Abstract
Background
Many studies have investigated the role of metals in various types of malignancies. Considering the wide range of studies conducted in this field and the achievement of different results, the presented systematic review was performed to obtain the results of investigations on the prevention and occurrence of various types of cancer associated with metal exposures.
Methods
In this review, research was conducted in the three databases: Scopus, PubMed, and Web of Science without historical restrictions until May 31, 2024. Animal studies, books, review articles, conference papers, and letters to the editors were omitted. The special checklist of Joanna Briggs Institute (JBI) was used for the quality assessment of the articles. Finally, the findings were classified according to the effect of the metal as preventive or carcinogenic.
Results
The total number of retrieved articles was 4695, and 71 eligible results were used for further investigation. In most studies, the concentration of toxic metals such as lead (Pb), chromium (Cr (VI)), arsenic (As), cadmium (Cd), and nickel (Ni) in the biological and clinical samples of cancer patients was higher than that of healthy people. In addition, the presence of essential elements, such as selenium (Se), zinc (Zn), iron (Fe), and manganese (Mn) in tolerable low concentrations was revealed to have anti-cancer properties, while exposure to high concentrations has detrimental health effects.
Conclusions
Metals have carcinogenic effects at high levels of exposure. Taking preventive measures, implementing timely screening, and reducing the emission of metal-associated pollutants can play an effective role in reducing cancer rates around the world.
Similar content being viewed by others
Introduction
Currently, one of the main causes of morbidity and mortality worldwide is cancer. More than 1 person out of 5 people get cancer [1]. Based on projections, the number of new cancer patients will increase from 14.1 million reported in 2012 to 21.6 million estimated in 2030 [2]. Lifestyle behaviors such as obesity, smoking, and unhealthy diet, genetic changes, chronic diseases, and environmental interactions play key roles in cancer etiopathogenesis [3, 4].
Cancer is a disease caused by the uncontrolled and unnormal cell growth, resulting in the possibility to invade and metastasize any part of the body and formation of a tumor [5]. There are several types of cancer, including carcinoma, sarcoma, lymphoma, and leukemia [6]. The causes of cancer are mutations in the genes responsible for cell growth and division control [6]. Among the causes of the mutations, genetic inheritance, hormonal imbalances, and exposure to environmental factors such as certain chemicals can be mentioned [6]. Cancer pathophysiology involves three stages. The first one is caused by the initiation of mutations in the cell DNA leading to the activation of cancer genes and the inactivation of tumor suppressor genes. In the second stage promotion of the mutation cell takes place, which is stimulated by the differentiation and rapid growth of the mutation cell, forming a small cluster of abnormal cells. In the third stage the progression takes place, where abnormal cells continue to divide and grow, forming tumors that invade the surrounding tissue, and spread to other parts of the body through the blood flow or lymph system [6].
Studies have shown that various metals can impact on cancer induction by the same mechanisms, such as manipulating the state of chromatin and gene expression [7] or producing ROS and increasing oxidative stress [8]. Nickel was found to activate the signal pathways induced by hypoxia, mediated by competition with Fe in the prolyl-hydroxylase [8]. Arsenic and Cd have also been shown to compete or replace important metals such as Zn and Ca in proteins as the main mechanisms of cell gene and cell toxicity [8]. Also, As and Cd were found to suppress cell autophagy, which is an important factor in tumor suppression [8]. Considering genetic damage through both oxidative and nonoxidative (DNA adducts) mechanisms, metals were revealed to cause significant changes in DNA methylation and histone modifications, that results in epigenetic silencing or reactivation of gene expression [9]. Moreover, it was revealed in in vitro genotoxicity experiments and animal carcinogenicity studies that metals can cause cocarcinogenic and comutagenic effects as metals are likely to interfere with DNA repair processes [9].
Existing reports estimate that environmental factors, such as insecticides and pesticides, pollutants in air, water, and soil, cause 24% of the global disease burden measured in healthy life years lost and 23% of all types of premature deaths [10]. Studies indicate that the difference in exposure to environmental-related risk factors and the ability to obtain adequate health care increase the environmental burden of the apperance of diseases in developing countries 15 times compared to developed countries [11,12,13]. Environmental anthropogenic changes, such as water, soil, and air pollution, the growth of industrial activities, agricultural practices, the use of chemical fertilizers and pesticides, and food processing, play an important role in increasing the incidence of cancer by disrupting the balance of trace elements and metals in the environment [14, 15].
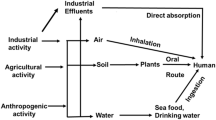
The mechanism of toxicity and carcinogenicity of toxic metals is presented in Fig. 1. After entering the body through exposure via digestion, inhalation, and dermal contact, metals can accumulate in the vital organs of the body such as the liver, kidney, and bones [16]. This feature can cause complications caused by the toxic effects of these elements in humans, including digestive system and kidney dysfunction, immune system dysfunction, nervous system disorders, vascular damage, birth defects, skin lesions, and epigenetic processes that lead to cancer [17, 18]. These processes lead to the deactivation of tumor suppressor genes, DNA repair enzymes, the transformation of proto-oncogenes into oncogenes, as well as changes in DNA methylation [19]. Metals also significantly affect the development of malignancies by activating redox-sensitive transcription factors, a protein that signals pathways involved in cell growth, apoptosis, disruption of cycle regulation in cells, as well as cell differentiation [20, 21], histone modifications and non-coding RNA expression [22, 23]. In addition, the initiation and progression of cancer have been found to be related to oxidative stress and the activation of inflammatory mediators. Connecting and activating transporters and cell surface receptors [24], activating metallothioneins [25] and specific enzymes and modulating selected intracellular kinases and phosphatases [26, 27] are other mechanisms of metal carcinogenesis. The following protein kinase: extracellular signal-regulated (ERK1/2) and protein kinase B (PKB or AKT) are the main elements of intracellular signaling that are effective in the cell proliferation regulation process [28, 29] and are sensitive to increasing/decreasing metal concentrations [29].
Although the carcinogenic role of metals has been proven in many studies [30,31,32], some researchers have reached contradictory results. The results of a recent case–control study showed that there was an inverse relationship between plasma Se levels and the chance of developing renal cell carcinoma [33]. Also, many studies reported higher concentrations of Mn and Fe in healthy people than in patients with various types of cancer such as lung [34], brain tumors [35], testicular [31], kidney [36], and non-melanoma skin [37].
However, many metals, such as copper (Cu), iron (Fe), selenium (Se), strontium (Sr), manganese (Mn), zinc (Zn), and molybdenum (Mo), are essential for life at low concentrations [38]. These metals have protective roles in processes such as chromosome damage and oxidation [38, 39]; and by regulating cell metabolism and DNA, RNA, and protein synthesis, they have preventive effects on cancer [40]. They also act as cofactors for some antioxidants such as superoxide dismutases [41], play a role in cell differentiation and apoptosis, and are essential for all stages of the cell cycle [42]. However, despite the evidence demonstrating their preventive role in cancer [33, 34, 43], if their concentration exceeds the body's homeostatic capacity, they can lead to degenerative conditions and even cancer [44].
The development of knowledge and technology in recent decades has led to the use of various solutions to prevent the spread of metals in the environment and reduce the adverse effects of exposure to these elements. Among these solutions, we can mention using Nanomaterials to reduce the consumption of substances containing heavy metals in the water remediation process [45] and chelating agents and barrier creams to prevent excessive exposure to heavy metals [46]. In addition, global removal of lead from gasoline, control of exposure to As in drinking water in Chile [47], and using bio-filters to remove these elements from wastewater [48] and landfill leachate [49] are among the preventive measures taken in this field. But considering the increasing spread of metals in the environment and the chronic exposure of people to these toxic elements, it seems that the measures taken are not enough.
Considering the broad exposure of humans to metals, the important role of these elements in cancer causation, and the contradictory results in various studies, our systematic review was performed to retrieve the scientific literature without historical restrictions until May 31, 2024. The main aims of this review were as follows: 1) evaluation of the types and concentrations of metals in the environmental or biological samples of exposed people, 2) investigation of the carcinogenic effect associated with metal concentrations, and 3) estimating the potential role of controlling metal exposures in cancer prevention.
Methods
Study protocol
The presented systematic review complies with the statement of Preferred Reporting Items for Systematic Review and Meta-analyses guidelines (PRISMA) [50] and fully adherence with the protocol that was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42023397867) on 8 February 2023.
PECO statement
To develop the research question, a Population, Exposure, Comparator, and Outcome (PECO) protocol was used, and the statement is presented in Table 1. In this statement, the exact purpose of the present systematic review, the terms of search, and the inclusion and exclusion criteria of studies related to the impact of exposure to different levels of metals and their impact on cancer occurrence or prevention were specified.
Search strategy and study selection
To access all studies presenting the effects of metals on the prevention or occurrence of cancer in humans, a systematic search without historical restrictions was conducted until May 31, 2024 in the following databases: Scopus, PubMed, and Web of Science for the following keywords: “heavy metal*”, “trace element*”, “trace metal*”, cancer, tumor, carcinogen, cancerogenic, neoplasm, oncogen*, neoplasia*, malignancy.
Criteria of study entering and extracting
In the review performed, studies focused on the effects of metals on cellular and biochemical changes (without examining the metal effect on the cancer appearance) were excluded. In addition, animal studies, books, review articles, conference papers, and letters to editors were omitted. In this systematic review, only original peer-reviewed articles in English were investigated. From the selected studies information on the name of the authors, publication year, study design, country, number of people in the investigated human subpopulation, their age, gender, type of metals, type of environmental and human samples, average metal concentration in samples, and type of cancer were gathered.
Quality assessment
The quality of the investigated studies was evaluated by two researches (M.M. and A.H.Kh.) independently, and the Joanna Briggs Institute (JBI) checklists for cohort studies, case–control studies, and analytical cross-sectional studies were used for this purpose. The JBI checklists assess the risk of bias in studies by asking 8 questions related to sample selection criteria, exposure assessment, confounding factors, and appropriate statistical analysis. According to the percentages assigned to each of the answers in the questionnaire (“yes”, “no”, “unclear”, or “not applicable”), the quality of the articles was determined at 3 levels, namely: 1) Q1 of high quality and low risk of bias (answer “yes” in ≥ 50–75%), 2) Q2 of average quality and unclear risk of bias (answer “unclear” in ≥ 50–75%), and 3) Q3 of low quality and high risk of bias (answer “no” in ≥ 50–75%) [51]. All articles that were of adequate quality were included in the study.
Result synthesis
Meta-analysis as quantitative synthesis was not suitable in this study, as we obtained a too diverse range of study designs and other heterogeneities were found in methodological and contextual aspects. Therefore, the results of the study, which included types of metals, the mean concentrations of metals in the samples, the type of cancer, and the role of metal in the prevention or appearance of cancer (Table S1), were narratively combined. A narrative synthesis was performed in two stages, including (1) initial synthesis using general grouping based on preventive/carcinogenic role and (2) exploration of associations within and between studies to investigate the relationship between metal exposure levels and severity of the outcome.
The entire process of the present systematic review which was carried out by the research team members shown in Fig. 2. This process includes 7 steps as follows: topic selection, keywords extraction, systematic search, screening and data extraction, evaluating risk of bias, resolving contradictions and ambiguities, and synthesizing results.
