Abstract
Enteroviruses (EVs) are the most prevalent viruses in humans. EVs can cause a range of acute symptoms, from mild common colds to severe systemic infections such as meningitis, myocarditis, and flaccid paralysis. They can also lead to chronic diseases such as cardiomyopathy. Although more than 280 human EV serotypes exist, only four serotypes have licenced vaccines. No antiviral drugs are available to treat EV infections, and global surveillance of EVs has not been effectively coordinated. Therefore, poliovirus still circulates, and there have been alarming epidemics of non-polio enteroviruses. Thus, there is a pressing need for coordinated preparedness efforts against EVs.
This review provides a perspective on recent enterovirus outbreaks and global poliovirus eradication efforts with continuous vaccine development initiatives. It also provides insights into the challenges and opportunities in EV vaccine development. Given that traditional whole-virus vaccine technologies are not suitable for many clinically relevant EVs and considering the ongoing risk of enterovirus outbreaks and the potential for new emerging pathogenic strains, the need for new effective and adaptable enterovirus vaccines is emphasized.
This review also explores the difficulties in translating promising vaccine candidates for clinical use and summarizes information from published literature and clinical trial databases focusing on existing enterovirus vaccines, ongoing clinical trials, the obstacles faced in vaccine development as well as the emergence of new vaccine technologies. Overall, this review contributes to the understanding of enterovirus vaccines, their role in public health, and their significance as a tool for future preparedness.
Similar content being viewed by others
Enteroviruses
Enteroviruses (EVs) constitute a genus of viruses belonging to the Picornaviridae family that consists of more than 280 viruses capable of infecting humans. The genus includes 15 species – the 12 Enterovirus species (A-L) and three Rhinovirus species (RV A-C) [1, 2]. The three poliovirus serotypes (poliovirus 1-3) are found within the species C Enterovirus and the rest of the enteroviruses are commonly referred to as non-polio enteroviruses (NPEVs). Seven out of the 15 NPEV species (EV A-D and RV A-C) infect humans, and NPEVs are among the most common human pathogens found worldwide, with infections particularly frequent in children. Examples of commonly circulating NPEVs are rhinoviruses, coxsackievirus (CV) A and B, EV-D68 and EV-A71 [1, 2].
EVs are non-enveloped viruses with a single stranded (ss) RNA molecule of about 7500 bases surrounded by an icosahedral capsid consisting of 60 copies each of the four viral proteins VP1-4 (Fig. 1). EV virions are very stable and most EVs have a strong resistance to acidic environments (down to pH 3.0). This enables many EVs to infect and replicate in the gastrointestinal tract, and faecal-oral spread is the primary route of transmission, but many EVs can also transmit via oral and respiratory droplets originating from infected individuals.
Enterovirus genome organisation and capsid structure (not to scale). A enterovirus genome encodes a single polyprotein (regions P1-P3) comprising a main open reading frame (ORF). Additional recently discovered [3, 4] ORF2 overlaps with the main ORF at the 5’ end. The ORF2 is not found from rhinoviruses or EV-D68 [4] B enterovirus capsid is formed by the four viral structural proteins VP1-VP4 of which the VP4 is located at the inner capsid, C while VP1-VP3 form a pseudo T=3 symmetry unit (highlighted with neon green) which assembles into pentamers 12 of which in turn form the outer capsid
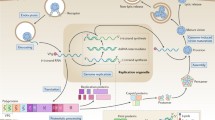
The EV replication cycle begins with virion binding to a cellular receptor followed by endocytic uptake of the virion. The virion then delivers the viral RNA genome to the cytosol where it is translated into a polyprotein. The polyprotein is proteolytically processed by viral proteases 3C and 2A into structural (capsid) and non-structural proteins, including the viral polymerase. Non-structural proteins then induce the formation of membrane structures (replication organelles) where genome replication takes place (Fig. 2). Subsequently, capsid proteins and genomic RNA self-assemble into virions that exit the host cell via cell lysis or within extracellular vesicles [5]. Transmission via filopodia has also been described [6, 7].
Enterovirus replication cycle (schematic representation). After receptor attachment and internalization by endocytosis, the genome is uncoated. As positive stranded RNA viruses, enteroviruses utilise host-cell machinery in genome replication – the replication takes place in a replication organelle, which are composed of cellular membranes prompted by the infection. After assembly the mature virions are released by either lytic or non-lytic pathways
While most NPEV infections result in mild respiratory illness, such as the common cold, some can lead to more severe illnesses. These include acute flaccid myelitis (AFM, a paralyzing illness), myocarditis, hand-foot-and-mouth disease (HFMD) and neonatal sepsis [8]. NPEV infections are also responsible for more than 50% of aseptic meningitis cases [9]. It is important to note that some complications of enterovirus infections may not be evident until years after the initial infection. For example, coxsackie B virus (CVB) infections are known to cause myocarditis, which can progress to chronic dilated cardiomyopathy [10] months or even years after acute infection [11]. NPEVs that affect the central nervous system and heart pose a particularly heavy burden on the health care system [8, 11]. Additionally, CVB infections have been associated with the autoimmune disease type 1 diabetes [6]. Thus, the disease and health care impacts of enterovirus infections are substantial (Table 1).
Since NPEVs are single-stranded RNA viruses with high mutation and recombination rates, this increases the risk of new pathogenic strains emerging in the future. Therefore, we think that it is critical to develop effective vaccines and adaptable vaccine platforms that can be rapidly deployed to respond to emerging or re-emerging enteroviruses. To our opinion it is particularly important to prioritize vaccine development concerning EVs associated with the most severe disease manifestations such as acute flaccid paralysis (AFP), AFM, meningitis, myocarditis, neonatal systemic illness as well as neurological complications. Thus, vaccines against emerging viruses (such as EV-B93, EV-D94, EV-D111) as well as re-emerging viruses (such as EV-A71, EV-D68, CVA1, CVA20, E11, E33, CVB1-6, EV-6, E9 and E30) should be actively progressed.
Additionally, the development of safe and effective antiviral drugs should be pursued – antivirals are an active area of research, especially against EV-A71, and are reviewed elsewhere [48,49,50,51,52,53,54,55,56,57]. Although five EV inhibitors targeting the EV capsid surface have been evaluated for safety and efficacy in clinical trials, a majority of the inhibitors were found to cause unwanted side effects and failed to meet their clinical endpoints [58]. Notably, inhibitors may lead to the development of drug-resistant strains; however, it has been observed that resistant strains typically exhibit lower levels of fitness than wild-type strains [58]. This review focuses on current and new enterovirus vaccines and vaccine technologies in development for poliovirus and other NPEV vaccines, while rhinovirus vaccines have been covered in reviews elsewhere [59, 60].
Enterovirus outbreaks are common
In recent years, several outbreaks of both polioviruses and NPEVs have occurred. While Afghanistan and Pakistan are the only countries where wild-type poliovirus remains endemic, in July 2024, there were 35 so-called ‘outbreak countries’ across five continents, implying the ongoing risk of imported wild-type poliovirus or the emergence of circulating vaccine-derived poliovirus (cVDPV) [61]. Poliovirus outbreaks resulting from mutations of the attenuated oral polio vaccine (OPV) are reported periodically, as in 2022, when cVDPV was detected in wastewater samples from New York, London and Jerusalem. Two cases of polio-related paralysis caused by cVDPV were reported in Israel and in the United States [62, 63]. According to the World Health Organization (WHO), one in 200 polio infections can lead to irreversible paralysis (typically affecting the legs) [64]. Therefore, every paralysis case suggests that many other people have been infected with polio and that the virus is circulating more widely. This is particularly alarming given that endemic poliovirus has largely been eradicated, and suboptimal vaccination coverage in at-risk populations poses a threat to the global polio eradication initiative.
NPEV outbreaks also pose a risk to human health. Between 2015 and 2017, 66 different NPEV types were identified circulating in 24 EU/EAA countries, leading to 68 deaths, 77 cases of paralysis and over 3000 neurological infections, 30% of which were paralytic [8]. EV-D68 gained attention in the United States in 2014 when it caused over 1300 confirmed cases, primarily among children, resulting in five deaths. The outbreak was also linked to several cases of polio-like syndrome [65]. Since then, EV-D68 has emerged as a biennial epidemic in the United States and Europe [66, 67]
After the 2014 outbreak of EV-D68 in North America, a study revealed that a majority of the children admitted to the hospital with AFM still presented persistent motor deficits after one year [68] and that EV-D68 continued to circulate after the initial outbreak [69]. A significant increase in the number of EV-D68-positive patients was again observed in September 2022 by the Johns Hopkins Hospital system, with 28% of patients requiring hospital admission and 49% of those requiring intensive care [70]. A similar unexpected outbreak of EV-D68 occurred in Finland during the same period and led to the hospitalization of several children [71]. This increase in EV-D68 cases is believed to account for the increase in cases of AFM every two years since 2014 [72,73,74]. The occurrence of AFM, characterized by sudden paralysis, follows the seasonal circulation pattern of enteroviruses, particularly that of EV-D68. Furthermore, a study from the United Kingdom revealed that the incidence of EV-D68 infections in young children was notably higher in 2016 compared to 2006, suggesting changes in population immunity, virus antigenicity, transmissibility, or cellular tropism [75]. The change in the clinical presentation of EV-D68 has been hypothesized to be caused by mutations, potentially allowing broader receptor binding, thus leading to increased virulence, transmissibility and infections at a younger age [76,77,78,79].
In addition to EV-D68, unexpected peaks of severe and fatal entero- and parechovirus infections were documented in the United States during 2022 [80]. Similarly, an increase in CVB-induced severe cases of myocarditis in neonates was reported in the United Kingdom between June 2022 and April 2023 [81]. Several European countries also reported numerous cases of echovirus 11 (E11)-related sepsis and meningoencephalitis in newborns between 2022 and the summer of 2023, some of which were fatal [82, 83]. Aseptic meningitis is one of the severe sequelae, caused by several different EVs (Table 1 above), such as CVBs and EV-A71. Enterovirus associated meningitis outbreaks have recently been reported in 2023 in Iraq (caused by both bacterial and enteroviral infections) [84], in 2019 in Mayotte French Comoros Island [85], and 2018-2019 in South Africa [86] A study found that in the United States between 2011-2014 almost 60% of studied meningitis and encephalitis cases were found to have enterovirus as an etiological agent [87]. In 2018, an increase in echovirus 30 (E30) infections associated with meningitis/meningoencephalitis were observed in Denmark, Germany, the Netherlands, Norway and Sweden [88], and a 2022 CVB2 outbreak caused meningoencephalitis in Israeli children [89], while in 2016 an outbreak of aseptic meningitis caused by E30 broke in Inner Mongolia – an autonomous region of China [90]. Cases of HFMD and neurological illness causing enteroviruses in Japan between 2012-2019 are reviewed at [91], while the circulation of non-polio enteroviruses in EU and EEA countries – resulting in e.g. HFMD, myocarditis and death – are studied at [8].
EV-A71 frequently causes outbreaks in East- and Southeast Asia [34, 92,93,94,95,96] (reviewed recently at [97]), and these outbreaks have been associated with several deaths in children [97, 98]. In 2011-2012 an EV-A71 outbreak resulted in fatalities in Vietnam’s largest outbreak of HFMD [99]. EV-A71 is not limited to Asia, as it has also been detected in European countries. In 2001, EV-A71 was found in Scottish blood donors [100]. Sporadic cases and epidemics of EV-A71 infections have been reported across Europe [101,102,103,104,105,106,107]. In 2016, Catalonia was hit by an outbreak that later spread across Spain, resulting in 57 children suffering from severe neurological disease instead of HMFD [17, 108]. The largest outbreak of EV-A71 in the Americas was observed in 2018, during which 43 children showed symptoms of meningitis, encephalitis, AFM or seizures [18].
EV-A71 is a major cause of HFMD along with CVA16, but the disease is caused by several EVs as indicated in Table 1 above. CVA6 has been proposed as a new emerging pathogen causing HFMD globally, as reviewed in [109]. HFMD is particularly troublesome in Asia-Pacific region, causing widespread outbreaks typically affecting young children [110, 111]. While the disease caused in majority of cases is mild, due to its prevalence HFMD contributes to mortality which interestingly was also found to be the major driver in bringing up the disability-adjusted life years (DALYs) associated with HFMD [112]. Thus it is suggested that HFMD associated DALYs could significantly be brought down by vaccination lowering mortality [112].
The closely associated enteroviruses EV-B93, EV-D94, and EV-D111 were suggested to be the cause of AFM outbreaks in both Egypt and the Democratic Republic of the Congo in the early 2000s [12]. Similarly, EV-B93 was identified in a patient who presented with AFP in Tibet in 1999 [13]. According to phylogenetic analysis, that particular EV-B93 strain had undergone recombination with EV-B107 [13]. Although it was discovered through seroepidemiology that EV-B93 had not caused an epidemic in Tibet, the EV-B93 strains identified in Tibet exhibited temperature resistance and prognosticative virulence, suggesting the potential for a large-scale outbreak [13].
A recent analysis revealed that EV-D111 strains isolated from human and non-human primate samples were not phylogenetically distinct, suggesting recent zoonotic transmission [113]. Evidence of intertypic genetic recombination events between EV-D111 and EV-D94 was also discovered, indicating a shared replication site in infected hosts [113]. Both EV-D94 and EV-D111 induce cytopathic effects in L20B cells commonly used to detect polioviruses [113]. It was hypothesized that this could lead to false-positive poliovirus detection, particularly in Central Africa, where EV-D111 circulates and is a key region for poliovirus eradication [113]. Taken together, these findings emphasize the continuous emergence of new enterovirus species and their zoonotic relatedness and potentially pathogenic nature, which call for epidemic preparedness. Although none of these NPEV outbreaks have yet led to disease on a global scale and have not been identified via clinical or laboratory-based enterovirus surveillance outside Africa, polio has taught us that enteroviruses possess both emerging and re-emerging potential.
