Abstract
Background
Butenyl-spinosyn produced by Saccharopolyspora pogona exhibits strong insecticidal activity and a broad pesticidal spectrum. Currently, important functional genes involve in butenyl-spinosyn biosynthesis remain unknown, which leads to difficulty in efficiently understanding its regulatory mechanism, and improving its production by metabolic engineering.
Results
Here, we identified a TetR family transcriptional regulator, SP_2854, that can positively regulate butenyl-spinosyn biosynthesis and affect strain growth, glucose consumption, and mycelial morphology in S. pogona. Using targeted metabolomic analyses, we found that SP_2854 overexpression enhanced glucose metabolism, while SP_2854 deletion had the opposite effect. To decipher the overproduction mechanism in detail, comparative proteomic analysis was carried out in the SP-2854 overexpressing mutant and the original strain, and we found that SP_2854 overexpression promoted the expression of proteins involved in glucose metabolism.
Conclusion
Our findings suggest that SP_2854 can affect strain growth and development and butenyl-spinosyn biosynthesis in S. pogona by controlling glucose metabolism. The strategy reported here will be valuable in paving the way for genetic engineering of regulatory elements in actinomycetes to improve important natural products production.
Similar content being viewed by others
Background
Butenyl-spinosyn is produced by Saccharopolyspora pogona (S. pogona) originally isolated from soil samples collected in Indiana, U.S.A., in 1990 [1, 2]. It is structurally and chemically related to spinosyn produced by Saccharopolyspora spinosa [2, 3]. Butenyl-spinosyn has excellent activity against various insects, such as Lepidoptera, Diptera, and Coleoptera, but has little or no effect on a broad range of nontarget insects and mammals, making it one of the most promising biological pesticides [4, 5]. At present, the butenyl-spinosyn biosynthetic gene cluster (bus cluster) and its biosynthetic pathway have been elaborated [2, 6]. Although much progress has been made in understanding the butenyl-spinosyn biosynthetic pathway, little is known about the regulation of butenyl-spinosyn biosynthesis. To date, only a few regulators have been identified as being involved in the regulation of butenyl-spinosyn production in S. pogona, including the SenX3/RegX3 system, Sp1418, Sp13016, SP_1288 and an AfsR regulatory factor [7,8,9,10,11]. A better understanding of the regulatory mechanisms of butenyl-spinosyn biosynthesis will provide important insights for strain improvement by metabolic engineering.
A number of regulatory genes with pleiotropic effects on the biosynthesis of natural products in actinomycetes have been identified and studied extensively [12,13,14]. Broadly speaking, we can divide the regulatory genes that control natural product biosynthesis into two types. First, some genes are located in natural product biosynthetic gene clusters together with genes for biosynthesis and secretion of natural products and are usually pathway-specific [15]. For example, the regulator genes actII-orf4, cdaR and redD exist in the Streptomyces coelicolor genome, and their coding proteins control actinorhodin, calcium-dependent antibiotic (CDA), and undecylprodigiosin biosynthesis, respectively [16, 17]. Second, other genes are global regulators that control multiple metabolic pathways and may not be linked to specific biosynthetic gene clusters [18]. For example, the two-component PhoR-PhoP system is well studied for the regulation of antibiotic biosynthesis induced by phosphate starvation [15]. TetR family regulatory factors (TFRs), a class of regulators commonly found in actinomycetes, participate in diverse cellular processes [19, 20]. In recent years, a variety of TFRs, as pathway-specific or global regulators, were identified as regulating the biosynthesis of natural products by binding to the promoter region of their target genes [21]. Whole genome sequencing results showed that at least 86 putative TFRs were encoded in the genome of S. pogona (CP031142). Therefore, the TFRs associated with butenyl-spinosyn production in S. pogona need to be identified to improve our understanding of the regulatory mechanism underlying butenyl-spinosyn biosynthesis.
The biosynthetic regulatory network of actinomycete natural products is robust and tightly regulated. Although previous studies have showed that metabolic engineering of key regulatory factors advances our understanding of natural product biosynthesis [22, 23], omics techniques are required to dissect detailed regulatory mechanisms that may reveal the natural product biosynthesis regulatory network [24,25,26]. Recently, proteomics has become an efficient and cost-effective strategy for dissecting the biological functions of specific regulatory factors [27]. For example, the regulatory effect of the GlnR-mediated response to nitrogen limitation in Streptomyces coelicolor M145 was described in detail following proteomic analysis of a wild-type and a mutant strain [28]. In addition to proteomics, metabolomics has also been widely used to reveal the role of regulatory factors in natural product biosynthesis by depicting intracellular metabolic profiles [29]. For example, introduction of adpA into Streptomyces ZYJ-6 significantly improved candicidin productivity to achieve a concentration of 9338 μg/mL. Combined metabolic flux and metabolomics analyses indicated that methylmalonyl-CoA played a central role in candicidin production and that the methB gene responsible for the biosynthesis of methylmalonyl-CoA might be a candidate gene target for the AdpA regulatory factor [30]. However, few studies have used proteomic and metabolomic analyses to reveal the regulatory mechanisms of TFRs in natural product biosynthesis.
Here, we identified a TFR, SP_2854, that positively regulates butenyl-spinosyn biosynthesis. A comparative proteomic and metabolomic approach confirmed that SP_2854, as the global regulatory factor, affects butenyl-spinosyn biosynthesis by regulating glucose metabolism in addition to affecting the transcription of the bus cluster. This work deepens our understanding of the butenyl-spinosyn regulatory mechanism and lays an important foundation for studying the regulatory mechanism of SP_2854 in natural product biosynthesis in other actinomycetes.
Results
Construction of SP_2854 deletion and overexpression mutants
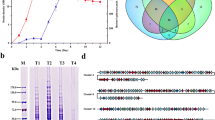
Whole genome sequencing results showed that at least 86 putative TFRs were encoded in the genome of S. pogona. Combined with previous comparative transcriptomic data mining studies, a new transcriptional regulator SP_2854, which was differentially expressed the strain growth logarithmic phase, attracted our attention. Therefore, SP_2854 was selected as a modified target to investigate whether it regulates primary metabolism and butenyl-spinosyn biosynthesis. According to the available genome sequence of S. pogona, SP_2854 consists of 213 amino acids with a molecular mass of approximately 24 kDa. qRT–PCR results showed that SP_2854 could be transcribed throughout the S. pogona growth cycle (Fig. 1a). Blast analysis revealed that SP_2854 homologues were widely distributed among actinomycetes, suggesting that this gene may have important biological functions in actinomycetes (Fig. 1b).
