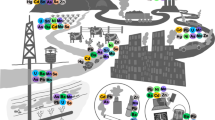
Abstract
The majority of the so-called heavy metals are suspected to be involved in a number of pathologies and play a role in human carcinogenesis. Some of them (i.e. arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg) and nickel (Ni)) have been defined as carcinogens, increasing the susceptibility of tumor development and progression in humans. Moreover, Ni, Cr, Cd, Hg, and Pb together with zinc (Zn) and iron (Fe), may be capable of stimulating the progression of breast cancer and reducing a patient’s sensitivity to treatment through alterations to DNA methylation. In patients with gastric cancers, levels of various heavy metals are augmented and hypothesized to amplify the expression of the human epidermal growth factor receptor type 2 gene. Cd may increase the risk of lung cancer development and have a negative impact on the overall survival of lung cancer patients. To investigate the relation between heavy metals in biological samples and risk, occurrence and survival cancer individuals, a comprehensive review work was performed, with a focus on breast, lung, prostate and gastric cancers. An extensive search strategy was devised to ensure relevant literature could be identified, with the PECO framework being adopted to facilitate this and identify key search terms. As evidenced in this review, there is substantial data to support the hypothesis that heavy metals influence tumor development and progression. Unluckily the number of papers dealing with the determination of metals directly in samples from cancer tissues is still rather limited, so we decided to expand the scope of this review also to analyses carried out on other biological samples, as urine, plasma, hair, nail, etc. The studies reviewed showed that several limitations and current knowledge gaps are present in the literature that require further investigation to improve our comprehension of the impact of different heavy metals on tumorigenesis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metals play many roles in the body, according to their nature. Some of them are the so called “essential” metals, belonging to the first (alkali) and second (alkaline earth) groups: sodium (Na) and potassium (K) are mainly charge carries; magnesium (Mg) and calcium (Ca) participate to the assembly of hard structures via bio-mineralization (teeth, bones etc.) and have structural functions in macromolecules (proteins, DNA); light, first-row transition metals, defined as “essential in traces” like iron (Fe), copper (Cu), or zinc (Zn), participate in prosthetic groups of proteins or enzymatic cofactors, with the redox-active ones busy in transferring electrons and catalyzing enzymatic redox reaction [1]. On the contrary, heavy metals have no biological function, and exert a toxic action on the body already at low concentrations. Even essential metals, in particular Fe, Zn, Cu, and to a lesser extent also Cr (in its + 3-oxidation state), can be noxious, when their amount in the organism exceeds the threshold limits: as Paracelsus said, “the dose makes the poison”.
Metal toxicity has long been recognized and studied for its consequences on human health. Non-essential metals such as Cd, Hg, Ni, and Pb, can be harmful mainly, but not only, because they interfere with metabolic processes by substituting essential divalent cations (e.g. Ca, Mg, Fe and Zn) in enzymes, proteins and hard structures, such as bones or teeth. Trace elements can be noxious to the human organism when their concentration exceeds precise physiological limits, and their toxicity is particularly related to their redox activity (except for Zn), which can induce oxidative stress, ROS production etc., in a Fenton (Fe) or Fenton-like (Cu, Cr, etc.) chain of reactions [1]. The cellular damage caused by heavy metals is linked to different characteristic of these elements, such as solubility, oxidation states, hard-soft character, binding attitudes, presence of different forms, which can influence their speciation in biological systems.
The speciation of an element is the chemical form(s) that it takes in solution, in this case biological fluids. Except for the nanoparticles of some noble elements (e.g. Ag, Au, Pt), metals in the body are never found in their metallic form, but as cations, bound in salts (e.g. sulfides, phosphates, etc.), oxides or complexes, and this is what we will refer to, when generically speaking of metals. Biotransformation mechanisms are able to convert inorganic forms of different cations into organometallic species, for instance through methylation processes [2]. Heavy metals can interact and make complexes with biological molecules such as DNA, enzymes, proteins and peptides (e.g. albumins, metallothioneins, glutathione, etc.) by binding to sulfur (e.g. cysteine) or nitrogen (e.g. histidine) groups, and this is the way in which they are generally found in cells and tissues. Free metal ions (aquo complexes) are infrequently found, and this is their most active and toxic form [1, 3,4,5].
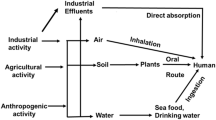
Metal exposition is not a rare event for humans. Metals are a core component of Earth and its crust and are present in every aspect of the environment [6]. Besides, heavy metals have multiple applications in industrial, domestic, agricultural, medical, and technological industries, causing concerns to be raised about their potential impact on human health [7, 8]. Collectively, this has triggered the absorption of such heavy elements in humans, primarily from crops, water, vegetables, and sediments [9, 10]. Although not widely comprehended, the toxicity induced by the accumulation of heavy metals has been established [11]. As just reminded, in the human body, these metallic elements can impair metabolism, cause oxidative stress, and result in the development of numerous diseases and conditions [12, 13]. For instance, Hg is able to induce neurological disorders and damage to the central nervous system (e.g. the mad hatter syndrome, also known as erethismus mercurialis [14], and the so-called Minamata disease, from the Hg poisoning among people from Minamata bay, in Japan [15]; Pb can lead to deficiencies in cognitive development in children [16] or to saturnism, a condition already known by the Romans, together with a series of other disfunctions such as anemia and neurological, respiratory, urinary, and cardiovascular disorders [17]; Cr(VI) causes oxidative damage that can lead to hemolysis and, ultimately, kidney and liver failure, but also gastrointestinal and skin disorders [18].
