpyrifos oxon in chlorinated water. Environ Toxicol Chem 22:261–264
Wu XY, Von Tiedemann A (2002) Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut 116:37–47
Wu GL, Cui EJ, Tao EL, Yang EH (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 19:24–132
Acknowledgments
First author is highly thankful to University Grants Commission (UGC), Government of India for providing fellowship during this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parween, T., Jan, S., Mahmooduzzafar et al. Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int. J. Environ. Sci. Technol. 9, 605–612 (2012). https://doi.org/10.1007/s13762-012-0095-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0095-x