Abstract
Background
Pepper is a popular ingredient in many Chinese households; however, anthracnose caused by Colletotrichum spp. has greatly decreased pepper production. The genus Bacillus is widely known for its important role in the development and protection of plants from phytopathogenic fungi.
Results
Fifty-eight endophytic strains were isolated from pepper leaves and tested for antifungal activity in this study. Specifically, L1-7 and L3-5 displayed growth inhibition rates of 79 and 80% against C. scovillei mycelium, respectively, while 25 of these strains all had growth inhibition rates of greater than 60%. Bacillus amyloliquefaciens and B. velezensis, based on culture and morphological identification with 16S rDNA and gyrB gene sequence analyses, were determined to be the respective species L1-7 and L3-5. Additionally, it was discovered that these two antagonistic endophytic bacteria could fix nitrogen, produce indoleacetic acid (IAA) and have a high salt tolerance. Pot experiments again showed excellent control of the pathogen C. scovillei by L1-7 and L3-5, with 80.64 and 73.39% control, respectively. Therefore, B. amyloliquefaciens (L1-7) and B. velezensis (L3-5) can be applied as biological control agents to protect peppers against C. scovillei-caused anthracnose.
Conclusion
Bacillus amyloliquefaciens (L1-7) and B. velezensis (L3-5) can be applied as biological control agents to protect peppers against C. scovillei-caused anthracnose. Thus, they can serve as promising biocontrol agents and plant growth promoters, and future research on the pertinent bacteria will serve as a useful guide for the creation of microbial resources.
Similar content being viewed by others
Background
Endophytes are mostly bacteria, fungi and actinomycetes. They are a diverse group of microorganisms that can live in healthy, living plant tissue without causing obvious pathological changes in the host plant (Hallmann et al. 1997). They are not only widely distributed in plants, but have also formed a mutually beneficial, symbiotic and interdependent relationship with plants over time, which can promote plant growth, endow plants with stress resistance, pest resistance and antagonistic functions (Bokhari et al. 2019), implying that plant endophytes have excellent biological control potential. Endophyte research is currently centered on promoting plant growth, acting as a nutrient and inhibiting the growth of pathogenic microorganisms (Egamberdieva et al. 2017). Pepper anthracnose causes significant production losses in all countries where pepper plants are grown (De Silva et al. 2019). In addition to affecting peppers, these fungi cause diseases in a variety of vegetables, fruits and other crops (Farr and Rossman 2023). As a result, preventing and treating pepper anthracnose caused by Colletotrichum are critical. Various methods are currently used to control various plant diseases. Chemical control is currently the most effective method for controlling plant diseases (Hirooka and Ishii 2013). However, the use of fungicides can pose a serious threat to the environment and cause harmful side effects in humans. The suppression of plant pathogen populations by microbial antagonists or the production of antimicrobials is referred to as the biocontrol of plant diseases. Therefore, microbial antagonists have been chosen as biocontrol agents as alternative to fungicides (Wonglom et al. 2019).
Furthermore, the use of endophytic strains to control pathogens is becoming more common (Bhattacharya et al. 2019). Many endophytic strains have been isolated and demonstrated to be capable of controlling a wide range of pathogens, including antagonistic bacteria, antagonistic fungi and antagonistic actinomycetes (Jiang and Song 2014). Endophytic strains have been used in biocontrol numerous times, but endophytic bacteria are the most commonly used (Asghari et al. 2019). According to an existing article, the dominant strains of endophytic bacteria isolated were: Bacillus, Pseudomonas and Streptomyces (Liu et al. 2016). Bacillus pumilus, an endophytic bacterium, caused the plants to produce a large amount of deposited tannins and phenols, which thickened the cell wall and prevented pathogens from invading between the outer layer and the cork layer (Benhamou et al. 1996). Streptomyces griseocarneus R132 inhibited plant pathogen growth by 57.24 ± 4.54% (Fusarium oxysporum) to 73.93 ± 3.71% (Botryosphaeria dothidea), which appears to have controlled the development of anthracnose symptoms caused by Colletotrichum gloeosporioides (Liotti et al. 2019). Despite numerous studies on antagonistic endophyte strain, there have been no reports on their ability to control C. scovillei.
In this study, endophytic bacteria were isolated from healthy pepper leaves, strains with good antagonistic effects on the pathogen C. scovillei were screened and identified, and their biological functions were determined. These results laid the groundwork for the biological control of pepper anthracnose and served as a guide for these endophytic strains to develop into excellent bacterial resources in plant disease control.