The lack and need for enterovirus epidemic preparedness
Epidemic preparedness requires a robust surveillance system to detect outbreaks early, sufficient healthcare infrastructure and clear plans on how to act in outbreak situations. Key elements of effective preparedness efforts include political commitment, public health communication plans, sustained funding of public health infrastructures as well as research and development of vaccines and therapeutic treatments. Additionally, international collaboration between countries, organizations, and health agencies facilitates a coordinated global response to prevent and manage outbreaks.
Rapid detection, characterization and control of circulating enteroviruses requires a proactive system based on regular sampling of appropriate patient cohorts and general surveillance on a global scale [21], which is currently lacking. Apart from EV-A71 vaccines used in China and Thailand, there are no approved vaccines for NPEVs. NPEV antivirals are also lacking [58, 114, 115]. As a result, the preparedness for NPEV outbreaks and epidemics is inadequate.
A recent article identified several knowledge gaps in enterovirus research that are crucial to pandemic preparedness [116]. These include, for example active surveillance strategies and comprehensive models of transmission, which are necessary for accurate disease outbreak predictions and evaluating interventions. Because enteroviruses are so common, many of them co-circulate which allows for recombination and thus leads to increased transmission potential [117,118,119,120,121,122,123]. Although the zoonosis of EVs has been poorly characterized, host transmission barrier is considered high for EVs. However, many EVs, including poliovirus, echoviruses, CVA and CVB have been shown to also infect non-human primates [124,125,126,127,128,129,130] and a novel viral recombinant between poliovirus and coxsackievirus displaying AFP symptoms was isolated from chimpanzees in sub-Saharan Africa [131]. Therefore, we think that integrating EV pandemic preparedness with One Health Concept would be crucial in enhancing overall public health outcomes against EVs.
EV-D68 has been suggested as a test case to establish an immunological surveillance program and develop countermeasures for future outbreaks, as the expected EV-D68 spike during 2020 was avoided, likely due to non-pharmaceutical interventions for COVID-19, but left behind a susceptible population [132]. In the United States, the National Institute of Health Vaccine Research Center’s PREMISE (Pandemic REsponse REpository through Microbial and Immune Surveillance and Epidemiology) program aims to address the problem of surveillance by 1) conducting immune analysis to detect reactivity against potentially pandemic viruses, 2) identifying immunogens for vaccine development and 3) developing monoclonal antibodies for prevention and therapy [133]. Similarly, in Europe, the European Non-Polio Enterovirus Network (ENPEN) aims to develop standardized protocols for hospital-based surveillance, diagnosis, detection and reporting of enterovirus-associated infections to establish the true burden of NPEV infections in Europe [134], while in Asia-Pacific region the Asia-Pacific Network for Enterovirus Surveillance (APNES) has been established with an analogous function [135].
Epidemic preparedness efforts against enteroviruses vary greatly between countries, largely reflecting the epidemiological situation and healthcare resources. The Global Polio Eradication Initiative (GPEI) is a collaborative effort aimed at eradicating polio worldwide through immunization, surveillance, and public health interventions [136]. Led by national governments and six core partners (the WHO, Rotary International, CDC, UNICEF, Bill & Melinda Gates Foundation, and Gavi, the Vaccine Alliance), the GPEI is a public‒private partnership. The WHO declared the European region free of wild-type poliovirus in 2002. However, the recent transmission of OPV-derived poliovirus in the United States, Europe and the Middle East [137], as well as the movement of refugees from war zones of Pakistan and Afghanistan, has increased the risk of new poliovirus outbreaks (either vaccine-derived or wild-type) globally. For example, a recent study conducted in Ethiopia revealed that only one-third of Ethiopian children receive all polio vaccine doses required for efficient protection against poliomyelitis [138]. Factors contributing to low vaccination coverage included limited access to health care facilities, dissatisfaction with vaccination services, low parental education and fear of vaccine-related side effects. As such, the study highlights the need for improved vaccination strategies among at-risk populations as well as the need to address barriers in both access and inequity in coverage. In addition, this underscores the need for continued vaccine vigilance as well as work to reduce vaccination hesitancy and barriers that lead to a lack of vaccination as a key approach to epidemic preparedness.
As part of the commitment to GPEI’s polio eradication strategy [139], the CDC and WHO have recommended enterovirus surveillance to 1) detect and control outbreaks, 2) conduct complete virological investigations and research for at least 80% of all AFP cases (surveillance indicators found at [140]), and 3) gather data for long-term public health planning. Several countries, including the United States [141], Australia [142], Germany [143], and 25 other European countries [144], have established surveillance systems that collect information on cases associated with enterovirus infections.
Current enterovirus surveillance systems primarily rely on passive methods, which involve detecting enteroviruses in diagnostic patient samples by PCR or serology. This approach is essential because of the large number of distinct enterovirus serotypes that can infect humans and the fact that the diseases they cause are often mild. However, in certain cases, additional laboratory surveillance of poliovirus and NPEV has been conducted among high-risk populations. This supplementary surveillance is necessary to meet the requirements set by the WHO, which include tracking the emergence of vaccine-derived polioviruses, the reappearance of wild polioviruses, or the disappearance of all vaccine-related strains [145].
To effectively eradicate polio and halt the transmission of NPEVs, the development of broadly reactive enterovirus vaccines that can be mass produced, supplied, and administered without the assistance of healthcare professionals would be the most effective approach. Vaccines should also be available to people in low- and middle-income countries and could thus realistically achieve global coverage. Investments in vaccine development are therefore crucial for epidemic preparedness efforts.
Existing enterovirus vaccines
Vaccination plays a crucial role in controlling and eradicating infectious diseases. The global battle against polio has witnessed remarkable advancements due to poliovirus vaccines. These vaccines have led to a 99.9% reduction in polio cases over the past three decades. There are two different types of poliovirus vaccines, the inactivated whole-virus vaccine (IPV) and the OPV. OPV has been instrumental in the fight against polio. It consists of a live-weakened form of poliovirus, is administered orally, replicates in the gut, and induces better mucosal immunity compared to IPV, which is administered intramuscularly. Moreover, OPV is more cost-effective and easier to administer, making it the preferred option in low-income countries.
OPV has enabled significant progress in the global eradication of polio, as in the past it was administered in now polio-free countries. However, the final stages of eradicating the disease have proven to be challenging, mainly due to persistent outbreaks of circulating OPV-derived polioviruses (cVDPVs). While wild-type polioviruses, particularly type 1 polioviruses, still pose a threat in countries including Afghanistan and Pakistan, outbreaks of all three poliovirus types can still occur. Currently, type 2 cVDPVs are the most prevalent variant of vaccine-derived viruses [146]. To overcome this, the WHO declared the Polio Eradication and Endgame Strategic Plan (2013-2018) in 2013. The plan outlined various goals, one of which was to cease the use of the oral polio vaccine containing all three poliovirus serotypes. This process began with the removal of the type 2 poliovirus from the OPV. In April 2016, the trivalent vaccine (tOPV), which contained all three serotypes, was replaced with the bivalent vaccine (bOPV), containing only serotypes 1 and 3. Currently, many countries use a combination of bOPV and IPV (types 1, 2 and 3) as part of their routine vaccination programs [147]. However, due to the eradication of wild-type poliovirus in high-income countries, OPV is no longer used for safety reasons.
Based on a database query on adisinsight.spinger.com (accessed 23.8.2023), there are currently 23 combination vaccines containing IPV on the market. These vaccines also include other antigens, such as diphtheria, tetanus, pertussis, and hepatitis B [148,149,150]. They typically contain inactivated virus, and their formulation varies based on location and intended age group. Combination vaccines have helped reduce the need for multiple injections and have contributed to widespread polio vaccine coverage. Well-known stakeholders such as Sanofi, AJ Vaccines, Intravacc, Sinovac, WHO, KM Biologics, and Mitsubishi have registered or preregistered three multivalent polio vaccines for use against multiple diseases.
As EV-A71-induced disease has become a major public health problem in China [151], three inactivated and alum-adjuvanted EV-A71 vaccines were introduced in China between 2015 and 2016. In 2022, the first EV-A71 vaccine was licenced in Thailand. EV-A71 vaccines are inactivated whole-virus vaccines that offer cross-protection among EV-A71 subgenotypes [151, 152], but these vaccines have not yet received regulatory approval outside of Asia. To be used worldwide, there is a need for global harmonization in terms of vaccine production, quality control, and standardization. Notably, the currently available EV-A71 vaccines are designed to target the C4 sub-genotype, which is the most prevalent sub-genotype circulating in China. If the Chinese vaccine quality regulations would comply globally, the safety and efficacy of these vaccines would need to be tested in the new intended target populations since the dominant EV-A71 strains are different outside of China [152]. The efficacy of the three existing vaccines after two vaccinations is very high, at more than 90%, and remains so at the two-year follow-up [151]. Candidate vaccines containing the B4 and B5 genogroups are in development elsewhere but have not yet reached the licensing stage [151].
Enterovirus vaccines in clinical trials
To map the current enterovirus vaccine landscape, we conducted a systematic search of all enterovirus vaccine trials through ClinicalTrials.gov (accessed 9.1.2024). This investigation showed that polio and EV-A71 vaccine trials still dominate the field (Fig. 3). There are 154 polio and 29 EV-A71 vaccine clinical trials, that have been completed, of which 51 and 10 are completed phase 4 studies for polio and EV-A71 vaccines respectively (Fig. 4). Of the 154 polio vaccine trials, 8 involve trials studying the efficacy of IPV and OPV, when administrated sequentially, whereas 120 involve trials on IPV and 23 studies on OPV (in mono- or multivalent formulations). Three of the vaccine trials involve comparison of a fractional IPV dose administered intradermally to a full dose administered intramuscularly. These studies involve the IPV vaccine shortage associated to the withdrawal of type 2 OPV in April, 2016, when trivalent OPV (containing types 1, 2, and 3) was replaced with bivalent OPV (containing types 1 and 3 [153]). Of the 29 completed EV-A71 vaccine trials 21 studies involve usage of vero-cell produced inactivated virus vaccine and three studies involve usage of human Diploid Cell (KMB-17) produced inactivated virus vaccine, whereas one study compared the efficacy of the vaccines produced in Vero vs. KMB-17 cells. Five of the studies did not reveal the vaccine technology or the production host. The completed polio vaccine clinical trials reflect the situation in the marketed polio vaccines. Most completed clinical trials for enterovirus vaccines have been done for combination vaccines where polio has been formulated together with diphtheria, tetanus and pertussis (e.g. study sponsored by GSK [154]). These clinical studies are necessary for evaluating whether antigenic interference occurs and for determining optimal vaccine dose for different groups of people. In addition to the polio and EV-A71 vaccine clinical trials, a first-in-human phase 1 study was recently conducted with a multivalent CVB inactivated virus vaccine. This vaccine, based on our vaccine platform and promising preclinical studies [155,156,157,158,159,160], targets several strains of CVBs associated with islet autoimmunity and type 1 diabetes (T1D). The phase 1 study suggested that the vaccine is well tolerated and elicits virus-neutralizing antibodies in vaccinated individuals [161].
The enterovirus vaccine landscape in clinical trials. Majority of enterovirus vaccine clinical trials are conducted for polio- and EV-A71 vaccines. In addition to those, single phase I clinical trial has been completed for CVB1 vaccine [79] (not shown in the figure). Based on www.clinicaltrials.cov search
The enterovirus vaccine landscape prior to clinical trials. The figure depicts enterovirus vaccines in A preclinical trials and B enterovirus vaccine technologies in preclinical trials. Based on www.adisinsight.com search
To enhance the safety of the oral poliovirus vaccine, a research team genetically modified type 2 poliovirus, increasing the stability of the new type 2 OPV (nOPV2) vaccine [162]. The team hypothesized that the modified weakened virus is less likely to mutate, evolve, or cause infection. After the assessment of phase II and phase III clinical trial data, as well as additional data on safety, efficacy, and manufacturing quality, the nOPV2 vaccine was granted emergency use listing (EUL) approval by the WHO in 2020. This marked a significant milestone, as it was the first ever vaccine to receive such approval [163]. Since the approval of the EUL, more than 600 million doses of the nOPV2 vaccine have been administered in 28 countries, primarily in response to polio outbreaks. Therefore, nOPV2 is currently in the wider roll-out phase, and with the increased use of nOPV2 in the last 2 years, both the incidence of cVDPV2 and the intensity of cVDPV2 transmission have decreased [164]. The nOPV2 was designed to prevent reversion to the virulent form but has failed in that respect. Seven cVDPV2 cases of nOPV2 originating from 61 paralytic cases and 39 environmental surveillance (sewage) samples were detected in six African countries during August 2021–July 2023, although the incidence of reversion is still approximately 10 times lower than that of the original type 2 monovalent oral polio vaccine (mOPV2) [165]. This demonstrates the high mutation and recombination capacity of enteroviruses and thus their epidemic potential.
The same research group modified the remaining two strains of poliovirus types 1 and 3 in OPV. Genetic modifications similar to those for type 2 poliovirus were made to these strains, resulting in the creation of new vaccine candidates. These vaccine candidates have shown great promise in mouse studies, particularly in terms of their immunogenicity when administered as monovalent and multivalent formulations [166]. Genetically modified novel live weakened type 1 and type 3 oral polio vaccines (nOPV1 and nOPV3) are currently undergoing phase I and II clinical studies to ensure that they are both effective and do not revert to dangerous forms in humans. Like for nOPV2, the first trials are being conducted to evaluate the safety, immunogenicity, shedding and genetic stability of the vaccine candidates in IPV-primed adults before the vaccines can be tested in a larger adult and adolescent (> 15 y of age) population or in young children or infants (in this order). If approved, the nOPV1 and nOPV3 vaccines can be combined into bivalent or trivalent combinations with nOPV2 and contribute to polio eradication.