Results and discussion
Study selection
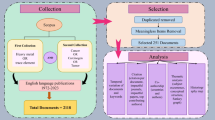
The process of articles selection performed in this study is presented in Fig. 3 using a PRISMA flow diagram. Through the systematic search, 4,695 articles were retrieved, of which 1558 were obtained from Web of Science, 2553 from the Scopus, and 584 from the PubMed database. After removing 983 duplicates, 3712 articles were screened by title and abstract. This step resulted in the exclusion of 3556 studies. Then, 156 full texts were subjected to additional check to assess the criteria of the entry and the exit, and quality assessment. Further 85 studies were excluded due to: lack of inclusion criteria (N = 25), in detail: not metal (7), benign tumor (4), not relevant (11), report on the cumulative effect of various contaminants on cancers (3), not determining and reporting the concentration of metals (self-reports) (N = 34), failure to report original data (N = 7), and investigating the effects of metals in cells on a laboratory scale (N = 19). Finally, in the current review, we included the total number of articles equal to 66 studies.
The studies in this review included 56 case–control studies [30,31,32,33,34,35,36, 52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99], 8 cohort studies [37, 100,101,102,103,104,105,106], 7 cross-sectional studies [107,108,109,110,111,112,113], and one observational study [114]. They were performed in various countries around the world: 8 in Pakistan, 7 in China, 6 in Taiwan, 5 in Sweden, 5 in USA, 4 in Iran, 6 in Poland, 3 in Turkey, 3 in Tunisia, 3 in Egypt, 2 in Spain, 2 in Romania, 2 in Iraq, 2 in India, 2 in Croatia, and one in Finland-Sweden-Iceland, Japan, Greece, the United Kingdom, New Zealand, United Arab Emirates, Ukraine, Italy, Nigeria, Russia, and Belgium. Four studies investigated metals in the natural environment and 67 in human biological samples such as tissue, serum, urine, blood, hair, and nails. The potential sources of exposure to metals are presented in Fig. 4.
The main analytical methods used to measure element concentrations were the Atomic Absorption Spectrophotometry (AAS) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS). A total of 1,369,887 people were examined in the 66 included studies. Table 2 presents a summary of the included 66 articles (for a complete table, see Table S1).
Toxic metals
The results of the majority of selected studies showed that metals such as Pb, As, Cr, Cd, and Ni act significantly in the development or progression of several types of cancer. Most toxic metals can damage cellular macromolecules such as DNA, RNA, proteins, and lipids by producing superoxide anion radical and hydroxyl radical reactive oxygen species (ROS) through the Fenton reaction and change cellular homeostasis [115]. In addition, oxidative stress caused by metals can induce genetic and epigenetic changes, abnormal cell signaling, increased micronuclei, chromosomal aberrations and mitotic index, and uncontrolled cell growth [116, 117]. In their role as endocrine disrupting chemicals [118], metals cause interference in the estrogen and androgen signaling pathways and affect the expression of genes involved in the growth and secretory function of the prostate gland [119].
Metals like Cd, Pb, As, Ni, and Cr(VI) compete with essential elements in the formation of ligands with enzymes and other proteins, including calcium-substituted lead, zinc-substituted cadmium, and aluminum-substituted mentioned with most of the rare elements [120]. These substitutions cause disturbances in important biochemical reactions, antioxidant imbalance, and adverse effects on various hormonal activities and the functioning of essential enzymes [121]. Gaman et al. (2021) reported that some metals can cross the blood–brain barrier through this replacement, accumulate in neurons and other brain cells, and potentially cause brain malignancies [35]. Despite the sensitivity of the placental barrier to toxic substances [122], metals can pass through the placental barrier by deceiving the transport proteins in the cell membrane of the placenta and causing important biochemical changes during fetal development [123]. The toxicity of each metal depends on its physicochemical characteristics, as well as on the biological properties of the target cells, in addition to the dose and duration of exposure [124].
The results of this systematic review showed that most of the examined people were elderly (> 60 years). Therefore, the age of exposed people can also play an important role in various malignancies [125]. The aging process in humans is associated with significant changes in cells, including telomere wear, genomic instability, epigenetic changes, cell aging, quantitative and qualitative changes in protein spectra, mitochondrial dysfunction, change in intercellular communication, and exhaustion of stem cells [125].
Cadmium
Studies show that an increase of 1 μg of Cd in urine, increases the risk of lung cancer by 1.25 times [94]. A case–control study in the Italian population showed that a 1.11-fold increase in Cd in the diet doubles the incidence of melanoma [126]. Men et al. (2020) showed that the concentration of Cd in the urine of breast cancer patients was almost twice that of the control population [89], which was consistent with the results of a previous epidemiological study [127]. Also, the study conducted by Abdul Qayyum et al. (2019) reported double the levels of this metal in the blood and scalp hair of patients with lymphoma [30]. Meanwhile, some researchers concluded that the levels of Cd in patients with stomach cancer were 1.37 times higher than those of the control group [91]. Although the difference in the concentration of this element between the group of patients and healthy people reported in [30] was higher than that of [91], the highest levels of Cd (case group) in these two studies were equal to 25.04 μg/dL and 344.6 μg/dL, respectively. In addition, the results of a recent case–control study showed that the Cd concentration in the whole blood of testicular cancer patients was 1.57 times higher than that of the control group [31]; which was consistent with the results obtained by Chang et al. (2016) [80]. The study examining the impact of heavy metals in urothelial carcinoma, revealed that the urinary concentration of Cd in patients were 1.52 times (1.53 μg/g CR) higher than in healthy people [80].
Many studies have proven the role of Cd in human lung, kidney, liver, breast, hematopoietic system, bladder, stomach, prostate, and pancreas cancers [128, 129]. Cadmium is a toxic metal with a long biological half-life, having low excretion levels related to the absence of efficient elimination mechanisms [130]. This element is spread in the environment through various sources, including industrial applications as stabilizers in PVC products, Ni–Cd batteries and pigments, fossil fuel combustion, use of phosphate fertilizers, electronic waste recycling, smoking, and volcanic activities and can cause chronic human exposure [131, 132].
Cadmium affects several cellular processes including cell cycle progression, proliferation, differentiation, DNA replication, and apoptosis [133], which may play a major role in Cd genotoxicity [134]. This process is also true for other diseases caused by exposure to Cd such as lung, prostate, or breast cancer [135], as well as non-cancerous diseases such as diabetes and cardiovascular diseases [136]. It can also replace essential elements like Fe, Cu, and Zn in various cytoplasmic and membrane proteins and cause oxidative stress through the Fenton reaction [90]. Chronic exposure to Cd, in addition to inhibiting the activity of superoxide dismutase, which is known as one of the strongest antioxidant enzymes [137], also causes dysplastic lesions in the gastric glands [138].
Studies have shown that Cd creates a key step in the initiation of cancer and tumor stimulation by disrupting E-cadherin at cell junctions [139]. This mechanism has been reported to accelerate cancer growth by activating proto-oncogenes and genes involved in cell proliferation, reducing p53 function, and inhibiting DNA methylation, causing the clonal expansion of damaged and mutated cells [140]. Studies have shown that increased oxidative stress caused by exposure to Cd may cause cancer [141]. Zhang et al. (2016) in an epidemiological study concluded that the concentration of Cd in prostate tissue and plasma from patients with prostate cancer is substantially higher compared to healthy individuals [142]; this is consistent with the study of Zimta et al. (2019) [143]. However, a recent meta-analysis did not find any epidemiological evidence of the effect of Cd exposure in the general or occupational population with an increased risk of prostate cancer [144]. Epidemiological studies indicate that exposure to this heavy metal can increase the risk of lung cancer [145]. Based on available evidence, a doubling of Cd concentration leads to an approximate 68% increase in the relative risk of lung cancer [146]. Chronic exposure to Cd causes a significant decrease in the amount of glomerular filtration [147] and kidney and bladder cancer [82]. However, some recent studies did not find any relationship between occupational exposure to Cd and kidney cancer [36]. The study of Madrigal et al. (2019) also revealed that the decrease in kidney function associated with occupational exposure to Cd differs according to gender and the presence of comorbidities, that is, diabetes mellitus and high blood pressure [148].
Lead
Lead is a key player for esophageal and stomach cancers [112], brain tumors [35], kidney [33], thyroid [91], and testicular cancer [31]. Metal plating industries, mines, paints, cosmetic products, batteries, electronic waste, and the combustion of fossil fuels containing Pb are among the most important sources of Pb emission in the environment [149]. Increasing levels of this element in the environment can increase levels of human exposure and cause adverse health effects.
So far, many studies have investigated the effect of Pb concentration on various types of malignancy [31, 92, 93]. In their study, Tariba Lovaković et al. [31] investigated the effect of heavy metal levels on testicular cancer and concluded that Pb levels in the whole blood of patients (30 μg/L) were 1.25 times those of healthy people (23.9 μg/L). In addition, the results of a case–control study in Pakistan showed that stomach cancer sufferers have 1.8 times more Pb in their blood [91]; which was consistent with the results of the study by Sarkar et al. (2020) [87]. In this research, 150 tissue samples (case = 50, control = 100) were evaluated in terms of the concentration of this element and showed that the Pb levels in the case group were 1.15 times higher than those in the control group [87]. Similar results were observed with other studies on thyroid cancer [86], breast cancer [84], and bladder carcinoma [82].
Furthermore, Chrysochou et al. (2021) observed that the serum Pb level in leukemia patients was 154 times higher than that of healthy individuals [93], which is consistent with the results of the study by Guzel et al. [150]. Guzel et al. (2012) indicated that the blood and prostatic Pb levels in patients with prostatic intraepithelial neoplasia are significantly higher than those with benign prostatic hypertrophy (BPH) [150]. Studies have found a positive correlation between Pb and Al elements with increased levels of methylated MGMT and methylated MLH1, DNA repair enzymes [88], which is consistent with the results obtained by Scanlon et al. (2017). This study points to an effective role for metals in silencing the MLH1 promoter and in initiating the carcinogenesis process [151]. Devóz et al. (2017) showed that occupational exposure to Pb causes changes in DNA methylation and gene expression regulation in exposed workers [152]. The BRAF and KRAS genes are important in the process of the RAS/RAF/MAPK signaling pathway and in this way can regulate cell growth, differentiation, proliferation and apoptosis in malignant and non-malignant cells [153]. In a study, Talaat Abd Elaziz et al. (2020) investigated the effect of occupational exposure to heavy metals on colorectal cancer in industrial workers in Egypt and concluded that compared to unexposed tissues, there was a statistically significant increase in the expression of the BRAF and KRAS genes in malignant tissues [88]. This relationship has a positive correlation with methylated repair enzymes MLH1 and MGMT and exposure to Pb and Al metals [88]. Some researchers reported that mutations in the KRAS and BRAF genes in patients with colorectal carcinoma play a role in disease progression and response to treatment [154]. Until now, many epidemiological studies have shown a relationship between workers' exposure to inorganic Pb and lung, kidney, and brain cancers [155, 156], but no correlation was found with prostate cancer [95]. Several factors potentially account for the differences in the studies, including genetics, sex, exposure dose, duration of exposure, bone accumulation over time, and also the number of people examined in each study.