Methods
Biological material
The endophytic strains were isolated from healthy leaves of sweet peppers (Capsicum annuum), which were grown in the Biocontrol Engineering Laboratory of Crop Diseases and Pests, College of Plant Protection, Gansu Agricultural University (November 2020). This laboratory also houses the pathogen Colletotrichum truncatum. Nutrient agar medium (NA), potato dextrose agar medium (PDA), Pikovaskaia’s medium (PKO), King’s medium, Mehknha medium and Ashby medium (Wei et al. 2018b) were used to grow the strains in the experiment. Sarkowski’s reagents (PC and S2) were used to determine the concentration of IAA (Glickmann and Dessaux 1995).
Isolation of endophytic bacteria
Endophytic strains were isolated (Separation place: Biocontrol Engineering Laboratory of Crop Diseases and Pests) from sweet pepper (Capsicum annuum) leaves using the traditional method of tissue separation (Vetrivelkalai et al. 2010). Based on the characteristics of colony size, morphology and color, 58 endophytic strains were isolated from pepper leaves and numbered as L1-7, L3-5, L5-3, L6-6, L7-5, L7-6, L7-7, L7-9, L7-10 and L7-13. Store the strains in test tubes and set them aside in a 4 °C refrigerator for backup.
Screening for endophytic strains with outstanding resistance to C. scovillei
The plate antagonism method (Skeen et al. 1995) was used to assess each isolated endophytic strain’s ability to suppress the pathogen C. scovillei’s mycelial growth. All endophytic strains stored in the refrigerator at 4 °C were inoculated into NA medium for 48 h, and then, these endophytic strains were inoculated on four symmetrical positions 2.5 cm from the center of the PDA medium, and the center of the PDA medium was inoculated with 0.6 cm diameter C. scovillei pathogenic fungi disks. Each biological treatment had four replicates, and the control was not inoculated with endophytic strains. They were placed in a constant temperature incubator at 25 °C for 7 days, and then, the colony diameters of the control were measured, as well as those of the pathogens inoculated with the endophytic strain. Finally, the strain inhibition rate was calculated using the equation below:
where Cc is the fungus diameter of control (cm), Cf is the fungus diameter of treatment (cm) and 0.6 is the diameter of the fungi disks.
Morphological identification of antagonistic strains
Antagonistic strains were inoculated onto NA medium and incubated at 28 °C for 18–24 h before Gram staining, observing bacterial morphology, measuring length and width, and photographing with a microscope. Endophytic strains with inhibition function were incubated at 28 °C for 72 h before colony morphology was examined.
Molecular identification of antagonistic strains
Using the Ezup Columnar Genomic DNA Extraction Kit (Solabao, Beijing, China), DNA was extracted from endophytic strains and stored as a backup at − 20 °C. In order to amplify gene fragments, the DNA was amplified using the universal primer set (27F: 5′-AGA GTT TGA TCM TGG CTC AG-3′, 1492R: 5′-TAC GGY TAC CTT GTT ACG ACT T-3′) and the gyrB-specific primer set (UP-1: 5′-GAA GTC ATC ATG ACC GTT CTG CAY GCN GGN AAR TTY GA-3′) (Bouaoud et al. 2018; Morris et al. 2008). The PCR amplification of both universal primer and specific primer was performed in a 50 μl mixture containing 25 μl PCR Mix (2×), 2 μl of each primer (10 μmol·l−1), 2 μl template DNA and 19 μl ddH2O, and the universal primer PCR conditions were pre-denaturation at 94 °C for 4 min, denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, extension at 72 °C for 90 s, 30 cycles and a final extension at 72 °C for 10 min. The specific primer’s PCR conditions were pre-denaturation at 94 °C for 4 min, denaturation at 94 °C for 1 min, annealing at 57 °C for 1 min, extension at 72 °C for 70 s, 35 cycles and a final extension at 72 °C for 10 min, with the amplified product stored at 4 °C after the reaction. Electrophoresis on a 1% agarose gel was used to detect PCR amplification. The amplified products were sequenced at Qingke Biology Company in Xi’an City, Shanxi Province, China. For approximate identification against the NCBI sequence database, the BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) algorithm with our gene sequences was used. The neighbor-joining (NJ) algorithm was used to construct a distance tree using the MEGA version 6 software.
Biological function determination of antagonistic strains
Determination of nitrogen fixation ability
The antagonistic strains were inoculated into an NA liquid medium and incubated in a thermostatic shaker for 24 h (28 °C, 180 r·min−1). Subsequently, 0.1 ml of the culture was inoculated into triangular flasks containing 100 ml of Ashby’s liquid medium without nitrogen. As a control, equal volumes of sterile distilled water were inoculated into Ashby medium. Three replicates for each biological treatment. Petri dishes and triangular flasks were incubated in a constant temperature incubator and oscillator at 28 °C for 7 days before being observed. If their colonies formed on Ashby’s medium or the liquid medium became turbid, it was demonstrated that the endophytic strain with inhibition strain function could also fix nitrogen (Cui et al. 2016).
