Abstract
Background
Sarcopenic obesity emerges as a risk factor for adverse clinical outcomes in non-hospitalized older adults, including physical disabilities, metabolic diseases, and even mortality. In this systematic review and meta-analysis, we investigated the overall SO prevalence in non-hospitalized adults aged ≥ 65 years and assessed the sociodemographic, clinicobiological, and lifestyle factors related to SO.
Methods
We searched the PubMed, Embase, Cochrane Library, and Web of Science databases for studies reporting the prevalence of SO from database inception to October 2023. Two researchers independently screened the literature, evaluated the study quality, and extracted the data. Both fixed- and random-effects models were used in the meta-analysis to estimate the pooled SO prevalence and perform subgroup analyses. Publication and sensitivity bias analyses were performed to test the robustness of the associations.
Results
Among 46 studies eligible for review and a total of 71,757 non-hospitalized older adults, the combined prevalence of SO was 14% (95% CI:11–17%, I2 = 99.5%, P < 0.01). Subgroup analysis according to lifestyle factors demonstrated that the SO prevalence was 17% (95% CI: 8–29%, I2 = 99.5%, P < 0.01) in older adults without exercise habits. Regarding clinicobiological factors, older adults with a history of falls (15% [95% CI: 10–22%, I2 = 82%, P < 0.01]), two or more chronic diseases (19% [95% CI: 10–29%, I2 = 97%, P < 0.01]), functional impairment (33% [95% CI: 29–37%, I2 = 0%, P = 0.95]), cognitive impairment (35% [95% CI: 9–65%, I2 = 83%, P = 0.02]), osteoporosis (20% [95% CI: 8–35%, I2 = 96%, P < 0.01]), high fasting glucose level (17% [95% CI: 1–49%, I2 = 98%, P < 0.01]), or the use of antipsychotics (13% [95% CI: 2–28%, I2 = 0%, P = 0.32]) exhibited a higher SO prevalence.
Conclusion
SO prevalence is high among non-hospitalized older adults, especially those with functional and cognitive impairments. Thus, SO is a potential problem for the aging population; implementation of planned interventions in the community is needed to reduce the prevalence and adverse outcomes of SO.
Similar content being viewed by others
Background
The coexistence of sarcopenia and increased fat mass is referred to as sarcopenic obesity (SO) [1]. Compared with sarcopenia or obesity alone, the synergistic effect of muscle loss and obesity leads to a higher risk of adverse outcomes such as falls, physical disabilities, and fractures and is closely related to the occurrence of metabolic diseases such as cardiovascular disease, diabetes, and non-alcoholic fatty liver disease, as well as death [2,3,4]. SO is an important health problem, and its prevalence and mortality are increasing worldwide, especially in the older adult population [5]. Apart from its impact on health status, SO also has considerable independent effects on healthcare expenses [6,7,8].
By recognizing the prevalence and risk factors for SO, healthcare professionals and primary care clinicians can provide early detection, diagnosis, and intervention for patients who potentially have SO [9–10]. However, the prevalence of SO is not yet clearly established and can vary by as much as 26, mainly because of the use of different evaluation methods, criteria, and cutoff points applied to determine muscle mass and fat mass [11]. The diagnostic criteria for SO currently used in research are based on the coexistence of sarcopenia and obesity, leading to the application of a diverse diagnostic criteria for sarcopenia and obesity [12]. A Korean cohort study using muscle mass and waist circumference to diagnose SO demonstrated an SO prevalence of 41.6%, with 35.2% prevalence in men and 48.2% in women [13]. A cross-sectional study in China defined sarcopenia as low muscle mass and strength and obesity as high body fat percentage; they showed an SO prevalence of only 4.0%, including 7.0% in men and 2.4% in women [14].
Furthermore, the prevalence rate of SO also differs when a different diagnostic criteria was adopted for the same population. A Brazilian longitudinal study included older adults aged > 65 years and found that the prevalence of SO using two diagnostic criteria for SO (muscle mass combined with body fat percentage vs. muscle mass and strength combined with body fat percentage) was 29.3% and 19.3%, respectively [7, 15]. In addition, the prevalence of SO is also affected by clinicobiological and lifestyle factors in older adults; older adults with chronic conditions were 1.8 times more likely to develop SO than the general population [16]. A study by Son et al. found that active physical activity may be negatively associated with the development of sarcopenia and sarcopenic obesity in older adults [17]. Based on the above research results, we hypothesized that there are differences in sociodemographic, clinicobiological, and lifestyle factors among older adults that would impact the corresponding SO prevalence.
Therefore, we conducted a systematic review and meta-analysis to investigate the overall prevalence of SO in non-hospitalized adults aged ≥ 65 years and examine the association of various health-related factors with the disease. Our results can provide information to ensure better allocation of healthcare resources and early healthcare decision making for older patients with SO.
Methods
Literature search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines were followed for the calculation and reporting of meta-analysis data [18]. Literature searches were conducted using the EMBASE, PubMed, Web of Science, and Cochrane Library databases; the search period was from database inception through October 2023. The following search terms were used: “Sarcopenia,” “Obesity,” “Sarcopenic Obesity,” “Aged,” and “Elderly.” The references identified in the relevant publications were also reviewed to identify additional studies. The detailed search strategy used for each database is presented in Additional File S1.
Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) participants: aged ≥ 65 years in nursing homes or communities, without sex, race, or regional restrictions; (2) exposure: SO (the patients should have both sarcopenia and obesity, and the diagnostic criteria and cutoff values for sarcopenia and obesity were not restricted); (3) outcome: SO prevalence (if there were any additional data required to confirm, we contacted the corresponding author of the study twice within a 1-month period); (4) study design: cohort studies and cross-sectional studies (baseline data were extracted from cohort studies); (5) there were no limitations on the language of publication, year of publication, or publication status. The study exclusion criteria were as follows: (1) studies that did not provide a clear diagnostic criterion of SO; (2) studies including participants with specific diseases; (3) reviews, lectures, case reports, or articles in which the data were evidently abnormal or missing (and the author could not be contacted).