Arsenic
Arsenic is very relevant because groundwater and drinking water supplies can exceed the Maximum Contaminant Level Goal (MCLG) of 10 µg/L (USAEPA) and indicate a health risk [31, 86, 92]. In the USA, around 7% of the wells contain As above the MCLG [157]. Sarkar et al. (2020) revealed that the mean level of As in the group of patients with colorectal cancer vs healthy people was 2.12 ± 1.04 and 1.43 ± 0.73 ppm, respectively, and this difference was statistically significant [87]. Also, exposure to this element at a sufficient dose and for a suitable period, by creating a dose–response relationship between As and cancer, leads to the progression of carcinogenesis [82]. In addition, the evaluation of serum levels of Cd and As with bladder cancer indicates that As levels in patients are 1.48 times those of healthy people [32]. Chrysochou et al. (2021) reported a 1440-fold difference in serum As concentration in Leukemia patients compared to the control group [93]. The results of this research were consistent with the study conducted by Chang et al. (2016) [80].
Furthermore, Chrysochou et al. (2021) showed that there is a strong relationship between As, Cd, Ni, and Pb in patients with chronic leukemia compared to the control group [93] and the twofold levels of As increase the risk of thyroid cancer 5.35 times [86]. Arsenic alters the transcription process in gene-related actions, inhibits glutathione, decreases antioxidant defense mechanisms, and increases free radicals [158, 159]. Talaat et al. (2011) [159] showed that Kindlin-2 protein is expressed in the stromal element of transplanted and archival samples of human bladder cancer associated with As. Prostate tissues are a target of As [105, 160]. Some studies do not support the relationship between As in prostate cancer and breast cancer [89, 95]. There are significant differences in tissue As concentrations. The reason could be the difference in the type of cancer and the accumulation of these metals in different tissues according to the biological characteristics of the involved cells [124]. Malandrino et al. (2020) [161] showed that the concentration of many elements such as As in thyroid tissue was higher than in sternothyroid muscle and subcutaneous neck fat, which could be a good explanation for this difference.
Today, there are many natural and human sources for exposure to As, which can increase the risk of various types of cancer in exposed people. Among the most important of these sources, the industries of metal smelting, fossil fuel combustion, coal power plants, industrial wastewater, pesticides, mining, and volcanic activities [162] are mentioned.
Nickel
In this review, 23 studies examined the relationship between Ni levels and cancer, and 78% of the studies reported elevated concentrations of this metal associated with several cancers. Concentrations of Ni in serum were different in acute and chronic leukemias vs the control group (22 and 2.2 times, respectively) [93]. These results are consistent with studies conducted in the breast [84], thyroid [91], bladder [82], lymphoma [30], prostate [96], and brain [35]. Lovaković et al. (2021) also showed that the total blood/serum Ni concentration of patients with testicular germ cell tumors (TGCT) was significantly higher than that of healthy subjects [31]. Nickel has various carcinogenic mechanisms, including induction of DNA changes, reduction in lymphocyte telomere length, inhibition of intercellular transfer mechanisms, inhibition of nucleotide excision maintenance, oxidative stress and DNA methylation, and endocrine disruptor.
Despite the evidence confirming the carcinogenicity of Ni, some studies have reported results different from those mentioned [31, 85, 94]. The accumulation and effects of Ni are different according to the type of cell/tumor [163]. Lee et al. (2022) in a recent case–control study in Taiwan showed that urinary Ni levels in patients with lung cancer were 8.72 μg/g CR, in patients with other malignancies were 5.9 μg/g CR, and in healthy subjects 11.63 μg/g CR [94]. The results of their study were consistent with the research conducted by Tariba Lovaković et al. (2021) [31]. Tariba Lovaković et al. in the study of the relationship between exposure to low levels of metals and testicular cancer concluded that although the urinary levels of Ni in healthy people are higher than in patients, this concentration was opposite in whole blood and serum samples [31]. The reason for this difference can be related to the physicochemical characteristics and the absorption and metabolism process of each metal in different target tissues.
In addition, increasing levels of exposure to Ni from volcanic activity, wild forest fires, windblown dust, fossil fuel combustion, commercial and industrial applications such as electroplating, stainless steel, battery manufacturing industries, industrial waste, stainless steel kitchen utensils, dental or orthopedic implants, tobacco, and cheap jewelry can increase the risk of various types of malignancies [164, 165].
Chromium
In this systematic review, 87% of Cr studies associated with cancer reported higher concentrations in cancer patients than in controls [93, 112]. The results of examination of the metal imbalance in the blood of patients with thyroid cancer showed that the levels of this element in the case group were 1.28 times those of the control group (757.9 vs 588.8 μg/dL) [91]. In addition, Afzal et al. (2020) achieved similar results in the study of stomach cancer [90]. The investigation of the effect of heavy metals on breast cancer progression also reported a 2.6-fold difference between sick and healthy people [54], which is consistent with the results of the study by Abdel-Gawad et al. (2016) [82] and Chang et al. (2016) [80]. Qayyum and Shah (2019) investigated the average Cr contents in blood and hair from lymphoma patients versus healthy controls and found a significant difference, 59.43 µg/dl vs 34.95 µg/dl, respectively [30].
Chromium levels can increase in the environment through mining, steel and metal alloy industries, paint production, wood and paper processing, burning coal ash, or the use of municipal waste for energy production and second generation fertilizer production [166]. Chromium is present in contaminated drinking water [167], and its effects other than oncological, include an endocrine disrupting capacity [168]. Das et al. (2015) reported mitochondrial-dependent apoptosis in male somatic cells and spermatogonial stem cells by hexavalent Cr(Cr (VI)), a pathway for reproductive abnormalities and infertility. Paternal exposure to heavy metals and solvents increases the risk of their sons developing a testicular germ cell tumor (TGCT) [169]. However, some previous evidence shows that there is no statistically significant risk between paternal exposure to heavy metals/welding fumes and boys suffering from TGCT, which requires more and more extensive studies to reach a definitive result [170]. A recent case–control study proved that maternal exposure to Cr increases the chance of Tenosynovial Giant Cell Tumor (TGCT) occurrence in male children [171]. Krstev et al. (2019) reported in a meta-analysis that occupational exposures to pesticides, Cr (VI), polycyclic aromatic hydrocarbons, diesel fumes, metal fabrication environments, vehicle batteries, flight personnel, jobs causing circadian disruption, firefighters, sewage and petroleum and gasoline workers increase the risk of prostate cancer [172]. In vitro and in vivo studies show that even exposure to low doses of Cr (VI) can affect the epithelial-mesenchymal pathway (EMT), cause the growth and migration of prostate cancer cells, and follow the progress of tumor and metastasis [173]. Rafnsson et al. (1997) reported the cancer incidence of an Iceland cohort of 1172 masons exposed to wet concrete aerosols and Cr (VI) exposures associated with cement. These masons had an increase in lung cancer with a standardized incidence ratio (SIR) of 1.69 and 1.77 after 30 years of work [174].
Mercury (Hg)
In this systematic review, 11 studies investigated the effect of Hg levels on thyroid [58, 86], breast [68, 89], lung [76, 94, 109], prostate [95], brain tumor [35], and other types of cancers [105]. The results showed that 62.5% of the case–control studies conducted in this field confirmed higher levels of Hg in the biological samples of patients compared to healthy people. Pizent et al. (2022) investigated the role of metal exposure in prostate cancer and concluded that the mean Hg levels in blood/serum of the case group were 2.43 times that of the control group [95]. In addition, some researchers have reported a twofold difference in the concentration of this metal in the urine of healthy people and those with thyroid cancer, confirming the existence of a direct relationship between exposure to heavy metals and the development of malignancies [86]. The results of these studies were consistent with the investigations conducted by Binkowski et al. (2015) [76] and Alatise et al. (2010) [68].
Cement, iron and steel, gold, and chloro-alkali industries, non-ferrous metal smelting, dental amalgam, forest fires, and volcanoes are among the main sources of Hg release in the environment and exposure of humans to this dangerous element [175]. Some laboratory studies concluded that Hg causes hypomethylation and hypermethylation of G protein signaling; therefore, it can act as a driving force for tumor growth [176]. One of the most important mechanisms in pathologies caused by Hg is oxidative stress [177]. Mercury has a strong ability to deplete intracellular thiols (especially glutathione) and bind to thiol groups on proteins. Although the exact mechanism of ROS production by Hg is not yet known, it seems that this mechanism depends on the physical and chemical form of the element [95]. For example, the detection of Hg in blood reveals exposure to organic methylmercury (MeHg) associated with eating seafood or inhaling elemental mercury vapor [95]. Studies have shown that the high affinity of this organic compound for selenohydryl groups, thiols, and selenides can disrupt the structure and function of antioxidant enzymes and proteins [178].
Although many researchers have proven the role of exposure to Hg in various types of malignancies, some studies have reached contradictory results. The results obtained from the study by Lee et al. (2022) showed that the urinary Hg concentration in patients with lung cancer was measured at 1.57 μg/g CR, while in healthy subjects urinary Hg concentration was equal to 2.3 μg/g CR [94]. In addition, a case–control study conducted in China showed that urinary Hg levels in the control group (24 μg/L) were 6 times higher than those with breast cancer (4 μg/L) [89]. The results of these two studies were consistent with the research conducted by Gaman et al. (2019) [35]. In this study, the concentration of Hg in tissue-blood samples from healthy people was higher than in patients with brain tumors [35].
The difference in the results of this section can be considered related to the difference in the type of biological sample examined and the duration of Hg detection (according to the Hg half-life). Studies have shown that Hg is distributed and accumulated in most vital organs, mainly in the central nervous system and kidneys [179]. Blood Hg concentration decreases with a half-life of nearly 50 days [180]. Researchers reported that 90% of this element is excreted through feces and only 10% through urine [181, 182].
Essential elements
Some of the metals such as Co, Cu, Fe, Mn, Cr (III), Zn, Se, and Mo also have a protective role in the processes of chromosome damage and oxidation [38, 39] and are vital for the body in low concentrations. Metals play key roles in antioxidant defense, hemoglobin and energy production in the aerobic respiration process, preserving normal nerve and muscle function, and aiding the immune system [183]. Some of these essential metals are also an integral part of antioxidant enzymes such as selenoenzymes, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) and are effective in maintaining sufficient amounts of metallothionein (MT), reducing ROS, and subsequently, oxidative DNA damage [184]. In addition, they can act as cofactors of DNA repair proteins and as essential components of ribonucleotide reductase in DNA synthesis and help in nucleotide acid metabolism [185].
The researchers showed that the increase in the concentration of essential elements in the body follows a U-shaped dose–response relationship. Accordingly, at very low doses of essential micronutrients, a high level of complications will occur (known as "deficiency"), which will decrease as the dose increases. In addition, very high doses also have the potential to cause high levels of side effects, which decrease as the dose is reduced. When the deficiency is removed by increasing the dose and no adverse reactions are detected, the organism reaches a state of homeostasis. Generally, Co, Cu, Fe, Mg, Mn, Mo, Se, and Zn are accepted as essential metals, each of which has a unique deficiency point and toxicity. This is happening while there is no deficiency and homeostasis area in the dose–response curve in toxic elements. In this situation, depending on the measured endpoint, the shape of this curve may be an inverted U or J shape. According to studies, if endpoints are related to growth, longevity, fertility, or cognitive function, the response is inverted U-shaped and in the case of disease, it is seen as J-shaped [186].