Determination of phosphorus solubilizing capacity
To test the ability of endogenous antagonistic strains to solubilize elemental phosphorus, they were coated on the surfaces of PKO medium, which contains inorganic phosphorus, and Mehknha medium, which contains organic phosphorus. Three times each biological treatment was used. The following equation was used to determine the elemental phosphorus solubilization capability (Yang et al. 2014).
Determination of IAA production capacity
The Salkowski colorimetric method (Dong and Cai 2001) was used to determine the ability of antagonistic endophytic strains to produce IAA. 0.1 ml of the antagonistic endophytic strain culture was inoculated into 300-ml triangular flasks containing 100 ml of King’s medium, which was divided into two types of medium, one without and with 100 mg·l−1 tryptophan. The same volume of sterile distilled water was added to the medium as a control, and the triangular flasks were incubated in a thermostatic shaker (28 °C, 120 r·min−1) for 12 days after spiking was completed. A 50 μl culture solution was drawn from each King medium, added to a 1.5-ml centrifuge tube containing an equal volume of Sakowski reagent (PC or S2) and left at room temperature for 15 min to observe the color reaction. Each biological treatment was replicated three times. When the color of all mixture turns red, it indicates that they have the function of secreting IAA.
The concentration of the produced IAA was then determined. Cultures were transferred to 10-ml centrifuge tubes and placed in a low-temperature centrifuge (4 °C, 10,000 r·min−1) for 10 min. The supernatant after centrifugation was pipetted 4 ml into a 10-ml centrifuge tube containing the same volume of colorimetric solution, and the two solutions were thoroughly mixed with a vortexer before being placed in a dark environment for 30 min. Each biological sample was replicated three times. The absorbance values of the mixed solutions were measured at 530 nm UV wavelength, with a control consisting of sterile distilled water and colorimetric solution. Using standard curves, IAA production secreted by endophytic strains was calculated.
Standards of IAA were used to prepare two groups of IAA standard solutions with different concentration gradients, each with seven different concentrations of IAA standard solutions (Group I: 2.5, 5, 7.5, 10, 12.5, 15, 17.5 μg·ml−1; Group II: 25, 50, 75, 100, 125, 150, 175 μg·ml−1). The absorbance value of the standard solution was determined using the same method as above. All data were processed in Microsoft Excel, and standard graphs were generated (Additional file 1: Fig. S1).
Determination of salt tolerance
Antagonistic endophytic strains were inoculated into 300-ml triangular flasks containing 100 ml of NA liquid medium containing 2, 5, 10, 15, 20 and 25% NaCl, respectively, with NaCl-free NA liquid medium serving as a control (Incubated at 28 °C for 24 h) (Chen et al. 2020). Each biological treatment was repeated three times. The absorbance values of the cultures were measured at 600 nm to determine the salt tolerance of the endophytic antagonist strains.
Potted plant experiment
Plant five pre-treated, sprouted pepper seeds per pot (Cui et al. 2020). When the tenth new leaf was grown, 12 pots of uniformly grown pepper leaves were selected for artificial wounding, nine of which were inoculated with the pathogen C. scovillei and three of which were not, and they were placed in an artificial climate chamber (temperature 25 °C, humidity 30%) for 48 h. This aided in the spread of the disease in pepper leaves. After 48 h, the peppers were removed and 3 pots of pepper leaves inoculated with the pathogen C. scovillei were sprayed with fermentation broth of strains L1-7 and L3-5 (50 ml, incubated for 48 h) each for biological control. The remaining 6 pots were used as controls, with 3 pots not inoculated with the pathogen C. scovillei and the 3 inoculated, but not sprayed with the fermentation solution. Finally, the disease grading standards for pepper anthracnose (Fang 1998; Holliday 1970) were as follows:
-
Level 0: No signs of disease.
-
Level 1: Only the tender shoots show signs of disease.
-
Level 2: Less than 25% of leaves, leaf buds, flower buds or fruits are susceptible to disease.
-
Level 3: 25–50% of leaves, leaf buds, flower buds or fruits are susceptible to disease.
-
Level 4: More than 50% of leaves, leaf buds, flower buds or fruits are susceptible to disease.