Study selection and data extraction
The identified studies were stored in a reference management software (EndNote, Clarivate, Philadelphia, PA, United States). Literature screening and data extraction were independently performed by two reviewers. If the included articles were not written in English or Chinese, the study team made a preliminary translation of the included documents with the help of translation software and invited translators to proofread and revise them before the study team performed the reading and data extraction. Any disagreements between the reviewers were resolved by discussion with a third reviewer. We extracted the first author’s name, year of publication, study name, country in which the study was conducted, sample size, diagnostic criteria of sarcopenia and obesity, body mass index (BMI) and other study parameters, and the prevalence of SO.
Quality assessment and publication bias
Two researchers independently evaluated the risk of bias in the included studies using the Joanna Briggs Institute’s Critical Appraisal Checklist for Prevalence Studies [19]. There were 9 items in total: (1) “Was the sample frame appropriate to address the target population?;” (2) “Were study participants sampled in an appropriate way?;” (3) “Was the sample size adequate?;” (4) “Were the study participants and setting described in detail?;” (5) “Was the data analysis conducted with sufficient coverage of the identified sample?;” (6) “Were valid methods used for identification of the condition?:” (7) “Was the condition measured in a standard, reliable way for all of the participants?;” (8) “Was there an appropriate statistical analysis?;” and (9) “Was the response rate adequate?.” For each item, the study was assigned a “yes,” “no,” “unclear,” or “not applicable” remark. The total number of “yes” answers was counted per study, with a greater number of “yes” answers indicating a higher quality of the study. Studies were eligible if more than five “yes” answers were achieved [5]. Any disagreements were resolved by discussion or through consultation with a third senior researcher. Publication bias was tested using Egger’s funnel plots.
Statistical analysis
We used the R software (version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria) for all statistical analyses. The combined prevalence and 95% confidence interval (95% CI) of SO in adults aged ≥ 65 years were calculated. Heterogeneity among the studies was assessed using Q and I2 statistic indices. A significant Q value (P < 0.1) indicated a lack of homogeneity among the studies; I2 = 0 indicated that the inconsistency among the results is not statistically different (I2 < 50% indicated low inconsistency, I2 ≥ 50% indicated high inconsistency). If the heterogeneity test results were P > 0.1 and I2 < 50%, the homogeneity of the study was considered good, and a fixed-effects model was adopted; otherwise, the random-effects model was adopted.
Subgroup analyses were performed based on the diagnostic criteria of SO, study design, geographical region, age, sex, race, education level, physical activity, fall history, number of chronic diseases, comorbidities, high fasting glucose level, and drug use.
Results
Characteristics of the included studies
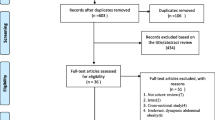
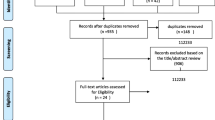
A flow chart of the study selection process and exclusion criteria is shown in Fig. 1. According to the search criteria, 6,910 articles were found during the literature search; after excluding duplicate references, 3,993 remained for further screening. We filtered the results by title, abstract, and full text. Finally, 46 studies met the inclusion and exclusion criteria. Among them, 17 [6, 9, 13, 15, 20,21,22,23,24,25,26,27,28,29,30,31,32] were cohort studies, and 29 [7–8, 10, 14, 17, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] were cross-sectional studies. Articles published in the last 3 years (post-2020) accounted for 28 [6–7, 9–10, 15, 20–21, 23, 26,27,28,29,30,31, 36, 39, 41, 44,45,46,47, 49–50, 52,53,54,55,56] studies. The total number of participants included in this review was 71,757 from studies with sample sizes ranging from 64 [34] to 7,852 [6]. Seven [31, 34–35, 37,38,39, 50] studies included only women, three [21, 24–25] included only men, and the remaining studies included participants of both sexes. The included studies used varying diagnostic criteria for sarcopenia (low muscle mass, low muscle strength, or low muscle strength and mass) combined with different obesity criteria (percentage of body fat [PBF], BMI, or waist circumference [WC]) to diagnose SO. Among them, 14 [6,7,8, 10, 13, 17, 20, 22, 28, 34, 45, 47, 51, 54] studies used low muscle mass as the diagnostic criterion for sarcopenia, 10 [9, 20, 26, 29, 40–41, 49–50, 55–56] studies used low muscle strength as the diagnostic criterion for sarcopenia, and 20 [14–15, 21,22,23,24,25, 27, 30–31, 35–36, 39, 40,43,44, 46, 48, 52–53] studies used low muscle mass plus low muscle strength as the diagnostic criterion for sarcopenia. In addition, one [6] study, a multicenter population study, did not provide a specific cutoff for their obesity diagnostic criteria (PBF). Two [37–38] studies used the appendicular fat-free mass (calculated as follows: -14.529 + [17.989 × height in meters] + [0.1307 × total fat mass in kg]) truncation value as the diagnostic criterion for SO and did not use the diagnostic method of sarcopenia combined with obesity. Detailed characteristics of the included studies are displayed in Additional File S2.
Study quality evaluation
Most studies were of fair [6, 8, 10, 2,3,4,5,6,7,8,9,10,11,12,13, 16, 19, 25–26, 29, 31, 32, 35,36,37, 42, 48, 51,52,53] or high [7, 9, 15, 21,22,23,24,25, 28–29, 31, 33, 35, 36, 40,41,42,43, 45, 49, 51, 35,53,54,55,56] quality because they scored “yes” for at least five items in the quality assessment checklist. Specifically, 26 [7, 9, 15, 20,22,23,24,25, 28–29, 31, 33, 35–36, 36,41,42,43, 43,46,47,48,49, 51–52, 56] studies scored “yes” for 8–9 items, 18 [6, 8, 13–14, 17, 20, 26–27, 30, 32, 34, 37, 39, 44, 50, 51,54,55] studies scored “yes” for 6–7 items, and 2 [38, 10] studies scored “yes” for 5 items. Detailed assessment results for the included studies are displayed in Fig. 2 and Additional File S3.