Deletion and overexpression of SP_2854. A Transcription levels of SP_2854 at different time points via qRT–PCR analysis. B Neighbour-joining (NJ) distance tree constructed using the amino acid sequence SP_2854 from actinomycetes using MEGA6. C Schematic diagrams of the S. pogona-ΔSP_2854 mutant. D Schematic diagrams of the S. pogona::SP_2854 mutant. E PCR identification of the S. pogona-ΔSP_2854 mutant. Wild-type S. pogona genomic DNA was used as the control. Lane M, DL5000 DNA marker; Lanes 1 and 2, S. pogona-ΔSP_2854 mutant; Lane 3, control. The tested transformant showed a 2.3-kb PCR amplicon, whereas the control showed no band in that location. F PCR identification of the S. pogona::SP_2854 mutant. Wild-type S. pogona genomic DNA was used as the control. Lane M, DL2000 DNA marker; Lanes 1–4, S. pogona::SP_2854 mutant; Lane 5, control. The tested transformant showed a 1.3-kb PCR amplicon, whereas the control showed no band that location. G Sequencing of the S. pogona-ΔSP_2854 mutant PCR amplicon
To define the role of SP_2854 in butenyl-spinosyn biosynthesis, plasmids pOJ260 and pKCcas9dO were selected as the editing tools for SP_2854 deletion and overexpression, respectively [31, 32]. To generate the SP_2854 deletion mutant, the recombinant plasmid pOJ260-UASP_2854-apr-DASP_2854 was constructed and then transferred into S. pogona through protoplast transformation (Fig. 1c). Since pOJ-260 is a suicide plasmid, it cannot be replicated in S. pogona and will eventually be lost as the strain grows. The resulting mutant, named S. pogona-ΔSP_2854, was verified by PCR with the primers FSP_2854up/Rapr for multiple independent transformants, and a 2.3-kb band was observed; no band was observed for the original strain of S. pogona (Fig. 1e). The results of Hind III/EcoR I double digestion were also in line with expectations (Fig. 1f). Sequencing of the PCR amplicons showed that the 668-bp DNA region was completely deleted (Fig. 1g). For SP_2854 overexpression, the recombinant plasmid pKCcas9-SP_2854 was constructed and then transferred into S. pogona through protoplast transformation (Fig. 1d). Since pKCcas9dO can be replicated in S. pogona, it was only necessary to verify the presence of the apr gene in transformants. The resulting mutant, named S. pogona::SP_2854, was verified by PCR with the primers Fapr/Rapr for multiple independent transformants, and a 1.3-kb band was observed; no band was observed for the original strain of S. pogona (Fig. 1h). qRT–PCR analysis revealed that the transcriptional level of SP_2854 in the S. pogona::SP_2854 mutant was significantly higher than that of the original strain, but no SP_2854 transcripts were detected in the mutant S. pogona-ΔSP_2854.
SP_2854 plays a positive regulatory role in the butenyl-spinosyn biosynthesis
To identify the effects of SP_2854 deletion and overexpression on butenyl-spinosyn biosynthesis, we estimated the production of the S. pogona-ΔSP_2854 and S. pogona::SP_2854 mutants by comparing their butenyl-spinosyn chromatographic peak areas with that of the original strain under the same conditions. Previous studies confirmed that the chromatographic peak at 13 min was the butenyl-spinosyn component spinosyn 6-methyl β [9]. Therefore, the peak at 13 min was collected and identified by LC–MS/MS, and the result showed that (M + H) + ions at m/z = 633.17 contained a rhamnose ion fragment of 189 molecular mass (Additional file 1: Fig. S1), which is consistent with previous data [9]. Next, the chromatographic peak at 13 min was used as a basis for comparing changes in butenyl-spinosyn production in different strains. The results showed that overexpression of SP_2854 significantly promoted butenyl-spinosyn biosynthesis, and its yield reached 65.12 ± 8.57 mg/L, which was obviously higher than that of the original strain (22.48 ± 4.53 mg/L). Only a small amount of butenyl-spinosyn was produced in S. pogona-∆SP_2854 (5.10 ± 0.32 mg/L) (Fig. 2a). To determine the effect of SP_2854 on butenyl-spinosyn biosynthesis in detail, the expression levels of the bus cluster in the mutants and the original strain were studied by qRT–PCR (Fig. 2b). We found that SP_2854 deletion resulted in a significant decrease in the expression of busA, busF, busG, busI, busP and busQ, but, except for busF, SP_2854 overexpression promoted their expression, indicating that SP_2854 had a positive effect on butenyl-spinosyn biosynthesis (Fig. 2c).