The results of the present study showed higher concentrations of these metals in the healthy control group than in the case group, which supports their preventive role in the onset and progression of cancer. Case–control studies indicate an inverse relationship between plasma Zn level and the risk of cancers of the lung [34], cervix [59], lung [34, 78], prostate [74, 75], thyroid [91], mesothelioma [92], and lymphoma [30] cancers. Under physiological conditions, Zn prevents the formation of free radicals, participates in the cellular immunity of T lymphocytes, and prevents tumor growth [187]. Zinc also plays a role in the antiangiogenic activity of endostatin cell proliferation and intracellular signaling pathways, and the serum level reduction can increase the risk of cancer [43].
Selenium is an important micronutrient for cancer prevention, which can effectively treat kidney cancer by inhibiting the proteins hypoxia-inducible factor 1- and 2-alpha (HIF 1α and HIF 2α) and the nuclear factor erythroid 2- (Nrf2) [188]. According to the studies, the presence of permissible concentrations of Se (230–250 ng/mL of blood) reduces the risk of cancer [189]. The results of the study by Hsueh et al. (2021) showed that higher plasma Se levels significantly reduce the chances of renal cell carcinoma (RCC) [33], which was consistent with the results of Bock et al. (2018). Chitta et al. (2013) showed that Se can reduce As toxicity by changing cytotoxicity, genotoxicity, and oxidative stress [190]. It also reduces the Cd oxidative stress by reducing serum malondialdehyde and increasing the activity of superoxide dismutase and glutathione peroxidase [191]. The mechanism of the Se effect against the reduction of As and Cd toxicity may be through Se-related antioxidant enzymes or the activation of the Nrf2 pathway [192].
Many studies in the present review showed that the concentration of Mn in the control group was higher than in the case group [31, 34, 35, 94, 112]. Manganese is also considered as an essential micronutrient for intracellular activities. Some studies have shown that nanoparticles of compounds of Mn suppress oxidative stress and DNA damage by imitating several enzymes [193]. It also acts as a cofactor for antioxidants such as Mn superoxide dismutase (MnSOD), which play a role in antioxidant defense [194]. Sohrabi et al. (2021) reported in a cross-sectional study that the Mn content in cancerous tissues is lower than in adjacent healthy tissues [112], which supports this mechanism.
Copper is an essential element and an integral constituent of various metalloenzymes. Studies have determined that the normal range of this element is 700–1400 ng/mL of blood [189]. Marzano et al. (2009) investigated various types of Cu complexes as possible antitumor agents [195]. Both decreased or increased Cu levels are associated with genetic disorders of Cu metabolism and with high environmental exposure resulting in severe pathologies [196]. Available reports indicate that the lack of trace elements such as Fe, Zn, and Cu is related to bladder cancer [197, 198].
The high concentration of Fe in healthy people compared to patients in some studies suggests a potential effect of Fe in preventing the occurrence or progression of cancer [34,35,36, 66, 81, 90]. Fonseca-Nunes et al. (2015) showed that higher serum Fe and ferritin is inversely related to the risk of stomach cancer [199]. Cook et al. (2012), however, reported that Fe metrics were not associated with neither gastric cardia or non-cardia cancers when taking into account the roles of Helicobacter pylori and gastric atrophy [200]. Puliyel et al. (2015) discussed Fe toxicity in children and adults undergoing treatments for neoplastic processes and the importance of transferrin saturation (TS), associated with toxic free Fe, and showed that high TS is associated with cancer development and indeed, lowering free Fe decreases the risk [201]. Cook et al. (2012) also obtained similar results [200]. Also, the investigations conducted revealed that the level of Fe in cancerous tissues was lower compared to healthy tissues [201], which could be due to the lower absorption of this metal in cancerous tissue. Fe is a vital micronutrient for oxygen transport and oxidative metabolism [202]. Deficiency of Fe, for example in Fe-deficient anemia, has a serious impact on physical and cognitive function with a severe reduction in quality of life [203]. It can also cause side effects such as increased DNA damage, decreased antioxidant defense, decreased enzyme activity, and subsequently increased genomic instability [203].
Trivalent cobalt (Co (III)) and chromium (Cr (III)) also participate in many biological mechanisms as essential elements. Cobalt constitutes the central atom of cobalamin (vitamin B12), and is also considered an important nutrient to maintain testicular function and normal fertility [204]. This element impacts DNA synthesis and cell division and participates in purines and pyrimidine production as a cofactor of methionine synthase [205]. Based on its capacity, Cr can be classified into an essential or toxic group. Trivalent chromium has an effective role in the metabolism of carbohydrates, proteins, and fats. However, Cr (VI) is classified as a toxic metal due to its angiogenic and carcinogenic activities [206, 207]. Bibi et al. (2020) investigated the imbalance of metals in the blood of thyroid cancer patients versus healthy individuals [91]. Concentrations of Pb (774.6 vs. 416.2 μg/dL), Cr (757.9 vs. 588.8 μg/dL), Cd (472.5 vs. 344.6 μg/dL) and Ni (360.5 vs. 200 μg/dL) were higher in cancer patients, while Co was higher in controls (2073 vs. 977 μg/dL).
There is a complex balance among essential elements in the prevention and progression of cancer, and several other factors, such as genetic protector and detrimental mechanisms, and the environment play a key role in the final outcomes [42, 44, 93].
Strengths and limitations
The strength of the present study is that it is the first systematic review that examines two opposite effects of metal exposure on carcinogenesis. This study provides new information on the toxicity of metals, their carcinogenic properties, and, on the other hand, their preventive properties against cancer at different doses. Furthermore, the selected studies were retrieved by a comprehensive systematic search, without restrictions on publication date, study type, or country of investigation. Adopting this approach allowed the highest number of relevant articles to be entered without the loss of scientific data. However, the limitation of this study was the lack of access to the full text of some articles and limiting the database search to studies in the English language only.
Gaps and recommendations
A comprehensive review of published studies shows the existence of some gaps in this important area of health, including the lack of information on some heavy metals. According to the available evidence, most studies published in this field have investigated the concentration of metals in only one type of biological sample, which may lack sufficient accuracy and representation. For example, measuring the levels of some metals in urine samples can reflect short-term exposure. Therefore, depending on the physicochemical characteristics and the unique absorption and metabolism processes of each metal in target tissues, detecting element levels only in one type of biological body sample may not be a suitable measure to reflect internal exposure levels for all types of metals. Therefore, more detailed studies are recommended to cover this important gap.
It is also recommended to design more prospective cohort studies with independent replications to investigate the role of prenatal exposure to toxic metals in the individual's incidence of various types of cancer during the years after birth. Investigating the unique characteristics and activities of vital body organs such as the thyroid, brain, and kidney, which can make them more susceptible to the carcinogenic activity of some toxic metals, seems necessary.
Considering the slow process of removing toxic metals from the body, it seems that using methods to accelerate this process can play an effective role in reducing the accumulation of these elements in the target organs and preventing adverse health effects. The surveys conducted indicate that few clinical trial studies have been conducted in this field [208]. Therefore, it is recommended to design and implement more clinical studies to achieve an effective method.
Conclusions
The presented review indicated that chronic exposure to metals, even in low concentrations, can be a potential risk factor for various types of cancer. The main elements found in the biological samples of the patients included Pb, Cr (VI), As, Cd, and Ni and these elements were identified in many studies. Generally, the levels of these metals in clinical cases were higher than in controls, which demonstrates the carcinogenicity of these metals. In addition, the investigation of the effect of essential metals such as Se, Zn, Fe, and Mn on the occurrence of adverse health effects revealed that low concentrations of these elements indicated anticancer properties and that their high concentrations could be the cause of biological toxicity. Therefore, according to the biological accumulation property of heavy metals in the vital organs of the body, regular monitoring of toxic metal concentrations in biological and clinical samples is recommended to identify possible sources of their exposure. In addition, authorities should adopt stricter laws to control and reduce metal emissions into the environment and to protect workers. This will significantly reduce the risk from dermal, inhalational, and ingestion exposure pathways from these metals and will play an effective role in reducing the cancer occurrence.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Stand together against cancer - World Cancer Day 2023 [https://www.who.int/multi-media/details/stand-together-against-cancer---world-cancer-day-2023]
Cancer prevention and control in the context of an integrated approach [ https://www.who.int/publications/i/item/cancer-prevention-and-control-in-the-context-of-an-integrated-approach]
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70(1):93–105.
Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, Galvano F, Giovannucci EL. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. 2017;75(6):405–19.
Kamal N, Ilowefah MA, Hilles AR, Anua NA, Awin T, Alshwyeh HA, Aldosary SK, Jambocus NG, Alosaimi AA, Rahman A, et al. Genesis and mechanism of some cancer yypes and an overview on the role of diet and nutrition in cancer prevention. Molecules. 2022;27:1–27.
Jones A. Pathology of Cancer: causes, pathophysiology, diagnosis, prevention and treatment. Int J Surg Pathol. 2023;8(2):1–2.
Chervona Y, Arita A, Costa M. Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics. 2012;4(7):619–27.
Zhu Y, Costa M. Metals and molecular carcinogenesis. Carcinogenesis. 2020;41(9):1161–72.
Salnikow K, Zhitkovich A. Genetic and Epigenetic Mechanisms in Metal Carcinogenesis and Cocarcinogenesis: Nickel, Arsenic, and Chromium. Chem Res Toxicol. 2008;21(1):28–44.
Bhandari P. Impacts on Environment and on Human Health. In: Leal Filho W, Azul AM, Brandli L, özuyar PG, Wall T, editors. Responsible Consumption and Production. Cham: Springer International Publishing; 2020. p. 349–57.
Landrigan PJ, Fuller R, Acosta NJ, Adeyi O, Arnold R, Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN. The Lancet Commission on pollution and health. Lancet. 2018;391(10119):462–512.
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–18.
Balakrishnan K, Dey S, Gupta T, Dhaliwal RS, Brauer M, Cohen AJ, Stanaway JD, Beig G, Joshi TK, Aggarwal AN, et al. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the Global Burden of Disease Study 2017. Lancet Planet Health. 2019;3(1):e26–39.
Mohammadzadeh M, Mirzaei N, Mostafaii G, Atoof F, Miranzadeh MB, Dehghani R. Determination of potentially toxic metals in depilatory products in the Iranian markets: human health risk assessment. Environ Sci Pollut Res. 2022;29(9):13756–65.
Martinez-Morata I, Bostick BC, Conroy-Ben O, Duncan DT, Jones MR, Spaur M, Patterson KP, Prins SJ, Navas-Acien A, Nigra AE. Nationwide geospatial analysis of county racial and ethnic composition and public drinking water arsenic and uranium. Nat Commun. 2022;13(1):7461.
Choiniere J, Wang L. Exposure to inorganic arsenic can lead to gut microbe perturbations and hepatocellular carcinoma. Acta Pharm Sin B. 2016;6(5):426–9.