Overall prevalence of SO in non-hospitalized older adults ≥ 65 years
The prevalence of SO in the 46 included studies varied from 3% [40] to 55% [6]. The pooled SO prevalence was 14% (95% CI: 11–17%), with a high level of heterogeneity (I2 = 99.5%, P < 0.01). Therefore, we used a random-effects model (Additional File S4).
Subgroup analysis of diagnostic criteria, study design, sociodemographic, lifestyle and clinicobiological factors
Diagnostic criteria of SO
We found a higher prevalence of SO when muscle mass alone was used to diagnose sarcopenia compared with that when muscle strength alone or muscle mass plus muscle strength was used. The pooled prevalence of SO diagnosed using low muscle mass combined with different diagnostic criteria for obesity was 21% (95% CI: 13–29%, I2 = 99.5%, P < 0.01, 14 studies [6,7,8, 10, 13, 17, 22, 28, 33–34, 45, 47, 51, 54]). Among them, the pooled prevalence of SO diagnosed using low muscle mass + BMI, low muscle mass + PBF, and low muscle mass + WC was 18% (95% CI: 9–29%, I2 = 99%, P < 0.01, 2 studies [33, 45]), 29% (95% CI: 13–49%, I2 = 98%, P < 0.01, 5 studies [6, 15, 34, 51–52]), 18% (95% CI: 7–32%, I2 = 98%, P < 0.01, 5 studies [8, 13, 17, 47, 54]), respectively (Table 1).
The pooled prevalence of SO diagnosed using low muscle strength combined with different diagnostic criteria for obesity was 12% (95% CI: 8–16%, I2 = 98%, P < 0.01, 10 studies [9, 20, 26, 29, 40–41, 49, 50, 55–56]). Among them, the pooled prevalence of SO diagnosed using low muscle strength + BMI was 11% (95% CI: 5–19%, I2 = 98%, P < 0.01, 5 studies [9, 29, 40, 50, 55]), whereas that diagnosed using low muscle strength + PBF was 8% (95% CI: 2–19%, I2 = 96%, P < 0.01, 2 studies [41, 49]) (Table 1).
The pooled prevalence of SO diagnosed using low muscle mass + low muscle strength combined with different diagnostic criteria for obesity was 10% (95% CI: 6–14%, I2 = 98%, P < 0.01, 20 studies [14–15, 20,22,23,24,25, 27, 30–31, 35–36, 39, 40,43,44, 46, 48, 52–53]). Among them, the pooled prevalence of SO diagnosed using low muscle mass + low muscle + PBF, low muscle mass + low muscle + BMI, and low muscle mass + low muscle + BMI + WC + PBF was 10% (95% CI: 6–14%, I2 = 98%, P < 0.01, 9 studies [14–15, 22, 24, 35, 42–43, 46, 52]), 18% (95% CI: 15–20%, I2 = 57%, P = 0.07, 4 studies [15, 31, 44, 48]), and 9% (95% CI: 9–17%, I2 = 94%, P < 0.01, 3 studies [27, 36, 53]), respectively (Table 1).
Study design
Our findings suggest that the study design (cross-sectional/cohort study) had no effect on the prevalence of SO. The pooled SO prevalence for cohort and cross-sectional studies was 13% (95% CI: 9–17%, I2 = 99%, P < 0.01) and 13% (95% CI: 10–16%, I2 = 99%, P < 0.01), respectively (Table 1).
Geographical region
The prevalence of SO is higher in South and North America than that in Asia, Europe, and Oceania. The pooled prevalence of SO in Asia, Europe, Eurasia, South America, North America, and Oceania was 12% (95% CI: 9–16%, I2 = 99%, P < 0.01, 22 studies [8, 10, 13–14, 17, 22, 26–27, 29, 33, 39,42,43,44,45,46,47, 49,52,53,54, 56]), 11% (95% CI: 7–15%, I2 = 97%, P < 0.01, 10 studies [23, 25, 28, 30, 31, 32,33,34,35, 36, 39,40,41,42,43,44,45,46,47,48, 49 ]), 14% (95% CI: 3–31%, I2 = 98%, P < 0.01, 2 studies [40, 55]), 22% (95% CI: 12–35%, I2 = 90%, P < 0.01, 6 studies [7, 15, 34, 37–38, 50]), 16% (95% CI: 10–24%, I2 = 98%, P < 0.01, 2 studies [9, 19]), and 8% (95% CI: 5–11%, I2 = 88%, P < 0.01, 2 studies [21, 24]), respectively (Table 1).
Age, sex, race, and education level
Subgroup analyses based on sociodemographic variables of the included study population revealed higher SO prevalence rates among those aged ≥ 85 years, females, Whites, and those with a high school or less than high school education. The pooled prevalence of SO in individuals aged 65–74, 75–84, and ≥ 85 years was 13% (95% CI: 9–18%, I2 = 98%, P < 0.01, 9 studies [6–7, 9, 15, 20, 23, 32, 46–47]), 17% (95% CI: 11–25%, I2 = 97%, P < 0.01, 7 studies [6–7, 9, 20, 23, 46–47]), and 23% (95% CI: 11–37%, I2 = 92%, P < 0.01, 4 studies [7, 9, 20, 23]), respectively (Table 1).
The pooled prevalence of SO in females and males was 15% (95% CI: 11–19%, I2 = 99.2%, P < 0.01, 38 studies [9–10, 2,14,15, 22, 23,24,25,26, 23,44,45, 52,53,54,55,56]) and 13% (95% CI: 10–17%, I2 = 99%, P < 0.01, 34 studies [6,7,8,9,10, 2,14,15, 19,21,22,23,24,25,26,27,28,29,30, 32–33, 36, 38,41,42,43, 43,46,47,48,49, 51–52, 56]), respectively (Table 1).