SP_2854 positively regulates butenyl-spinosyn production in S. pogona. A Comparison of butenyl-spinosyn production in wild-type S. pogona and the S. pogona-ΔSP_2854 and S. pogona::SP_2854 mutants via HPLC analysis. Eight-day fermentation liquid was pretreated for HPLC analysis. B Genetic organization of the bus cluster in S. pogona. C Effects of SP_2854 deletion and overexpression on the transcription level of the bus cluster. The cells of the different strains were inoculated into SFM and cultured at 30 °C for 4 days. Total RNA was then isolated and used for qRT–PCR assays. The control strain was wild-type S. pogona. 16S rRNA served as the normalization control
SP_2854 regulates strain growth and development, glucose consumption and mycelial morphology but not sporulation
TFRs often play much broader roles, such as in growth and development, nutrition, metabolism, and morphological differentiation [14], so we compared the mutants and the original strain in growth density, glucose consumption, mycelial morphology and sporulation. The results showed that the S. pogona::SP_2854 mutant and the original strain had similar growth rates in early growth, but bacterial density in the stationary phase and decline phase was higher in the mutant than in the original strain. The S. pogona-ΔSP_2854 mutant, however, had a significantly lower growth rate than that of the original strain (Fig. 3a). Glucose consumption analysis showed that the changing trend was consistent with growth density, which was reduced during the logarithmic phase and was almost exhausted late in the stationary phase. We found that the S. pogona::SP_2854 mutant and the original strain had similar glucose consumption rates; however, the S. pogona-ΔSP_2854 mutant required more time to complete glucose consumption in the environment than the original strain (8.5 days vs. 5 days) (Fig. 3b). To explain the effect of SP_2854 deletion on glucose uptake in strain, we performed qRT–PCR analysis on mutant S. pogona-ΔSP_2854 and the original strain to detect the mRNA levels for ptsH, ptsI, ptsIIA and ptsIIBC (phosphoenolpyruvate-dependent glucose phosphotransferase system genes, PTSGlc), which are important genes involved in glucose transport. The result showed that SP_2854 deletion resulted in a significant decrease in the expression of these genes (Fig. 3c). Scanning Electronic Microscopy (SEM) observation showed that there was no significant difference between S. pogona::SP_2854 and the original strain in mycelial morphology. However, some mycelia of the S. pogona-ΔSP_2854 mutant presented short rods or ellipsoid shapes in early growth (2 days) and exhibited obvious protrusions on the surface of mycelia at the stationary phase (4 days and 6 days) (Fig. 3d). With regard to sporulation, the mutants and the original strain had no obvious difference (Additional file 1: Fig. S2). These results indicate that SP_2854 can regulate strain growth, glucose consumption and mycelial morphology but is not involved in sporulation.
Effects of SP_2854 deletion and overexpression on strain growth, glucose consumption and mycelial morphology. A Growth curve analysis of wild-type S. pogona and the S. pogona-ΔSP_2854 and S. pogona::SP_2854 mutants in SFM. B Comparison of glucose utilization in wild-type S. pogona and the S. pogona-ΔSP_2854 and S. pogona::SP_2854 mutants in SFM. C Effects of SP_2854 deletion on the transcription levels of PTSGlc genes involved in glucose uptake. The cells of the different strains were inoculated into SFM and cultured at 30 °C for 4 days. Total RNA was then isolated and used for qRT–PCR assays. The control strain was wild-type S. pogona. 16S rRNA served as the normalization control. D Scanning electron micrographs of wild-type S. pogona and the S. pogona-ΔSP_2854 and S. pogona::SP_2854 mutants. Comparison of mycelial morphology between the mutants and wild-type S. pogona on the 2nd, 4th and 6th days
SP_2854 can enhance acetyl-CoA supply to promote butenyl-spinosyn biosynthesis
Butenyl-spinosyn production is not only affected by the expression level of bus clusters but also related to the supply capacity of precursors. Under nutrient- and oxygen-replete conditions, acetyl-CoA is predominantly derived from glucose and is the most important precursor in butenyl-spinosyn biosynthesis. Glucose determination and qRT-PCR analysis results showed that a change in SP_2854 expression could affect the glucose uptake and the expression of PTSGlc genes and finally affect the glucose metabolism and the acetyl-CoA supply. To further investigate whether SP_2854 can influence butenyl-spinosyn production by regulating the supply of acetyl-CoA, we performed a targeted metabolomics analysis for specific metabolites. As a result, 27 intracellular metabolites associated with acetyl-CoA metabolism were identified and quantified by LC–MS/MS (Fig. 4, Additional file 2: DataSet S1). KEGG pathway analysis showed that these metabolites mainly participated in glycolysis and the TCA cycle. Further analysis showed that the S. pogona::SP_2854 mutant experienced significant increases in the abundance of several metabolites in the logarithmic phase and stationary phase, such as glucose 6-phosphate, fructose 6-phosphate, dihydroxyacetone phosphate, pyruvate, acetyl-CoA, citrate, alpha-ketoglutarate, fumarate and malate, suggesting that SP_2854 overexpression enhanced glucose metabolism and acetyl-CoA synthesis, which finally promoted butenyl-spinosyn biosynthesis (Fig. 5). For the S. pogona-ΔSP_2854 mutant, most metabolites involved in glycolysis and the TCA cycle, such as glucose 6-phosphate, fructose 6-phosphate, dihydroxyacetone phosphate, phosphoenolpyruvate, pyruvate, oxaloacetate, citrate, alpha-ketoglutarate and malate, were lower in abundance during the logarithmic phase compared with their abundance in the original strain, suggesting that SP_2854 deletion inhibited glucose metabolism, which was consistent with lower rates of growth and glucose consumption of the S. pogona-ΔSP_2854 mutant in this phase (Fig. 6). However, the abundance of these metabolites in the stationary phase or decline phase was significantly higher than their abundance in the original strain. Combined with the growth curve and glucose consumption analysis, we speculate that the S. pogona-ΔSP_2854 mutant still exhibited a high concentration of glucose in the extracellular environment on the 4th day of fermentation compared with that of the original strain, which enabled it to continue to use extracellular glucose for catabolism and synthesize other intermediate metabolites. Meanwhile, SP_2854 deletion caused a decrease in cellular glucose metabolism, which eventually led to the abundance of some intermediate metabolites being higher than they were in the original strain. Both mutants showed different metabolic profiles compared with the original strain, which could be due to SP_2854 being a regulatory factor that can control the expression of certain key genes that are important to enhance acetyl-CoA supply.
Comparative proteomic analysis of the high-production mutant S. pogona::SP_2854 and the original strain
Based on morphological, physiological and biochemical characteristics, and targeted metabolome analysis, we found that SP_2854 exhibited multiple regulatory functions in the growth and development, nutrient metabolism and butenyl-spinosyn biosynthesis of S. pogona. In particular, SP_2854 overexpression significantly promoted butenyl-spinosyn biosynthesis. To reveal the regulatory mechanism of SP_2854, proteomic comparison between the S. pogona::SP_2854 mutant and the original strain was performed by using quantitative proteomics analysis based on iTRAQ labelling. It has been reported that butenyl-spinosyn is synthesized mainly in the stationary phase [31]; therefore, samples on Day 6 were collected for proteomic analysis. A total of 2910 proteins were identified and present in all biological replicates (Fig. 7a, Additional file 3: DataSet S2). A total of 230 proteins were differentially expressed (fold change > 1.33 or < 0.75, P value < 0.05), including 97 upregulated and 133 downregulated proteins, which were classified according to KEGG pathways (Fig. 7b, Additional file 4: DataSet S3). These differentially expressed proteins were involved in multiple biological processes, such as glycolysis, the pentose phosphate pathway (PP pathway), the TCA cycle, oxidative phosphorylation, amino acid metabolism, a two-component system, and ABC transporters, which suggests that SP_2854 is likely to be a global regulator (Fig. 7c).