The disease index and control efficiency were calculated as follows:
where Dpl is the diseased plants at all levels, Gd is the Grade of disease, Tip is the total number of investigated plants and Md is the maximum disease grade.
where Cd is the diseased index of the control area and Td is the disease index of the treatment area.
Statistical analysis
Variance analysis was used to analyze the data. The software used for this task was IBM SPSS Statistics 25. Data are presented as mean ± standard error (SE), based on three replicates. Means with the same lowercase letters are not significantly different (p < 0.05) according to Duncan’s multiple range tests. The experimental data were counted and calculated by Microsoft Excel to clarify the function and fungistatic effect of endophytic strains.
Results
Determination of antagonistic ability
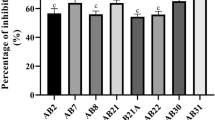
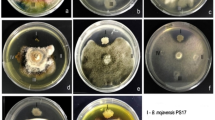
Antagonistic potentials of 58 endophytic strains against the pathogen C. scovillei were studied (Additional file 2: Table S1). Eight of these endophytic strains prevented the pathogen’s mycelial growth to a level of 70–80%, while only two strains (L1-7 and L3-5) prevented a level of more than 80%. The mycelial growth of C. scovillei was suppressed by 33 endophytic strains by less than 60% and by 15 endophytic strains between 60 and 70%. To further study the stability of their capacity to suppress infections, endophytic strains with more than 70% inhibition of pathogenic fungal mycelium development in the initial round of screening were once again chosen (Additional file 3: Table S2). It was found that strains L1-7 and L3-5 once again inhibited C. scovillei mycelial development by more than 79% (Fig. 1). The results of the two screening tests showed that L1-7 and L3-5 inhibited C. scovillei consistently and persistently. The effect of L1-7 antagonistic on the pathogenic fungus C. scovillei’s mycelium resulted in a milky white, fluffy appearance, showing that L1-7 hindered the pathogenic fungus’s morphological transition in the early stages of its development. However, following exposure to L3-5, the mycelium of the pathogenic fungus C. scovillei was gray–green fluffy, indicating that the endophyte had a significant inhibitory effect on the mycelium’s normal growth as opposed to having any influence on the pathogenic fungus’s development of its developmental cycle. This conclusion was made in light of the C. scovillei mycelium’s early manifestation of a creamy white fluffy morphology and later change to a gray–green fluffy morphology in an advantageous growth environment. This discovery suggests that the two antagonistic endophyte strains target the pathogenic fungus through different nodes.
Identification of morphological characteristics
The L1-7 colony had a spherical or nearly circular colony form and was milky white. The colony’s surface was initially smooth and opaque, but as it grew, the edge developed clearly serrated wrinkles. The colony was encircled by a translucent, colorless halo. The L3-5 colony was irregularly shaped, yellowish in color, with a dry, wrinkled, dull surface. Gram-positive bacteria L1-7 and L3-5 are both rod-shaped, with L1-7 measuring 0.71–2.25 μm and L3-5 measuring 0.86–2.23 μm and 0.24–0.51 μm, respectively (Fig. 2).
Gene sequence analysis
When the 16S rDNA gene sequence obtained by sequencing was compared to other sequences in the GenBank database, it was discovered that the two antagonistic endophytic bacteria were both Bacillus. L1-7 and L3-5, in contrast, were found to be 100% identical to Bacillus spp. (Figs. 3 and 4). Using the same universal primer (27F, 1492R), L1-7 and L3-5 were amplified yielding DNA fragments of 1433 bp and 1401 bp in length, respectively. L1-7 and L3-5 were both submitted to GenBank (MW672320 for L1-7, MW672321 for L3-5). By amplification and sequencing with the Bacillus-specific primer gyrB, the L1-7 and L3-5 antagonistic endophytic strains were identified. When the sequenced gene fragments were compared in the GenBank database, it was discovered that both strains were related to the Bacillus species B. amyloliquefaciens and B. velezensis. However, L1-7 and L3-5 culture characteristics matched those of B. amyloliquefaciens and B. velezensis, respectively, with a milky white culture characteristic and a yellowish culture characteristic. Therefore, in order to construct a phylogenetic tree, the gene sequences of the strains that matched the cultural characteristics of each of the two strains were selected. L1-7 and L3-5 were clustered with B. amyloliquefaciens (HM585078) and B. velezensis (MK203036), respectively, on the phylogenetic tree, and their similarity was 99% (Figs. 3 and 4). By combining culture and morphological traits with the findings of 16S rDNA and gyrB gene sequence analysis, L1-7 was therefore determined to be B. amyloliquefaciens, and L3-5 was determined to be B. velezensis. L1-7 and L3-5 were amplified, using the required primers (UP-1 and UP-2r), producing DNA fragments of 1195 bp and 1198 bp, respectively. L1-7 and L3-5 have been added to GenBank (MZ209100 for L1-7, MZ209101 for L3-5).