Cancer Research institution (UK) reported an estimated 18.1 million new cancer cases worldwide in 2020, with 10 million deaths, of which 33% were caused by smoke [19]. In a similar way the National Center for Health Statistics reported that, in 2019, there were 1,918,030 new cancer cases and 609,360 cancer death in the United States [20]. This includes an estimated 350 deaths per day from lung cancer, one of the most prevalent malignancies and causes of cancer death. Long-term exposure to some heavy metals and their compounds has been linked to the development of cancer and tumor formation. For example, Cd is proposed to mimic estrogenic effects, as a result of its ability to form a high-affinity complex with the hormone binding domain of the estrogen receptor, promoting the development of breast cancer [21].The accumulation of Cd has also been found to increase the risk of lung cancer occurrence, with high exposure to this heavy metal resulting in poor prognosis in patients with lung cancer [22]. Additionally, numerous heavy metals, including Cr, Ni, and Cd, are proposed to destroy the gastric mucosa barrier, triggering inflammation and tissue damage, and leading to gastric cancers [23]. The International Agency for Research on Cancer has classified As, Cd, Cr and Ni as group one carcinogens [24,25,26]. These elements and their compounds induce oxidative stress, DNA damage, and cell death processes, augmenting the risk of cancer and cancer-related conditions such as high blood pressure, arthritis, diabetes, lung disease, heart disease, or kidney disease [27]. Collectively, these metals represent a profound environmental risk factor for the development of several malignancies [28]. In patients with malignancies, the possible noxious role of heavy metals can be reflected in the biomarkers present in blood, tissues, skin, and nails, such as those related to ROS production, as evidenced in studies carried out on several species, from humans to aquatic moss [29,30,31]. This review aims to investigate the relation between heavy metals in biological samples and occurrence and survival cancer individuals, by summarizing the most recent evidence on the implications of repeated heavy metal exposure as an environmental risk factor for cancer development and progression, together with related evidences in other species and organisms that can be relevant for the discussion. The malignancies of focus for the purpose of this review are lung cancer, breast cancer, prostate cancer, and gastric cancer.
Results and discussion
The results of the search strategy identified four prevalent malignancies in the current literature that are influenced by the presence of heavy metals in biological samples. The results and discussion of this review will, therefore, comprise research on heavy metals in the biological samples of lung, breast, prostate and gastric cancer patients.
Lung cancer
Lung cancer is a prevalent malignancy that can be attributed to many environmental and genetic risk factors, particularly smoking [32] (Table 1). However, the role of prolonged heavy metal exposure as an environmental factor has been recently established [26, 31]. A 2022 study by Lee et al. carried out on urine samples from population residing close to a petrochemical industrial area with high air Cd concentration noted that air exposure to this metal increases the risk of lung cancer development and has a negative impact on the overall survival of lung cancer patients [22]. It has also been suggested that a relationship exists between the levels of soil contamination by heavy metals and lung cancer incidence [33, 34]. Tran et al. investigated the prevalence of Cd, As, Hg, and Pb in postsurgical tissues from individuals with and without non-small cell lung cancer (NSCLC). Two groups of biological samples were explored. In the first group, tissue samples from 15 patient cases with stage I and stage II NSCLC who underwent surgical resection were assessed. The findings indicate that the amount of Pb and Hg observed was not significant. On the other hand, Cd was the most prevalent heavy metal identified, and As was observed in moderate amounts. In the second group, 28 patients with a confirmed NSCLC, including adenocarcinoma, squamous cell carcinoma, or large cell carcinoma, and nine noncancer benign lung tissue samples were evaluated. In all tissue samples obtained from NSCLC patients, Cd was observed, and its levels were significantly higher in noncancer benign lung samples than NSCLC patients. Additionally, As was observed in moderate amounts in NSCLC patients, whilst both Hg and Pb amounts were negligible. These findings warrant the hypothesis that Cd and As may have an impact on the development of NSCLC; however, further studies are necessitated to corroborate these results [34, 35]. The presence of heavy metals in biological samples from lung cancer patients was reinforced by Pietrzak et al., who assessed the blood concentrations of As, Cd, Hg, and Pb in 336 patients with lung cancer. The relationship between these concentrations and overall survival was also reported. It was observed that Cd was the only heavy metal that significantly influenced overall survival in these patients. The findings also revealed that low blood.