In the case of EV-A71 vaccines, five are combination vaccines, where inactivated EV-A71 virus is formulated together with one or several of the following vaccines: inactivated CVA16 virus, split influenza virus vaccine, hepatitis B vaccine, meningococcal polysaccharide vaccine, mumps and rubella, Japanese encephalitis vaccine, or weakened measles virus. For completed clinical studies on EV-A71 combination vaccines, the seroconversion rates of antibodies against EV-A71 have been more than 94%, and combination vaccines have induced non-inferior responses to monovalent EV-A71 vaccines [167].
Most of the clinical trials on EV-A71 vaccines have focused on the three inactivated vaccines that are currently marketed in China. These vaccines differ from each other in terms of the vaccine strain, manufacturing cells (human diploid cells vs Vero cells), and antigen dose. However, clinical studies have demonstrated that the immunogenicity of these vaccines is nearly the same in children between the ages of 6 and 35 months [152]. This suggests that EV-A71 vaccines are equally effective in stimulating an immune response in young children, which is a critical target population for EV-A71 vaccination.
As EVs have caused several outbreaks over time globally and the number and diversity of EVs is high, each human has contracted several symptomatic or asymptomatic EV-infections during their lifetime. Therefore, if a new EV vaccine is developed and analyzed in clinical trials, assessing its efficacy becomes possible by examining correlates of protection. These correlates include vaccine-specific antibody levels in samples from the vaccinated individuals. However, because of the existing immunity against EVs circulating globally and locally, the efficacy of the vaccine needs to be determined e.g. by comparing the antibody-levels from pre- and post-immunization samples to see if the new vaccine has been able to induce immunity. If the new vaccine is approved for clinical use, we recommend prioritizing both young and elderly individuals for vaccination because infections are most common and most severe at the extremes of age, where the immune system is the most vulnerable.
Enterovirus vaccines in research and development phase
To date, virtually all existing vaccine technologies and approaches for the development of enterovirus vaccines have been explored (Table 2). The vaccine technologies differ in terms of production method and the immune response inducing antigen. According to a database query made from adisinsight.spinger.com (accessed 23.8.2023), the majority of enterovirus vaccines in the research and development phase are EV-A71 and poliovirus candidate vaccines. However, there have also been developments in CVA, CVB and EV-D68 virus vaccines of varying valencies (Fig. 4A). In total, there were 22 multivalent vaccines, 11 monovalent vaccines, and five vaccines with unknown valency in the research and development phase. Combination vaccines have been considered a solution to the issue of increased numbers of injections during single clinic visits. Previous evaluations have been conducted on the safety and efficacy of vaccines containing diphtheria, tetanus, pertussis, hepatitis B, Haemophilus influenzae and type B with polio. While the formulation of a combination vaccine encompassing seven vaccines has been discontinued, there are several similar multivalent vaccine formulations in clinical use. In terms of enterovirus vaccine technologies (Fig. 4A), 12 vaccines were based on inactivated viruses, and seven utilized proprietary technologies. However, a total of eight different technologies have been employed for enterovirus vaccine development (Fig. 4B). Because the number of EVs infecting human is high, but the cross-reactivity of the immune response against EVs is low, multivalent vaccine formulations containing vaccine antigens against the most prevalent and severe disease causing EVs should be developed. We and others have shown previously that the multivalent vaccine approach is feasible as immunity against the six CVBs [158] or 50 different rhinoviruses [168] could be successfully achieved with an approach combining 6 or 50 inactivated viruses in one vaccine formulation. However, the vaccines were based on traditional inactivated viruses, which is not a feasible approach against several EVs. Therefore, new vaccine technologies combining an optimal formulation of vaccine antigens should be developed in order to produce a multivalent EV vaccine. Efforts to develop vaccines against CVB [169], enterovirus A species and EV-A71 [170, 171] have been extensively reviewed elsewhere [169,170,171,172,173]. As noted, essentially all currently existing vaccine technologies have been employed to develop enterovirus vaccines, as demonstrated with the examples of vaccine development studies below.
Inactivated virus vaccines
Inactivated virus vaccines are a ‘traditional’ vaccine technology and have been in use for over a century. The technology is based on production of inactive viruses where the infectivity of the virus is destroyed either by a chemical (such as formalin [155] or beta-propiolactone [184]) or irradiation. We have developed inactivated virus vaccines for the six CVBs and demonstrated their safety and efficacy as monovalent [155,156,157,158,159,160] and multivalent [158] vaccines in animal models. Based on these studies, a double-blind randomised placebo-controlled Phase I trial for multivalent CVB vaccine targeting serotypes associated with type 1 diabetes has been completed [161]. Inactivated virus vaccines have been developed also for CVB3 (reviewed in [169]), CVA5 [185], CVA6, CVA10 [184, 186], CVA16 [187], CVA2 [188] and EV-D68 [189]. In 2001 researchers studied different approaches for developing a EV-A71 vaccine based on passive immunization [178]. In lethal challenge studies the antibodies transferred from mothers immunized with inactivated virus provided most protection for the suckling mice when the level of protection was compared to subunit based and DNA vector based vaccines [178]. Although it seems unlikely that inactivated virus vaccines could solve EV problem, the advantage of the technology is existing manufacturing infrastructure in the countries that have active vaccine industry.
Weakened virus vaccines
Weakened virus (also known as attenuated) vaccines are the other ‘traditional’ vaccine technology and based on reducing the infectivity of the pathogen by either mutations or deletions. Often weakened virus vaccines are developed by serial passaging in cell cultures over multiple generations until the virulence of the virus has been weakened, while still being immunogenic. Weakened virus vaccine against EV-A71 [190, 191] and CVB3 (reviewed in [169]) have been produced, while attenuating mutations that render CVA6 less virulent have been recently described [192]. Although weakened virus vaccines have proven to be very efficient against poliovirus as well as many other pathogens, more safe and applicable vaccine technologies should be developed.
Virus-like particle vaccines
Virus-like particles (VLPs) are a vaccine technology based on producing vaccines by using one or several structural proteins (capsid proteins) of the virus. The proteins are produced recombinantly, typically in insect-, yeast-, or bacterial cells, but also mammalian cells are used. Experimental VLP based vaccines have been developed for EV-A71 [193], CVA6 [194], CVA10 [195], CVA16 [176] and EV-D68 [196, 197] as well as for CVB1 and CVB3 which we have developed [172, 173]. Additionally, immunogenic but unstable poliovirus-like particles have been produced previously [198, 199]. To combat the instability and improve the immunogenicity of these VLPs, chemical crosslinking and genetically engineered disulfides have been utilized, and these stabilized particles have been shown to generate high levels of neutralizing antibodies in animal models [200,201,202,203,204,205]. The most distinct advantages of VLP-based vaccines are their safety and applicability against different pathogens, as well as the cost-effective manufacturing processes that can be optimized for several different expression hosts.
Recombinant protein- subunit and peptide vaccines
Like in the case of VLP-based vaccines, in the recombinant, subunit or peptide-based vaccine approaches virus components are produced recombinantly, for example in insect-, mammalian-, or bacterial cells. These types of vaccines are considered to be much safer for at-risk populations compared to traditional whole virus technologies. In the case of EV-A71 vaccines based on VP1 recombinant protein, DNA-vector and inactivated virus, the VP1 recombinant protein vaccine provided protection against lethal challenge only at the lowest challenge dose. However, the subunit vaccine demonstrated better efficacy than the DNA vector [178]. Although the inactivated EV-A71 vaccine elicited a stronger immune response compared to the other vaccines tested, protein-based vaccine technologies show promise for future vaccine development [178]. Recombinant subunit vaccines developed against CVB3 are reviewed in [169].
Vector vaccines
Virus vector vaccines utilise a second virus – often adenovirus – as a delivery vector or platform for the target antigen. For virus vector vaccines the virus genome is mutated, and it cannot replicate. Virus vector technologies have been explored for the development of EV-A71 vaccine utilizing adenovirus as a vector [179]. The researchers inserted the EV-A71 P1 and 3CD genes into the E1/E3-deleted adenoviral genome and in immunogenicity studies the vaccine candidates were shown to provide immunity against EV-A71 in a mouse challenge model [179]. Vector vaccines developed against CVB3 are reviewed in [169].
DNA vaccines
In DNA-vector based vaccine technology the DNA vector is produced by inserting the viral target gene (e.g. VP1) into eucaryotic expression vector (e.g. pVAX1). Upon vaccination the DNA vector enters host cell nuclei for mRNA transcription, and the antigen is produced in target cells. In the EV-A71 vaccine study, a VP1 DNA vaccine was tested along with inactivated virus and subunit vaccine, and the DNA vector based vaccine provided some protection in lethal challenge studies, but overall performed weakest compared to inactivated virus and VP1 recombinant protein vaccines [178]. Another study also looked at constructing a EV-A71 VP1 DNA vector vaccine using pVAX1 expression vector into which the VP1 gene was cloned [181]. The VP1 DNA vector vaccine elicited an immune response in a mouse model, but further development improving the expression and immunogenicity of the vaccine would be required [181]. DNA vaccines developed against CVB3 are reviewed in [169].
mRNA vaccines
For mRNA-based approaches, mRNA of the target antigen gene(s) is synthesized and packed into lipid nanoparticles. Upon vaccination the mRNA utilises host cell machinery in antigen production, inducing an immune response. mRNA vaccines developed against CVB3 are reviewed in [169].
Exosome vaccines
Exosomes are extracellular vesicles of endosomal origin, and they are produced by almost all cell types [206]. In exosome-based vaccine development strategies exosomes are produced by target virus producing cell lines, and the exosomes are released into the supernatant. Recently, an exosome based antigen delivery strategy was tried for CVB3 VP1 vaccine, and was shown to enhance resistance to CVB3 induced myocarditis in a mouse model – more interestingly the study found the exosome based vaccine to be more potent compared to recombinant protein VP1 subunit vaccine the group had created previously [183, 207].
What has been learned from immune responses elicited during enterovirus infection and preclinical and clinical enterovirus vaccine trials
Enterovirus infections induce the production of virus-neutralizing antibodies, which provide long-term protection against reinfection by the same serotype. Sera have therefore long been used to define virus serotypes, which is also the reason why infection with one serotype does not confer protection from another serotype. Therefore, it is currently believed that effective enterovirus vaccines should elicit serotype-specific antibodies. However, partial neutralization of related enterovirus serotypes has been observed when studying serotype-specific sera [208], and several monoclonal antibodies (mAbs) that bind and neutralize multiple poliovirus serotypes have been isolated from infected humans [209]. These antibodies exhibit varying affinities for the three poliovirus serotypes, and two of them, which have cross-serotype capabilities, can block infection by poliovirus serotypes 1 and 2 in cell culture, while the third poliovirus serotype remains unaffected by these antibodies. These data indicate that poliovirus antibody cross-neutralization involves binding to highly conserved structures within the canyon that bind to the cellular receptor [209] and demonstrate that perhaps not all virus-neutralizing antibodies are solely serotype specific. Although polio vaccine coverage has increased over 90% in the US, biennial outbreaks causally associated to AFM have been caused by EV-D68 for unknown reason [210]. Likewise, epidemiological and surveillance studies demonstrated that CVA6 has replaced EV-A71 as the major cause of hand, foot and mouth disease in Nanchang China following the deployment of an EV-A71 vaccine, suggesting that vaccination against one enterovirus may lead to replacement by another [211].
Research has demonstrated that neutralizing antibody levels above a protective threshold are essential, and effective memory CD4+ T-cell responses confer long-term protection against enteroviruses [212]. Although the humoral immune response is important for controlling enterovirus infections, it has also been suggested that inadequate cellular immunity is associated with more severe clinical outcomes of these infections. Decreased cellular immunity and lower levels of interferon-gamma (IFN-γ) have been found to correlate with the severity of EV-A71 infection [213]. Whether cellular immunity (e.g., CD8+ T-cell responses) and/or IFN-γ contributes to robust immunity against enterovirus infections after vaccination is not fully understood. However, studies on IPV and OPV have provided some interesting insights and accumulating evidence suggest the importance of CD4+ and CD8+ T-cells for optimal protective immunity against EVs induced by vaccination [174].
The immune responses elicited by IPV and OPV are different. IPV provides systemic humoral immunity and only modest intestinal immunity to all three types of poliovirus [214]. Vaccination does not prevent infection but stops poliovirus from reaching the central nervous system, thus preventing paralytic poliomyelitis. The presence of virus-neutralizing antibodies in plasma/serum after IPV vaccination correlates with protection against paralytic disease. In contrast to IPV, OPV induces both systemic and mucosal humoral immunity, providing additional local protection at the gastrointestinal tract, which is effective in interrupting infection and transmission of the virus. Furthermore, OPV vaccination seems to induce long-term CD4+ and CD8+ cytotoxic T-cell responses [174]. Cross-reactive T cells have been shown to contribute to host protection against viral infections, including those induced by influenza and SARS-CoV-2 [215], and it is possible that a similar phenomenon occurs after OPV vaccination, which could provide partial protection against other enteroviruses. Consistent with this, several studies have demonstrated that vaccine induced CD8+ T-cell priming may improve the efficacy of immunization against influenza [216] and SARS-CoV2 [217, 218]. Research has indicated that there are cross-reactive B- and T-cell responses to conserved enteroviral epitopes in healthy individuals vaccinated against polio [219, 220]. Moreover, an immune response to a conserved enteroviral B-cell epitope on the major capsid VP1 protein has been associated with a decreased risk of cardiovascular disease [220]. Additionally, human T cells have been found to recognize group-specific enteroviral antigen(s) [221,222,223]. Identifying motifs that elicit cross-reactive B and T cells and incorporating these residues into future vaccines could provide a measure of protection against EVs with epidemic potential or accelerated clearance. How the cellular immune response contributes to robust intestinal immunity and adequate virus-specific IgA levels in the mucosa and in the serum remains to be fully understood. A recent study investigating how CVB affects human β cells and anti-CVB T-cell responses demonstrated that CVB-infection induces limited antiviral CD8+ T-cell responses [223]. Therefore, we anticipate that inducing CD8+ T-cell responses through vaccination might be beneficial in promoting antigen cross-presentation, but also for being essential in promoting the early protective effects during the first two weeks after the initial vaccination, when neutralizing antibody levels are still too low for protecting against the virus.