Comparative proteomic analysis of the S. pogona::SP_2854 mutant and wild-type S. pogona. A Venn diagram of protein identification in three biological replicates. The number of proteins is shown in each area. B Statistics for differentially expressed proteins. C KEGG pathway analysis for differentially expressed proteins
A detailed metabolic network diagram was constructed to reveal the specific metabolic differences between the S. pogona::SP_2854 mutant and the original strain based on an analysis of KEGG metabolic pathways (Fig. 8). We found that the abundances of PTS glucose transporter subunit IIA (ptsIIA), phosphoenolpyruvate phosphotransferase (ptsI), and their catalytic product, glucose 6-phosphate, were higher in the S. pogona::SP_2854 mutant than in the original strain. Glycolysis and the PP pathway are two main mechanisms whereby microorganisms metabolize glucose [33]. Throughout glycolysis, the abundance of pyruvate dehydrogenase (aceE, bkdC1) in the S. pogona::SP_2854 mutant was higher than that in the original strain; this enzyme is involved in the conversion of pyruvate to acetyl-CoA [34]. Other enzymes involved in glycolysis (class II fructose-bisphosphate aldolase (fba) and enolase (eno)) were also observed, and their abundance in the S. pogona::SP_2854 mutant was also higher than that in the original strain. Additionally, their catalytic products, such as dihydroxyacetone phosphate, phosphoenolpyruvate and acetyl-CoA, displayed high abundance in the S. pogona::SP_2854 mutant, indicating that SP_2854 overexpression had a significant stimulative effect on the generation of acetyl-CoA from glucose. For the PP pathway [33], however, we found no other differentially expressed proteins related to this pathway in S. pogona::SP_2854 except for ribose 5-phosphate isomerase (rpi). The acetyl-CoA produced through glycolysis will enter the TCA cycle to produce energy and several metabolic precursors for cell growth and the synthesis of other metabolites [34]. Succinyl-CoA synthetase (sucC) catalyses the reversible reaction of succinyl-CoA to succinate. It plays a key role as one of the catalysts involved in the TCA cycle, a central pathway in cellular metabolism [35]. Malate dehydrogenase (mdh) is an enzyme that reversibly catalyses the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH [36]. The two enzymes and the relevant metabolites, succinate, malate, oxaloacetate and NADH, were all present at higher levels under SP_2854 overexpression conditions. These data suggest that the S. pogona::SP_2854 mutant shows a greater metabolic capacity than the original strain in terms of glycolysis and the TCA cycle, so it can highly efficiently utilize nutrients in the extracellular environment during the analysis phase to promote butenyl-spinosyn biosynthesis in S. pogona (Fig. 9).
Effect of SP_2854 deletion on primary metabolism in S. pogona. This regulatory network is based on the analysis of differentially expressed proteins and metabolites associated with glucose transport, glycolysis, the pentose phosphate pathway and the TCA cycle. Reactions are reported according to KEGG metabolic pathway databases. A triangle represents a change in protein abundance, and an arrow represents a change in metabolite abundance
Discussion
As a large and important family of one-component signal transduction systems, TFRs are widely associated with metabolism, antibiotic production, quorum sensing, and multidrug resistance [20,21,22,23]. Natural products, such as avermectin and calcium-dependent antibiotics, have been reported to exhibit pathway-specific regulators in their gene clusters, such as aveR and cdaR [37, 38]. Further studies have demonstrated that some TFRs directly control the expression of these genes to regulate corresponding target product biosynthesis. Unlike these natural products, no pathway-specific regulators existed in or near the bus cluster [2]. Therefore, the elucidation of regulatory genes with important roles in butenyl-spinosyn biosynthesis has become a research hotspot.
In this study, we characterized a TFR, SP_2854, in S. pogona and found that a change in its expression level can affect butenyl-spinosyn biosynthesis. The inactivation of SP_2854 decreased the production of butenyl-spinosyn by 77.3%, while its overexpression increased the production of butenyl-spinosyn by 289.7%. The differential transcription of busA, busF, busG, busI, busP and busQ in mutants S. pogona::SP_2854 and S. pogona-ΔSP_2854 further suggests that SP_2854 positively regulates butenyl-spinosyn biosynthesis. It was previously reported that some TFRs can affect other morphological, physiological and biochemical characteristics in actinomycetes [14]. In our further analysis, SP_2854 deletion also inhibited strain growth, glucose consumption and mycelial morphology. These data suggest that SP_2854 has important biological significance for maintaining normal growth and development and nutritional metabolism of S. pogona.
We present the first results using a targeted metabolomic platform to better understand detectable alterations in the intracellular metabolome caused by SP_2854 deletion and overexpression compared with the original strain. As a secondary metabolite, butenyl-spinosyn possesses a complex biosynthetic pathway, and its synthetic precursors include acetyl-CoA, malonyl-CoA, methylmalonyl-CoA, rhamnose, forosamine and S-adenosyl methionine [2]. As shown by the growth curve and targeted metabolome analysis, the promotion of butenyl-spinosyn by SP_2854 overexpression was due to an enhancement in cell growth and an enrichment of the precursors required. The two factors are correlated with each other since the precursors are derived from growth-related metabolic intermediates, such as acetyl-CoA, which was distributed in the complex metabolic network of S. pogona, as shown in Fig. 5. For example, the S. pogona::SP_2854 mutant showed significant increases in the abundance of several metabolites from glycolysis and the TCA cycle in the logarithmic phase and stationary phase, such as glucose 6-phosphate, fructose 6-phosphate, dihydroxyacetone phosphate, pyruvate, acetyl-CoA, citrate, alpha-ketoglutarate, fumarate and malate. For SP_2854 deletion, most metabolites involved in glycolysis and the TCA cycle in the S. pogona-ΔSP_2854 mutant, such as glucose 6-phosphate, fructose 6-phosphate, dihydroxyacetone phosphate, phosphoenolpyruvate, pyruvate, oxaloacetate, citrate, alpha-ketoglutarate and malate, exhibited lower abundance in the logarithmic phase compared with their abundance in the original strain. It has been demonstrated that there is a novel link between primary metabolism and butenyl-spinosyn biosynthesis, suggesting that SP_2854 is tightly linked to butenyl-spinosyn biosynthesis by regulating the primary metabolic pathway except for controlling the transcription of the bus cluster.