Cd levels, defined as < 1.47 µg/L, may result in improved overall survival in treated patients with stage IA disease [36]. Although not widely investigated, it has also been suggested that serum levels of heavy metals may influence the development and progression of lung cancer. 440 patients were investigated by Bai et al. for the presence of 11 metals (Cu, Zn, Fe, Co, Mn, Mo, Rb, Se, Sn, Sr and V) in their blood samples, but only Zn seemed related to lung cancer, as high plasma levels of this metal were associated with lower incident risk of lung cancer, probably due to Zn slowing down telomere attrition and regulating the expressions of cancer-related genes [37]. Cu was, for a long time, considered as an active site metabolic cofactor solely [38], but lately it started to appear as an essential nutrient for tumour growth and metastasis that have an increased requirement of this metal, and the group of C. Chang has recently introduced the term “cuproplasia”, within the wider frame of “metalloplasias”, to indicate copper-dependent cell proliferation, akin to ferroptosis as an iron-dependent form of cell death [39]. In animal models (Drosophila melanogaster and mouse), Cu emerged in the evidence that is a dynamic signaling metal and metalloallosteric regulator, as for copper-dependent phosphodiesterase 3B (PDE3B) in lipolysis [40], mitogen-activated protein kinase 1 (MEK1) and mitogen-activated pro tein kinase 2 (MEK2) in cell growth and proliferation and the unc-51 like kinase 1 (ULK1) and unc-51 like kinase 2 (ULK2) in autophagy [41, 42]. Within the frame of the aforementioned cuproplasia, cuproptosis was investigated as novel copper-dependent cell death (in analogy with ferroptosis) to evaluate the association between single-nucleotide polymorphisms in cuproptosis-related genes and lung cancer risk. Some of the minor alleles examined correlated with an increased risk of lung cancer, while some others were associated with lower risk, evidencing a relation between cuproptosis-related genes an lung cancer risk [43]. Cobanoglu et al. observed that Zn may behave as a protective agent against lung cancer, whilst low Zn levels may facilitate the pathogenesis of tumor development [44]. Also, dysregulation of iron metabolic proteins is closely associated with the initiation and development of lung cancer [45]. The serum, bronchoalveolar lavage fluid, of patients with lung cancer contain elevated ferritin levels [46,47,48]; moreover lung cancer cells could increase iron intake by enhancing the effects of the transferrin protein (TF) and transferrin receptor (TfR) [48, 49]. Different authors boldly guess than an increase in Fe intake by lung cancer cells could be related to ferroptosis, one of the potential forms of death for lung cancer cells [50,51,52].
Breast cancer
Trace elements and heavy metals have been widely observed in biological samples of breast cancer patients and have been the focus of several research investigations over the past two decades [53,54,55,56] (Table 2). It has been evidenced that through multiple pathogenetic links, heavy metals are capable of stimulating the progression of breast cancer and reducing a patient’s sensitivity to treatment [57,58,59]. A prospective study by Cihan et al. analyzed the hair levels of 36 elements in 52 patients with stage III breast cancer, comparing them to those of healthy individuals. There were notable differences between the two patient groups; a higher level of Fe was observed in cancer patients, whilst the control participants had higher levels of Ca. The primary difference observed between the two groups concerned increased levels of heavy metals in the hair samples of cancer patients [32]. Likewise, Ionescu et al. observed a pathological accumulation of transition metals in breast tissue. This study analyzed the concentration of transition metals in 20 breast cancer biopsies, comparing the findings to the levels observed in eight healthy biopsies. Significantly higher levels of Fe, Ni, Cr, Zn, Cd, Hg, and Pb were observed in the cancer biopsies compared to the healthy ones. Hence, it was concluded that these metals might be involved in the malignant growth process [60]. Li studied the association between plasma heavy metals and the metabolome in patients with breast cancer, and the association with cancer development. In these patients, Cd was significantly ~ 15-fold higher in the plasma of patients with breast cancer compared with that in the control population, but also Cr, As and Pb were elevated by ~ 3.24, 2.14 and 1.52-fold, respectively. Moreover, small molecules, including amino acids and salts, were altered in the plasma of patients with breast cancer compared with the control population. Multivariate analysis has suggested that exposure to heavy metals, including Cd, As, Cr and Pb, may influence blood lipid levels and other small molecule metabolites, which in turn may be involved in breast cancer development [61].
A similar study, but on urine, investigated the correlation between heavy metals detected in urine and the urine metabolome of patients with breast cancer, and their association with cancer development [61]. As in plasma samples, the results demonstrated that Cd, Cr and As, were markedly increased in the urine of patients with breast cancer compared with the control population. Moreover, small molecule metabolites were altered in the urine of patients with breast cancer and the results were very similar to previous reports, indicating that environmental exposure to Cd, As, or Cr could be involved in breast cancer development [61]. O’Brien et al. have studied toenail-based metal concentrations in relation to young-onset breast cancer (diagnosis age < 50 years) of 1,217 disease-discordant sister pairs in the US-based Sister and Two Sister. They studied cadmium as a metalloestrogen, together with 9 other metals, such as As, Co, Cr, Cu, Mo, Pb, Sn, and V. Cd was associated with a small increase in risk, with no evidence of a dose-response relationship. In case-control studies, no association was observed between young-onset breast cancer and toenail concentrations of any metal evaluated [62]. A case-control study within the ORDET cohort by Pala et al. has observed that Cu is high and Zn low in blood and urine of women in 496 breast cancer cases compared with controls; in addition, increased Cu/Zn ratio in plasma and urine may both be an early marker of, and a risk factor for, breast cancer development [63]. The role of heavy metals in the air and the risk of postmenopausal breast cancer have also been discussed. In a study evaluating the association between air toxics, including heavy metals, and breast density, increased levels of Hg, Cd and Pb were found to be associated with postmenopausal breast cancer. The odds ratio was 1.1 in a cohort comprising 2587 breast cancer cases [64]. The rationale for this increase may lie with the hypothesized role of Cd and Ni as metalloestrogens, as a result of their ability to form high-affinity complexes with the hormone binding domain of the estrogen receptor thus mimicking the action of estrogens [21, 58].