The proximity of enterovirus T-cell epitopes to B-cell antigenic sites in human poliovirus suggests that there may also be a requirement for spatial proximity of T- and B-cell epitopes to attain efficient protection against enteroviruses [222]. Nevertheless, there are notable gaps in the knowledge surrounding what constitutes an optimal immune response to enteroviruses and how this response can be induced by vaccines. According to studies on polio- and EV-A71-vaccinated or infected individuals, it seems that the optimal immune response requires balanced humoral, cellular and mucosal immune responses in addition to correct spatial T- and B-cell epitope proximity, which might also be needed.
Challenges and opportunities in enterovirus vaccine development
Traditional vaccine production methods, particularly for enteroviruses, pose several challenges. One issue is safety concerns, as vaccines based on whole-virus particles may not be adequately inactivated, and live-weakened virus vaccines may revert to a virulent form. Another challenge for inactivated whole-virus particle vaccines is the time-consuming and costly production process, which is unlikely to meet the demand during a global pandemic. The successful growth of large quantities of virus particles is required, but this is difficult or even impossible for certain EVs due to their inefficient growth in standard cell culture systems. Our own unpublished research suggests that the production of vaccines against some enteroviruses, such as CVA6, CVA10, CVA16, EV-A71, and EV-D68, face challenges in this regard. Additionally, traditional vaccine production methods can lead to the loss of immunogenicity, and the risk of the introduction of mutations is high when viruses are cultured in cells (our unpublished results). In the case of an inactivated virus vaccine, there is also uncertainty about its ability to induce sufficient intestinal immunity to protect populations where faecal-oral transmission is predominant. Another major limitation of traditional enterovirus vaccines is their specificity for a particular serotype, although some cross-protection has been found. Currently, the inactivated EV-A71 vaccines produced in China demonstrate high effectiveness, with vaccine efficacy exceeding 90% against the C4 sub-genogroup included in the vaccine. In addition to T lymphocytes, neutralizing antibody responses have been shown to be equally important in the protection of mice from EV-A71 infection [212]. Similar to poliovirus vaccines, EV-A71 clinical trials have shown that although high levels of neutralizing antibodies could be sufficient for protection, poor cellular immunity might increase the risk of vaccine failure, severe neurological complications and death [212]. Therefore, the results from EV-A71 mouse infection models have indicated that humoral immunity protects mice from lethal EV-A71 challenge, but for optimal immunity against enteroviruses, better cellular immunity is desirable [212]. Studies have demonstrated that the cross-neutralizing activity of EV-A71 vaccines (based on subgenotype C4) can provide broad cross-protection against different EV-A71 subgenotypes, but ongoing research and development are essential to address the challenges posed by the genetic evolution and immunogenicity of EV-A71 vaccines [224].
Evidence for the ability of cross-serotype anti-enterovirus antibodies to block infection have been gathered since 1920s when monkeys were infected with three different poliovirus isolates and were challenged with infection by heterologous isolates [225,226,227] and some cross-protection was found. Moreover, monoclonal antibody A12 has been shown to bind all three serotypes of poliovirus [228]. However, the binding to different serotypes is not similar and this antibody blocks the infection of cells in culture for serotypes 1 and 2 but not for serotype 3. Moreover, a recent study demonstrated that anti-enterovirus antibodies can inhibit infections by heterologous enteroviruses, when sera from mice immunized with the EV-D68 protected cells from infection poliovirus type 1/Mahoney. The same study demonstrated that sera from mice immunized with poliovirus type 1/Mahoney protected cells against infection by EV-D68, EV-A71 and HRV1A and weakly against EV-D94 [219].
Given the large number of enterovirus serotypes capable of infecting humans and the high likelihood of new strains emerging, it is practically impossible to develop vaccines against all enterovirus serotypes using traditional methods. Therefore, to better prepare for future epidemics, it is crucial to establish vaccine platforms that can promptly address the demand for adaptable and rapid responses during outbreaks. These vaccines should be easy and quick to produce, have a high degree of safety, and have a well-characterized composition that can be easily modified to meet specific requirements in the case of re-emergence of enteroviruses or the appearance of new pathogenic strains. Furthermore, these vaccines must be cost-effective to manufacture and supply. For this purpose, the development of vaccine technologies that incorporate scalable production hosts and scalable manufacturing and purification methods is necessary. These advancements will enable vaccines to be quickly mass-produced and readily available. Additionally, the design of these vaccines should prioritize safety, effectiveness, and the ability to be transported without the need for ultralow temperatures. Like marketed vaccines, multivalent vaccines in the development phase are administered parenterally, which means that these vaccines are not very effective at inducing mucosal immune responses. As a result, while these vaccines can help alleviate the severity of enterovirus disease, they typically cannot completely stop the spread of the virus. Considering this limitation, it is important to shift the focus from the exploitation of traditional vaccine technologies and immunization routes to new technologies and ways to administer vaccines (e.g., mucosal administration). OPV has shown that vaccine administration to mucosal surfaces can create immunity at the site of virus entry, thereby effectively stopping the infection. If such a vaccine was to be translated into clinical use, achieving a vaccination rate of approximately 60-70% of the human population could potentially create herd immunity.
A change in the enterovirus vaccine development landscape also requires a change in the interface of the academic and industrial vaccine development sectors since bringing promising candidate vaccines from preclinical studies into clinical trials is very expensive and requires expertise in vaccine manufacturing process optimization, quality control and regulatory aspects. Researchers at universities should consider these aspects while the development work is ongoing. The patenting of new technologies or manufacturing processes will also facilitate the transition from preclinical to clinical studies, as buying or licencing intellectual property rights is key for the pharmaceutical industry to make investments.
Conclusions
Vaccination has long been recognized as the most effective method for preventing the spread of viral diseases on a global scale. It has played a significant role in reducing morbidity and mortality associated with various infectious diseases. However, the lack or ineffectiveness of vaccines has been identified as a major contributing factor to the emergence and propagation of epidemics and pandemics. Poliovirus still maintains its status as a Public Health Emergency of International Concern (PHEIC) – declared by the WHO in 2014 – despite the significant progress made in the eradication program over the past 35 years. While outbreaks of circulating variant poliovirus have decreased in the Eastern Mediterranean Region, both Afghanistan and Pakistan remain endemic countries for wild poliovirus.
There are more than 280 known enterovirus serotypes, several of which cause acute infections in humans, occasionally with short- and long-term life-threatening complications. Enteroviruses can mutate, recombine and cause devastating epidemics. However, the only available vaccines are against the three poliovirus serotypes and EV-A71. Furthermore, current enterovirus vaccines are mainly serotype specific, associated with high production costs and, in the case of poliovirus, are at risk of the emergence of circulating vaccine-derived viruses. The absence of adaptable vaccine technologies is a significant barrier to combatting re-emerging and new enterovirus strains. In addition, many low- and middle-income countries face substantial challenges in establishing and maintaining vaccine manufacturing capacity. This limitation further exacerbates the global problem of vaccine shortages and leaves vulnerable populations without access to life-saving immunizations.
The development and production of vaccines require a high level of expertise, resources, and infrastructure. With respect to enteroviruses, it is critical to develop new vaccine platforms that can rapidly address the demand for new vaccines during outbreaks. These vaccines must be easy and quick to produce, have a high degree of safety, and have a well-characterized composition that can be easily modified to meet specific requirements in the case of re-emergence of enteroviruses or the appearance of new pathogenic strains. In addition, these vaccines must be cost-effective to manufacture and deliver. With such measures, we will be in a better position to react to future enterovirus epidemics.
Availability of data and materials
References for this review were identified through a search of PubMed for articles published by use of the terms “enterovirus” and “vaccine” or any specific enterovirus serotype, e.g. “Enterovirus 71”. Other relevant references were identified from key online sources (e.g., WHO, CDC, clinicaltrials.gov, adisinsight.com). Only articles published in English were included.
References
Simmonds P, Gorbalenya AE, Harvala H, Hovi T, Knowles NJ, Lindberg AM, et al. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch Virol. 2020;165:793–7. https://doi.org/10.1007/s00705-019-04520-6.
International Committee on Taxonomy of Viruses: ICTV. Available at https://ictv.global/report/chapter/picornaviridae/picornaviridae/enterovirus.
Lulla V, Dinan AM, Hosmillo M, Chaudhry Y, Sherry L, Irigoyen N, et al. An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells. Nat Microbiol. 2019;4:280–92. https://doi.org/10.1038/s41564-018-0297-1.
Guo H, Li Y, Liu G, Jiang Y, Shen S, Bi R, et al. A second open reading frame in human enterovirus determines viral replication in intestinal epithelial cells. Nat Commun. 2019;10:4066. https://doi.org/10.1038/s41467-019-12040-9.
Laitinen OH, Svedin E, Kapell S, Nurminen A, Hytönen VP, Flodström-Tullberg M. Enteroviral proteases: structure, host interactions and pathogenicity. Rev Med Virol. 2016;26:251–67. https://doi.org/10.1002/rmv.1883.
Carré A, Vecchio F, Flodström-Tullberg M, You S, Mallone R. Coxsackievirus and type 1 diabetes: diabetogenic mechanisms and implications for prevention. Endocr Rev. 2023;44:737–51. https://doi.org/10.1210/endrev/bnad007.
Paloheimo O, Ihalainen TO, Tauriainen S, Välilehto O, Kirjavainen S, Niskanen EA, et al. Coxsackievirus B3-induced cellular protrusions: structural characteristics and functional competence. J Virol. 2011;85:6714–24. https://doi.org/10.1128/JVI.00247-10.
Bubba L, Broberg EK, Jasir A, Simmonds P, Harvala H, Redlberger-Fritz M, et al. Circulation of non-polio enteroviruses in 24 EU and EEA countries between 2015 and 2017: a retrospective surveillance study. Lancet Infect Dis. 2020;20:350–61. https://doi.org/10.1016/S1473-3099(19)30566-3.
Gundamraj V, Hasbun R. Viral meningitis and encephalitis: an update. Curr Opin Infect Dis. 2023;36:177–85. https://doi.org/10.1097/QCO.0000000000000922.
Marjomäki V, Flodström-Tullberg M. Coxsackie B virus. Trends Microbiol. 2022;30:606–7. https://doi.org/10.1016/j.tim.2022.01.016.
Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–93. https://doi.org/10.1038/s41569-020-00435-x.
Junttila N, Lévêque N, Kabue JP, Cartet G, Mushiya F, Muyembe-Tamfum J-J, et al. New enteroviruses, EV-93 and EV-94, associated with acute flaccid paralysis in the Democratic Republic of the Congo. J Med Virol. 2007;79:393–400. https://doi.org/10.1002/jmv.20825.
Zhang M, Zhang Y, Hong M, Xiao J, Han Z, Song Y, et al. Molecular typing and characterization of a novel genotype of EV-B93 isolated from Tibet China. PLoS One. 2020;15:e0237652. https://doi.org/10.1371/journal.pone.0237652.
Mao Q, Wang Y, Bian L, Xu M, Liang Z. EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg Microbes Infect. 2016;5:e75. https://doi.org/10.1038/emi.2016.73.
Liu X, Tao Z, Wang H, Lin X, Song L, Li Y, et al. Complete genome analysis of human enterovirus B73 isolated from an acute flaccid paralysis patient in Shandong China. Virus Genes. 2014;49:38–44. https://doi.org/10.1007/s11262-014-1077-5.
Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis. 2016;16:e64-75. https://doi.org/10.1016/S1473-3099(15)00543-5.
González-Sanz R, Casas-Alba D, Launes C, Muñoz-Almagro C, Ruiz-García MM, Alonso M, et al. Molecular epidemiology of an enterovirus A71 outbreak associated with severe neurological disease, Spain, 2016. Euro Surveill. 2019;24:1800089. https://doi.org/10.2807/1560-7917.ES.2019.24.7.1800089.
Messacar K, Spence-Davizon E, Osborne C, Press C, Schreiner TL, Martin J, et al. Clinical characteristics of enterovirus A71 neurological disease during an outbreak in children in Colorado, USA, in 2018: an observational cohort study. Lancet Infect Dis. 2020;20:230–9. https://doi.org/10.1016/S1473-3099(19)30632-2.
Kapadia RK, Gill CM, Baca C, McMenamin C, Kannappan A, Niehaus WN, et al. Enterovirus A71 causing meningoencephalitis and acute flaccid myelitis in a patient receiving rituximab. J Neuroimmunol. 2021;358:577639. https://doi.org/10.1016/j.jneuroim.2021.577639.
Esposito S, Chidini G, Cinnante C, Napolitano L, Giannini A, Terranova L, et al. Acute flaccid myelitis associated with enterovirus-D68 infection in an otherwise healthy child. Virol J. 2017;14:4. https://doi.org/10.1186/s12985-016-0678-0.
Fischer TK, Simmonds P, Harvala H. The importance of enterovirus surveillance in a post-polio world. Lancet Infect Dis. 2022;22:e35-40. https://doi.org/10.1016/S1473-3099(20)30852-5.
Joo CH, Ahn J, Seo I, Kim YK, Kim D, Hong H, et al. Characterization of nonpolio enteroviruses recovered from patients with aseptic meningitis in Korea. Intervirology. 2005;48:97–103. https://doi.org/10.1159/000081735.
Wong AH, Lau CS, Cheng PKC, Ng AYY, Lim WWL. Coxsackievirus B3-associated aseptic meningitis: an emerging infection in Hong Kong. J Med Virol. 2011;83:483–9. https://doi.org/10.1002/jmv.21998.
Muehlenbachs A, Bhatnagar J, Zaki SR. Tissue tropism, pathology and pathogenesis of enterovirus infection. J Pathol. 2015;235:217–28. https://doi.org/10.1002/path.4438.