Phylogram generated from neighbor-joining analysis based on alignment of partial sequences of 16S rDNA and gyrB gene, showing the phylogenetic relationships of Bacillus species to the isolate L1-7. Among them, ‘L1-7 MW672320’ was the sequence of amplification by the universal primer. ‘L1-7 MZ209100’ was the sequence of amplification by the gyrB primer
Phylogram generated from neighbor-joining analysis based on alignment of partial sequences of 16S rDNA and gyrB gene, showing the phylogenetic relationships of Bacillus species to the isolate L3-5. Among them, ‘L3-5 MW672321’ was the sequence of amplification by the universal primer. ‘L3-5 MZ209101’ was the sequence of amplification by the gyrB primer
Biological function test
Biofunctional experiments revealed that strains L1-7 and L3-5 could not fix elemental nitrogen, but could solubilize elemental phosphorus. IAA could be secreted by L1-7 and L3-5, although tryptophan in the medium had no effect on IAA production (Table 1). The versatility and high potential of strains L1-7 and L3-5 in preventing plant diseases and fostering plant growth are revealed by these findings.
L1-7 grew best in a medium containing 2% salt, while L3-5 grew best in a medium containing 5% salt. Both considerably differed (p < 0.05) from other biological treatments. Both endophytic strains could also continue to grow in media with a 10% salt content (Fig. 5). According to this finding, L1-7 and L3-5 both had a good salt tolerance. These two antagonistic strains may one day be used to treat plant diseases in locations with salinized soil.
Salt tolerance of antagonistic bacterial strains L1-7 and L3-5. A Salt tolerance of strain L1-7; B salt tolerance of strain L3-5. Data are presented as mean ± standard error (SE), based on three replicates. Means with the same lowercase letters are not significant different (p < 0.05) according to Duncan’s multiple range tests
Potted control effect of antagonistic strains
The pathogen-inoculated pepper leaves that were not sprayed with the fermentation solution had the most spots and the most severe disease, according to the results of the controlled indoor pot trials (Fig. 6). The frequency of spots and intensity of the symptoms on pepper leaves treated with L1-7 and L3-5 sprays were much less frequent and severe compared to pepper leaves not treated with fermentation solutions. The harmful fungus was successfully eradicated by the biocontrol strain, as cured by a comparison of pepper leaves with and without the infection implanted. Based on the incidence data on the leaves, L1-7 and L3-5 were shown to be 80.64 and 73.39% effective against the pathogen C. scovillei, respectively. Therefore, L1-7 and L3-5 are unquestionably better biological control agents.
Results of pot experiment. A a: After inoculation with pathogenic fungi, the leaves treated with fermentation liquid L1-7 was sprayed; b: after inoculation with pathogenic fungi, the leaves treated with fermentation liquid L3-5 was sprayed; c: leaves untreated after inoculation with pathogenic fungi; d: a blank control without any treatment. B Data are presented as mean ± standard error (SE), based on three replicates. Means with the same lowercase letters are not significant different (p < 0.05) according to Duncan’s multiple range tests
Discussion
Plant endophytes are a group of microorganisms that colonize plant tissues organs or intercellular spaces at a specific stage or throughout their life cycle (Choub et al. 2021). Plant endophytes have been shown to perform a variety of biological functions, including plant hormone secretion, plant resistance induction, antifungal metabolite production and nitrogen fixation (Amaresan et al. 2012). In this study, two endophytic bacterial strains from healthy pepper were isolated and identified. These strains had good antagonistic effects on C. scovillei leaves. They were identified as B. amyloliquefaciens and B. velezensis, respectively, and it was shown that they both possessed excellent salt tolerance, nitrogen fixation and IAA secretion abilities. As previously reported by (Reyes-Estebanez et al. 2020), B. subtilis, B. amyloliquefaciens and B. velezensis were good antagonists against agents for controlling Colletotrichum. Since C. scovillei is a pathogen that was only recently found in China (Zhao et al. 2016), there have been no research on how endophytic Bacillus strains can control this pathogen. Therefore, the two endophytic strains identified in this study are essential for controlling the pathogen C. scovillei.