The pooled prevalence of SO in Whites and Blacks was 23% (95% CI: 10–38%, I2 = 97%, P < 0.01, 3 studies [7, 15, 20]) and 17% (95% CI: 12–23%, I2 = 71%, P = 0.02, 4 studies [7, 15, 20, 31]), respectively (Table 1).
The pooled prevalence of SO in individuals with educational levels < high school, high school to some college, and college or more was 16% (95% CI: 9–24%, I2 = 99%, P < 0.01, 7 studies [6,7,8, 20, 42, 47, 56]), 14% (95% CI: 6–24%, I2 = 98%, P < 0.01, 6 studies [6,7,8, 20, 42, 47]), and 11% (95% CI: 5–19%, I2 = 96%, P < 0.01, 8 studies [6,7,8, 19,20,21, 42, 47, 51]), respectively (Table 1).
Physical activity
Among older adults, those who do not exercise regularly tend to have a higher prevalence of SO compared with those who maintain a consistent exercise routine. The pooled prevalence of SO in individuals whose physical activity levels were moderate, vigorous, and not much/not at all was 15% (95% CI: 8–23%, I2 = 99.5%, P < 0.01, 13 studies [6,7,8, 15, 17, 20, 26, 43,46,47, 51, 54, 56]), 12% (95% CI: 4–23%, I2 = 98%, P < 0.01, 4 studies [8, 17, 46–47]), and 17% (95% CI: 8–29%, I2 = 99.5%, P < 0.01, 7 studies [6, 10, 14, 44–45, 49, 52]), respectively (Table 1).
Fall history
Older adults with a history of falls were more likely to experience SO than those without such history. The pooled prevalence of SO in older adults with and without a history of falls was 15% (95% CI: 10–22%, I2 = 82%, P < 0.01, 5 studies [9, 21, 27, 46, 51]) and 9% (95% CI: 9–14%, I2 = 98%, P < 0.01, 5 studies [9, 21, 27, 46, 51]), respectively (Table 1).
Number of chronic diseases
Subgroup analyses showed that older adults with no chronic disease or only one chronic disease had lower SO prevalence than those with multiple chronic diseases. The pooled prevalence of SO for individuals with 0, 1, and ≥ 2 chronic diseases was 16% (95% CI: 3–37%, I2 = 99.5%, P < 0.01, 3 studies [6, 45, 52]), 10% (95% CI: 2–23%, I2 = 99%, P < 0.01, 4 studies [6, 8, 45, 52]), and 19% (95% CI: 10–29%, I2 = 97%, P < 0.01, 4 studies [6, 8, 45, 52]), respectively (Table 1).
Comorbidities
Subgroup analysis based on disease type demonstrated higher SO prevalence among older adults with functional or cognitive impairment and osteoporosis. The pooled prevalence of SO in individuals with cancer, lung disease, hypertension, dyslipidemia, functional disabilities, osteoporosis, arthritis, probable dementia, cerebrovascular disease, diabetes, heart disease, and depressive symptoms was 8% (95% CI: 2–18%, I2 = 95%, P < 0.01, 2 studies [20, 42]), 16% (95% CI: 9–25%, I2 = 85%, P < 0.01, 5 studies [9, 20, 23, 26, 39]), 12% (95% CI: 8–17%, I2 = 98%, P < 0.01, 10 studies [8–9, 15, 20, 22, 27, 29, 41, 47, 51]), 15% (95% CI: 6–27%, I2 = 99%, P < 0.01, 6 studies [8, 29, 33, 41, 47, 51]), 33% (95% CI: 29–37%, I2 = 0%, P = 0.95, 2 studies [34, 37]), 20% (95% CI: 8–35%, I2 = 96%, P < 0.01, 4 studies [8–9, 25–26]), 17% (95% CI: 10–25%, I2 = 95%, P < 0.01, 6 studies [7, 9, 19, 25–26, 49]), 35% (95% CI: 9–65%, I2 = 83%, P = 0.02, 2 studies [9, 22], 9% (95% CI: 5–14%, I2 = 71%, P < 0.01, 5 studies [19, 22, 28, 40, 49]), 14% (95% CI: 10–19%, I2 = 95%, P < 0.01, 14 studies [7,8,9, 14, 19, 22–23, 26, 28, 39–40, 45, 49, 53]), 14% (95% CI: 9–19%, I2 = 92%, P < 0.01, 10 studies [9, 14, 19, 22, 25, 28, 37, 39–40, 49]), and 16% (95% CI: 7–28%, I2 = 96%, P < 0.01, 4 studies [9, 22, 28, 40]), respectively (Table 1).
High fasting glucose levels
Higher fasting glucose levels in older adults were associated with a higher incidence of SO. The pooled prevalence of SO in individuals with and without high fasting glucose levels was 17% (95% CI: 1–49%, I2 = 98%, P < 0.01, 2 studies [7, 45]) and 12% (95% CI: 1–42%, I2 = 98%, P < 0.01, 2 studies [7, 45]), respectively (Table 1).
Drug use
We also analyzed the impact of medication usage on the prevalence of SO among older adults and found that those using antipsychotics had a higher rate of SO occurrence. The pooled prevalence of SO in individuals using oral hypoglycemic agents, anti-psychotics, and statins was 11% (95% CI: 6–17%, I2 = 66%, P = 0.09, 2 studies [23, 39]), 13% (95% CI: 2–28%, I2 = 0%, P = 0.32, 2 studies [26, 40]), and 6% (95% CI: 4–7%, I2 = 0%, P = 0.53, 3 studies [23, 40, 49]), respectively (Table 1).
Table 1 Subgroup analysis of diagnostic criteria, study design, sociodemographic, lifestyle and clinicobiological factors.
Publication bias
A funnel plot was created to represent the total prevalence of SO; the plot showed an asymmetric distribution of the study points. Egger’s test results (P = 0.05) also suggested the possibility of a publication bias. A nonparametric shear complement method was used to estimate the number of missing studies and evaluate the influence of publication bias on the results, which showed significant differences in the results before and after splicing. The prevalence of SO, calculated before and after trimming, was 14% (95% CI: 11–17%) and 20% (95% CI: 16–24%), respectively, suggesting that publication bias had a great influence on the stability of the results (Fig. 3).