Systematic Reviews and Meta-analyses have studied the association between heavy metals and breast cancer and reviewed the potential mechanisms systematically [65, 66]. 36 studies in different biological samples, comprising 4151 individuals from five continents around the world were included. It was found that especially in plasma and serum, Cu, Cd, and Pb concentrations were higher in patients with breast cancer, while Zn (in hair) and Mn concentrations were lower [65, 67]. Another systematic review and meta-analysis evaluated the associations between Fe and breast carcinogenesis. 23 studies were included assessing iron intake and/or biomarkers of iron status in relation to breast cancer risk, and dose-response meta-analyses were also performed to investigate linear and nonlinear associations. Heme iron intake was significantly associated with increased breast cancer risk, whereas no associations were found for dietary Fe, data that may have an impact and public health implications given the widespread consumption of (heme) iron-rich foods [68].
Prostate cancer
Prostate cancer represents one of the most prevalent malignancies in men, particularly in industrialized countries [69]. Several heavy metals such as Cd, As, Zn and Fe have been indicated to play a role in the various biochemical processes underpinning the pathogenesis of this disease [70] (Table 3). This includes disruption of protection against oxidative damage in cellular respiration, genomic stability, immunity, apoptosis, and cell signaling [71, 72]. A 2019 study by Lim conducted a multiple metal analysis to explore the association between serum heavy metals and the occurrence of prostate cancer. A total of 141 cases were included in this study and were matched to 114 controls, with the concentrations of ten heavy metals being determined. The results identified four heavy metals, specifically As, Zn, Mn, and Sb that were significantly and positively associated with prostate cancer occurrence. Additional analyses revealed As and Zn to have the most significant impact on the risk of prostate cancer when all other metals were held fixed [73]. Likewise, Sarafanov et al. found that there is an association between Zn and Fe prostate tissue levels and the development of prostate cancer, since patients with biochemical (PSA) recurrence of disease showed 12% lower median Fe (95 µg/g vs. 111 µg/g) and 21% lower Zn (279 µg/g vs. 346 µg/g) concentrations in the normal-appearing tissue immediately adjacent to cancer areas [74]. Wu’s group conducted a study to investigate whether there was a relationship between heavy metal exposure and prostate-specific antigen (PSA). It was examined whether men without prostate cancer, aged ≥ 40 years, identified by the National Health and Nutrition Examination Survey 2003–2010, had a correlation between levels of total urinary As as urinary dimethylarsonic acid, blood Cd, blood Pb and total blood Hg levels and elevated PSA levels. It was found that men with elevated PSA had higher blood Cd and blood Pb levels than men with normal PSA. This significant relationship disappeared after adjusting for age, race/ethnicity, body mass index, smoking and education [51]. Besides heavy metal levels, associations have also been found between trace elements and heavy metal concentrations in biological samples of those with prostate cancer. A 2012 study by Karimi explored the relationship between heavy metals including Se, Zn, Cu, Mn, and Fe with prostate cancer [75]. A case-control study analyzing hair and nail samples from 100 patients revealed significantly lower mean levels of Se and Zn compared to controls. Conversely, elevated levels were observed for Fe and Mn [75]. Neslund-Dudas et al. described that Cd exposure from smoking may play a role in disease progression. Metal concentrations were measured for biochemical and distant disease recurrence in neoplastic tissues in paraffin. It was found that smokers had significantly higher Cd and lower Zn in non-neoplastic tissue than non-smokers [70].
Gastric cancer
The levels of Zn, Cr, Mn, Sn, Cu, Pb, Al, and Fe were investigated in 50 esophagus and gastric cancer tissue samples in a study by Sohrabi et al., with these findings being compared to that of healthy tissues. The samples were taken from 13 patients with esophageal cancer and 37 patients with gastric cancer. Significant differences in Zn, Cr, and Sn were observed between cancer and healthy tissues. Moreover, the researchers observed differences in the levels of some trace elements and heavy metals between genders, a finding which necessities further consideration in future investigations [76] (Table 4). In addition, Wang et al. observed a relationship between the concentration of heavy metals and human epidermal growth factor receptor type 2 (HER2) gene amplification in 105 gastric cancer patients. In this study, the concentration of As, Cr, Cu, Hg, Mn, Sb, Se, Pb, Sr, Tl, V, Sn and Zn significantly differed between gastric cancer patients and the 62 healthy controls. In addition, it was observed that Hg, Se, and Tl levels were noticeably increased in the HER2 positive group when compared to the HER2 negative group [77]. Feng et al. have detected the concentrations of 17 metals in gastric tissues, which have been associated with investigated rDNA copy number in gastric cancers and its link with existing biomarkers. This study was performed on paired tumor and adjacent normal tissues obtained from 65 gastric cancer patients, revealing high concentrations of As, Cd, Cr, Cu and Fe in both pathological and adjacent healthy tissues [78]. Moreover, rDNA copy number variation was related with the concentrations of certain metals and may be associated with metal exposure [78]. Fonseca-Nunes e al. have conducted a study case-control in the multicentric European Prospective Investigation into Cancer and Nutrition (EPIC) study, that included 456 primary incident gastric adenocarcinoma cases and 900 controls during 11 years of follow-up. They showed a decreased risk of gastric cancer related to higher body iron stores as measured by serum iron and ferritin [79].