Hodgkin DC, Kamper J, Mackay M, Pickworth J, Trueblood KN, White JG. Structure of vitamin B 12. Nature. 1956;178:64–6.
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol. 2021;12:1–19.
Bond CE, Liu C, Kawamata F, McKeone DM, Fernando W, Jamieson S, Pearson S-A, Kane A, Woods SL, Lannagan TRM, et al. Oncogenic BRAF mutation induces DNA methylation changes in a murine model for human serrated colorectal neoplasia. Epigenetics. 2018;13(1):40–8.
Mortoglou M, Manić L, Buha Djordjevic A, Bulat Z, Đorđević V, Manis K, Valle E, York L, Wallace D, Uysal-Onganer P. Nickel’s Role in Pancreatic Ductal Adenocarcinoma: Potential Involvement of microRNAs. Toxics. 2022;10(3):148.
Wallace DR, Taalab YM, Heinze S, Tariba Lovaković B, Pizent A, Renieri E, Tsatsakis A, Farooqi AA, Javorac D, Andjelkovic M, et al. Toxic-Metal-Induced Alteration in miRNA Expression Profile as a Proposed Mechanism for Disease Development. Cells. 2020;9(4):901.
Wang Z, Yang C. Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis. Semin Cancer Biol. 2019;57:95–104.
Cowley M, Skaar DA, Jima DD, Maguire RL, Hudson KM, Park SS, Sorrow P, Hoyo C. Effects of Cadmium Exposure on DNA Methylation at Imprinting Control Regions and Genome-Wide in Mothers and Newborn Children. Environ Health Perspect. 2018;126(3):037003.
Nuttall JR, Oteiza PI. Zinc and the ERK Kinases in the Developing Brain. Neurotox Res. 2012;21(1):128–41.
Peris-Díaz MD, Guran R, Domene C, de los Rios V, Zitka O, Adam V, Krężel A. An integrated mass spectrometry and molecular dynamics simulations approach reveals the spatial organization impact of metal-binding sites on the stability of metal-depleted metallothionein-2 species. J Am Chem Soc. 2021;143(40):16486–501.
Qian Y, Castranova V, Shi X. New perspectives in arsenic-induced cell signal transduction. J Inorg Biochem. 2003;96(2–3):271–8.
Singh KB, Maret W. The interactions of metal cations and oxyanions with protein tyrosine phosphatase 1B. Biometals. 2017;30(4):517–27.
Donaldson K, Stone V, Borm PJA, Jimenez LA, Gilmour PS, Schins RPF, Knaapen AM, Rahman I, Faux SP, Brown DM, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic Biol Med. 2003;34(11):1369–82.
Barthel A, Ostrakhovitch EA, Walter PL, Kampkötter A, Klotz L-O. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: Mechanisms and consequences. Arch Biochem Biophys. 2007;463(2):175–82.
Qayyum MA, Shah MH. Disparities in the concentrations of essential/toxic elements in the blood and scalp hair of lymphoma patients and healthy subjects. Sci Rep. 2019;9(1):15363.
Tariba Lovaković B, Živković Semren T, Safner T, Gamulin M, Soče M, Pizent A. Is low-level metal exposure related to testicular cancer?. J Environ Sci Health C. 2021;39(1):87–107.
Moazed V, Jafari E, Ebadzadeh MR, Pourzare A, Gharehchahi HR, Mangeli F, Jamali E. Association between serum cadmium and arsenic levels with bladder cancer: a case-control study. Int J Cancer Manag. 2021;14(7):1–5.
Hsueh Y-M, Lin Y-C, Huang Y-L, Shiue H-S, Pu Y-S, Huang C-Y, Chung C-J. Effect of plasma selenium, red blood cell cadmium, total urinary arsenic levels, and eGFR on renal cell carcinoma. Sci Total Environ. 2021;750:141547.
Bai Y, Wang G, Fu W, Lu Y, Wei W, Chen W, Wu X, Meng H, Feng Y, Liu Y, et al. Circulating essential metals and lung cancer: Risk assessment and potential molecular effects. Environ Int. 2019;127:685–93.
Gaman L, Radoi MP, Delia CE, Luzardo OP, Zumbado M, Rodríguez-Hernández Á, Stoian I, Gilca M, Boada LD, Henríquez-Hernández LA. Concentration of heavy metals and rare earth elements in patients with brain tumours: analysis in tumour tissue, non-tumour tissue, and blood. Int J Environ Health Res. 2021;31(7):741–54.
Michalek IM, Martinsen JI, Weiderpass E, Hansen J, Sparen P, Tryggvadottir L, Pukkala E. Heavy metals, welding fumes, and other occupational exposures, and the risk of kidney cancer: a population-based nested case-control study in three Nordic countries. Environ Res. 2019;173:117–23.
Rhee J, Vance TM, Lim R, Christiani DC, Qureshi AA, Cho E. Association of blood mercury levels with nonmelanoma skin cancer in the U.S.A. using National Health and Nutrition Examination Survey data (2003–2016). Br J Dermatol. 2020;183(3):480–7.
Bai Y, Feng W, Wang S, Zhang X, Zhang W, He M, Zhang X, Wu T, Guo H. Essential metals zinc, selenium, and strontium protect against chromosome damage caused by polycyclic aromatic hydrocarbons exposure. Environ Sci Tech. 2016;50(2):951–60.
Xu P, Chen Z, Chen Y, Feng L, Wu L, Xu D, Wang X, Lou X, Lou J. Body burdens of heavy metals associated with epigenetic damage in children living in the vicinity of a municipal waste incinerator. Chemosphere. 2019;229:160–8.
Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9(7):775–806.
Baek Y, Woo T-G, Ahn J, Lee D, Kwon Y, Park B-J, Ha N-C. Structural analysis of the overoxidized Cu/Zn-superoxide dismutase in ROS-induced ALS filament formation. Commun Biol. 2022;5(1):1085.
Hrabeta J, Eckschlager T, Stiborova M, Heger Z, Krizkova S, Adam V. Zinc and zinc-containing biomolecules in childhood brain tumors. J Mol Med. 2016;94(11):1199–215.
Stepien M, Hughes DJ, Hybsier S, Bamia C, Tjønneland A, Overvad K, Affret A, His M, Boutron-Ruault M-C, Katzke V, et al. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br J Cancer. 2017;116(5):688–96.
Scharf B, Clement CC, Zolla V, Perino G, Yan B, Elci SG, Purdue E, Goldring S, Macaluso F, Cobelli N, et al. Molecular analysis of chromium and cobalt-related toxicity. Sci Rep. 2014;4(1):5729.
Mathur J, Goswami P, Gupta A, Srivastava S, Minkina T, Shan S D, Rajput V. Nanomaterials for water remediation: an efficient strategy for prevention of metal(loid) hazard. Water. 2022;14(24):3998.
Mahato M, Sherman NE, Kiran Kumar Mudnakudu N, Joshi N, Briand E, Karp JM, Vemula PK. Prevention of metal exposure: chelating agents and barrier cream. In: Chen JK, Thyssen JP, editors. Metal allergy: from dermatitis to implant and device failure. Cham: Springer International Publishing; 2018. p. 227–46.
Landrigan Pj, Lucchini RG, Kotelchuck D, Grandjean P. Chapter 24 - Principles for prevention of the toxic effects of metals. In: Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the toxicology of metals (Fourth Edition). San Diego: Academic Press; 2015. p. 507–28.
Awan SA, Khan I, Rizwan M, Ali Z, Ali S, Khan N, Arumugam N, Almansour AI, Ilyas N. A new technique for reducing accumulation, transport, and toxicity of heavy metals in wheat (Triticum aestivum L.) by bio-filtration of river wastewater. Chemosphere. 2022;294:133642.
Bilardi S, Calabrò PS, Greco R, Moraci N. Selective removal of heavy metals from landfill leachate by reactive granular filters. Sci Total Environ. 2018;644:335–41.
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, Blunt H, Brigham T, Chang S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39.
Yu L, Wang B, Cheng M, Yang M, Gan S, Fan L, Wang D, Chen W. Association between indoor formaldehyde exposure and asthma: a systematic review and meta-analysis of observational studies. Indoor Air. 2020;30(4):682–90.
Hammer D, Calocci A, Hasselblad V, Williams M, Pinkerson C. Cadmium and lead in autopsy tissues. J Occup Med. 1973;15(12):956–63.
Hojo Y. Subject groups high and low in urinary selenium levels: workers exposed to heavy metals and patients with cancer and epilepsy. Bull Environ Contam Toxicol. 1981;26(4):466–71.
Gerhardsson L, Wester PO, Nordberg GF, Brune D. Chromium, cobalt and lanthanum in lung, liver and kidney tissue from deceased smelter workers. Sci Total Environ. 1984;37(2–3):233–46.
Gerhardsson L, Brune D, Nordberg GF, Wester PO. Selenium and other trace elements in lung tissue in smelter workers relationship to the occurrence of lung cancer. Acta Pharmacol Toxicol (Copenh). 1986;59(Suppl 7):256–9.
Gerhardsson L, Brune D, Nordberg GF, Wester PO. Distribution of cadmium, lead and zinc in lung, liver and kidney in long-term exposed smelter workers. Sci Total Environ. 1986;50:65–85.
Gerhardsson L, Brune D, Nordberg GF, Wester PO. Multielemental assay of tissues of deceased smelter workers and controls. Sci Total Environ. 1988;74:97–110.
Zaichick VY, Tsyb AF, Vtyurin BM. Trace elements and thyroid cancer. Analyst. 1995;120(3):817–21.
Cunzhi H, Jiexian J, Xianwen Z, Jingang G, Shumin Z, Lili D. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res. 2003;94(2):113–22.
Yaman M, Atici D, Bakırdere S, Akdeniz İ. Comparison of trace metal concentrations in malign and benign human prostate. J Med Chem. 2005;48(2):630–4.
Kriegel AM, Soliman AS, Zhang Q, El-Ghawalby N, Ezzat F, Soultan A, Abdel-Wahab M, Fathy O, Ebidi G, Bassiouni N, et al. Serum cadmium levels in pancreatic cancer patients from the East Nile delta region of Egypt. Environ Health Perspect. 2006;114(1):113–9.
Kuo C-Y, Wong R-H, Lin J-Y, Lai J-C, Lee H. Accumulation of chromium and nickel metals in lung tumors from lung cancer patients in Taiwan. J Toxicol Environ Health - A. 2006;69(14):1337–44.
Kellen E, Zeegers M, Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case–control study on bladder cancer. Int J Urol. 2006;13(9):1180–4.
Pasha Q, Malik SA, Iqbal J, Shah MH. Characterization and distribution of the selected metals in the scalp hair of cancer patients in comparison with normal donors. Biol Trace Elem Res. 2007;118(3):207–16.
Kazi TG, Memon AR, Afridi HI, Jamali MK, Arain MB, Jalbani N, Sarfraz RA. Determination of cadmium in whole blood and scalp hair samples of Pakistani male lung cancer patients by electrothermal atomic absorption spectrometer. Sci Total Environ. 2008;389(2):270–6.
Pasha Q, Malik SA, Shah MH. Statistical analysis of trace metals in the plasma of cancer patients versus controls. J Hazard Mater. 2008;153(3):1215–21.