Bacillus is often employed as a biocontrol agent in agricultural settings because of its powerful ability to inhibit plant pathogenic bacteria (Mishra et al. 2022). According to a similar study, both endophytes are the main strains employed as bioorganic pesticides or biofertilizers to control plant diseases and strains that promote plant development (Wei et al. 2018a). In other studies, the bacillin D produced by B. amyloliquefaciens prevented pathogenic mycelium from developing normally, so controlling the spread of the disease (Luna-Bulbarela et al. 2018). Additional studies have also demonstrated that it can secrete a certain antifungal substance that makes the pathogenic fungus stop growing (Chen et al. 2018). In addition, B. amyloliquefaciens HAB-2 produces a metabolite n-butanol that deforms and swells the mycelium, thus preventing the pathogen from harming the plant (Wei et al. 2018b). The previous literature also contains information on B. velezensis as a biocontrol agent, demonstrating its capacity to suppress a number of fungal infections, such as Arthrinium phaeospermum, Fusarium oxysporum, Cylindrocladium scoparium, Botrytis cinerea and Penicillium (Sun et al. 2018). However, while exerting their effects against pathogenic fungi, many endophytic strains exhibit a variety of reactions and distinct modes of action (Gomes et al. 2023).
In addition to controlling pathogens that harm plants, biocontrol strains increase the bioavailability of essential nutrients and improve plant growth and performance (Younas et al. 2023). In the present study, Bacillus strains L1-7 and L3-5 possessed these same functions, such as nitrogen fixation, IAA secretion and higher salt tolerance. Similar studies have reported that B. velezensis can secrete IAA to promote plant root development and nutrient uptake to promote plant growth and improve plant resistance (Cai et al. 2018). In addition, B. amyloliquefaciens K103 was shown to have the ability to fix nitrogen and dissolve organic phosphorus (Dong et al. 2018), but the strains we tested could not dissolve organic phosphorus. The differences in the function of endophytic strains may be due to the fact that they were isolated from different hosts and the hosts live in very different environments, thus resulting in different functions of bacteria of the same species. Global land management is facing significant challenges due to soil salinization (Xun et al. 2015). Research and development of biocontrol strains with salt tolerance are required to successfully prevent and control diseases in saline soil locations. Both Bacillus strains demonstrated increased salt tolerance in the current experiment, indicating greater ecological flexibility. In highly salinized environments, they can function better as antagonists.
In order to manage the pathogenic fungus C. scovillei, pot experiments for the endophytic strains L1-7 and L3-5 were designed, and they demonstrated 80.64 and 73.39% control in potted circumstances, respectively. Similar results were shown in a similar experiment on the control of early leaf spot disease by B. amyloliquefaciens (Ahsan et al. 2022). However, the method described in this report was different than the one that used, where they sprayed the fermentation solution before the pathogen infection. Additionally, the final control efficiency was higher (92.00%) than that of spraying following the commencement of the disease. Therefore, prevention before infection with the disease is also possible based on the benefits of biocontrol microorganisms that are favorable to the environment. According to the findings, not all diseases can be controlled by Bacillus species, despite the fact that many of them have been tested for pathogen control. Therefore, it would be worthwhile and helpful to reexamine the greater resistance of the screened-out Bacillus to the pathogen C. scovillei.
Conclusion
A total of 58 endophytic strains was examined, and anti-pathogen testing revealed that two Bacillus strains, B. amyloliquefaciens (L1-7) and B. velezensis (L3-5) showed outstanding control over the anthracnose fungus C. scovillei. Additionally, both of them were capable of fixing nitrogen and secreting IAA, and also had a good salt tolerance. Pot studies confirmed their exceptional antagonistic potential against the pathogen C. scovillei.
Availability of data and materials
The sequencing data generated and analyzed in this study are available in the NCBI Sequence Read Archive database (https://www.ncbi.nlm.nih.gov/), Accession Number: MW672320 and MZ209100 for L1-7, MW672321 and MZ209101 for L3-5.