Materials and methods
PubMed, Web of Science, and Scopus were systematically searched to identify relevant literature published up until the end of 2022 on the presence of heavy metals in biological samples of tumor patients. The search strategy was formulated according to the PECO guideline[80]. The key search terms used in the search strategy included “heavy metals” or “trace elements” combined with “cancer” or “tumor” or “carcinoma”. Additionally, all results reported in previous reviews and relevant meta-analyses were searched.
Study selection and data collection
Identified studies were only included if they were compliant with the following standards: (1) the population (P) was restricted to patients with a confirmed cancer diagnosis; (2) exposure (E) to heavy metals, evidenced in biological samples; (3) the comparator (C) was specified in the research, including individuals without cancer; (4) the outcome (O) was cancer prevalence; (5) only studies involving human participants were included; and (6) the studies that were available in the English language. In some instances, this study incorporated research that did not present original data, such as review articles, if deemed relevant to the examined topics. However, the primary focus was on original works. Conversely, studies that did not assess heavy metals in biological samples were excluded. Subsequently, data were collected and categorized based on the malignancy of the patients.
Conclusions
Examination of the literature available on the topic of metal-induced carcinogenesis indicates that there is enough substantial data to support the hypothesis of a direct relationship between the presence of heavy metals within the tissues and the increased risk of tumorigenesis. The levels and the type of metal involved in such processes differ substantially amongst lung, breast, prostate and gastric cancer patients; however, there is a collective agreement amongst the current literature that the levels of heavy metals are augmented in biological samples of tumor patients. However, although several mechanisms for this relationship were proposed, such as genotoxicity, oxidative stress, inhibition of DNA repair, just to quote some, there are current knowledge gaps within the literature that call for further research.
Data collected during this review work indicate the number of papers dealing with metals determined directly in cancer tissues (biopsies) is still rather limited, while this should be one of the main points to be addressed while trying to unveil the relation between heavy metals and cancer. We encourage researchers to explore this field in order to help understand such delicate aspect.
Moreover, it must be said it is rather difficult to compare data relative to different metals involved in the carcinogenesis of a given organ when their concentrations are measured in different biological samples (e.g. biopsies, adjacent normal tissues, plasma, urine, hair, nail, etc.), as the accumulation of metals can normally vary from tissue to tissue. Even the same metal can be unevenly distributed in different parts of the body. The significance of a study, in our opinion, could be increased when metals are determined in various biological samples, and not only in one. This could add soundness to the conclusion drawn from the collection of these data.
What is important, however, is that the awareness on the relationship between heavy metals and increased cancer risk, and the relative consequences for human health has been raised, and that significant counter-measures can be undertaken in order to reduce human exposure to these pollutants both in the environment and in the working place. Moreover, effective therapies should be available for those who have been exposed to chronic and acute contamination, although being the latter normally less dangerous, especially through detoxification of the organism with selective chelating agents or analogous treatments.
In conclusion, the presence of heavy metals in biological samples from tumor patients represents a critical area of study that requires ongoing research and a multidisciplinary approach to better understand the intricate relationships between heavy metal exposure and cancer. These insights hold the promise of improving our understanding of cancer etiology and potentially influencing public health measures to mitigate heavy metal exposure and reduce cancer risk.
As we continue to explore the complex network of connections between heavy metals and cancer, we hope that these discoveries will ultimately lead to more effective preventive measures, earlier detection, and improved treatment strategies for individuals affected by these devastating diseases. The collaborative efforts of researchers, clinicians, and policymakers are essential in addressing this urgent issue and enhancing the overall well-being of cancer patients and the general population.
Data availability
Not applicable.
References
Peana M, Pelucelli A, Medici S, Cappai R, Nurchi VM, Zoroddu MA (2021) Metal toxicity and speciation: a review. Curr Med Chem 28:7190–7208
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60
Aaseth J, Gerhardsson L, Skaug MA, Alexander J (2016) General chemistry of metal toxicity and basis for metal complexation. Chelation therapy in the treatment of metal intoxication. Academic Press, pp 1–33
Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M (2022) Essential metals in health and disease. Chemico-Biol Interact. https://doi.org/10.1016/j.cbi.2022.110173
Islam MR, Akash S, Jony MH, Alam MN, Nowrin FT, Rahman MM, Rauf A, Thiruvengadam M (2023) Exploring the potential function of trace elements in human health: a therapeutic perspective. Mol Cell Biochem. https://doi.org/10.1007/s11010-022-04638-3
Kumar A, Misra BB (2019) Challenges and opportunities in cancer metabolomics. Proteomics 19:e1900042. https://doi.org/10.1002/pmic.201900042
Bradl H (2005) Sources and origins of heavy metals. Interface science and technology, 6th edn. Elsevier, pp 1–27
Azevedo JA, Carter BS, Meng F, Turner DL, Dai M, Schatzberg AF, Barchas JD, Jones EG, Bunney WE, Myers RM (2016) The microrna network is altered in anterior cingulate cortex of patients with unipolar and bipolar depression. J Psychiatri Res. https://doi.org/10.1016/j.jpsychires.2016.07.012
Ahmad M, Islam S, Rahman S, Haque M, Islam M. (2010) Heavy metals in water, sediment and some fishes of buriganga river, bangladesh.