In addition, proteomic analyses were used to compare the protein expression differences between the S. pogona::SP_2854 mutant and original strain. A total of 97 proteins were upregulated, while 133 proteins were downregulated in the S. pogona::SP_2854 mutant. These differentially expressed proteins are involved in multiple biological processes, such as glycolysis, the PP pathway, the TCA cycle, oxidative phosphorylation, amino acid metabolism, a two-component system, and ABC transporters, suggesting that SP_2854 may act as a pleiotropic regulator to control butenyl-spinosyn biosynthesis. Combined with contemporaneous metabolome data, we found that most proteins and metabolites involved in primary metabolism, among which the upregulated proteins not only promote the conversion of extracellular glucose to phosphorylated glucose but also enhance glycolysis and the TCA cycle, provided sufficient precursors for other biological processes. These results may explain why the S. pogona::SP_2854 mutant exhibited high butenyl-spinosyn production.
Conclusions
We have shown that SP_2854 plays a very important role in the regulation of growth and development and butenyl-spinosyn biosynthesis in S. pogona. Our present findings provide a strategy for the improvement of butenyl-spinosyn production based on the engineering of SP_2854, which may be applied to the augmentation of other antibiotics produced by actinomycetes that have SP_2854 homologues.
Methods
Bacterial strains, plasmids, and media
The bacterial strains and plasmids used in this study are listed in Table 1. The media and culture conditions used in this study are described in the Supplementary Materials and Methods.
SP_2854 deletion and overexpression
Targeted gene deletion mediated by homologous recombination was used to generate an SP_2854 null mutant. With S. pogona genomic DNA as a template, a 1.0-kb fragment upstream (UASP_2854) of SP_2854 was amplified with primers FSP_2854up/RSP_2854up, and a 1.0-kb fragment downstream (DASP_2854) of SP_2854 was amplified with primers FSP_2854down/RSP_2854down. With pOJ260 plasmid DNA as template, a 1.3-kb apramycin resistance gene (apr) was amplified with primers Fapr/Rapr. The 5′-ends of RSP_2854up and FSP_2854down have a 35-nt overlapping sequence, allowing the joining of UASP_2854, apr and DASP_2854 in a subsequent fusion PCR. The resulting 3.3 kb fusion fragment was digested with Hind III and EcoR I and cloned into the corresponding restriction sites of pOJ260, yielding pOJ260-UASP_2854-apr-DASP_2854. Then, this plasmid was transformed into S. pogona by protoplast transformation [39]. Transformants were selected directly on R5 plates containing 50 µg/mL apramycin. Since pOJ-260 is a suicide plasmid, it cannot be replicated in S. pogona and will eventually be lost as the strain grows. Therefore, the chromosome structure at the SP_2854 locus of several ApraR colonies was analysed by PCR using primers FSP_2854up/Rapr and by sequencing (Sangon Biotech, Shanghai), and the mutant that was successfully verified was named S. pogona-ΔSP_2854.
For SP_2854 overexpression, plasmid pKCcas9dO with the synthetic promoter J23119 was selected as the editing tool [32]. S. pogona NRRL 30141 was used as the template for SP_2854 amplification by PCR, and the primers were FSP_2854/RSP_2854. The resulting 703-bp fragment was digested with Spe I and Hind III and cloned into the corresponding restriction sites of pKCcas9dO, yielding pKCcas9-SP_2854. Then, this plasmid was transformed into S. pogona by protoplast transformation. Transformants were selected directly on R5 plates containing 50 µg/mL apramycin. The chromosome structure of several ApraR colonies was analysed by PCR using primers Fapr/Rapr, and the mutant that was successfully verified was named S. pogona::SP_2854.
Cultivation profile analysis of the S. pogona-ΔSP_2854 and S. pogona::SP_2854 mutants
The cells were collected in SFM at 8th day to determine the butenyl-spinosyn production by using an HPLC system (1290, Agilent, USA). The sample processing and testing methods as described previously [9]. The samples were cultured with ethyl acetate at a 1:1 volumetric ratio, incubated for 48 h, and then centrifuged at 9000×g for 15 min. The supernatants were filtered through 0.22 µm Millipore filters. After filtration, a 20 µL aliquot of each supernatant was loaded onto a C18 column (AQ12S05-1546WT, YMC, Kyoto, Japan) and eluted with elution buffer (buffer A: 10% (v/v) acetonitrile, buffer B: 90% (v/v) acetonitrile) at 1.0 mL/min. The following gradient of buffer B was applied: 0 min, 0%; 2 min, 0%; 20 min, 100%; 22 min, 100%; 23 min, 0%; and 25 min, 0%. The detection wavelength was set to 250 nm during the analysis. Spinosyn 6-methyl β, which was previously purified and identified in our laboratory, was used as an internal standard because it fulfilled the requirements in terms of chemical similarity to the analyte.
An optical density of 600 nm (OD600) was used to determine the cell concentration. Cells were collected every 12 h during fermentation for growth curve measurements by UV scanning, and a certain dilution ratio was chosen to ensure that the measured value ranged from 0.2 to 0.8. Supernatants were collected every 12 h during fermentation to determine the glucose concentration by using a glucose assay kit (Sangon Biotech, Shanghai) until the glucose was fully consumed.