Sensitivity analysis
To assess the stability of the results, we performed a sensitivity analysis on the 46 included studies by sequentially excluding individual studies. After arbitrarily excluding one study, the combined conversion rate based on the random-effects model was 14% (95% CI: 11–17%), indicating that it had little influence on the combined effect size. Therefore, the results of our meta-analysis are stable and reliable (Additional File S5).
Discussion
We assessed the overall prevalence of SO in non-hospitalized adults aged ≥ 65 years and comprehensively assessed the sociodemographic, clinicobiological, and lifestyle factors associated with SO. The global prevalence of SO was 14%, higher than the 11% prevalence reported by Gao et al. [5], which may be due to the older age of our study population. Moreover, the prevalence of SO was higher when muscle mass measurements alone were used to diagnose sarcopenia compared with when muscle strength alone (12%) or muscle mass plus muscle strength (10%) was used for diagnosis. Study design (cross-sectional study/cohort study) had no effect on the prevalence of SO. Furthermore, the prevalence of SO in South (22%) and North America (16%) was higher than that in Asia (12%), Europe (11%), and Oceania (8%), which may be because the South and North American studies included in this study mainly used muscle mass to diagnose sarcopenia and the higher rates of obesity in North America and South America [55].Various factors, including comorbidities, resulted in the SO prevalence varying broadly from 6–35%.
Consistent with multiple study findings [20, 27, 34, 37], our review shows that the prevalence of SO is influenced by its diagnostic criteria. When muscle mass alone was used to diagnose sarcopenia, the prevalence of SO was higher than that when muscle strength alone or the combined diagnosis of muscle mass and strength was used. The prevalence of SO was the lowest when diagnosed using muscle strength combined with PBF and highest when diagnosed using muscle mass combined with PBF. Different diagnostic criteria for sarcopenia have been developed for different ethnic groups [56,57,58,59]. These diagnostic criteria comprehensively evaluate muscle mass, strength, and function to diagnose sarcopenia and suggest a joint definition of sarcopenia using appendicular skeletal muscle mass and one or two functional parameters (grip strength and/or gait speed). However, there are still some studies that diagnose sarcopenia solely based on muscle mass [6, 27, 43, 45, 52]. The application of consistent definitions would improve the comparability of sarcopenia prevalence in future cohort studies.
Obesity is typically diagnosed using BMI, WC, or PBF; the prevalence of obesity as defined using BMI is lower than that defined using WC or waist-to-hip ratio [60]. Fat accumulation and redistribution associated with muscle loss do not necessarily increase BMI [61]. Therefore, when BMI is used for diagnosis, the prevalence of obesity is relatively low, and missed diagnosis may occur. WC (abdominal fat accumulation), the only measure of body fat distribution independently associated with impaired mobility, had a better correlation with SO [62]. Additionally, follicle-stimulating hormone levels increase along with the rapid decline of estrogen in postmenopausal females, leading to increased visceral fat accumulation [63]. This transfer of fat deposits to the center of the body can increase WC in females [64]. Therefore, the WC method is more sensitive to the diagnosis of obesity in females than in males. When obesity was defined by PBF, the prevalence of SO also increased with age [24, 65]. This explains the major age-related changes in body composition, including increased body fat and sarcopenia. Finally, regardless of the diagnostic method for SO, low muscle mass, low muscle strength, and obesity are significant risk factors for disability and increased mortality [14, 20, 22]. Therefore, prevention of sarcopenia and obesity in the relatively healthy older adult population should be a major goal.
The prevalence of SO varies demographically. In this study, the prevalence in individuals aged 75–84 years was 17%; in those ≥ 85 years, it was as high as 23%. Aging increases body fat and insulin resistance, leading to chronic diseases as well as decreased muscle strength and mass [66]. Therefore, advanced age is associated with a higher prevalence of SO. There was no significant sex difference in the prevalence of SO, potentially because male muscle mass gradually decreases with age, whereas female muscle mass does not decrease significantly with age [67]. However, in early menopause, female muscle mass and function decline significantly owing to a significant decrease in estrogen [68]. Additionally, our results showed that the prevalence of SO was higher in Whites than in Blacks. Different cultural backgrounds, dietary patterns, and physical activity levels may contribute to racial differences in the prevalence of SO. Moreover, lower education levels were associated with a higher SO prevalence. Education level is a predictor of employment type and health behavior. Therefore, providing health education regarding SO to older people of lower socioeconomic status is essential
Regarding lifestyle, consistent with findings from multiple studies [8, 45, 49], older adults who were moderately and intensely active had a lower prevalence of SO than those who were inactive. Park et al. [45] demonstrated that all types of exercises were beneficial to SO and observed that moderate-to-high intensity exercise was highly correlated with skeletal muscle index and grip strength. A meta-analysis of randomized controlled trials showed that exercise, particularly resistance exercise, is critical for improving body composition and physical performance in patients with muscle-reducing obesity [69]. Another study showed that post-exercise macronutrient supplementation (equivalent to 200 kcal) during home-based interval walking training enhanced skeletal muscle mass and strength compared with exercise alone [70]. Calorie restriction combined with moderate aerobic exercise was shown to reduce muscle mass loss in older adults with obesity [71]. These findings support the importance of exercise in the intervention of sarcopenic obesity. Future studies are needed to longitudinally compare the combined effects of nutrition and exercise interventions in sarcopenia and SO.