Wang Y, Liu Y, Zhan W, Zheng K, Wang J, Zhang C, Chen R (2020) Stabilization of heavy metal-contaminated soils by biochar: challenges and recommendations. Sci Total Environ 729:139060
Sardar K, Ali S, Hameed S, Afzal S, Fatima S, Shakoor MB, Bharwana SA (2013) Heavy metals contamination and what are the impacts on living organisms. Greener J Environ Manag Public Safety 2:172–179
Jaishankar M, Tseten T, Anbalagan N (2014) Mathew and K. N. J. I. t. Beeregowda. Toxic Mechanism Health Eff some Heavy Met 7:60
Fu Z, Xi S (2020) The effects of heavy metals on human metabolism. Toxicol Mech Methods 30:167–176
O’Carroll RE, Masterton G, Dougall N, Ebmeier KP, Goodwin GM (1995) The neuropsychiatric sequelae of mercury poisoning. The mad hatter’s disease revisited. Br J Psychiatry 167:95–98
Harada M (1995) Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25:1–24
Canfield, R., T. Jusko and K. J. R. Kordas (2005) Environmental lead exposure and children’s cognitive function. 31: 293
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972. https://doi.org/10.3389/fphar.2021.643972
Hossini H, Shafie B, Niri AD, Nazari M, Esfahlan AJ, Ahmadpour M, Nazmara Z, Ahmadimanesh M (2022) Makhdoumi and N. Mirzaei. A comprehensive review on human health effects of chromium: insights on induced toxicity. Environ Sci Pollut Res 29:70686–70705
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F (2021) Cancer statistics for the year 2020: an overview. Int J Cancer 149:778–789
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Lappano R, Malaguarnera R, Belfiore A, Maggiolini M (2017) Recent advances on the stimulatory effects of metals in breast cancer. Mol Cell Endocrinol 457:49–56.
Lee NW, Wang HY, Du CL, Yuan TH, Chen CY, Yu CJ, Chan CC (2022) Air-polluted environmental heavy metal exposure increase lung cancer incidence and mortality: a population-based longitudinal cohort study. Sci Total Environ 810:152186.
Yuan W, Yang N, Li X (2016) Advances in understanding how heavy metal pollution triggers gastric cancer. BioMed Res Int. https://doi.org/10.1155/2016/7825432
Boffetta P (1993) Carcinogenicity of trace elements with reference to evaluations made by the international agency for research on cancer. Scandinavian J Work, Environ Health 19:67–70
Kim HS, Kim YJ, Seo YR (2015) An overview of carcinogenic heavy metal molecular toxicity mechanism and prevention. J Cancer Prev 20:232–240
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–64. https://doi.org/10.1007/978-3-7643-8340-4_6
Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M (2023) Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol 97:2499–2574
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Mol, Clinical Environ Toxicol: Environ Toxicol 3:133–64
Ohsawa M (1997) Biomarkers for responses to heavy metals. Cancer Causes Control 8:514–517
Farombi E, Adelowo O, Ajimoko Y (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun river. Int J Environ Res Public Health 4:158–165
Maresca V, Fusaro L, Sorbo S, Siciliano A, Loppi S, Paoli L, Monaci F, Piscopo M, Guida M, Galdiero E (2018) Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in southern europe. Ecotoxicol Environ Safety 163:665–673
Malhotra J, Malvezzi M, Negri E, Vecchia CL, Boffetta P (2016) Risk factors for lung cancer worldwide. Eur Respir J 48:889–902.
Huang HH, Huang JY, Lung CC, Wu CL, Ho CC, Sun YH, Ko PC, Su SY, Chen SC, Liaw YP (2013) Cell-type specificity of lung cancer associated with low-dose soil heavy metal contamination in Taiwan: an ecological study. BMC Public Health 13:330. https://doi.org/10.1186/1471-2458-13-330
Hong Y-S, Song K-H, Chung JY (2014) Health effects of chronic arsenic exposure. J Prev Med Public Health 47:245
Tran JQ, Dranikov A, Iannucci A, Wagner WP, LoBello J, Allen J, Weiss GJ (2014) Heavy metal content in thoracic tissue samples from patients withand without NSCLC. Lung Cancer Int. https://doi.org/10.1155/2014/853158
Pietrzak S, Wójcik J, Baszuk P, Marciniak W, Wojtyś M, Dębniak T, Cybulski C, Gronwald J, Alchimowicz J, Masojć BJB (2021) Influence of the levels of arsenic, cadmium, mercury and lead on overall survival in lung cancer. Biomolecules 11:1160
Bai Y, Wang G, Fu W, Lu Y, Wei W, Chen W, Wu X, Meng H, Feng Y, Liu Y et al (2019) Circulating essential metals and lung cancer: risk assessment and potential molecular effects. Environ Int 127:685–693.
Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L (2014) Copper active sites in biology. Chem Rev 114:3659–3853.
Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S et al (2022) Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer 22:102–113.
Krishnamoorthy L, Cotruvo JA Jr., Chan J, Kaluarachchi H, Muchenditsi A, Pendyala VS, Jia S, Aron AT, Ackerman CM, Wal MN et al (2016) Copper regulates cyclic-amp-dependent lipolysis. Nat Chem Biol 12:586–592.
Turski ML, Brady DC, Kim HJ, Kim BE, Nose Y, Counter CM, Winge DR, Thiele DJ (2012) A novel role for copper in ras/mitogen-activated protein kinase signaling. Mol Cell Biol 32:1284–1295. https://doi.org/10.1128/MCB.05722-11
Tsang T, Posimo JM, Gudiel AA, Cicchini M, Feldser DM, Brady DC (2020) Copper is an essential regulator of the autophagic kinases ulk1/2 to drive lung adenocarcinoma. Nature Cell Biol 22:412–24. https://doi.org/10.1038/s41556-020-0481-4
Yun YH, Wang Y, Yang ED, Jing X (2022) Cuproptosis-related gene-slc31a1 fdx1 and atp7b-polymorphisms are associated with risk of lung cancer. Pharmacogenomics Personalized Med 15:733
Cobanoglu U, Demir H, Sayir F, Duran M, Mergan D (2010) Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pacific J Cancer Prev 11:1383–88
Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM (2018) Iron and cancer. Annu Rev Nutr 38:97–125
Alemán MR, Santolaria F, Batista N, Marıa J, González-Reimers E, Milena A, Llanos M, Gómez-Sirvent JC (2002) Leptin role in advanced lung cancer. A mediator of the acute phase response or a marker of the status of nutrition? Cytokine 19:21–26
Fracchia A, Ubbiali A, El Bitar O, Pacetti M, Sommariva E, Arreghini M, Longhini E, Bonalumi GP (1999) A comparative study on ferritin concentration in serum and bilateral bronchoalveolar lavage fluid of patients with peripheral lung cancer versus control subjects. Oncology 56:181–188.
Shi HB, Li XD, Jiang JT, Zhao WQ, Ji M, Wu CP (2014) Serum ferritin is elevated in advanced non-small cell lung cancer patients and is associated with efficacy of platinum-based chemotherapy. J Cancer Res Ther 10:2014. https://doi.org/10.4103/0973-1482.139156
Xiong R, He R, Liu B, Jiang W, Wang B, Li N, Geng Q (2021) Ferroptosis: a new promising target for lung cancer therapy. Oxid Med Cell Longev 2021:8457521.
Kukulj S, Jaganjac M, Boranic M, Krizanac S, Santic Z, Poljak-Blazi M (2010) Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer. Med Oncol 27:268–277.
Wu HK, Wang M, Raman JD, McDonald AC (2021) Association between urinary arsenic, blood cadmium, blood lead, and blood mercury levels and serum prostate-specific antigen in a population-based cohort of men in the United States. PLoS ONE 16:e0250744
Coradduzza D, Congiargiu A, Chen Z, Zinellu A, Carru C, Medici S (2023) Ferroptosis and senescence: a systematic review. Int J Mol Sci 24:3658.
Benderli Cihan Y, Sozen S, Ozturk Yildirim S (2011) Trace elements and heavy metals in hair of stage iii breast cancer patients. Biol Trace Elem Res 144:360–79.
Khalaf EA, Abduljaleel SA, Al-Jassani HMJTI (2021) Appraisal of trace elements and heavy metals levels in breast cancer patients of Basrah Province. Toxicol Int 28:8–14
Siddiqui MKJ, Jyoti S, Singh PK, Mehrotra K, Singh Sarangi R (2006) Comparison of some trace elements concentration in blood, tumor free breast and tumor tissues of women with benign and malignant breast lesions: an Indian study. Environ Int 32:630–37. https://doi.org/10.1016/j.envint.2006.02.002
Marouf BH (2018) Association between serum heavy metals level and cancer incidence in Darbandikhan and Kalar area, Kurdistan region, Iraq. Nigerian J Clin Pract 21:766–71. https://doi.org/10.4103/njcp.njcp_384_16
Aquino NB, Sevigny MB, Sabangan J, Louie MC (2012) The role of cadmium and nickel in estrogen receptor signaling and breast cancer: metalloestrogens or not? J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 30:189–224. https://doi.org/10.1080/10590501.2012.705159
Grattan BJ (2012) Freake. Zinc and cancer: implications for liv-1 in breast cancer. Nutrients 4:648–675.
Ionescu JG (2006) Heavy metal accumulation in malignant tumours as basis for a new integrative therapy model. Anti-Aging Ther 9:189–201
Li L, Zhang MH, Men YH, Wang W, Zhang WD (2020) Heavy metals interfere with plasma metabolites, including lipids and amino acids, in patients with breast cancer. Oncol Lett 19:2925–33. https://doi.org/10.3892/ol.2020.11402
Men Y, Li L, Zhang F, Kong X, Zhang W, Hao C, Wang G (2020) Evaluation of heavy metals and metabolites in the urine of patients with breast cancer. Oncol Lett 19:1331–1337.