Lee KY, Lee MS, Kim DB. Neurologic Manifestations of Enterovirus 71 Infection in Korea. J Korean Med Sci. 2016;31:561. https://doi.org/10.3346/jkms.2016.31.4.561.
Berger JR, Chumley W, Pittman T, Given C, Nuovo G. Persistent Coxsackie B encephalitis: report of a case and review of the literature. J Neurovirol. 2006;12:511–6. https://doi.org/10.1080/13550280601090546.
Verma NA, Zheng XT, Harris MU, Cadichon SB, Melin-Aldana H, Khetsuriani N, et al. Outbreak of life-threatening Coxsackievirus B1 myocarditis in neonates. Clin Infect Dis. 2009;49:759–63. https://doi.org/10.1086/605089.
Kim K-S, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Rev Med Virol. 2001;11:355–68. https://doi.org/10.1002/rmv.326.
de Graaf H, Pelosi E, Cooper A, Pappachan J, Sykes K, MacIntosh I, et al. Severe enterovirus infections in hospitalized children in the South of England. Pediatr Infect Dis J. 2016;35:723–7. https://doi.org/10.1097/INF.0000000000001093.
Bissel SJ, Winkler CC, DelTondo J, Wang G, Williams K, Wiley CA. Coxsackievirus B4 myocarditis and meningoencephalitis in newborn twins. Neuropathol. 2014;34:429–37. https://doi.org/10.1111/neup.12121.
Huber S, Ramsingh AI. Coxsackievirus-induced pancreatitis. Viral Immunol. 2004;17:358–69. https://doi.org/10.1089/vim.2004.17.358.
Zhang Y-F, Deng H-L, Fu J, Zhang Y, Wei J-Q. Pancreatitis in hand-foot-and-mouth disease caused by enterovirus 71. World J Gastroenterol. 2016;22:2149–52. https://doi.org/10.3748/wjg.v22.i6.2149.
Zhang T, Du J, Xue Y, Su H, Yang F, Jin Q. Epidemics and frequent recombination within species in outbreaks of human enterovirus B-associated hand, foot and mouth disease in Shandong China in 2010 and 2011. PloS One. 2013;8:e67157. https://doi.org/10.1371/journal.pone.0067157.
Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, et al. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PloS One. 2014;9:e98888. https://doi.org/10.1371/journal.pone.0098888.
Hu Y-Q, Xie G-C, Li D-D, Pang L-L, Xie J, Wang P, et al. Prevalence of Coxsackievirus A6 and enterovirus 71 in hand, foot and mouth disease in Nanjing, China in 2013. Pediatr Infect Dis J. 2015;34:951. https://doi.org/10.1097/INF.0000000000000794.
Aswathyraj S, Arunkumar G, Alidjinou EK, Hober D. Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med Microbiol Immunol. 2016;205:397–407. https://doi.org/10.1007/s00430-016-0465-y.
Yao X, Bian L-L, Lu W-W, Li J-X, Mao Q-Y, Wang Y-P, et al. Epidemiological and etiological characteristics of herpangina and hand foot mouth diseases in Jiangsu, China, 2013–2014. Hum Vaccin Immunother. 2016;13:823–30. https://doi.org/10.1080/21645515.2016.1236879.
de Graaf H, Pelosi E, Cooper A, Pappachan J, Sykes K, MacIntosh I, et al. Severe enterovirus infections in hospitalized children in the South of England: clinical phenotypes and causative genotypes. Pediatr Infect Dis J. 2016;35:723–7. https://doi.org/10.1097/INF.0000000000001093.
Orbach R, Mandel D, Lubetzky R, Ovental A, Haham A, Halutz O, et al. Pulmonary hemorrhage due to Coxsackievirus B infection—A call to raise suspicion of this important complication as an end-stage of enterovirus sepsis in preterm twin neonates. J Clin Virol. 2016;82:41–5. https://doi.org/10.1016/j.jcv.2016.07.003.
Laitinen OH, Honkanen H, Pakkanen O, Oikarinen S, Hankaniemi MM, Huhtala H, et al. Coxsackievirus B1 is associated with induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014;63:446–55. https://doi.org/10.2337/db13-0619.
Stone VM, Hankaniemi MM, Svedin E, Sioofy-Khojine A, Oikarinen S, Hyoty H, et al. A Coxsackievirus B vaccine protects against virus-induced diabetes in an experimental mouse model of type 1 diabetes. Diabetologia. 2018;61:476–81. https://doi.org/10.1007/s00125-017-4492-z.
Jaïdane H, Sané F, Gharbi J, Aouni M, Romond MB, Hober D. Coxsackievirus B4 and type 1 diabetes pathogenesis: contribution of animal models. Diabetes Metab Res Revs. 2009;25:591–603. https://doi.org/10.1002/dmrr.995.
Esposito S, Zampiero A, Ruggiero L, Madini B, Niesters H, Principi N. Enterovirus D68-associated community-acquired pneumonia in children living in Milan. Italy J Clin Virol. 2015;68:94–6. https://doi.org/10.1016/j.jcv.2015.05.017.
Zhou J, Li Y, Yin Q, Vinturache A, Ding G. Coxsackievirus A6 pneumonia in a child. Lancet Infect Dis. 2023;23:e567. https://doi.org/10.1016/S1473-3099(23)00576-5.
Li H, Lao Q. The pulmonary complications associated with EV71-infected hand–foot–mouth disease. Radiol Infect Dis. 2017;4:137–42. https://doi.org/10.1016/j.jrid.2017.01.001.
Chia J, Chia A, Wang D, El-Habbal R, Sinkowitz D. Chronic enterovirus D68 bronchiolitis causing severe respiratory insufficiency. Open J Respir Dis. 2016;06:47–51. https://doi.org/10.4236/ojrd.2016.63007.
Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis. 2016;16:64. https://doi.org/10.1016/S1473-3099(15)00543-5.
Liu Y, Xi Y, Ji L, Shen Q, Zhang W, Xue M. Antiviral Drugs (Synthetic Small Molecule Inhibitors and Nature Drugs) Against EV71 in Enteroviruses: Advances and Perspectives. CCMP. 2023;3:100099. https://doi.org/10.1016/j.ccmp.2023.100099.
Lalani S, Gew LT, Poh CL. Antiviral peptides against Enterovirus A71 causing hand, foot and mouth disease. Peptides. 2021;136:170443. https://doi.org/10.1016/j.peptides.2020.170443.
Wei Y, Liu H, Hu D, He Q, Yao C, Li H, et al. Recent advances in enterovirus A71 infection and antiviral agents. Lab Investig. 2024;104:100298. https://doi.org/10.1016/j.labinv.2023.100298.
Lin J-Y, Kung Y-A, Shih S-R. Antivirals and vaccines for Enterovirus A71. J Biomed Sci. 2019;26:65. https://doi.org/10.1186/s12929-019-0560-7.
Wang S, Pang Z, Fan H, Tong Y. Advances in anti-EV-A71 drug development research. J Adv Res. 2024;56:137–56. https://doi.org/10.1016/j.jare.2023.03.007.
Diarimalala RO, Hu M, Wei Y, Hu K. Recent advances of enterovirus 71 3Cpro targeting Inhibitors. Virol J. 2020;17:173. https://doi.org/10.1186/s12985-020-01430-x.
Lalani S, Poh CL. Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses. 2020;12:184. https://doi.org/10.3390/v12020184.
Tammaro C, Guida M, Appetecchia F, Biava M, Consalvi S, Poce G. Direct-acting antivirals and host-targeting approaches against enterovirus B infections: recent advances. Pharmaceuticals. 2023;16:203. https://doi.org/10.3390/ph16020203.
Hu Y, Musharrafieh R, Zheng M, Wang J. Enterovirus D68 antivirals: past, present, and future. ACS Infect Dis. 2020;6:1572–86. https://doi.org/10.1021/acsinfecdis.0c00120.
Sun J, Hu X-Y, Yu X-F. Current Understanding of Human Enterovirus D68. Viruses. 2019;11:490. https://doi.org/10.3390/v11060490.
Anasir MI, Zarif F, Poh CL. Antivirals blocking entry of enteroviruses and therapeutic potential. J Biomed Sci. 2021;28:10. https://doi.org/10.1186/s12929-021-00708-8.
Glanville N, Johnston SL. Challenges in developing a cross-serotype rhinovirus vaccine. Curr Opin Virol. 2015;11:83–8. https://doi.org/10.1016/j.coviro.2015.03.004.
McLean GR. Developing a vaccine for human rhinoviruses. J Vaccines Immun. 2014;2:16–20. https://doi.org/10.14312/2053-1273.2014-3.
GPEI: Outbreak Countries, https://polioeradication.org/where-we-work/polio-outbreak-countries/. Accessed 31 May 2024.
Link-Gelles R, Lutterloh E, Schnabel Ruppert P, Backenson PB, St. George K, Rosenberg ES, et al. Public Health Response to a Case of Paralytic Poliomyelitis in an Unvaccinated Person and Detection of Poliovirus in Wastewater — New York, June–August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1065–8. https://doi.org/10.15585/mmwr.mm7133e2.
Kalkowska DA, Badizadegan K, Routh JA, Burns CC, Rosenberg ES, Brenner IR, et al. Modeling undetected poliovirus circulation following the 2022 outbreak in the United States. Expert Rev Vacc. 2024;23:186–95. https://doi.org/10.1080/14760584.2023.2299401.
WHO: Poliomyelitis. https://www.who.int/news-room/fact-sheets/detail/poliomyelitis. Accessed 4 March 2024.
Messacar K, Schreiner TL, Maloney JA, Wallace A, Ludke J, Oberste MS, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015;385:1662–71. https://doi.org/10.1016/S0140-6736(14)62457-0.
Gilrane VL, Zhuge J, Huang W, Nolan SM, Dhand A, Yin C, et al. Biennial Upsurge and Molecular Epidemiology of Enterovirus D68 Infection in New York, USA, 2014 to 2018. J Clin Microbiol. 2020;58:e00284-20. https://doi.org/10.1128/JCM.00284-20.
Christy A, Messacar K. Acute flaccid myelitis associated with enterovirus D68: a review. J Child Neurol. 2019;34:511–6. https://doi.org/10.1177/0883073819838376.
Martin JA, Messacar K, Yang ML, Maloney JA, Lindwall J, Carry T, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology. 2017;89:129–37. https://doi.org/10.1212/WNL.0000000000004081.
Messacar K, Robinson CC, Pretty K, Yuan J, Dominguez SR. Surveillance for enterovirus D68 in Colorado children reveals continued circulation. J Clin Virol. 2017;92:39–41. https://doi.org/10.1016/j.jcv.2017.05.009.
Fall A, Han L, Abdullah O, Norton JM, Eldesouki RE, Forman M, et al. An increase in enterovirus D68 circulation and viral evolution during a period of increased influenza like illness, The Johns Hopkins Health System, USA, 2022. J Clin Virol. 2023;160:105379. https://doi.org/10.1016/j.jcv.2023.105379.
Peltola V, Österback R, Waris M, Ivaska L, Tähtinen PA, Laine M, et al. Enterovirus D68 Outbreak in Children, Finland, August–September 2022. Emerg Infect Dis. 2023;29:1258–61. https://doi.org/10.3201/eid2906.221795.
Uprety P, Curtis D, Elkan M, Fink J, Rajagopalan R, Zhao C, et al. Association of enterovirus D68 with acute flaccid myelitis, Philadelphia, Pennsylvania, USA, 2009–2018. Emerg Infect Dis. 2019;25:1676. https://doi.org/10.3201/eid2509.190468.
Murphy OC, Messacar K, Benson L, Bove R, Carpenter JL, Crawford T, et al. Acute flaccid myelitis: cause, diagnosis, and management. Lancet. 2021;397:334–46. https://doi.org/10.1016/S0140-6736(20)32723-9.
Cao RG, Mejias A, Leber AL, Wang H. Clinical and molecular characteristics of the 2022 Enterovirus-D68 outbreak among hospitalized children, Ohio, USA. J Clin Vir. 2023;169:105618. https://doi.org/10.1016/j.jcv.2023.105618.
Kamau E, Harvala H, Blomqvist S, Nguyen D, Horby P, Pebody R, et al. Increase in enterovirus D68 infections in young children, United Kingdom, 2006–2016. Emerg Infect Dis. 2019;25:1200–3. https://doi.org/10.3201/eid2506.181759.
Baggen J, Thibaut HJ, Staring J, Jae LT, Liu Y, Guo H, et al. Enterovirus D68 receptor requirements unveiled by haploid genetics. PNAS. 2016;113:1399–404. https://doi.org/10.1073/pnas.1524498113.
Hixon AM, Yu G, Leser JS, Yagi S, Clarke P, Chiu CY, et al. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 2017;13:e1006199. https://doi.org/10.1371/journal.ppat.1006199.
Pons-Salort M, Grassly NC. Serotype-specific immunity explains the incidence of diseases caused by human enteroviruses. Science. 2018;361:800–3. https://doi.org/10.1126/science.aat6777.
Pons-Salort M, Lambert B, Kamau E, Pebody R, Harvala H, Simmonds P, et al. Changes in transmission of Enterovirus D68 (EV-D68) in England inferred from seroprevalence data. Elife. 2023;12:e76609. https://doi.org/10.7554/eLife.76609.
Tao L. Notes from the Field: Cluster of Parechovirus Central Nervous System Infections in Young Infants — Tennessee, 2022. MMWR Morb Mortal Wkly Rep. 2022;71. https://doi.org/10.15585/mmwr.mm7130a5.
Singanayagam A, Moore C, Froude S, Celma C, Stowe J, Hani E, et al. Increased reports of severe myocarditis associated with enterovirus infection in neonates, United Kingdom, 27 June 2022 to 26 April 2023. Euro Surveill. 2023;28:2300313. https://doi.org/10.2807/1560-7917.ES.2023.28.39.2300313.
Enterovirus-Echovirus 11 Infection - the European Region. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON474. Accessed 15 Nov 2023.