Chiang C-T, Chang T-K, Hwang Y-H, Su C-C, Tsai K-Y, Yuan T-H, Lian I-B. A critical exploration of blood and environmental chromium concentration among oral cancer patients in an oral cancer prevalent area of Taiwan. Environ Geochem Health. 2011;33(5):469–76.
Alatise OI, Schrauzer GN. Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol Trace Elem Res. 2010;136(2):127–39.
Klatka J, Remer M, Dobrowolski R, Pietruszewska W, Trojanowska A, Siwiec H, Charytanowicz M. The content of cadmium, cobalt and nickel in laryngeal carcinoma. Arch Med Sci. 2011;7(3):517–22.
Khlifi R, Olmedo P, Gil F, Hammami B, Chakroun A, Rebai A, Hamza-Chaffai A. Arsenic, cadmium, chromium and nickel in cancerous and healthy tissues from patients with head and neck cancer. Sci Total Environ. 2013;452–453:58–67.
Khlifi R, Olmedo P, Gil F, Feki-Tounsi M, Chakroun A, Rebai A, Hamza-Chaffai A. Blood nickel and chromium levels in association with smoking and occupational exposure among head and neck cancer patients in Tunisia. Environ Sci Pollut Res. 2013;20(11):8282–94.
Ene CD, Nicolae I, Musetescu A, Tampa M, Matei C, Georgescu SR. Exposure to heavy metals in plastics industry and dyes-risk factor in the development of skin cancer. Mater Plast. 2014;51(2):180–4.
Khlifi R, Olmedo P, Gil F, Feki-Tounsi M, Hammami B, Rebai A, Hamza-Chaffai A. Risk of laryngeal and nasopharyngeal cancer associated with arsenic and cadmium in the Tunisian population. Environ Sci Pollut Res. 2014;21(3):2032–42.
Neslund-Dudas C, Kandegedara A, Kryvenko ON, Gupta N, Rogers C, Rybicki BA, Dou QP, Mitra B. Prostate tissue metal levels and prostate cancer recurrence in smokers. Biol Trace Elem Res. 2014;157(2):107–12.
Qayyum MA, Shah MH. Comparative study of trace elements in blood, scalp hair and nails of prostate cancer patients in relation to healthy donors. Biol Trace Elem Res. 2014;162(1):46–57.
Binkowski ŁJ, Rogoziński P, Roychoudhury S, Bruliński K, Kucharzewski M, Łaciak T, Massanyi P, Stawarz R. Accumulation of metals in cancerous and healthy tissues of patients with lung cancer in Southern Poland. J Environ Sci Health A. 2015;50(1):9–15.
Sherief LM, Abdelkhalek ER, Gharieb AF, Sherbiny HS, Usef DM, Almalky MAA, Kamal NM, Salama MA, Gohar W. Cadmium status among pediatric cancer patients in Egypt. Medicine (Baltimore). 2015;94(20):e740.
Kazi TG, Wadhwa SK, Afridi HI, Talpur FN, Tuzen M, Baig JA. Comparison of essential and toxic elements in esophagus, lung, mouth and urinary bladder male cancer patients with related to controls. Environ Sci Pollut Res. 2015;22(10):7705–15.
Peng L, Huang Y, Zhang J, Peng Y, Lin X, Wu K, Huo X. Cadmium exposure and the risk of breast cancer in Chaoshan population of southeast China. Environ Sci Pollut Res. 2015;22(24):19870–8.
Chang C-H, Liu C-S, Liu H-J, Huang C-P, Huang C-Y, Hsu H-T, Liou S-H, Chung C-J. Association between levels of urinary heavy metals and increased risk of urothelial carcinoma. Int J Urol. 2016;23(3):233–9.
Klimczak M, Dziki A, Kilanowicz A, Sapota A, Duda-Szymańska J, Daragó A. Concentrations of cadmium and selected essential elements in malignant large intestine tissue. Prz Gastroenterol. 2016;11(1):24–9.
Abdel-Gawad M, Elsobky E, Shalaby MM, Abd-Elhameed M, Abdel-Rahim M, Ali-El-Dein B. Quantitative evaluation of heavy metals and trace elements in the urinary bladder: comparison between cancerous, adjacent non-cancerous and normal cadaveric tissue. Biol Trace Elem Res. 2016;174(2):280–6.
Ostadrahimi A, Payahoo L, Somi MH, Hashemzade SH, Esfahani A, Asgharijafarabadi M, Mobasseri M, Samadi N, Faraji S, KhajeBishak Y. The association between blood cadmium levels and the risk of gastrointestinal cancer in Tabriz, northwest of Iran. Pol Ann Med. 2017;24(2):133–7.
Romaniuk A, Lyndin M, Sikora V, Lyndina Y, Romaniuk S, Sikora K. Heavy metals effect on breast cancer progression. J Occup Med Toxicol. 2017;12(1):32.
Hussein HH, Alsabari EK, Kadhim BA, Hatif KH, AL-Khafaji QS, Hamidi SAK. Study the impact of the trace elements between the healthy females and who take chemotherapy for samples of Sera. Res J Pharm Technol. 2017;10(10):3323–5.
Zhang Q, Hu M, Wu H, Niu Q, Lu X, He J, Huang F. Plasma polybrominated diphenyl ethers, urinary heavy metals and the risk of thyroid cancer: A case-control study in China. Environ Pollut. 2021;269:116162.
Sarkar S, Mukherjee SK, Roy K, Ghosh P. Evaluation of Role of Heavy Metals in Causation of Colorectal Cancer. J Evol Med Dent Sci. 2020;9(13):1036–9.
Abd Elaziz AT, Elzahed HM, Hassan Ahmed S, Fathy W. Occupational Exposure to Heavy Metal Initiate Carcinogenesis Though BRAF/KRAS Over Expression and DNA Methylation. J Chem Health Risks. 2020;10(2):81–91.
Men Y, Li L, Zhang F, Kong X, Zhang W, Hao C, Wang G. Evaluation of heavy metals and metabolites in the urine of patients with breast cancer. Oncol Lett. 2020;19(2):1331–7.
Afzal A, Qayyum MA, Shah MH. Study of Trace Metal Imbalances in the Scalp Hair of Stomach Cancer Patients with Different Types and Stages. Biol Trace Elem Res. 2020;196(2):365–74.
Bibi K, Shah MH. Appraisal of Metal Imbalances in the Blood of Thyroid Cancer Patients in Comparison with Healthy Subjects. Biol Trace Elem Res. 2020;198(2):410–22.
Menevse E, Metin B, Sivrikaya A, Intepe YS, Gocmen AY. Importance of elements, vitamin D levels, and DNA damage in patients with asbestosis and mesothelioma. Trace Elem Electroly. 2021;38(1):21.
Chrysochou E, Koukoulakis K, Kanellopoulos PG, Sakellari A, Karavoltsos S, Dassenakis M, Minaidis M, Maropoulos G, Bakeas E. Human serum elements’ levels and leukemia: A first pilot study from an adult Greek cohort. J Trace Elem Med Biol. 2021;68:126833.
Lee N-W, Wang H-Y, Du C-L, Yuan T-H, Chen C-Y, Yu C-J, Chan C-C. Air-polluted environmental heavy metal exposure increase lung cancer incidence and mortality: A population-based longitudinal cohort study. Sci Total Environ. 2022;810: 152186.
Pizent A, Anđelković M, Tariba Lovaković B, Živković Semren T, Buha Djordjevic A, Gamulin M, Bonderović V, Aćimović M, Bulat Z. Environmental Exposure to Metals, Parameters of Oxidative Stress in Blood and Prostate Cancer: Results from Two Cohorts. Antioxidants. 2022;11(10):2044.
Alegre-Martínez A, Martínez-Martínez MI, Rubio-Briones J, Cauli O. Plasma Nickel Levels Correlate with Low Muscular Strength and Renal Function Parameters in Patients with Prostate Cancer. Diseases. 2022;10(3):39.
Hu D, Zhang L, Qin B, Wang N, Li X, Shi W. Association between Urinary Lead and Female Breast Cancer: A Population-Based Cross-Sectional Study. Discov Med. 2023;35(179):1177–89.
Essa HO, Al-Attiyah KH, Ali A-H. Estimation of Uranium Concentration of Cancer Patients’ Blood in Babylon Province. Iraq Pollution. 2024;10(1):236–47.
Singh V, Madeshiya AK, Ansari NG, Singh MK, Abhishek A. CYP1A1 gene polymorphism and heavy metal analyses in benign prostatic hyperplasia and prostate cancer: An explorative case-control study. Urol Oncol. 2023;41(8):355.e9-.e17.
Gray MA, Centeno JA, Slaney DP, Ejnik JW, Todorov T, Nacey JN. Environmental Exposure to Trace Elements and Prostate Cancer in Three New Zealand Ethnic Groups. Int J Environ Res Public Health. 2005;2(3):374–84.
Núñez O, Fernández-Navarro P, Martín-Méndez I, Bel-Lan A, Locutura JF, López-Abente G. Arsenic and chromium topsoil levels and cancer mortality in Spain. Environ Sci Pollut Res. 2016;23(17):17664–75.
Guo J, Su L, Zhao X, Xu Z, Chen G. Relationships between urinary antimony levels and both mortalities and prevalence of cancers and heart diseases in general US population, NHANES 1999–2010. Sci Total Environ. 2016;571:452–60.
Nyqvist F, Helmfrid I, Augustsson A, Wingren G. Increased cancer incidence in the local population around metal-contaminated glassworks sites. Occup Environ Med. 2017;59(5):e84–90.
Bai Y, Yang A, Pu H, Dai M, Cheng N, Ding J, Li J, Li H, Hu X, Ren X, et al. Cohort Profile: The China Metal-Exposed Workers Cohort Study (Jinchang Cohort). Int J Epidemiol. 2016;46(4):1095–1096e.
Yuan T-H, Shen Y-C, Shie R-H, Hung S-H, Chen C-F, Chan C-C. Increased cancers among residents living in the neighborhood of a petrochemical complex: A 12-year retrospective cohort study. J Hyg Environ. 2018;221(2):308–14.
Kiljańczyk A, Matuszczak M, Marciniak W, Derkacz R, Stempa K, Baszuk P, Bryśkiewicz M, Lubiński K, Cybulski C, Dębniak T, et al. Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers. Nutrients. 2024;16(9):1–9.
Stocks P. On the relations between atmospheric pollution in urban and rural localities and mortality from cancer, bronchitis and pneumonia, with particular reference to 3: 4 benzopyrene, beryllium, molybdenum, vanadium and arsenic. Br J Cancer. 1960;14(3):397.
Mohajer R, Salehi MH, Mohammadi J, Emami MH, Azarm T. The status of lead and cadmium in soils of high prevalenct gastrointestinal cancer region of Isfahan. J Res Med Sci. 2013;18(3):210–4.
Buononato EV, De Luca D, Galeandro IC, Congedo ML, Cavone D, Intranuovo G, Guastadisegno CM, Corrado V, Ferri GM. Assessment of environmental and occupational exposure to heavy metals in Taranto and other provinces of Southern Italy by means of scalp hair analysis. Environ Monit Assess. 2016;188(6):337.