We found that the prevalence of SO in older adults with a history of falls was 15%. Depending on the number of chronic diseases and medication usage, the prevalence of SO is between 6% and 35%. Fall history was independently related to balance confidence and fall risk [33]. Balance confidence and fear of falling lead to further self-restriction and avoidance of activities of daily living, with reduced physical activity, whereas sedentary behavior is associated with obesity and low muscle strength in older adults. Additionally, falls also cause dysfunction in older adults, and dysfunction is a risk factor for SO [25, 28]. Correspondingly, we found that the prevalence of SO among disabled older adults was high at 33%. The main reason may be related to low physical activity due to physical limitations. A prospective study of 1,851 Japanese residents aged 65 years and older showed that, in addition to aging, major factors associated with sarcopenia were depressed mood and cognitive impairment [72]. We found that the prevalence of SO in older adults with depression and those with cognitive impairment was 16% and 35%, respectively. Correspondingly, the prevalence of SO in older adults using antipsychotic drugs was higher than that in those taking oral hypoglycemic drugs and statins. Similarly, in obese women, increased grip strength is associated with a reduced cognitive impairment risk [73]. The synergistic effect between cognitive impairment and SO is unclear. Chronic inflammation is a basic common pathology of dementia, obesity, and sarcopenia [28, 74–75]. Patients with SO may be in a chronic inflammatory state, leading to a strong correlation between SO and dementia. Additionally, because cognitive and motor performance depend on the nervous system, nervous system damage may lead to both cognitive and motor function decline [76].
Further prospective and interventional studies are needed to clarify the causal relationship between SO prevalence and cognitive impairment. We found that the prevalence of SO was as high as 20% in the older adult population with osteoporosis. This may be related to the loss of muscle mass caused by osteoporosis. Additionally, women with SO are more likely to have high blood sugar, whereas men with SO are more likely to have osteoporosis and dyslipidemia [13]. Further research is needed to establish the causal pathways and identify mediators of the association, particularly modifiable factors, to prevent comorbidities and sarcopenic obesity. Additionally, men and women with SO-related adverse outcomes should be addressed differently
Our study had certain strengths. First, our study highlights the particular importance of sociodemographic, clinicobiological, and lifestyle factors in the prevalence of SO among non-hospitalized older adults. Second, we conducted an extensive literature search and included high-quality studies that produced reliable results. Nevertheless, our study also had some limitations. First, heterogeneity remained high after subgroup analysis; in addition to racial and ethnic differences, different definitions or diagnostic parameters may also contribute to heterogeneity. Several examples for the varying definitions were those for the criteria (gait speed and/or grip strength) and calculation of skeletal muscle mass index (ASMM/height2 or ASMM/BMI) and cutoff points between the morphometric and functional criteria (grip strength 30 kg–26 kg; gait speed 0.8 m/s or 1.0 m/s) [56,57,58,59]. However, heterogeneity is often unavoidable in meta-analyses of observational studies and does not necessarily invalidate meta-analysis results [77]. Second, nutritional status and nutrient intake are closely related to the metabolism of muscle and fat; however, because the nutrition-related data reported in studies included in our review were mostly continuous data, a single rate meta-analysis was not possible. Therefore, the relationship between nutritional status and nutrient intake and the prevalence of SO needs further exploration
Conclusions
The higher prevalence of SO in non-hospitalized older adults, especially among those with dysfunction and cognitive impairment, is a potential problem for the aging population. Our findings provide valuable information to clinicians who plan community interventions, as they can address these risk factors and thus reduce the prevalence of SO. Owing to the diversity of SO diagnostic criteria and demarcation points, the comparability of data is limited. Therefore, our findings provide a useful basis for future researchers to work from as they build a consensus on the diagnosis of SO.
Data availability
All extracted data used in this review has been reported in the text, fgures, tables, and Additional file.
Abbreviations
- BMI:
-
body mass index
- 95% CI:
-
95% confidence interval
- PBF:
-
percentage of body fat
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SO:
-
sarcopenic obesity
- WC:
-
waist circumference
References
Park MJ, Choi KM. Interplay of skeletal muscle and adipose tissue: sarcopenic obesity. Metabolism. 2023;144:155577.
Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010;33(7):1652–4.
Kim TN, Yang SJ, Yoo HJ, et al. Prevalence of Sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond). 2009;33(8):885–92.
Baek SJ, Nam GE, Han KD, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008–2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Invest. 2014;37(3):247–60.
Gao Q, Mei F, Shang Y, et al. Global prevalence of sarcopenic obesity in older adults: a systematic review and meta-analysis. Clin Nutr. 2021;40(7):4633–41.
Daskalopoulou C, Wu YT, Pan W, et al. Factors related with Sarcopenia and sarcopenic obesity among low- and middle-income settings: the 10/66 DRG study. Sci Rep. 2020;10(1):20453.
de Campos GC, Lourenço RA, Lopes CS. Prevalence of Sarcopenic Obesity and its Association with functionality, lifestyle, biomarkers and morbidities in older adults: the FIBRA-RJ Study of Frailty in older Brazilian adults. Clin (Sao Paulo). 2020;75:e1814.
Lim HS, Park YH, Suh K, et al. Association between Sarcopenia, Sarcopenic Obesity, and Chronic Disease in Korean Elderly. J Bone Metab. 2018;25(3):187–93.
Dondero KR, Falvey JR, Beamer BA, et al. Geriatric vulnerabilities among obese older adults with and without Sarcopenia: findings from a nationally Representative Cohort Study. J Geriatr Phys Ther. 2022;46(3):168–73.
Hwang J, Park S. Gender-specific prevalence and risk factors of sarcopenic obesity in the Korean Elderly Population: a nationwide cross-sectional study. Int J Environ Res Public Health. 2023;20(2):1140.
Batsis JA, Barre LK, Mackenzie TA, et al. Variation in the prevalence of Sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61(6):974–80.
Donini LM, Busetto L, Bauer JM, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–88.
Du Y, Wang X, Xie H, et al. Sex differences in the prevalence and adverse outcomes of Sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr Disord. 2019;25(1):109.
Campos GC, Lourenço RA, Molina MDCB. Mortality, sarcopenic obesity, and Sarcopenia: Frailty in Brazilian older people Study - FIBRA - RJ. Rev Saude Publica. 2021;55:75.
Tyrovolas S, Koyanagi A, Olaya B, et al. Factors associated with skeletal muscle mass, Sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7(3):312–21.