Grapin M, Mirand A, Pinquier D, Basset A, Bendavid M, Bisseux M, et al. Severe and fatal neonatal infections linked to a new variant of echovirus 11, France, July 2022 to April 2023. Euro Surveill. 2023;28:2300253. https://doi.org/10.2807/1560-7917.ES.2023.28.22.2300253.
Qurbani K, Hussein S, Ahmed SK. Recent meningitis outbreak in Iraq: a looming threat to public health. Ther Adv Infect Dis. 2023;10:20499361231216584. https://doi.org/10.1177/20499361231216586.
Fourgeaud J, Mirand A, Demortier J, Kamus L, Collet L, Olivier S, et al. Enterovirus meningitis in Mayotte French Comoros Island, March-June 2019. J Clin Vir. 2022;150–151:105154. https://doi.org/10.1016/j.jcv.2022.105154.
Nkosi N, Preiser W, Van Zyl G, Claassen M, Cronje N, Maritz J, et al. Molecular characterisation and epidemiology of enterovirus-associated aseptic meningitis in the Western and Eastern Cape Provinces, South Africa 2018–2019. J Clin Vir. 2021;139:104845. https://doi.org/10.1016/j.jcv.2021.104845.
Hasbun R, Wootton SH, Rosenthal N, Balada-Llasat JM, Chung J, Duff S, et al. Epidemiology of Meningitis and Encephalitis in Infants and Children in the United States, 2011–2014. Pediatr Infect Dis. 2019;38:37. https://doi.org/10.1097/INF.0000000000002081.
Broberg EK, Simone B, Jansa J. Upsurge in echovirus 30 detections in five EU/EEA countries, April to September, 2018. Euro Surveill. 2018;23:1800537. https://doi.org/10.2807/1560-7917.ES.2018.23.44.1800537.
Kriger O, Abramovich A, Fratty IS, Leshem E, Amit S, Stein M, et al. An Outbreak of Coxsackievirus B Type 2 Acute Meningoencephalitis in Children, Israel, July–September 2022. Pediatr Infect Dis. 2023;42:e177. https://doi.org/10.1097/INF.0000000000003876.
Tian X, Han Z, He Y, Sun Q, Wang W, Xu W, et al. Temporal phylogeny and molecular characterization of echovirus 30 associated with aseptic meningitis outbreaks in China. Virol J. 2021;18:118. https://doi.org/10.1186/s12985-021-01590-4.
Gonzalez G, Carr MJ, Kobayashi M, Hanaoka N, Fujimoto T. Enterovirus-associated hand-foot and mouth disease and neurological complications in Japan and the rest of the world. Int J Mol Sci. 2019;20:5201. https://doi.org/10.3390/ijms20205201.
Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H, et al. The persistent circulation of enterovirus 71 in People’s Republic of China: causing emerging nationwide epidemics since 2008. PLoS One. 2011;6:e25662. https://doi.org/10.1371/journal.pone.0025662.
Baek K, Yeo S, Lee B, Park K, Song J, Yu J, et al. Epidemics of enterovirus infection in Chungnam Korea, 2008 and 2009. Virol J. 2011;8:297. https://doi.org/10.1186/1743-422X-8-297.
Liu D-P, Wang T-A, Huang W-T, Chang L-Y, Wang E-T, Cheng S-H, et al. Disease burden of enterovirus infection in Taiwan: Implications for vaccination policy. Vaccine. 2016;34:974–80. https://doi.org/10.1016/j.vaccine.2015.12.026.
Kang HJ, Yoon Y, Lee Y-P, Kim H-J, Lee D-Y, Lee J-W, et al. A Different Epidemiology of Enterovirus A and Enterovirus B Co-circulating in Korea, 2012–2019. JPIDS. 2021;10:398–407. https://doi.org/10.1093/jpids/piaa111.
Chavan NA, Lavania M, Shinde P, Sahay R, Joshi M, Yadav PD, et al. The 2022 outbreak and the pathobiology of the coxsackie virus [hand foot and mouth disease] in India. Infect Genet Evol. 2023;111:105432. https://doi.org/10.1016/j.meegid.2023.105432.
Puenpa J, Wanlapakorn N, Vongpunsawad S, Poovorawan Y. The history of enterovirus A71 outbreaks and molecular epidemiology in the Asia-Pacific Region. J Biomed Sci. 2019;26:75. https://doi.org/10.1186/s12929-019-0573-2.
Chan LG, Parashar UD, Lye MS, Ong FGL, Zaki SR, Alexander JP, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis. 2000;31:678–83. https://doi.org/10.1086/314032.
Donato C, Hoi LT, Hoa NT, Hoa TM, Van Duyet L, Dieu Ngan TT, et al. Genetic characterization of Enterovirus 71 strains circulating in Vietnam in 2012. Virology. 2016;495:1–9. https://doi.org/10.1016/j.virol.2016.04.026.
Welch J, Maclaran K, Jordan T, Simmonds P. Frequency, viral loads, and serotype identification of enterovirus infections in Scottish blood donors. Transfusion. 2003;43:1060–6. https://doi.org/10.1046/j.1537-2995.2003.00463.x.
Huemer HP, Ortner B, Huang C-W, Schmid D, Mutz I, Wewalka G, et al. Isolating Asian enterovirus 71 subgenogroup C4 in two Austrian clinical samples from 2004. Euro Surveill. 2008;13:18922. https://doi.org/10.2807/ese.13.28.18922-en.
van der Sanden S, Koopmans M, Uslu G, van der Avoort H. Epidemiology of Enterovirus 71 in The Netherlands, 1963 to 2008. J Clin Microbiol. 2009;47:2826–33. https://doi.org/10.1128/JCM.00507-09.
Badran SA, Midgley S, Andersen P, Böttiger B. Clinical and virological features of enterovirus 71 infections in Denmark, 2005 to 2008. Scand J Infect Dis. 2011;43:642–8. https://doi.org/10.3109/00365548.2011.577094.
Fischer TK, Nielsen AY, Sydenham TV, Andersen PH, Andersen B, Midgley SE. Emergence of enterovirus 71 C4a in Denmark, 2009 to 2013. Euro Surveill. 2014;19:20911. https://doi.org/10.2807/1560-7917.ES2014.19.38.20911.
Hassel C, Mirand A, Lukashev A, TerletskaiaLadwig E, Farkas A, Schuffenecker I, et al. Transmission patterns of human enterovirus 71 to, from and among European countries, 2003 to 2013. Euro Surveill. 2015;20:30005. https://doi.org/10.2807/1560-7917.ES.2015.20.34.30005.
Akhmadishina LV, Govorukhina MV, Kovalev EV, Nenadskaya SA, Ivanova OE, Lukashev AN. Enterovirus A71 Meningoencephalitis Outbreak, Rostov-on-Don, Russia, 2013 - Volume 21, Number 8—August 2015 - Emerging Infectious Diseases journal - CDC. Emerg Infect Dis. 2015;21:1440–3. https://doi.org/10.3201/eid2108.141084.
Midgley SE, Nielsen AG, Trebbien R, Poulsen MW, Andersen PH, Fischer TK. Co-circulation of multiple subtypes of enterovirus A71 (EV- A71) genotype C, including novel recombinants characterised by use of whole genome sequencing (WGS), Denmark 2016. Euro Surveill. 2017;22:30565. https://doi.org/10.2807/1560-7917.ES.2017.22.26.30565.
Wörner N, Rodrigo-García R, Antón A, Castellarnau E, Delgado I, Vazquez È, et al. Enterovirus-A71 Rhombencephalitis Outbreak in Catalonia: characteristics, management and outcome. Pediatr Infect Dis J. 2021;40:628–33. https://doi.org/10.1097/INF.0000000000003114.
Bian L, Wang Y, Yao X, Mao Q, Xu M, Liang Z. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti-Infect Ther. 2015;13:1061–71. https://doi.org/10.1586/14787210.2015.1058156.
Noisumdaeng P, Korkusol A, Prasertsopon J, Sangsiriwut K, Chokephaibulkit K, Mungaomklang A, et al. Longitudinal study on enterovirus A71 and coxsackievirus A16 genotype/subgenotype replacements in hand, foot and mouth disease patients in Thailand, 2000–2017. Int J Infect Dis. 2019;80:84–91. https://doi.org/10.1016/j.ijid.2018.12.020.
Zhu P, Ji W, Li D, Li Z, Chen Y, Dai B, et al. Current status of hand-foot-and-mouth disease. J Biomed Sci. 2023;30:15. https://doi.org/10.1186/s12929-023-00908-4.
Koh WM, Badaruddin H, La H, Chen MIC, Cook AR. Severity and burden of hand, foot and mouth disease in Asia: a modelling study. BMJ Glob Health. 2018;3:e000442.
Sadeuh-Mba SA, Joffret M-L, Mazitchi A, Endegue-Zanga M-C, Njouom R, Delpeyroux F, et al. Genetic and phenotypic characterization of recently discovered enterovirus D type 111. PLoS Negl Trop Dis. 2019;13:e0007797. https://doi.org/10.1371/journal.pntd.0007797.
Bauer L, Lyoo H, van der Schaar HM, Strating JR, van Kuppeveld FJ. Direct-acting antivirals and host-targeting strategies to combat enterovirus infections. Curr Opin Virol. 2017;24:1–8. https://doi.org/10.1016/j.coviro.2017.03.009.
Wang J, Hu Y, Zheng M. Enterovirus A71 antivirals: past, present, and future. Acta Pharm Sin B. 2022;12:1542–66. https://doi.org/10.1016/j.apsb.2021.08.017.
Andino R, Kirkegaard K, Macadam A, Racaniello VR, Rosenfeld AB. The Picornaviridae family: knowledge gaps, animal models, countermeasures, and prototype pathogens. J Infect Dis. 2023;228:S427-45. https://doi.org/10.1093/infdis/jiac426.
Liu Y, Zhou J, Ji G, Gao Y, Zhang C, Zhang T, et al. A novel subgenotype C6 Enterovirus A71 originating from the recombination between subgenotypes C4 and C2 strains in mainland China. Sci Rep. 2022;12:593. https://doi.org/10.1038/s41598-021-04604-x.
Midgley SE, Benschop K, Dyrdak R, Mirand A, Bailly J-L, Bierbaum S, et al. Co-circulation of multiple enterovirus D68 subclades, including a novel B3 cluster, across Europe in a season of expected low prevalence, 2019/20. Euro Surveill. 2020;25:1900749. https://doi.org/10.2807/1560-7917.ES.2020.25.2.1900749.
Xie J, Yang X-H, Hu S-Q, Zhan W-L, Zhang C-B, Liu H, et al. Co-circulation of coxsackieviruses A-6, A-10, and A-16 causes hand, foot, and mouth disease in Guangzhou city China. BMC Infect Dis. 2020;20:271. https://doi.org/10.1186/s12879-020-04992-x.
Junttila N, Lévêque N, Magnius LO, Kabue JP, Muyembe-Tamfum JJ, Maslin J, et al. Complete coding regions of the prototypes enterovirus B93 and C95: Phylogenetic analyses of the P1 and P3 regions of EV-B and EV-C strains. J Med Virol. 2015;87:485–97. https://doi.org/10.1002/jmv.24062.
Lévêque N, Jacques J, Renois F, Antona D, Abely M, Chomel J-J, et al. Phylogenetic analysis of Echovirus 30 isolated during the 2005 outbreak in France reveals existence of multiple lineages and suggests frequent recombination events. J Clin Virol. 2010;48:137–41. https://doi.org/10.1016/j.jcv.2010.03.011.
Bouslama L, Nasri D, Chollet L, Belguith K, Bourlet T, Aouni M, et al. Natural Recombination Event within the Capsid Genomic Region Leading to a Chimeric Strain of Human Enterovirus B. J Virol. 2007;81:8944–52. https://doi.org/10.1128/JVI.00180-07.
Lindberg AM, Andersson P, Savolainen C, Mulders MN, Hovi T. Evolution of the genome of Human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J Gen Virol. 2003;84:1223–35. https://doi.org/10.1099/vir.0.18971-0.
He W, Lu H, Song D, Zhao K, Gai X, Wang X, et al. The evidence of Coxsackievirus B3 induced myocarditis as the cause of death in a Sichuan snub-nosed monkey (Rhinopithecus roxellana). J Med Primatol. 2009;38:192–8. https://doi.org/10.1111/j.1600-0684.2008.00336.x.
He W, Lu H, Zhao K, Song D, Gai X, Gao F. Complete Genome Sequence of a Coxsackievirus B3 Isolated from a Sichuan Snub-Nosed Monkey. J Virol. 2012;86:13134–5. https://doi.org/10.1128/JVI.02365-12.
Harvala H, Van Nguyen D, McIntyre C, Ahuka-Mundeke S, Ngole EM, Delaporte E, et al. Co-circulation of enteroviruses between apes and humans. J Gen Virol. 2014;95:403–7. https://doi.org/10.1099/vir.0.059048-0.
Harvala H, McIntyre CL, Imai N, Clasper L, Djoko CF, LeBreton M, et al. High Seroprevalence of enterovirus infections in apes and old world monkeys. Emerg Infect Dis. 2012;18:283–6. https://doi.org/10.3201/eid1802.111363.
Amona I, Medkour H, Akiana J, Davoust B, Tall ML, Grimaldier C, et al. Enteroviruses from humans and Great Apes in the Republic of Congo: recombination within enterovirus C serotypes. Microorganisms. 2020;8:1779. https://doi.org/10.3390/microorganisms8111779.
Sereme Y, Zarza SM, Medkour H, Amona I, Fenollar F, Akiana J, et al. Stool serology: development of a Non-invasive immunological method for the detection of enterovirus-specific antibodies in Congo Gorilla Faeces. Microorganisms. 2021;9:810. https://doi.org/10.3390/microorganisms9040810.
Sadeuh-Mba SA, Bessaud M, Joffret M-L, Endegue Zanga M-C, Balanant J, Mpoudi Ngole E, et al. Characterization of enteroviruses from non-human primates in Cameroon revealed virus types widespread in humans along with candidate new types and species. PLoS Negl Trop Dis. 2014;8:e3052. https://doi.org/10.1371/journal.pntd.0003052.