O’Brien KM, White AJ, Jackson BP, Karagas MR, Sandler DP, Weinberg CRJ (2019) Toenail-based Metal Concentrations and young-onset Breast cancer 188:646–655
Pala V, Agnoli C, Cavalleri A, Rinaldi S, Orlandi R, Segrado F, Venturelli E, Vinceti M, Krogh V, Sieri S (2022) Prediagnostic levels of copper and zinc and breast cancer risk in the ordet cohort. Cancer Epidemiol Biomarkers Prev 31:1209–1215.
White AJ, Weinberg CR, O’Meara ES, Sandler DP, Sprague BL (2019) Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res 21:24.
Liu L, Chen J, Liu C, Luo Y, Chen J, Fu Y, Xu Y, Wu H, Li X, Wang H (2022) Relationships between biological heavy metals and breast cancer: a systematic review and meta-analysis. Front Nutr 9:838762
Jouybari L, Saei Ghare Naz M, Sanagoo A, Kiani F, Sayehmiri F, Sayehmiri K, Hasanpour Dehkordi A (2018) Toxic elements as biomarkers for breast cancer: a meta-analysis study. Cancer Manage Res. https://doi.org/10.2147/CMAR.S151324
Jouybari L, Kiani F, Islami F, Sanagoo A, Sayehmiri F, Hosnedlova B, Dosa MD, Kizek R, Chirumbolo S, Bjorklund G (2020) Copper concentrations in breast cancer: a systematic review and meta-analysis. Curr Med Chem 27:6373–6383.
Chang VC, Cotterchio M, Khoo E (2019) Iron intake, body iron status, and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer 19:543.
Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10:63–89.
Neslund-Dudas C, Kandegedara A, Kryvenko ON, Gupta N, Rogers C, Rybicki BA, Dou QP, Mitra B (2014) Prostate tissue metal levels and prostate cancer recurrence in smokers. Biol Trace Elem Res 157:107–112
Vella V, Malaguarnera R, Lappano R, Maggiolini M, Belfiore A (2017) Recent views of heavy metals as possible risk factors and potential preventive and therapeutic agents in prostate cancer. Mol Cell Endocrinol 457:57–72
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107.
Lim JT, Tan YQ, Valeri L, Lee J, Geok PP, Chia SE, Ong CN, Seow WJ (2019) Association between serum heavy metals and prostate cancer risk-a multiple metal analysis. Environ Int 132:105109
Sarafanov AG, Todorov TI, Centeno JA, Macias V, Gao W, Liang WM, Beam C, Gray MA, Kajdacsy-Balla AA (2011) Prostate cancer outcome and tissue levels of metal ions. Prostate 71:1231–1238
Karimi G, Shahar S, Homayouni N, Rajikan R, Bakar NFA, Othman MS (2012) Association between trace element and heavy metal levels in hair and nail with prostate cancer. Asian Pac J Cancer Prev 13:4249–4253
Sohrabi M, Nikkhah M, Sohrabi M, Farimani AR, Shahi MM, Ziaie H, Shirmardi S, Kohi Z, Salehpour D, Tameshkel FS et al (2021) Evaluating tissue levels of the eight trace elements and heavy metals among esophagus and gastric cancer patients: a comparison between cancerous and non-cancerous tissues. J Trace Elem Med Biol 68:126761
Wang L, Miao C, He Y, Li H, Zhang S, Li K, Liu H, Li W, Zhao J, Xu Y et al (2022) The influence of heavy metals on gastric tumorigenesis. J Oncol 2022:6425133. https://doi.org/10.1155/2022/6425133
Feng L, Du J, Yao C, Jiang Z, Li T, Zhang Q, Guo X, Yu M, Xia H, Shi L (2020) Ribosomal DNA copy number is associated with p53 status and levels of heavy metals in gastrectomy specimens from gastric cancer patients. Environ Int 138:105593
Fonseca-Nunes A, Agudo A, Aranda N, Arija V, Cross AJ, Molina E, Sanchez MJ, Bueno-de-Mesquita HB, Siersema P, Weiderpass E et al (2015) Body iron status and gastric cancer risk in the eurgast study. Int J Cancer 137:2904–2914. https://doi.org/10.1002/ijc.29669
Morgan RL, Whaley P, Thayer KA, Schunemann HJ (2018) Identifying the peco: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 121:1027–1031.
Acknowledgements
The images are original and created with BioRender.com.
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement. This research was funded by FAR 2019 of Serenella Medici and Ciriaco Carru.
Author information
Authors and Affiliations
Contributions
Conceptualization, DC and SM; methodology, DC and SM; software, DC; for-mal analysis, DC and AC, IMAM; investigation, DC and SM and AC; resources, CC; writing original draft preparation, DC, AC, and SM; writing—review and editing, SM, and CC; supervision, CC; funding acquisition, SM. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coradduzza, D., Congiargiu, A., Azara, E. et al. Heavy metals in biological samples of cancer patients: a systematic literature review. Biometals (2024). https://doi.org/10.1007/s10534-024-00583-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10534-024-00583-4