References
Ahsan T, Zang CQ, Yu SY, Pei X, Xie JH, Lin Y, Liu XZ, Liang CH (2022) Screening, and optimization of fermentation medium to produce secondary metabolites from Bacillus amyloliquefaciens, for the biocontrol of early leaf spot disease, and growth promoting effects on Peanut (Arachis hypogaea L.). J Fungi (basel) 8(11):1223
Amaresan N, Jayakumar V, Kumar K, Thajuddin N (2012) Endophytic bacteria from tomato and chilli, their diversity and antagonistic potential against Ralstonia solanacearum. Arch Phytopathol Pflanzenschutz 45(3):344–355
Asghari S, Harighi B, Mozafari AA, Esmaeel Q, Barka EA (2019) Screening of endophytic bacteria isolated from domesticated and wild growing grapevines as potential biological control agents against crown gall disease. Biocontrol 64(6):723–735
Benhamou N, Kloepper JW, Quadt-Hallman A, Tuzun S (1996) Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol 112(3):919–929
Bhattacharya A, Giri VP, Singh SP, Pandey S, Chauhan P, Soni SK, Srivastava S, Singh PC, Mishra A (2019) Intervention of bio-protective endophyte Bacillus tequilensis enhance physiological strength of tomato during Fusarium wilt infection. Biol Control 139:104074
Bokhari A, Essack M, Lafi FF, Andres-Barrao C, Jalal R, Alamoudi S, Razali R, Alzubaidy H, Shah KH, Siddique S, Bajic VB, Hirt H, Saad MM (2019) Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci Rep-Uk 9(1):18154
Bouaoud Y, Troulet C, Foughalia A, Berge O, Aissat K, Bardin M (2018) A multi-criteria approach for the selection of efficient biocontrol agents against Botrytis cinerea on tomato in Algeria. Biocontrol 63(2):299–311
Cai GL, Zhang F, Ouyang YX, Zhao CS, Peng XH, Jiang AM (2018) Research progress on Bacillus velezensis. Beifang Yuanyi 12:162–167
Chen NN, Yin JY, Wang Y, Qin PW, Shi TR, Liu Y (2018) Identification and physicochemical properties of pntifungal pubstances of Bacillus amylolyticus SSY2. Zhongguo Xu Mu Shou Yi 45(11):3221–3228
Chen L, Mi GH, Li KK, Shao H, Hu D, Yang JP, Sui XH, Chen WX (2020) Effects of multifunctional plant rhizosphere promoting bacteria on maize growth in black soil areas in Northeast China. Ying Yong Sheng Tai Xue Bao 31(8):2759–2766
Choub V, Ajuna HB, Won S-J, Moon J-H, Choi S-I, Maung CEH, Kim C-W, Ahn YS (2021) Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of Walnut (Juglans regia L.) trees. Int J Mol Sci 22(19):10438
Cui YZ, Yang XL, Yang CD, Xue L, Zhang JL, Yao YL (2016) Identification and determination of biological functions of endophytic bacteria from alpine pasture against Phytophthora infestans. J Plant Prot 43(5):789–795
Cui LX, Yang CD, Wei LJ, Li TH, Chen XY (2020) Isolation and identification of an endophytic bacteria Bacillus velezensis 8–4 exhibiting biocontrol activity against potato scab. Biol Control 141:104156
De Silva DD, Groenewald JZ, Crous PW, Ades PK, Nasruddin A, Mongkolporn O, Taylor PWJ (2019) Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus 10(1):8
Dong XZ, Cai MY (2001) Manual of system identification of common bacteria. China Science Press, Beijing
Dong CJ, Wang LL, Li L, Qin YX, Li PL, Shang QM (2018) Growth-promoting effects of Bacillus amyloliquefaciens K103 on cucumber plug seedlings. Yuan Yi Xue Bao 45(11):2199–2208
Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd Allah EF (2017) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under Salt Stress. Front Microbiol 8:1887
Fang ZD (1998) Methods of research on plant diseases, 3rd edn. Agricultural Publishing House, Beijing
Farr DF, Rossman AY (2023) Fungal databases, U.S. National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/. Accessed 6 Jan 2023
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic com-pounds produced by phytopathogenic bacteria. Appl Environ Microbiol 619(2):793–796
Gomes AAM, Paes SA, Ferreira APS, Pinho DB, de Lourdes Cardeal Z, Menezes HC, Cardoso PG, Pereira OL (2023) Endophytic species of Induratia from coffee and carqueja plants from Brazil and its potential for the biological control of toxicogenic fungi on coffee beans by means of antimicrobial volatiles. Braz J Microbiol. 54(1):349–360
Hallmann J, Mahaffee WF, Kloepper JW, Quadthallmann A (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hirooka T, Ishii H (2013) Chemical control of plant diseases. J Gen Plant Pathol 79(6):390–401
Holliday P (1970) An introduction to plant diseases. Transact Bri Mycolo Soc 54(3)
Jiang GF, Song L (2014) Advances on biologically controling pepper anthracnose. Hubei Nong Ye Ke Xue 53(11):2481–2485