Mombo IM, Berthet N, Lukashev AN, Bleicker T, Brünink S, Léger L, et al. First detection of an enterovirus C99 in a captive chimpanzee with acute flaccid paralysis, from the Tchimpounga Chimpanzee Rehabilitation Center Republic of Congo. PLoS One. 2015;10:e0136700. https://doi.org/10.1371/journal.pone.0136700.
Nguyen-Tran H, Park SW, Messacar K, Dominguez SR, Vogt MR, Permar S, et al. Enterovirus D68: a test case for the use of immunological surveillance to develop tools to mitigate the pandemic potential of emerging pathogens. Lancet Microbe. 2022;3:e83-5. https://doi.org/10.1016/S2666-5247(21)00312-8.
Douek DC. PREMISE (Pandemic REsponse REpository through Microbial and Immune Surveillance and Epidemiology) program. https://www.niaid.nih.gov/research/premise. Accessed 12 Aug 2023.
Harvala H, Benschop KSM, Berginc N, Midgley S, Wolthers K, Simmonds P, et al. European Non-Polio Enterovirus Network: Introduction of Hospital-Based Surveillance Network to Understand the True Disease Burden of Non-Polio Enterovirus and Parechovirus Infections in Europe. Microorganisms. 2021;9:1827. https://doi.org/10.3390/microorganisms9091827.
Chiu M-L, Luo S-T, Chen Y-Y, Chung WY, Duong V, Dussart P, et al. Establishment of Asia-Pacific Network for Enterovirus Surveillance. Vaccine. 2020;38:1–9. https://doi.org/10.1016/j.vaccine.2019.09.111.
GPEI-Who we are. https://polioeradication.org/who-we-are/. Accessed 22 Sept 2023.
Ledford H. Spate of polio outbreaks worldwide puts scientists on alert. Nature. 2022;609:20–1. https://doi.org/10.1038/d41586-022-02233-6.
Gebremedhin S, Shiferie F, Tsegaye DA, Alemayehu WA, Wondie T, Donofrio J, et al. Oral and inactivated polio vaccine coverage and determinants of coverage inequality among the most at-risk populations in Ethiopia. Am J Trop Med Hyg. 2023;109:1148–56. https://doi.org/10.4269/ajtmh.23-0319.
GPEI: Polio Eradication Strategy 2022-2026, Geneva: World Health Organisation; 2021 Available at. https://polioeradication.org/gpei-strategy-2022-2026/
GPEI: Surveillance Indicators. https://polioeradication.org/polio-today/polio-now/surveillance-indicators/. Accessed 2 Dec 2023.
National Enterovirus Surveillance System (NESS) | CDC 2023. https://www.cdc.gov/surveillance/ness/index.html. Accessed 22 Sept 2023.
Protection AGD of HO of H. Australian National Enterovirus Reference Laboratory (ANERL) [poliovirus surveillance] annual reports. Available from https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-polioanrep.htm
Keeren K, Böttcher S, Diedrich S. Enterovirus Surveillance (EVSurv) in Germany. Microorganisms. 2021;9:2005. https://doi.org/10.3390/microorganisms9102005.
Harvala H, Jasir A, Penttinen P, Pastore Celentano L, Greco D, Broberg E. Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European Economic Area, 2016. Euro Surveill 2017;22. https://doi.org/10.2807/1560-7917.ES.2017.22.45.16-00807.
Pogka V, Labropoulou S, Emmanouil M, Voulgari-Kokota A, Vernardaki A, Georgakopoulou T, et al. Laboratory surveillance of polio and other enteroviruses in high-risk populations and environmental samples. Appl Environ Microbiol. 2017;83:e02872-16. https://doi.org/10.1128/AEM.02872-16.
Hampton LM. Immunization Systems Management Group of the Global Polio Eradication Initiative. Introduction of Inactivated Poliovirus Vaccine and Switch from Trivalent to Bivalent Oral Poliovirus Vaccine - Worldwide, 2013-2016. MMWR Morb Mortal Wkly Rep. 2015;64:699–702. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6425a4.htm
Hampton LM. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine — Worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65. https://doi.org/10.15585/mmwr.mm6535a3.
Hib-DTaP-hepatitis B-poliovirus vaccine - GSK - AdisInsight. https://adis.springer.com/drugs/800004693 Accessed 17 Oct 2023.
Hib-DTaP-Hepatitis-B-Poliovirus-vaccine - Merck - AdisInsight. https://adis.springer.com/drugs/800037616. Accessed 17 Oct 2023.
Tregnaghi MW, Zambrano B, Santos-Lima E. Immunogenicity and Safety of an Investigational Hexavalent Diphtheria-tetanus-acellular Pertussis-inactivated Poliovirus-hepatitis B-Haemophilus influenzae B Conjugate Combined Vaccine in Healthy 2-, 4-, and 6-month-old Argentinean Infants. J Pediatr Infect Dis. 2011;30:e88. https://doi.org/10.1097/INF.0b013e318212eb80.
WHO: Enterovirus 71 https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/enterovirus-71. Accessed 21 Sept 2023.
Li Y, Gao F, Wang Y, Li J, Zhang Y, Lv H, et al. Immunogenicity and safety of inactivated enterovirus A71 vaccines in children aged 6-35 months in China: a non-inferiority, randomised controlled trial. Lancet Reg Health West Pac. 2021;16. https://doi.org/10.1016/j.lanwpc.2021.100284.
Snider CJ, Zaman K, Estivariz CF, Yunus M, Weldon WC, Wannemuehler KA, et al. Immunogenicity of full and fractional dose of inactivated poliovirus vaccine for use in routine immunisation and outbreak response: an open-label, randomised controlled trial. Lancet. 2019;393:2624–34. https://doi.org/10.1016/S0140-6736(19)30503-3.
GlaxoSmithKline. Evaluation of GSK Biological’s dTpa-IPV Booster Vaccine in Children and Adolescents, 5 Years After Previous dTpa-IPV Boosting. Available at https://clinicaltrials.gov/study/NCT00635128
Hankaniemi MM, Laitinen OH, Stone VM, Sioofy-Khojine A, Maatta JAE, Larsson PG, et al. Optimized production and purification of Coxsackievirus B1 vaccine and its preclinical evaluation in a mouse model. Vaccine. 2017;35:3718–25. https://doi.org/10.1016/j.vaccine.2017.05.057.
Hankaniemi MM, Stone VM, Sioofy-Khojine A-B, Heinimäki S, Marjomäki V, Hyöty H, et al. A comparative study of the effect of UV and formalin inactivation on the stability and immunogenicity of a Coxsackievirus B1 vaccine. Vaccine. 2019;37:5962–71. https://doi.org/10.1016/j.vaccine.2019.08.037.
Stone VM, Hankaniemi MM, Svedin E, Sioofy-Khojine A, Oikarinen S, Hyoty H, et al. A Coxsackievirus B vaccine protects against virus-induced diabetes in an experimental mouse model of type 1 diabetes. Diabetologia. 2017. https://doi.org/10.1007/s00125-017-4492-z.
Stone VM, Hankaniemi MM, Laitinen OH, Sioofy-Khojine AB, Lin A, Diaz Lozano IM, et al. A hexavalent Coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates. Sci Adv. 2020;6:eaaz2433. https://doi.org/10.1126/sciadv.aaz2433.
Stone VM, Butrym M, Hankaniemi MM, Sioofy-Khojine A-B, Hytönen VP, Hyöty H, et al. Coxsackievirus B Vaccines Prevent Infection-Accelerated Diabetes in NOD Mice and Have No Disease-Inducing Effect. Diabetes. 2021;70:2871–8. https://doi.org/10.2337/db21-0193.
Stone VM, Utorova R, Butrym M, Sioofy-Khojine A-B, Hankaniemi MM, Ringqvist EE, et al. Coxsackievirus B infections are common in Cystic Fibrosis and experimental evidence supports protection by vaccination. iScience. 2022;25:105070. https://doi.org/10.1016/j.isci.2022.105070.
Hyöty H, Kääriäinen S, Laiho JE, Comer GM, Tian W, Härkönen T, et al. Safety, tolerability and immunogenicity of PRV-101, a multivalent vaccine targeting coxsackie B viruses (CVBs) associated with type 1 diabetes: a double-blind randomised placebo-controlled Phase I trial. Diabetologia. 2024. https://doi.org/10.1007/s00125-024-06092-w.
Yeh MT, Bujaki E, Dolan PT, Smith M, Wahid R, Konz J, et al. Engineering the live-attenuated polio vaccine to prevent reversion to virulence. Cell Host Microbe. 2020;27:736-751.e8. https://doi.org/10.1016/j.chom.2020.04.003.
WHO: First ever vaccine listed under WHO emergency use https://www.who.int/news/item/13-11-2020-first-ever-vaccine-listed-under-who-emergency-use. Accessed 7 Dec 2023.
WHO: Poliovirus vaccines https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/poliovirus-vaccines. Accessed 7 Dec 2023.
Davlantes E. Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use — Six African Countries, 2021–2023. MMWR Morb Mortal Wkly Rep. 2023;72. https://doi.org/10.15585/mmwr.mm7238a4.
Yeh MT, Smith M, Carlyle S, Konopka-Anstadt JL, Burns CC, Konz J, et al. Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature. 2023;619:135–42. https://doi.org/10.1038/s41586-023-06212-3.
Liu X, Chang S, Wang R, Xiao Y, Li F, Xu Q, et al. Immunogenicity and Safety of an Inactivated Enterovirus 71 Vaccine Administered Simultaneously with Hepatitis B Virus Vaccine, Group A Meningococcal Polysaccharide Vaccine, Measles-Rubella Combined Vaccine and Japanese Encephalitis Vaccine: A Multi-Center, Randomized Controlled Clinical Trial in China. Vaccines (Basel). 2022;10:895. https://doi.org/10.3390/vaccines10060895.
Lee S, Nguyen MT, Currier MG, Jenkins JB, Strobert EA, Kajon AE, et al. A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques. Nat Commun. 2016;7:12838. https://doi.org/10.1038/ncomms12838.
Mone K, Lasrado N, Sur M, Reddy J. Vaccines against Group B Coxsackieviruses and Their Importance. Vaccines. 2023;11:274. https://doi.org/10.3390/vaccines11020274.
Bello AM, Roshorm YM. Recent progress and advances towards developing enterovirus 71 vaccines for effective protection against human hand, foot and mouth disease (HFMD). Biologicals. 2022;79:1–9. https://doi.org/10.1016/j.biologicals.2022.08.007.
Fang C-Y, Liu C-C. Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease. Expert Rev Vacc. 2018;17:819–31. https://doi.org/10.1080/14760584.2018.1510326.
Hankaniemi MM, Stone VM, Andrejeff T, Heinimäki S, Sioofy-Khojine A-B, Marjomäki V, et al. Formalin treatment increases the stability and immunogenicity of coxsackievirus B1 VLP vaccine. Antiviral Res. 2019;171:104595. https://doi.org/10.1016/j.antiviral.2019.104595.
Hankaniemi MM, Baikoghli MA, Stone VM, Xing L, Vaatainen O, Soppela S, et al. Structural Insight into CVB3-VLP Non-Adjuvanted Vaccine. Microorganisms. 2020;8: https://doi.org/10.3390/microorganisms8091287.
Wahid R, Cannon MJ, Chow M. Virus-Specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J Virol. 2005;79:5988–95. https://doi.org/10.1128/JVI.79.10.5988-5995.2005.
Kasstan B, Mounier-Jack S, Chantler T, Masters N, Flores SA, Stokley S, et al. Poliovirus outbreak in New York State, August 2022: qualitative assessment of immediate public health responses and priorities for improving vaccine coverage. Epidemiol Infect. 2023;151:e120. https://doi.org/10.1017/S0950268823001127.
Zhang W, Dai W, Zhang C, Zhou Y, Xiong P, Wang S, et al. A virus-like particle-based tetravalent vaccine for hand, foot, and mouth disease elicits broad and balanced protective immunity. Emerg Microbes Infect. 2018;7:94. https://doi.org/10.1038/s41426-018-0094-1.
Lim P-Y, Hickey AC, Jamiluddin MF, Hamid S, Kramer J, Santos R, et al. Immunogenicity and performance of an enterovirus 71 virus-like-particle vaccine in nonhuman primates. Vaccine. 2015;33:6017–24. https://doi.org/10.1016/j.vaccine.2015.05.108.
Wu CN, Lin YC, Fann C, Liao NS, Shih SR, Ho MS. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine. 2001;20:895–904. https://doi.org/10.1016/s0264-410x(01)00385-1.
Tsou Y-L, Lin Y-W, Shao H-Y, Yu S-L, Wu S-R, Lin H-Y, et al. Recombinant adeno-vaccine expressing enterovirus 71-like particles against hand, foot, and mouth disease. PLoS Negl Trop Dis. 2015;9:e0003692. https://doi.org/10.1371/journal.pntd.0003692.
Sekaly R-P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. https://doi.org/10.1084/jem.20072681.
Tung WS, Bakar SA, Sekawi Z, Rosli R. DNA vaccine constructs against enterovirus 71 elicit immune response in mice. Genet Vaccines Ther. 2007;5:6. https://doi.org/10.1186/1479-0556-5-6.
Kimura T, Leal JM, Simpson A, Warner NL, Berube BJ, Archer JF, et al. A localizing nanocarrier formulation enables multi-target immune responses to multivalent replicating RNA with limited systemic inflammation. Mol Ther. 2023;31:2360–75. https://doi.org/10.1016/j.ymthe.2023.06.017.
Zhang C, Zhang Y, Li Y, Lu J, Xiong S, Yue Y. Exosome-based delivery of VP1 protein conferred enhanced resistance of mice to CVB3-induced viral myocarditis. Virology. 2023;579:46–53. https://doi.org/10.1016/j.virol.2022.12.015.