Son J, Yu Q, Seo JS. Sarcopenic obesity can be negatively associated with active physical activity and adequate intake of some nutrients in Korean elderly: findings from the Korea National Health and Nutrition Examination Survey (2008–2011). Nutr Res Pract. 2019;13(1):47–57.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53.
Batsis JA, Haudenschild C, Roth RM, et al. Incident impaired cognitive function in sarcopenic obesity: data from the National Health and Aging trends Survey. J Am Med Dir Assoc. 2021;22(4):865–e8725.
Scott D, Blyth F, Naganathan V, et al. Sarcopenia prevalence and functional outcomes in older men with obesity: comparing the use of the EWGSOP2 Sarcopenia versus ESPEN-EASO sarcopenic obesity consensus definitions. Clin Nutr. 2023;42(9):1610–8.
Kim JE, Choi J, Kim M, et al. Assessment of existing anthropometric indices for screening sarcopenic obesity in older adults. Br J Nutr. 2023;129(5):875–87.
Ulugerger Avci G, Bektan Kanat B, Can G, et al. The impact of Sarcopenia and obesity on mortality of older adults: five years results. Ir J Med Sci. 2023;192(5):2209–16.
Scott D, Cumming R, Naganathan V, et al. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: the Concord Health and Ageing in Men Project. Exp Gerontol. 2018;108:99–105.
Kemmler W, Teschler M, Weißenfels A, et al. Prevalence of Sarcopenia and sarcopenic obesity in older German men using recognized definitions: high accordance but low overlap! Osteoporos Int. 2017;28(6):1881–91.
Morikawa M, Lee S, Makino K, et al. Sarcopenic obesity and risk of disability in Community-Dwelling Japanese older adults: a 5-Year longitudinal study. J Am Med Dir Assoc. 2023;24(8):1179–e11841.
Peng TC, Chen WL, Chen YY, et al. Associations between different measurements of sarcopenic obesity and health outcomes among non-frail community-dwelling older adults in Taiwan. Br J Nutr. 2021;126(11):1749–57.
Rossi AP, Urbani S, Fantin F, et al. Worsening disability and hospitalization risk in Sarcopenic obese and dynapenic abdominal obese: a 5.5 years Follow-Up study in Elderly men and women. Front Endocrinol (Lausanne). 2020;11:314.
Someya Y, Tamura Y, Kaga H, et al. Sarcopenic obesity is associated with cognitive impairment in community-dwelling older adults: the Bunkyo Health Study. Clin Nutr. 2022;41(5):1046–51.
von Berens Å, Obling SR, Nydahl M, et al. Sarcopenic obesity and associations with mortality in older women and men - a prospective observational study. BMC Geriatr. 2020;20(1):199.
Mendham AE, Goedecke JH, Micklesfield LK, et al. Understanding factors associated with sarcopenic obesity in older African women from a low-income setting: a cross-sectional analysis. BMC Geriatr. 2021;21(1):247.
Moreno-Franco B, Pérez-Tasigchana RF, Lopez-Garcia E, et al. Socioeconomic determinants of sarcopenic obesity and frail obesity in community-dwelling older adults: the Seniors-ENRICA Study. Sci Rep. 2018;8(1):10760.
Nascimento DDC, Oliveira SDC, Vieira DCL, et al. The impact of sarcopenic obesity on inflammation, lean body mass, and muscle strength in elderly women. Int J Gen Med. 2018;22:11:443–9.
Aibar-Almazán A, Martínez-Amat A, Cruz-Díaz D, et al. Sarcopenia and sarcopenic obesity in Spanish community-dwelling middle-aged and older women: Association with balance confidence, fear of falling and fall risk. Maturitas. 2018;107:26–32.
Diago-Galmés A, Guillamón-Escudero C, Tenías-Burillo JM, et al. Salivary testosterone and cortisol as biomarkers for the diagnosis of Sarcopenia and sarcopenic obesity in Community-Dwelling older adults. Biology (Basel). 2021;10(2):93.
dos Santos EP, Gadelha AB, Safons MP, et al. Sarcopenia and sarcopenic obesity classifications and cardiometabolic risks in older women. Arch Gerontol Geriatr. 2014;59(1):56–61.
Dutra MT, Gadelha AB, Nóbrega OT, et al. Body Adiposity Index, but not visceral Adiposity Index, correlates with inflammatory markers in Sarcopenic obese Elderly women. Exp Aging Res. 2017;43(3):291–304.
Fonfría-Vivas R, Pérez-Ros P, Barrachina-Igual J, et al. Assessing quality of life with SarQol is useful in screening for Sarcopenia and sarcopenic obesity in older women. Aging Clin Exp Res. 2023;35(10):2069–79.
Halil M, Ulger Z, Varlı M, et al. Sarcopenia assessment project in the nursing homes in Turkey. Eur J Clin Nutr. 2014;68(6):690–4.
Ida S, Kaneko R, Imataka K, et al. Incidence of sarcopenic obesity in older patients with diabetes and association between sarcopenic obesity and higher-level functional capacity: evaluation based on a consensus statement. Endocr J. 2023;70(6):591–9.
Ishii S, Chang C, Tanaka T, et al. The Association between SarcopenicObesity and depressive symptoms in older Japanese adults. PLoS ONE. 2016;11(9):e0162898.
Kera T, Kawai H, Hirano H, et al. Differences in body composition and physical function related to pure Sarcopenia and sarcopenic obesity: a study of community-dwelling older adults in Japan. Geriatr Gerontol Int. 2017;17(12):2602–9.
Kim J, Baek Y, Jeong K, et al. Association of Dietary factors with grip Strength, Body Fat, and prevalence of sarcopenic obesity in rural Korean Elderly with Cardiometabolic Multimorbidity. Front Nutr. 2022;9:910481.
Lee JH, Park HM, Lee YJ. Using Dietary Macronutrient patterns to predict sarcopenic obesity in older adults: a Representative Korean Nationwide Population-based study. Nutrients. 2021;13(11):4031.
Lu L, He X, Song Y, et al. Prevalence and risk factors of Sarcopenia without obesity and sarcopenic obesity among Chinese community older people in suburban area of Shanghai: a cross-sectional study. Front Aging Neurosci. 2022;14:1034542.