Li H, Li X, Liu J, Jin L, Yang F, Wang J, Wang O, Gao Y. Correlation between serum lead and thyroid diseases: papillary thyroid carcinoma, nodular goiter, and thyroid adenoma. Int J Environ Health Res. 2017;27(5):409–19.
Adilay U, Gunal M, Tanriverdi O, Guler AK, Güçlü B, Demirgil B. Quantitative Analysis of Lead and Nickel in Benign Meningioma and Glioblastoma Multiforme. Med J Bakirkoy. 2017;13(4):190–4.
Sohrabi M, Nikkhah M, Sohrabi M, Rezaee Farimani A, Mirasgari Shahi M, Ziaie H, Shirmardi S, Kohi Z, Salehpour D, Safarnezhad Tameshkel F, et al. Evaluating tissue levels of the eight trace elements and heavy metals among esophagus and gastric cancer patients: A comparison between cancerous and non-cancerous tissues. J Trace Elem Med Biol. 2021;68: 126761.
Michalczyk K, Kupnicka P, Witczak G, Tousty P, Bosiacki M, Kurzawski M, Chlubek D, Cymbaluk-Płoska A. Assessment of Cadmium (Cd) and Lead (Pb) Blood Concentration on the Risk of Endometrial Cancer. Biology. 2023;12(5):1–15.
Szymanska-Chabowska A, Antonowicz-Juchniewicz J, Andrzejak R. The concentration of selected cancer markers (TPA, TPS, CYFRA 21–1, CEA) in workers occupationally exposed to arsenic (As) and some heavy metals (Pb, Cd) during a two-year observation study. Int J Occup Med Environ Health. 2007;20(3):229.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol Rev. 2014;94(2):329–54.
Pagano G, Guida M, Tommasi F, Oral R. Health effects and toxicity mechanisms of rare earth elements—Knowledge gaps and research prospects. Ecotoxicol Environ Saf. 2015;115:40–8.
Lee J-C, Son Y-O, Pratheeshkumar P, Shi X. Oxidative stress and metal carcinogenesis. Free Radic Biol Med. 2012;53(4):742–57.
Baralić K, Javorac D, Marić Đ, Đukić-Ćosić D, Bulat Z, Antonijević Miljaković E, Anđelković M, Antonijević B, Aschner M, Buha DA. Benchmark dose approach in investigating the relationship between blood metal levels and reproductive hormones: Data set from human study. Environ Int. 2022;165:107313.
Benbrahim-Tallaa L, Webber MM, Waalkes MP. Mechanisms of Acquired Androgen Independence during Arsenic-Induced Malignant Transformation of Human Prostate Epithelial Cells. Environ Health Perspect. 2007;115(2):243–7.
Mukke V, Chinte D. Impact of heavy metal induced alterations in lipase activity of fresh water crab, Barytelphusa guerini. 2012;4(5):2763–66.
Abdelhameed SAM, de Azambuja F, Vasović T, Savić ND, Ćirković Veličković T, Parac-Vogt TN. Regioselective protein oxidative cleavage enabled by enzyme-like recognition of an inorganic metal oxo cluster ligand. Nat Commun. 2023;14(1):486.
Zheng G, Zhong H, Guo Z, Wu Z, Zhang H, Wang C, Zhou Y, Zuo Z. Levels of Heavy Metals and Trace Elements in Umbilical Cord Blood and the Risk of Adverse Pregnancy Outcomes: a Population-Based Study. Biol Trace Elem Res. 2014;160(3):437–44.
Nuttall JR. The plausibility of maternal toxicant exposure and nutritional status as contributing factors to the risk of autism spectrum disorders. Nutr Neurosci. 2017;20(4):209–18.
Gianì F, Masto R, Trovato MA, Malandrino P, Russo M, Pellegriti G, Vigneri P, Vigneri R. Heavy Metals in the Environment and Thyroid Cancer. Cancers. 2021;13(16):4052.
Smetana K, Lacina L, Szabo P, DvoŘÁNkovÁ B, BroŽ P, ŠEdo A. Ageing as an Important Risk Factor for Cancer. Anticancer Res. 2016;36(10):5009.
Filippini T, Malagoli C, Wise L, Malavolti M, Pellacani G, Vinceti M. Dietary cadmium intake and risk of cutaneous melanoma: An Italian population-based case-control study. J Trace Elem Med Biol. 2019;56:100–6.
Filippini T, Torres D, Lopes C, Carvalho C, Moreira P, Naska A, Kasdagli M-I, Malavolti M, Orsini N, Vinceti M. Cadmium exposure and risk of breast cancer: a dose-response meta-analysis of cohort studies. Environ Int. 2020;142: 105879.
Djordjevic VR, Wallace DR, Schweitzer A, Boricic N, Knezevic D, Matic S, Grubor N, Kerkez M, Radenkovic D, Bulat Z. Environmental cadmium exposure and pancreatic cancer: Evidence from case control, animal and in vitro studies. Environ Int. 2019;128:353–61.
Park E, Kim S, Song S-H, Lee C-W, Kwon J-T, Lim MK, Park EY, Won Y-J, Jung K-W, Kim B. Environmental exposure to cadmium and risk of thyroid cancer from national industrial complex areas: a population-based cohort study. Chemosphere. 2021;268: 128819.
Cui Z-G, Ahmed K, Zaidi SF, Muhammad JS. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat Res Commun. 2021;27: 100372.
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. The Effects of Cadmium Toxicity. Int J Environ Res Public Health. 2020;17(11):1–24.
Ghaderi A, Mohammadzadeh M, Banafshe HR, Mousavi SGA, Mirzaei N, Parmoozeh Z, Mostafaei G, Rasouli-Azad M, Ghalerashidi HM, Fouladi-Fard R. The carcinogenic and non-carcinogenic risk assessment of heavy metals from the butts of smoked and non-smoked cigarettes. Hum ecol risk assess. 2023;29(1):187–201.
Luo T, Yu Q, Zou H, Zhao H, Gu J, Yuan Y, Zhu J, Bian J, Liu Z. Role of poly (ADP-ribose) polymerase-1 in cadmium-induced cellular DNA damage and cell cycle arrest in rat renal tubular epithelial cell line NRK-52E. Environ Pollut (Barking, Essex: 1987). 2020;261:114149.
Nagaraju R, Kalahasthi R, Balachandar R, Bagepally BS. Cadmium exposure and DNA damage (genotoxicity): a systematic review and meta-analysis. Crit Rev Toxicol. 2023;52(10):786–98.
Kiran Kumar KM, Naveen Kumar M, Patil RH, Nagesh R, Hegde SM, Kavya K, Babu RL, Ramesh GT, Sharma SC. Cadmium induces oxidative stress and apoptosis in lung epithelial cells. Toxicol Mech Methods. 2016;26(9):658–66.
Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjørklund G, Gatiatulina ER, Popova EV, Nemereshina ON, Huang P-T. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ Res. 2018;162:240–60.
Polykretis P, Cencetti F, Donati C, Luchinat E, Banci L. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 2019;21:101102.
Nai GA, Gonçalves Filho MA, Estrella MPS, Teixeira LDS. Study of the influence of the ph of water in the initiation of digestive tract injury in cadmium poisoning in rats. Toxicol Rep. 2015;100(2):1033–8.
Pearson C, Prozialeck W. E-cadherin, β-catenin and cadmium carcinogenesis. Med Hypotheses. 2001;56(5):573–81.
Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie. 2006;88(11):1549–59.
Branca JJV, Fiorillo C, Carrino D, Paternostro F, Taddei N, Gulisano M, Pacini A, Becatti M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants. 2020;9(6):492.
Zhang L, Zhu Y, Hao R, Shao M, Luo Y. Cadmium Levels in Tissue and Plasma as a Risk Factor for Prostate Carcinoma: a Meta-Analysis. Biol Trace Elem Res. 2016;172(1):86–92.
Zimta A-A, Schitcu V, Gurzau E, Stavaru C, Manda G, Szedlacsek S, Berindan-Neagoe I. Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ Res. 2019;178:108700.
Chen C, Xun P, Nishijo M, Carter S, He K. Cadmium exposure and risk of prostate cancer: a meta-analysis of cohort and case-control studies among the general and occupational populations. Sci Rep. 2016;6(1):1–7.
Chen C, Xun P, Nishijo M, He K. Cadmium exposure and risk of lung cancer: a meta-analysis of cohort and case–control studies among general and occupational populations. J Expo Sci Environ Epidemiol. 2016;26(5):437–44.
Nawrot TS, Martens DS, Hara A, Plusquin M, Vangronsveld J, Roels HA, Staessen JA. Association of total cancer and lung cancer with environmental exposure to cadmium: the meta-analytical evidence. Cancer Causes Control. 2015;26(9):1281–8.
Satarug S, Boonprasert K, Gobe GC, Ruenweerayut R, Johnson DW, Na-Bangchang K, Vesey DA. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin Kidney J. 2018;12(4):468–75.
Madrigal JM, Ricardo AC, Persky V, Turyk M. Associations between blood cadmium concentration and kidney function in the US population: Impact of sex, diabetes and hypertension. Environ Res. 2019;169:180–8.
Omar S, Muhamad MS, Te Chuan L, Hadibarata T, Teh ZC. A Review on Lead Sources, Occurrences, Health Effects, and Treatment Using Hydroxyapatite (HAp) Adsorbent Made from Fish Waste. Wat Air and Soil Poll. 2019;230(12):275.
Guzel S, Kiziler L, Aydemir B, Alici B, Ataus S, Aksu A, Durak H. Association of Pb, Cd, and Se Concentrations and Oxidative Damage-Related Markers in Different Grades of Prostate Carcinoma. Biol Trace Elem Res. 2012;145(1):23–32.
Scanlon SE, Scanlon CD, Hegan DC, Sulkowski PL, Glazer PM. Nickel induces transcriptional down-regulation of DNA repair pathways in tumorigenic and non-tumorigenic lung cells. Carcinogenesis. 2017;38(6):627–37.
Devóz PP, Gomes WR, De Araújo ML, Ribeiro DL, Pedron T, Greggi Antunes LM, Batista BL, Barbosa F, Barcelos GRM. Lead (Pb) exposure induces disturbances in epigenetic status in workers exposed to this metal. J Toxicol Environ Health - A. 2017;80(19–21):1098–105.
Houssaini M, Damou M, Guessous F, Ismaili N. Mutational status in Ras/Raf/MAPK signaling pathway in Moroccan colorectal cancer patients. Ann Oncol. 2020;8(4):S206.
Wang J, Yi Y, Xiao Y, Dong L, Liang L, Teng L, Ying JM, Lu T, Liu Y, Guan Y, et al. Prevalence of recurrent oncogenic fusion in mismatch repair-deficient colorectal carcinoma with hypermethylated MLH1 and wild-type BRAF and KRAS. Mod Pathol. 2019;32(7):1053–64.
Liao LM, Friesen MC, Xiang Y-B, Cai H, Koh D-H, Ji B-T, Yang G, Li H-L, Locke SJ, Rothman N, et al. Occupational Lead Exposure and Associations with Selected Cancers: The Shanghai Men’s and Women’s Health Study Cohorts. Environ Health Perspect. 2016;124(1):97–103.