For observations of mycelium morphology, cells were collected on the 2nd, 4th and 6th days and soaked with 2.5% glutaraldehyde at 4 °C overnight and then subjected to gradient dehydration using ethanol solutions (30%, 50%, 70%, 90%, and 100%). The samples were dried naturally, sprayed with platinum, and subsequently observed by scanning electron microscopy (Hitachi SU8010, Japan). For sporulation, equal strains were streaked on CSM, BHI and TSM solid media and incubated at 30 °C for observation of sporulation for 5 days.
All of the experiments were performed in triplicate.
Determination of intracellular metabolites associated with energy metabolism by LC–MS/MS
For targeted metabolomic analysis, samples were collected on Day 2, Day 4, Day 6 and Day 8, and the sample treatment methods used were as described by Yang et al. with some modifications [40]. Ten millilitres of culture broth was collected and centrifuged, and the samples were transferred to EP tubes and mixed with 400 μL of methanol/acetonitrile (1:1, v/v). The tubes were vortexed for 30 s, incubated for 10 min at − 20 °C, and then centrifuged at 14,000g for 15 min at 4 °C. The supernatants were collected and dried with nitrogen, and the lyophilized powder was stored at − 80 °C prior to analysis. Targeted metabolite analysis was performed using an LC–MS/MS system. The dried metabolites were dissolved in 100 μL of acetonitrile/H2O (1:1, v/v) and centrifuged (13,000g) for 15 min. Electrospray ionization was conducted with an Agilent 1290 Infinity chromatography system and AB Sciex QTRAP 5500 mass spectrometer. NH4COOH (15 mM) and acetonitrile were used as mobile phases A and B, respectively. A binary solvent gradient was used as follows: A, NH4COOH; B, 0–18 min at 90% to 40% acetonitrile; 18–18.1 min at 40% to 90% acetonitrile; and 18.1–23 min at 90% acetonitrile. The LC–MS/MS was operated in negative mode under the following conditions: source temperature, 450 °C; ion source gas 1, 45; ion source gas 2, 45; curtain gas, 30; and ion spray voltage floating (ISVF), − 4500 V. All of the samples were performed in quadruplicate.
Extraction, preparation and LC–MS/MS analysis of whole proteins
The cells of the S. pogona-ΔSP_2854 mutant and the original strain were harvested (centrifugation at 9000g for 10 min at 4 °C) after 6 days of culture, washed four times by resuspending the cell pellet in 20 mL fresh PBS (10 mM, pH 7.8, prechilled at 4 °C), and quickly frozen in liquid nitrogen (three independent repeats). Protein extraction and 2D LC–MS/MS analysis were performed as described previously with slight modifications [41]. Detailed procedures are described in the Additional file 1.
LC–MS/MS data were analysed for protein identification and quantification using ProteinPilot software v.4.5 (Sciex Inc., USA). The utilized protein database was the protein sequence set of all Saccharopolyspora strains. The false discovery rate (FDR) was estimated via a reverse database strategy, and only proteins below the threshold of 1% of FDR were further considered. The differentially abundant proteins (DAPs) were defined in the iTraq experiment by the following criteria: unique peptides ≥ 1, P value < 0.05, and fold change > 1.33 or < 0.75. Only proteins present in all replicate samples were considered for subsequent analysis. The metabolic pathway analysis of DAPs was conducted using the KEGG database (http://www.genome.jp/kegg/).
RNA isolation, cDNA synthesis, and quantitative reverse transcription PCR (qRT–PCR)
The total RNAs of the WT strain and mutants were separately isolated by using TRIzol Reagent (Sangon Biotech, Shanghai). cDNA synthesis was performed by using a High-Capacity cDNA Archive Kit (Fermentas) in accordance with the manufacturer’s instructions. Real-time qPCR amplification was performed using Power SYBRR Green PCR Master Mix (Applied Biosystems) as previously described [9]. The primer sequences used in qRT–PCR were designed with Primer Premier 5.0 and are listed in Additional file 1: Table S2. The transcript generated from the 16S rRNA gene was used for normalization. The results are expressed as the means from three replicate experiments.
Statistical analysis
All data in this study were stated as means ± standard error of mean (SEM), and analysis by Student’t t-test, with *p < 0.05, **p < 0.01, ns, no significant.
Abbreviations
- CDA:
-
Calcium-dependent antibiotic
- TFRs:
-
TetR family regulatory factors
- qRT–PCR:
-
Quantitative reverse transcription PCR
- SEM:
-
Scanning electronic microscopy
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- TCA:
-
Tricarboxylic acid
- iTRAQ:
-
Isobaric tags for relative and absolute quantification
- ABC:
-
ATP -binding cassette
- PTS:
-
Phosphotransferase system
- PP:
-
Pentose phosphate
- apr :
-
Apramycin resistance gene
- HPLC:
-
High performance liquid chromatography
- SFM:
-
Synthetic fermentation medium
- CSM:
-
Complete synthetic medium
- FDR:
-
False discovery rate
- DAPs:
-
Differentially abundant proteins
References
Paul L, Donald RH, Laura LK, Dennis OD, Jeffrey RG, Gary DC, Thomas W, Thomas CS, Pat MRE, Paul RG. Discovery of the butenyl-spinosyn insecticides: novel macrolides from the new bacterial strain Saccharopolyspora pogona. Bioorg. Med Chem. 2009;17:4185–96.
Huang KX, Xia LQ, Zhang YM, Ding XZ, Zahn AJ. Recent advances in the biochemistry of spinosyns. Appl Microbiol Biotechnol. 2009;82:12–23.
Sheng Z, Chen K, Li X. Advances in the biosynthesis of spinosad—a review. Wei Sheng Wu Xue Bao. 2016;56:397–405.
Araujo RDS, Lopes MP, Barbosa WF, Gonçalves WG, Fernandes KM, Martins GF, Tavares MG. Spinosad-mediated effects on survival, overall group activity and the midgut of workers of Partamona helleri (Hymenoptera: Apidae). Ecotoxicol Environ Saf. 2019;175:148–54.
Mendonça TP, Davi de Aquino J, Junio da Silva W, Mendes DR, Campos CF, Vieira JS, Barbosa NP, Carvalho Naves MP, Olegário de E, Alves de Rezende AA, Spanó MA, Bonetti AM, Vieira Santos VS, Pereira BB, Resende de Morais C. Genotoxic and mutagenic assessment of spinosad using bioassays with Tradescantia pallida and Drosophila melanogaster. Chemosphere. 2019;222:503–10.