Park JE, Lee S, Kim K. The effect of combining nutrient intake and physical activity levels on central obesity, Sarcopenia, and sarcopenic obesity: a population-based cross-sectional study in South Korea. BMC Geriatr. 2023;23(1):119.
Pedrero-Chamizo R, Gómez-Cabello A, Meléndez A, et al. Higher levels of physical fitness are associated with a reduced risk of suffering sarcopenic obesity and better perceived health among the elderly: the EXERNET multi-center study. J Nutr Health Aging. 2015;19(2):211–7.
Schluessel S, Huemer MT, Peters A, et al. Sarcopenic obesity using the ESPEN and EASO consensus statement criteria of 2022 - results from the German KORA-Age study. Obes Res Clin Pract. 2023;17(4):349–52.
Teixeira LAC, Avelar NCP, Parentoni AN, et al. Inflammatory biomarkers in older women with obesity, Sarcopenia, and sarcopenic obesity. J Am Med Dir Assoc. 2023;24(10):1562–4.
Yang CW, Li CI, Li TC, et al. Association of Sarcopenic Obesity with higher serum high-sensitivity C-Reactive protein levels in Chinese older Males–A Community-based study (Taichung Community Health Study-Elderly, TCHS-E). PLoS ONE. 2015;10(7):e0132908.
Yang M, Hu M, Zhang Y, et al. Sarcopenic obesity is associated with frailty among community-dwelling older adults: findings from the WCHAT study. BMC Geriatr. 2022;22(1):863.
Mo YH, Yang C, Su YD, et al. Prevalence and diagnostic agreement of sarcopenic obesity with different definitions among Chinese community-dwelling older adults. Age Ageing. 2022;51(1):afab272.
Lee DY, Shin S. Sarcopenic obesity is associated with coffee intake in elderly koreans. Front Public Health. 2023;11:990029.
Altinkaynak M, Ozturk CK, Goksoy Y, et al. The prevalence of sarcopenic obesity and its relationship with type 2 diabetes in a nursing home. Eur Rev Med Pharmacol Sci. 2023;27(7):2858–64.
Jang W, Kim H. Association of socioeconomic factors and dietary intake with sarcopenic obesity in the Korean older population. Asia Pac J Clin Nutr. 2023;32(3):348–55.
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metab Clin Exp. 2019;92:6e10.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older people. Age Ageing. 2010;39(4):412–23.
Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–e3072.
Studenski SA, Peters KW, Alley DE et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547– 58.
Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on Sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56.
Xu W, Zhang H, Paillard-Borg S, et al. Prevalence of overweight and obesity among Chinese adults: role of Adiposity indicators and Age. Obes Facts. 2016;9(1):17–28.
Molino S, Dossena M, Buonocore D, et al. Sarcopenic obesity: an Appraisal of the current status of knowledge and management in Elderly people. J Nutr Health Aging. 2016;20(7):780–8.
Bouchard DR, Choquette S, Dionne IJ, et al. Is fat mass distribution related to impaired mobility in older men and women? Nutrition as a determinant of successful aging: the Quebec longitudinal study. Exp Aging Res. 2011;37(3):346–57.
Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–12.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37.
Liu X, Hao Q, Yue J, et al. Sarcopenia, obesity and Sarcopenia obesity in comparison: Prevalence, Metabolic Profile, and key differences: results from WCHAT Study. J Nutr Health Aging. 2020;24(4):429–37.
Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18(5):388–95.
Shimokata H, Ando F, Yuki A, et al. Age-related changes in skeletal muscle mass among community-dwelling Japanese: a 12-year longitudinal study. Geriatr Gerontol Int. 2014;14(Suppl 1):85–92.
Tiidus PM. Benefits of estrogen replacement for skeletal muscle mass and function in post-menopausal females: evidence from human and animal studies. Eurasian J Med. 2011;43(2):109–14.
Trouwborst I, Verreijen A, Memelink R, et al. Exercise and Nutrition Strategies to counteract sarcopenic obesity. Nutrients. 2018;10(5):605.
Okazaki K, Yazawa D, Goto M, et al. Effects of macronutrient intake on thigh muscle mass during home-based walking training in middle-aged and older women. Scand J Med Sci Sports. 2013;23(5):e286–92.
Chomentowski P, Dubé JJ, Amati F, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol Biol Sci Med Sci. 2009;64(5):575–80.
Kitamura A, Seino S, Abe T, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;12(1):30–8.
Jeong SM, Choi S, Kim K, et al. Association among handgrip strength, body mass index and decline in cognitive function among the elderly women. BMC Geriatr. 2018;18(1):225.
Gustafson D, Lissner L, Bengtsson C, et al. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876–81.
Gómez-Apo E, Mondragón-Maya A, Ferrari-Díaz M, et al. Structural brain changes Associated with overweight and obesity. J Obes. 2021;2021:6613385.
Ho AJ, Raji CA, Becker JT, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010;31(8):1326–39.
Noubiap JJ, Balti EV, Bigna JJ, et al. Dyslipidaemia in Africa-comment on a recent systematic review - authors’ reply. Lancet Glob Health. 2019;7(3):e308–9.
Acknowledgements
Not applicable.
Funding
The work was supported by the Capital clinical diagnosis and treatment technology research and transformation application project [ grant number Z201100005520006].
Author information
Authors and Affiliations
Contributions
All authors have contributed sufficiently to the manuscript to be included as authors. L: Conceptualization. W: Conceptualization, Investigation, Writing - original draft. T: Data curation. X: Data curation. Z: Data curation. H: Data curation. L: Writing - original draft Investigation. X: Data curation. Binru Han: Conceptualization, Writing - original draft. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, Y., Wang, Y., Tang, S. et al. Prevalence of sarcopenic obesity in the older non-hospitalized population: a systematic review and meta-analysis. BMC Geriatr 24, 357 (2024). https://doi.org/10.1186/s12877-024-04952-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-04952-z