Ahn J, Park MY, Kang M-Y, Shin I-S, An S, Kim H-R. Occupational Lead Exposure and Brain Tumors: Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2020;17(11):3975.
National Primary Drinking Water Regulations [https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations]
Hu Y, Li J, Lou B, Wu R, Wang G, Lu C, Wang H, Pi J, Xu Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020;10(2):240.
Talaat S, Somji S, Toni C, Garrett SH, Zhou XD, Sens MA, Sens DA. Kindlin-2 expression in arsenite-and cadmium-transformed bladder cancer cell lines and in archival specimens of human bladder cancer. Urology. 2011;77(6):1507.e1501-1507.
Kohzadi S, Sheikhesmaili F, Rahehagh R, Parhizkar B, Ghaderi E, Loqmani H, Shahmoradi B, Mohammadi E, Maleki A. Evaluation of trace element concentration in cancerous and non-cancerous tissues of human stomach. Chemosphere. 2017;184:747–52.
Malandrino P, Russo M, Ronchi A, Moretti F, Gianì F, Vigneri P, Masucci R, Pellegriti G, Belfiore A, Vigneri R. Concentration of metals and trace elements in the normal human and rat thyroid: comparison with muscle and adipose tissue and volcanic versus control areas. Thyroid. 2020;30(2):290–9.
Patel KS, Pandey PK, Martín-Ramos P, Corns WT, Varol S, Bhattacharya P, Zhu Y. A review on arsenic in the environment: contamination, mobility, sources, and exposure. RSC Adv. 2023;13(13):8803–21.
Matés JM, Segura JA, Alonso FJ, Márquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med. 2010;49(9):1328–41.
Begum W, Rai S, Banerjee S, Bhattacharjee S, Mondal MH, Bhattarai A, Saha B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022;12(15):9139–53.
Das KK, Reddy RC, Bagoji IB, Das S, Bagali S, Mullur L, Khodnapur JP, Biradar M. Primary concept of nickel toxicity–an overview. J Basic Clin Physiol Pharmacol. 2019;30(2):141–52.
Tumolo M, Ancona V, De Paola D, Losacco D, Campanale C, Massarelli C, Uricchio VF. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int J Environ Res Public Health. 2020;17(15):1–25.
Proctor DM, Suh M, Mittal L, Hirsch S, Valdes Salgado R, Bartlett C, Van Landingham C, Rohr A, Crump K. Inhalation cancer risk assessment of hexavalent chromium based on updated mortality for Painesville chromate production workers. J Expo Sci Environ Epidemiol. 2016;26(2):224–31.
Remy LL, Byers V, Clay T. Reproductive outcomes after non-occupational exposure to hexavalent chromium, Willits California, 1983–2014. Environ Health. 2017;16(1):18.
Das J, Kang M-H, Kim E, Kwon D-N, Choi Y-J, Kim J-H. Hexavalent chromium induces apoptosis in male somatic and spermatogonial stem cells via redox imbalance. Sci Rep. 2015;5(1):13921.
Togawa K, Le Cornet C, Feychting M, Tynes T, Pukkala E, Hansen J, Olsson A, Oksbjerg Dalton S, Nordby K-C, Uuksulainen S, et al. Parental Occupational Exposure to Heavy Metals and Welding Fumes and Risk of Testicular Germ Cell Tumors in Offspring: A Registry-Based Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2016;25(10):1426–34.
Olsson A, Togawa K, Schüz J, Le Cornet C, Fervers B, Dalton SO, Pukkala E, Feychting M, Skakkebæk NE, Hansen J. Parental occupational exposure to solvents and heavy metals and risk of developing testicular germ cell tumors in sons (NORD-TEST Denmark). Scand J Work Environ Health. 2018;44(6):658–69.
Krstev S, Knutsson A. Occupational Risk Factors for Prostate Cancer: A Meta-analysis. J Cancer Prev. 2019;24(2):91–111.
Zhang C, Cai K, Feng Q, Xu Y, Zhang Z. Chromium(VI) promotes cell migration through targeting epithelial-mesenchymal transition in prostate cancer. Toxicol Lett. 2019;300:10–7.
Rafnsson V, Gunnarsdottir H, Kiilunen M. Risk of lung cancer among masons in Iceland. Occup Environ Med. 1997;54(3):184–8.
Pavithra KG, SundarRajan P, Kumar PS, Rangasamy G. Mercury sources, contaminations, mercury cycle, detection and treatment techniques: A review. Chemosphere. 2023;312: 137314.
Zefferino R, Piccoli C, Ricciardi N, Scrima R, Capitanio N. Possible Mechanisms of Mercury Toxicity and Cancer Promotion: Involvement of Gap Junction Intercellular Communications and Inflammatory Cytokines. Oxid Med Cell Longev. 2017;2017:7028583.
Valko M, Morris H, Cronin M. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–208.
Fujimura M, Usuki F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants. 2020;9(10):1–20.
Belsten J, Wright A, Van den Berg H, Velthuis-te Wierik E, Cavadini C, Farre R, Favier A, Richard M, Lamand M, Sandstrom B. European Community-FLAIR common assay for whole-blood glutathione peroxidase (GSH-Px); Results of an inter-laboratory trial. Eur J Clin Nutr. 1995;49(12):921–7.
Miyake H, Hara I, Kamidono S, Eto H. Oxidative dna damage in patients with prostate cancer and its response to treatment. J Urol Balt. 2004;171(4):1533–6.
Ohtake S, Kawahara T, Ishiguro Y, Takeshima T, Kuroda S, Izumi K, Miyamoto H, Uemura H. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol Clin Oncol. 2018;9(3):302–4.
Shukla S, Srivastava JK, Shankar E, Kanwal R, Nawab A, Sharma H, Bhaskaran N, Ponsky LE, Fu P, MacLennan GT, et al. Oxidative Stress and Antioxidant Status in High-Risk Prostate Cancer Subjects. Diagnostics. 2020;10(3):1–11.
Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82(8):493–512.
De Silva V, Woznichak MM, Burns KL, Grant KB, May SW. Selenium Redox Cycling in the Protective Effects of Organoselenides against Oxidant-Induced DNA Damage. J Am Chem Soc. 2004;126(8):2409–13.
Elledge SJ, Zhou Z, Allen JB. Ribonucleotide reductase: regulation, regulation, regulation. T Trends Biochem Sci. 1992;17(3):119–23.
Moffett DB, Mumtaz MM, Sullivan DW, Whittaker MH. Chapter 13 - General considerations of dose-effect and dose-response relationships∗. In: Handbook on the Toxicology of Metals (Fifth Edition). edn. Edited by Nordberg GF, Costa M. Cambridge, MA, USA: Academic Press; 2022. p. 299–317.
Baltaci AK, Dundar TK, Aksoy F, Mogulkoc R. Changes in the Serum Levels of Trace Elements Before and After the Operation in Thyroid Cancer Patients. Biol Trace Elem Res. 2017;175(1):57–64.
Garje R, An JJ, Sanchez K, Greco A, Stolwijk J, Devor E, Rustum Y, Zakharia Y. Current Landscape and the Potential Role of Hypoxia-Inducible Factors and Selenium in Clear Cell Renal Cell Carcinoma Treatment. Int J Mol Sci. 2018;19(12):3834.
Saravanabhavan G, Werry K, Walker M, Haines D, Malowany M, Khoury C. Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013. J Hyg Environ. 2017;220(2, Part A):189–200.
Chitta KR, Landero Figueroa JA, Caruso JA, Merino EJ. Selenium mediated arsenic toxicity modifies cytotoxicity, reactive oxygen species and phosphorylated proteins†. Metallomics. 2013;5(6):673–85.
Hossain KFB, Rahman MM, Sikder MT, Saito T, Hosokawa T, Kurasaki M. Inhibitory effects of selenium on cadmium-induced cytotoxicity in PC12 cells via regulating oxidative stress and apoptosis. Food Chem Toxicol. 2018;114:180–9.
Zwolak I. The Role of Selenium in Arsenic and Cadmium Toxicity: an Updated Review of Scientific Literature. Biol Trace Elem Res. 2020;193(1):44–63.
Singh N, Savanur MA, Srivastava S, D’Silva P, Mugesh G. A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale. 2019;11(9):3855–63.
Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci - Landmark. 2018;23(9):1655–79.
Marzano C, Pellei M, Tisato F, Santini C. Copper complexes as anticancer agents. Anti-cancer Agents Med Chem (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). 2009;9(2):185–211.
Kim B-E, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176–85.
Barrera-García A, O’Hara T, Galván-Magaña F, Méndez-Rodríguez LC, Castellini JM, Zenteno-Savín T. Trace elements and oxidative stress indicators in the liver and kidney of the blue shark (Prionace glauca). Comp Biochem Physiol A Mol Integr Physiol. 2013;165(4):483–90.
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–90.
Fonseca-Nunes A, Agudo A, Aranda N, Arija V, Cross AJ, Molina E, Sanchez MJ, Bueno-de-Mesquita HB, Siersema P, Weiderpass E, et al. Body iron status and gastric cancer risk in the EURGAST study. Int J Cancer. 2015;137(12):2904–14.
Cook MB, Kamangar F, Weinstein SJ, Albanes D, Virtamo J, Taylor PR, Abnet CC, Wood RJ, Petty G, Cross AJ. Iron in Relation to Gastric Cancer in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention StudyIron and Gastric Cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2033–42.
Puliyel M, Mainous AG, Berdoukas V, Coates TD. Iron toxicity and its possible association with treatment of Cancer: Lessons from hemoglobinopathies and rare, transfusion-dependent anemias. Free Radic Biol Med. 2015;100(79):343–51.
Prá D, Rech Franke SI, Pêgas Henriques JA, Fenech M. A Possible Link Between Iron Deficiency and Gastrointestinal Carcinogenesis. Nutr Cancer. 2009;61(4):415–26.
Stein J, Connor S, Virgin G, Ong DE, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22(35):7908–25.
Banihani SA. Vitamin B12 and Semen Quality. Biomolecules. 2017;7(2):42.
O’Leary F, Samman S. Vitamin B12 in Health and Disease. Nutrients. 2010;2(3):299–316.
Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis—Part II: Cr, Si, Zn, Cu, and S. Rev Oncol Hematol. 2015;96(1):143–55.
Mulware SJ. Trace elements and carcinogenicity: a subject in review. 3 Biotech. 2013;3(2):85–96.
Benli AR, Ersoy S. The effect of wet cupping therapy on heavy metal levels: a single-arm clinical trial. Iran Red Crescent Med J. 2020;22(4):e96348.
Acknowledgements
Not applicable.
Funding
No funding was secured for this study.
Author information
Authors and Affiliations
Contributions
A.H.Kh: Investigation/ systematic search/ extraction/ Supervision/ Review and Editing/ Data Curation. M.M: Investigation/ systematic search/ extraction/ Writing Original draft/ Data Curation/ Review and Editing. A.G.K: Review and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khoshakhlagh, A.H., Mohammadzadeh, M. & Gruszecka-Kosowska, A. The preventive and carcinogenic effect of metals on cancer: a systematic review. BMC Public Health 24, 2079 (2024). https://doi.org/10.1186/s12889-024-19585-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19585-5