Hahn DR, Gustafson G, Waldron C, Bullard B, Jackson JD, Mitchell J. Butenyl-spinosyns, a natural example of genetic engineering of antibiotic biosynthetic genes. J Ind Microbiol Biotechnol. 2006;33:94–104.
Rang J, He HC, Chen JM, Hu JJ, Tang JL, Liu ZD, Xia ZY, Ding XZ, Zhang YM, Xia LQ. SenX3-RegX3, an important two-component system, regulates strain growth and butenyl-spinosyn biosynthesis in Saccharopolyspora pogona. iScience. 2020;23:101398.
He HC, Yuan SQ, Hu JJ, Chen JM, Rang J, Tang JL, Liu ZD, Xia ZY, Ding XZ, Hu SB, Xia LQ. Effect of the TetR family transcriptional regulator Sp1418 on the global metabolic network of Saccharopolyspora pogona. Microb Cell Fact. 2020;19:27.
Tang JL, He HC, Li YL, Liu ZD, Xia ZY, Cao L, Zhu ZR, Shuai L, Liu Y, Wan QQ, Luo YW, Zhang YM, Rang J, Xia LQ. Comparative proteomics reveals the effect of the transcriptional regulator Sp13016 on butenyl-spinosyn biosynthesis in Saccharopolyspora pogona. J Agric Food Chem. 2021;69:12554–65.
Rang J, Zhu ZR, Li YL, Cao L, He HC, Tang JL, Hu JJ, Chen JM, Hu SB, Huang WT, Yu ZQ, Ding XZ, Sun YJ, Xie QJ, Xia LQ. Identification of a TetR family regulator and a polyketide synthase gene cluster involved in growth development and butenyl-spinosyn biosynthesis of Saccharopolyspora pogona. Appl Microbiol Biotechnol. 2021;105:1519–33.
Li L, Gong L, He HC, Liu ZD, Rang J, Tang JL, Peng SN, Yuan SQ, Ding XZ, Yu ZQ, Xia LQ, Sun YJ. AfsR is an important regulatory factor for growth and butenyl-spinosyn biosynthesis of Saccharopolyspora pogona. Ann Microbiol. 2019;69:809–18.
Hwang S, Lee N, Jeong Y, Lee Y, Kim W, Cho S, Palsson BO, Cho BK. Primary transcriptome and translatome analysis determines transcriptional and translational regulatory elements encoded in the Streptomyces clavuligerus genome. Nucleic Acids Res. 2019;47:6114–29.
Martínez-Burgo Y, Álvarez-Álvarez R, Rodríguez-García A, Liras P. The pathway-specific regulator ClaR of Streptomyces clavuligerus has a global effect on the expression of genes for secondary metabolism and differentiation. Appl Environ Microbiol. 2015;81:6637–48.
Lu FJ, Hou YY, Zhang HM, Chu YW, Xia HY, Tian YQ. Regulatory genes and their roles for improvement of antibiotic biosynthesis in Streptomyces. 3 Biotech. 2017;7:250.
Lu W, Alanzi AR, Abugrain ME, Ito T, Mahmud T. Global and pathway-specific transcriptional regulations of pactamycin biosynthesis in Streptomyces pactum. Appl Microbiol Biotechnol. 2018;102:10589–601.
Uguru GC, Stephens KE, Stead JA, Towle JE, Baumberg S, McDowall KJ. Transcriptional activation of the pathway-specific regulator of the actinorhodin biosynthetic genes in Streptomyces coelicolor. Mol Microbiol. 2005;58:131–50.
Shu D, Chen L, Wang WH, Yu ZY, Ren C, Zhang WW, Yang S, Lu YH, Jiang WH. afsQ1-Q2-sigQ is a pleiotropic but conditionally required signal transduction system for both secondary metabolism and morphological development in Streptomyces coelicolor. Appl Microbiol Biotechnol. 2009;81:1149–60.
Bednarz B, Kotowska M, Pawlik KJ. Multi-level regulation of coelimycin synthesis in Streptomyces coelicolor A3(2). Appl Microbiol Biotechnol. 2019;103:6423–34.
Wang K, Sybers D, Maklad HR, Lemmens L, Lewyllie C, Zhou X, Schult F, Bräsen C, Siebers B, Valegård K, Lindås AC, Peeters E. A TetR-family transcription factor regulates fatty acid metabolism in the archaeal model organism Sulfolobus acidocaldarius. Nat Commun. 2019;10:1542.
Xu YR, Ke ML, Li J, Tang YQ, Wang N, Tan GQ, Wang YS, Liu RH, Bai LQ, Zhang LX, Wu H, Zhang BC. TetR-type regulator SLCG_2919 is a negative regulator of lincomycin biosynthesis in Streptomyces lincolnensis. Appl Environ Microbiol. 2018;85:e02091-e2118.
Cuthbertson L, Nodwell JR. The TetR family of regulators. Microbiol Mol Biol Rev. 2013;77:440–75.
Yuan PH, Zhou RC, Chen X, Luo S, Wang F, Mao XM, Li YQ. DepR1, a TetR family transcriptional regulator, positively regulates daptomycin production in an industrial producer, Streptomyces roseosporus SW0702. Appl Environ Microbiol. 2016;82:1898–905.
Liu WS, Zhang QL, Guo J, Chen Z, Li JL, Wen Y. Increasing avermectin production in Streptomyces avermitilis by manipulating the expression of a novel TetR-family regulator and its target gene product. Appl Environ Microbiol. 2015;81:5157–73.
Palazzotto E, Tong Y, Lee SY, Weber T. Synthetic biology and metabolic engineering of actinomycetes for natural product discovery. Biotechnol Adv. 2019;37: 107366.
Tan GY, Deng KH, Liu XH, Tao H, Chang YY, Chen J, Chen K, Sheng Z, Deng ZX, Liu TG. Heterologous biosynthesis of spinosad: an omics-guided large polyketide synthase gene cluster reconstitution in Streptomyces. ACS Synth Biol. 2017;6:995–1005.