Shen C, Liu Q, Zhou Y, Ku Z, Wang L, Lan K, et al. Inactivated coxsackievirus A10 experimental vaccines protect mice against lethal viral challenge. Vaccine. 2016;34:5005–12. https://doi.org/10.1016/j.vaccine.2016.08.033.
Jin W-P, Lu J, Zhang X-Y, Wu J, Wei Z-N, Mai J-Y, et al. Efficacy of Coxsackievirus A5 Vaccine Candidates in an Actively Immunized Mouse Model. J Virol. 2021;95:https://doi.org/10.1128/jvi.01743-20.
Zhang Z, Dong Z, Wang Q, Carr MJ, Li J, Liu T, et al. Characterization of an inactivated whole-virus bivalent vaccine that induces balanced protective immunity against coxsackievirus A6 and A10 in mice. Vaccine. 2018;36:7095–104. https://doi.org/10.1016/j.vaccine.2018.09.069.
Sun Y-S, Xia Y, Xu F, Lu H-J, Mao Z-A, Gao M, et al. Development and evaluation of an inactivated coxsackievirus A16 vaccine in gerbils. Emerg Microbes Infect. 11:1994–2006. https://doi.org/10.1080/22221751.2022.2093132.
Hu G, Jin W-P, Yang Z-H, Lv S-Y, Wu J, Yu Y-T, et al. Efficacy of Coxsackievirus A2 vaccine candidates correlating to humoral immunity in mice challenged with a mouse-adapted strain. Vaccine. 2022;40:4716–25. https://doi.org/10.1016/j.vaccine.2022.06.021.
Raychoudhuri A, Naru AK, Kanubothula SR, Uddala R. Development of an experimental inactivated vaccine from Vero cell adapted Enterovirus D68. Virus Res. 2021;304:198528. https://doi.org/10.1016/j.virusres.2021.198528.
Yee PTI, Tan SH, Ong KC, Tan KO, Wong KT, Hassan SS, et al. Development of live attenuated Enterovirus 71 vaccine strains that confer protection against lethal challenge in mice. Sci Rep. 2019;9:4805. https://doi.org/10.1038/s41598-019-41285-z.
Meng T, Wong S-M, Chua K-B. A Novel Attenuated Enterovirus A71 Mutant with VP1-V238A,K244R Exhibits Reduced Efficiency of Cell Entry/Exit and Augmented Binding Affinity to Sulfated Glycans. J Virol. 2021;95:https://doi.org/10.1128/jvi.01055-21. https://doi.org/10.1128/jvi.01055-21.
Wang R, Sun Q, Xiao J, Wang C, Li X, Li J, et al. Effects of glycine 64 substitutions in RNA-dependent RNA polymerase on ribavirin sensitivity and pathogenicity of coxsackievirus A6. Virus Res. 2023;339:199268. https://doi.org/10.1016/j.virusres.2023.199268.
Yang Z, Gao F, Wang X, Shi L, Zhou Z, Jiang Y, et al. Development and characterization of an enterovirus 71 (EV71) virus-like particles (VLPs) vaccine produced in Pichia pastoris. Hum Vaccin Immunother. n.d.;16:1602–10. https://doi.org/10.1080/21645515.2019.1649554.
Zhou Y, Shen C, Zhang C, Zhang W, Wang L, Lan K, et al. Yeast-produced recombinant virus-like particles of coxsackievirus A6 elicited protective antibodies in mice. Antiviral Res. 2016;132:165–9. https://doi.org/10.1016/j.antiviral.2016.06.004.
Zhou Y, Zhang C, Liu Q, Gong S, Geng L, Huang Z. A virus-like particle vaccine protects mice against coxsackievirus A10 lethal infection. Antiviral Res. 2018;152:124–30. https://doi.org/10.1016/j.antiviral.2018.02.016.
Krug PW, Wang L, Shi W, Kong W-P, Moss DL, Yang ES, et al. EV-D68 virus-like particle vaccines elicit cross-clade neutralizing antibodies that inhibit infection and block dissemination. Sci Adv. 2023;9:eadg6076. https://doi.org/10.1126/sciadv.adg6076.
Zhang C, Zhang X, Zhang W, Dai W, Xie J, Ye L, et al. Enterovirus D68 virus-like particles expressed in Pichia pastoris potently induce neutralizing antibody responses and confer protection against lethal viral infection in mice. Emerg Microbes Infect. 2018;7:3. https://doi.org/10.1038/s41426-017-0005-x.
Urakawa T, Ferguson M, Minor PD, Cooper J, Sullivan M, Almond JW, et al. Synthesis of Immunogenic, but Non-infectious, Poliovirus Particles in Insect Cells by a Baculovirus Expression Vector. J Gen Virol. 1989;70:1453–63. https://doi.org/10.1099/0022-1317-70-6-1453.
Rombaut B, Jore JP. Immunogenic, non-infectious polio subviral particles synthesized in Saccharomyces cerevisiae. J Gen Virol. 1997;78(Pt 8):1829–32. https://doi.org/10.1099/0022-1317-78-8-1829.
Frietze KM, Peabody DS, Chackerian B. Engineering virus-like particles as vaccine platforms. Curr Opin Virol. 2016;18:44–9. https://doi.org/10.1016/j.coviro.2016.03.001.
Rohovie M, Nagasawaa M, Swartz J. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng Transl Med. 2016;2:43–57. https://doi.org/10.1002/btm2.10049.
Adeyemi OO, Nicol C, Stonehouse NJ, Rowlands DJ. Increasing Type 1 Poliovirus Capsid Stability by Thermal Selection. J Virol. 2017;91:e01586-16. https://doi.org/10.1128/JVI.01586-16.
Fox H, Knowlson S, Minor PD, Macadam AJ. Genetically thermo-stabilised, immunogenic poliovirus empty capsids; a strategy for non-replicating vaccines. PLoS Pathog. 2017;13:e1006117. https://doi.org/10.1371/journal.ppat.1006117.
Xu Y, Ma S, Huang Y, Chen F, Chen L, Ding D, et al. Virus-like particle vaccines for poliovirus types 1, 2, and 3 with enhanced thermostability expressed in insect cells. Vaccine. 2019;37:2340–7. https://doi.org/10.1016/j.vaccine.2019.03.031.
Sherry L, Bahar MW, Porta C, Fox H, Grehan K, Nasta V, et al. Risk-free polio vaccine: Recombinant expression systems for production of stabilised virus-like particles 2024. bioRxiv 2024.05.13.593909; https://doi.org/10.1101/2024.05.13.593909.
Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 2023;23:236–50. https://doi.org/10.1038/s41577-022-00763-8.
Gao Y, Yue Y, Xiong S. An Albumin-Binding Domain Peptide Confers Enhanced Immunoprotection Against Viral Myocarditis by CVB3 VP1 Vaccine. Front Immunol. 2021;12. https://doi.org/10.3389/fimmu.2021.666594.
Emini EA, Jameson BA, Wimmer E. Priming for and induction of anti-poliovirus neutralizing antibodies by synthetic peptides. Nature. 1983;304:699–703. https://doi.org/10.1038/304699a0.
Puligedda RD, Kouiavskaia D, Al-Saleem FH, Kattala CD, Nabi U, Yaqoob H, et al. Characterization of human monoclonal antibodies that neutralize multiple poliovirus serotypes. Vaccine. 2017;35:5455–62. https://doi.org/10.1016/j.vaccine.2017.03.038.
Kidd S, Lopez AS, Konopka-Anstadt JL, Nix WA, Routh JA, Oberste MS. Enterovirus D68–associated acute flaccid myelitis, United States, 2020. Emerg Infect Dis. 2020;26:e201630. https://doi.org/10.3201/eid2610.201630.
He F, Rui J, Deng Z, Zhang Y, Qian K, Zhu C, et al. Surveillance, Epidemiology and Impact of EV-A71 Vaccination on Hand, Foot, and Mouth Disease in Nanchang, China, 2010–2019. Front Microbiol. 2022;12. https://doi.org/10.3389/fmicb.2021.811553.
Lim HX, Poh CL. Insights into innate and adaptive immune responses in vaccine development against EV-A71. Ther Adv Vaccines Immunother. 2019;7:2515135519888998. https://doi.org/10.1177/2515135519888998.
Chang L-Y, Hsiung CA, Lu C-Y, Lin T-Y, Huang F-Y, Lai Y-H, et al. Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatr Res. 2006;60:466–71. https://doi.org/10.1203/01.pdr.0000238247.86041.19.
Brickley EB, Wieland-Alter W, Connor RI, Ackerman ME, Boesch AW, Arita M, et al. Intestinal Immunity to poliovirus following sequential trivalent inactivated polio vaccine/bivalent oral polio vaccine and trivalent inactivated polio vaccine-only immunization schedules: analysis of an open-label, randomized, controlled trial in Chilean infants. Clin Infect Dis. 2018;67:S42-50. https://doi.org/10.1093/cid/ciy603.
Gaevert JA, Luque Duque D, Lythe G, Molina-París C, Thomas PG. Quantifying T cell cross-reactivity: influenza and coronaviruses. Viruses. 2021;13:1786. https://doi.org/10.3390/v13091786.
Mohn KGI, Brokstad KA, Islam S, Oftung F, Tøndel C, Aarstad HJ, et al. Early induction of cross-reactive CD8+ T-cell responses in tonsils after live-attenuated influenza vaccination in children. J Infect Dis. 2020;221:1528–37. https://doi.org/10.1093/infdis/jiz583.
Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597:268–73. https://doi.org/10.1038/s41586-021-03841-4.
Chen M, Venturi V, Munier CML. Dissecting the protective effect of CD8+ T cells in response to SARS-CoV-2 mRNA vaccination and the potential link with lymph node CD8+ T CELLS. Biology. 2023;12:1035. https://doi.org/10.3390/biology12071035.
Rosenfeld AB, Shen EQL, Melendez M, Mishra N, Lipkin WI, Racaniello VR. Cross-reactive antibody responses against nonpoliovirus enteroviruses. Bio. 2022;13:e03660-21. https://doi.org/10.1128/mbio.03660-21.
Pupina N, Avarlaid A, Sadam H, Pihlak A, Jaago M, Tuvikene J, et al. Immune response to a conserved enteroviral epitope of the major capsid VP1 protein is associated with lower risk of cardiovascular disease. EBioMedicine. 2022;76:103835. https://doi.org/10.1016/j.ebiom.2022.103835.
Beck MA, Tracy SM. Evidence for a group-specific enteroviral antigen(s) recognized by human T cells. J Clin Microbiol. 1990;28:1822–7. https://doi.org/10.1128/jcm.28.8.1822-1827.1990.
Graham S, Wang EC, Jenkins O, Borysiewicz LK. Analysis of the human T-cell response to picornaviruses: identification of T-cell epitopes close to B-cell epitopes in poliovirus. J Virol. 1993;67:1627–37. https://doi.org/10.1128/jvi.67.3.1627-1637.1993.
Vecchio F, Carré A, Korenkov D, Zhou Z, Apaolaza P, Tuomela S, et al. Coxsackievirus infection induces direct pancreatic β-cell killing but poor anti-viral CD8+ T-cell responses. bioRxiv. 2023.08.19.553954. https://doi.org/10.1101/2023.08.19.553954.
Liu P, Yuan Y, Cui B, Huo Y, Bian L, Chen L, et al. Cross-Antigenicity between EV71 Sub-Genotypes: Implications for Vaccine Efficacy. Viruses. 2021;13:720. https://doi.org/10.3390/v13050720.
Paul JR, Trask JD. The neutralization test in poliomyelitis : comparative results with four strains of the virus. J Exp Med. 1935;61:447–64. https://doi.org/10.1084/jem.61.4.447.
Stewart FW, Rhoads CP. Intradermal versus subcutaneous immunization of monkeys against poliomyelitis. J Exp Med. 1929;49:959–73. https://doi.org/10.1084/jem.49.6.959.
Burnet FM, Macnamara J. Immunological Differences Between Strains of Poliomyelitic Virus. Br J Exp Pathol. 1931;12:57–61. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2048137/.
Chen Z, Fischer ER, Kouiavskaia D, Hansen BT, Ludtke SJ, Bidzhieva B, et al. Cross-neutralizing human anti-poliovirus antibodies bind the recognition site for cellular receptor. Proc Natl Acad Sci U S A. 2013;110:20242–7. https://doi.org/10.1073/pnas.1320041110.
Acknowledgements
We wish to thank Dr. Heli Harvala (Nuffield Department for Clinical Laboratory Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, UK, and Microbiology Services, NHS Blood and Transplant, Colindale, UK) for the valuable comments, which improved the manuscript. Figures 1A and 2 were made partially using BioRender, and we wish to thank Samu Voutilainen for the help with Figures 1B and 1C. Also, we wish to thank Erja Janhonen for the help with literature search.
Funding
This work was supported by Tampere University Graduate School (MJ), Allergy Research Foundation (MJ), Tampere Tuberculosis Foundation (MJ) and Research Council of Finland (#355414, MMH). Research in the MMH laboratory is also supported by grants from Tampere Institute of Advanced Studies, Jane and Aatos Erkko Foundation, Diabetes Research Foundation, Business Finland and Finnish Cultural Foundation. Research in the MFT laboratory is supported by grants from Karolinska Institutet, Sweden, including the Strategic Research Programme in Diabetes, the Swedish Child Diabetes Foundation, the Swedish Diabetes Foundation, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
Author information
Authors and Affiliations
Contributions
MJ, MFT and MMH wrote the manuscript. MJ performed database searches for the manuscript, and made Figs. 1 and 2, whereas MMH made Figs. 3 and 4 and Tables 1 and 2 for the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MJ, MFT and MMH have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jartti, M., Flodström-Tullberg, M. & Hankaniemi, M.M. Enteroviruses: epidemic potential, challenges and opportunities with vaccines. J Biomed Sci 31, 73 (2024). https://doi.org/10.1186/s12929-024-01058-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-024-01058-x