Liotti RG, Figueiredo MDJ, Soares MA (2019) Streptomyces griseocarneus R132 controls phytopathogens and promotes growth of pepper (Capsicum annuum). Biol Control 138:104065
Liu YH, Guo JW, Salam N, Li L, Zhang YG, Han J, Mohamad OA, Li WJ (2016) Culturable endophytic bacteria associated with medicinal plant Ferula songorica: molecular phylogeny, distribution and screening for industrially important traits. Biotech 6:209
Luna-Bulbarela A, Tinoco-Valencia R, Corzo G, Kazuma K, Konno K, Galindo E, Serrano-Carreon L (2018) Effects of bacillomycin D homologues produced by Bacillus amyloliquefaciens 83 on growth and viability of Colletotrichum gloeosporioides at different physiological stages. Biol Control 127:145–154
Mishra A, Bhattacharya A, Chauhan P, Pandey S, Dwivedi A (2022) Phenotype microarray analysis reveals the biotransformation of Fusarium oxysporum f.sp. lycopersici influenced by Bacillus subtilis PBE-8 metabolites. FEMS Microbiol Ecol 98(10):fiac102
Morris CE, Sands DC, Vinatzer BA, Glaux C, Guilbaud C, Buffière A, Yan S, Dominguez H, Thompson BM (2008) The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J 2(3):321–334
Reyes-Estebanez M, Sanmartín P, Camacho-Chab JC, De la Rosa-García SC, Chan-Bacab MJ, Águila-Ramírez RN, Carrillo-Villanueva F, De la Rosa-Escalante E, Arteaga-Garma JL, Serrano M, Ortega-Morales BO (2020) Characterization of a native Bacillus velezensis-like strain for the potential biocontrol of tropical fruit pathogens. Biol Control 141:104127
Skeen RS, Valentine NB, Hooker BS, Petersen JN (1995) Kinetics of nitrate inhibition of carbon tetrachloride transformation by a denitrifying consortia. Biotechnol Bioeng 45(3):279–284
Sun PP, Cui JC, Jia XH, Wang WH (2018) Complete genome analysis of Bacillus velezensis L-1 and its inhibitory effect on pear gray and blue mold. Wei Sheng Wu Xue Bao 58(9):1637–1646
Vetrivelkalai P, Sivakumar M, Jonathan E (2010) Biocontrol potential of endophytic bacteria on Meloidogyne incognita and its effect on plant growth in bhendi. J Biofertil Biopestic 3:452–457
Wei DD, Miao WG, Sun QQ, Wu GL, Liu WB, Jin PF (2018a) Analysis of the active components of Bacillus amyloliquefaciens HAB-2 against Colletotrichum gloeosporioides. Guoshu Xuebao 35(10):1253–1261
Wei LJ, Wang YQ, Yang CD, Xue L (2018b) Biological function identification and determination of endophytic bacteria in leaves of Kobresia capillifoliain Haiyan County, Qinghai Province. Cao Di Xue Bao 26(3):764–769
Wonglom P, Suwannarach N, Lumyong S, Ito S, Matsui K, Sunpapao A (2019) Streptomyces angustmyceticus NR8-2 as a potential microorganism for the biological control of leaf spots of Brassica rapa subsp. pekinensis caused by Colletotrichum sp. and Curvularia lunata. Biol Control 138:104046
Xun FF, Xie BM, Liu SS, Guo CH (2015) Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ Sci Pollut Res 22(1):598–608
Yang CD, Wang Y, Wang YQ, Yao YL, Xue L, Xu CL, Chen XR (2014) Identification and determination of biological functions of endophytic bacteria from Achnatherum inebrians in Alpine grassland of East Qilian Mountains. Cao Ye Xue Bao 23(5):249–255
Younas H, Nazir A, Bareen FE, Thies JE (2023) Metabolic profile and molecular characterization of endophytic bacteria isolated from Pinus sylvestris L. with growth-promoting effect on sunflower. Environ Sci Pollut Res Int 30:40147–40161
Zhao W, Wang T, Chen QQ, Chi YK, Swe TM, Qi RD (2016) First report of Colletotrichum scovillei causing anthracnose fruit rot on pepper in Anhui Province, China. Plant Dis 100(10):2168–2168
Acknowledgements
Not applicable.
Funding
This study was supported by the project of National Natural Science Foundation of China (Grant No: 31660148) and by the Science and Technology Innovation Fund of Gansu Agricultural University (Grant No. GAU-XKJS-2018-148).
Author information
Authors and Affiliations
Contributions
WLJ conceived and designed the study, drafted the manuscript, and analyzed and interpreted the data; WLJ, CLX and JMJ acquired the data; YCD and OR revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have given their consent to publish the submitted manuscript as an ‘Original paper’ in EJBPC.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, L., Yang, C., Cui, L. et al. Bacillus spp. isolated from pepper leaves and their function and inhibition of the fungal plant pathogen Colletotrichum scovillei. Egypt J Biol Pest Control 33, 46 (2023). https://doi.org/10.1186/s41938-023-00686-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00686-z