Hwang KS, Kim HU, Charusanti P, Palsson BØ, Lee SY. Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnol Adv. 2014;32:255–68.
Wang K, Chen XA, Li YQ, Mao XM. Identification of a secondary metabolism-responsive promoter by proteomics for over-production of natamycin in Streptomyces. Arch Microbiol. 2019;201:1459–64.
Tiffert Y, Franz-Wachtel M, Fladerer C, Nordheim A, Reuther J, Wohlleben W, Mast Y. Proteomic analysis of the GlnR-mediated response to nitrogen limitation in Streptomyces coelicolor M145. Appl Microbiol Biotechnol. 2011;89:1149–59.
Wang C, Liu J, Liu HH, Liang SX, Wen JP. Combining metabolomics and network analysis to improve tacrolimus production in Streptomyces tsukubaensis using different exogenous feedings. J Ind Microbiol Biotechnol. 2017;44:1527–40.
Liu XY, Sun XJ, He WM, Tian XW, Zhuang YP, Chu J. Dynamic changes of metabolomics and expression of candicidin biosynthesis gene cluster caused by the presence of a pleiotropic regulator AdpA in Streptomyces ZYJ-6. Bioprocess Biosyst Eng. 2019;42:1353–65.
Li L, Rang J, He HC, He SY, Liu ZD, Tang JL, Xiao J, He L, Hu SB, Yu ZQ, Ding XZ, Xia LQ. Impact on strain growth and butenyl-spinosyn biosynthesis by overexpression of polynucleotide phosphorylase gene in Saccharopolyspora pogona. Appl Microbiol Biotechnol. 2018;102:8011–21.
Huang H, Zheng GS, Jiang WH, Hu HF, Lu YH. One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim Biophys Sin. 2015;47:231–43.
Nikel PI, Chavarría M, Fuhrer T, Sauer U, de Lorenzo V. Pseudomonas putida KT2440 strain metabolizes glucose through a cycle formed by enzymes of the Entner-Doudoroff, Embden-Meyerhof-Parnas, and Pentose phosphate pathways. J Biol Chem. 2015;290:25920–32.
Vuoristo KS, Mars AE, Sanders JPM, Eggink G, Weusthuis RA. Metabolic engineering of TCA cycle for production of chemicals. Trends Biotechnol. 2017;34:191–7.
Bridger WA, Wolodko WT, Henning W, Upton C, Majumdar R, Williams SP. The subunits of succinyl-coenzyme A synthetase–function and assembly. Biochem Soc Symp. 1987;54:103–11.
Goward CR, Nicholls DJ. Malate dehydrogenase: a model for structure, evolution, and catalysis. Protein Sci. 1994;3:1883–8.
Barreiro C, Martínez-Castro M. Regulation of the phosphate metabolism in Streptomyces genus: impact on the secondary metabolites. Appl Microbiol Biotechnol. 2019;103:1643–58.
Kitani S, Ikeda H, Sakamoto T, Noguchi S, Nihira T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl Microbiol Biotechnol. 2009;82:1089–96.
Ma Z, Liu JX, Liu XZ, Shentu XP, Bian YL, Yu XP. Formation, regeneration, and transformation of protoplasts of Streptomyces diastatochromogenes 1628. Folia Microbial. 2014;59:93–7.
Yang J, Fu MM, Ji C, Huang YC, Wu YR. Maize oxalyl-CoA decarboxylase1 degrades oxalate and affects the seed metabolome and nutritional quality. Plant Cell. 2018;30:2447–62.
Yang Q, Ding XZ, Liu XM, Liu S, Sun YJ, Yu ZQ, Hu SB, Rang J, He H, He L, Xia LQ. Differential proteomic profiling reveals regulatory proteins and novel links between primary metabolism and spinosad production in Saccharopolyspora spinosa. Microb Cell Fact. 2014;13:27.
Acknowledgements
We also thank Shanghai Applied Protein Technology, Co. and Shanghai Sangon Biotech for providing technical support.
Funding
This work was supported by funding from the National Natural Science Foundation of China (31770106, 3107006), the Natural Science Foundation of Hunan Province (2021JJ40341), the Cooperative Innovation Center of Engineering and New Products for Developmental Biology of Hunan Province (20134486).
Author information
Authors and Affiliations
Contributions
LX conceived the project. LX and JR generated the concepts and designed the research. JR JT and ZX performed mutants construction. JT, LS, XL and LC performed HPLC and data analysis. YL, JX, YL, QX and SH performed LC–MS/MS and data analysis. JR, QX and LX wrote the manuscript. All authors discussed the results. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Oligonucleotides used in this study. Table S2. qRT-PCR primers used in this study. Figure S1. Mass spectrum identification of butenyl-spinosyn. (A) Chromatographic peak detection spectrum in 13.0 min. (B) Chromatographic peak detection spectrum of secondary spectrum in 13.0 min. MS identification results showed that (M + H) + ions at m/z = 633.17 (black arrow) contained a rhamnose ion fragment of 189 molecular mass, which was confirmed as a butenyl-spinosyn component spinosyn 6-methyl β. Figure S2. Sporulation observation of the S. pogona-ΔSP_2854 (1), S. pogona::SP_2854 (2), and S. pogona (3) in the BHI, CSM and TSB solid media. The strains were grown on the BHI, CSM and TSB solid media and photographed on days 5.
Additional file 2: DataSet S1.
Transcriptomic data of mutants S. pogona-ΔSP_2854 and S. pogona::SP_2854.
Additional file 3: DataSet S2.
Comparative proteomic analysis of the S. pogona::SP_2854 mutant and S. pogona at 6th day.
Additional file 4: DataSet S3.
Differentially expressed proteins analysis of the S. pogona::SP_2854 mutant and wild-type S. pogona at 6th day.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rang, J., Xia, Z., Shuai, L. et al. A TetR family transcriptional regulator, SP_2854 can affect the butenyl-spinosyn biosynthesis by regulating glucose metabolism in Saccharopolyspora pogona. Microb Cell Fact 21, 83 (2022). https://doi.org/10.1186/s12934-022-01808-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-022-01808-2